Summary
One of the most fundamental problems in immunology is the seemingly schizophrenic ability of the immune system to launch robust immunity against pathogens, while acquiring and maintaining a state of tolerance to the body’s own tissues and the trillions of commensal microorganisms and food antigens that confront it every day. A fundamental role for the innate immune system, particularly dendritic cells (DCs), in orchestrating immunological tolerance has been appreciated, but emerging studies have highlighted the nature of the innate receptors and the signaling pathways that program DCs to a tolerogenic state. Furthermore, several studies have emphasized the major role played by cellular interactions, and the microenvironment in programming tolerogenic DCs. Here we review these studies and suggest that the innate control of tolerogenic responses can be viewed as different hierarchies of organization, in which DCs, their innate receptors and signaling networks, and their interactions with other cells and local microenvironments represent different levels of the hierarchy.
Keywords: dendritic cells, innate immunity, tolerance
Introduction
An extraordinary feature of the mammalian immune system is its capacity to generate a repertoire of T and B-cell receptors that can recognize virtually any antigen in the universe. Stochastically, some of these receptors recognize antigens within our own bodies, so called ‘self antigens’, so nature has evolved several mechanisms to regulate the unbridled activation of these autoreactive clones and to prevent Ehrlich’s ‘horror autotoxicus’ (1–3). This delicate balance of respondingto foreign antigens while remaining tolerant to self-antigens is critical, because its breakdown can lead to autoimmunity which results from a failure of tolerogenic mechanisms and excessive inflammatory responses, or to chronic infections and tumors, caused by insufficient immunity and excessive tolerance. Research over the last decade has revealed a fundamental role for innate immune system in initiating protective responses against different pathogens as well as in tuning the quality of such responses. Emerging studies are now beginning to reveal a key role for the innate immune system in regulating immunity against self components such as tissue antigens, food antigens, and commensals (4–7). Although there has been much progress in understanding the role of innate immunity in inducing protective responses against pathogens, very little is known about its role in promoting ‘tolerogenic’ responses and suppressing autoimmune responses.
Immunological tolerance is often considered to occur by two processes, namely central tolerance and peripheral tolerance (2, 3, 8). Central tolerance operates mainly in thymus and bone marrow, where most of the self-reactive T cells and B cells are deleted at an immature stage of their development in either thymus (T cells) or the bone marrow (B cells). However, potentially harmful self-reactive B and T cells often slip through the bulwark of central tolerance and can be found in the peripheral tissues of normal individuals (2, 3, 8). Peripheral tolerance serves as a backup mechanism to promote systemic tolerance to such autoreactive immune cells. In addition, peripheral tolerance is also critical in suppressing immune responses to innocuous external antigens or non-pathogenic organisms in the lung and digestive tract as well as at the immune privileged areas such as the brain, the anterior chamber of the eye, the testis, and the fetus. Breakdown of central or peripheral tolerance leads to autoimmune diseases such as type 1 diabetes (T1D), multiple sclerosis, inflammatory bowel disease (IBD), and rheumatoid arthritis. Emerging studies are beginning to provide insights into the mechanisms by which the innate immune system regulates the process of immunological tolerance. The innate immune system consists of a diverse array of cells, including dendritic cells (DCs), macrophages, natural killer cells, mast cells, basophils, neutrophils, eosinophils, and γδ T cells (9). DCs, the most efficient antigen-presenting cells in the immune system, have emerged as key players in initiating and regulating adaptive immune responses (4–7). Furthermore, emerging evidence also suggest that DCs are also critical in suppressing immune responses, and maintaining peripheral tolerance, through the generation of anergic and/or regulatory T cells and fine-tuning the response by altering the T-helper 1 (Th1)/Th2/Th17 balance. In addition to their well-established role in adaptive immunity, Th1 and Th17 cells play an important role in the pathogenesis of several autoimmune diseases. Here, we review our current knowledge of the role of dendritic cells in regulating tolerogenic responses in various tissue environments and highlight some unanswered questions.
Mechanisms of tolerance induction by DCs
Central tolerance
T-cell tolerance to self-antigens is established in the thymus, where thymocytes undergo negative selection to eliminate autoreactive clones. DCs play a vital role in mediating negative selection of T cells in the thymus. Brocker et al. (10)demonstrated that targeted expression of major histocompatibility complex (MHC) class II molecules on thymic DCs, (which are localized mainly in the thymic medulla), but not on cortical or medullary epithelial cells, B cells or macrophages, was sufficient to negatively select I-E reactive CD4+ T cells, and to a less complete extent, CD8+ T cells. Furthermore, McCaughtry etal. (11) demonstrated that thymocytes undergoing clonal deletion were preferentially associated with rare CD11c+ cortical DCs, and elimination of such DCs impaired deletion of T cells. In addition, a role for thymic DCs in the induction of T-regulatory cells has been demonstrated, both in mice and in humans. Bonasio etal. (12)demonstrated that antigen loaded exogenous DCs injected intomice, were recruited to the thymus, and resulted in the deletion of antigen-specific CD4+ T cells in the thymic medulla. Consistent with this, Proietto et al.(13)demonstrated that CD8loSirpa+ DCs in the thymus, or their immediate precursors in the blood which can migrate to the thymus, efficiently induce generation of T-regulatory cells and negative selection. Importantly, in humans it was recently shown that Hassall’s corpuscles express thymic stromal lymphopoeitin (TSLP) and instruct thymic CD11c+ DCs to induce Foxp3+ T-regulatory cells(14). Taken together, these findings establish a key role for DCs in regulating emergence of self-reactive T cells in the thymus.
Peripheral tolerance
Despite the effectiveness of negative selection in the thymus, significant numbersof self-reactive T cells bearing low-affinity TCR to tissue-antigens still escape to the periphery and cause autoimmune diseases when immune regulation goes awry. In addition to its well-established role in central tolerance, DCs play a major role in the induction of peripheral tolerance and maintenance of immune homeostasis. DCs promote peripheral tolerance by several mechanisms such as generation of regulatory T cells and induction of T-cell anergy/deletion.
What roles DCs play in maintain immunological tolerance in the periphery? A recent study demonstrates that constitutive ablation of DCs in mice, breaks self-tolerance of CD4+ T cells and results in spontaneous fatal autoimmunity, demonstrating an essential role for DCs in maintaining immunological tolerance (15). In contrast however, an independent study using a different mouse model of constitutive DC ablation did not reveal spontaneous autoimmunity (16). However, in the latter study, plasmacytoid DCs (pDCs) and Langerhans cells were not depleted (16), which could in part account for the discrepancies between the two studies.
The tolerogenic properties of DCs can depend on their maturation state, exposure to anti-inflammatory and immunosuppressive agents, the nature of the microbial stimuli, and environmental cues from the tissue microenvironment (6, 7, 17, 18). The maturation state of DCs is a critical determinant of their tolerogenic capacity.
Maturation stage of DCs
It has been postulated that immature DCs promote tolerogenic responses, whereas mature DCs promote immunogenic responses (4, 5). Under homeostatic conditions, peripheral DCs typically display an ‘immature’ phenotype characterized by expression of low surface levels of MHC II and costimulatory molecules and induce suboptimal T-cell priming, often leading to T-cell anergy or tolerance. Immature DCs promote tolerance in vivo by either deleting antigen-specific T cells or by expanding regulatory T cells (19–22). In the eye, an immature DC subset that expresses low levels of MHC II but lacks the expression of costimulatory molecules is critical in promoting tolerance or anergy (23, 24). It is generally believed that maturation stimuli promote immunogenic DCs. Upon stimulation, DCs undergo maturation characterized by expression of high levels of MHC II and costimulatory molecules and induce robust T cell activation and effector differentiation. However, certain stimuli can promote DCs activation and maturation and yet induce tolerogenic T cells. For example, disruption of E-cadherin-mediated DC-DC interaction promotes DC maturation including upregulation of costimulatory molecules, MHC class II and chemokine receptors but the DCs fail to secrete pro-inflammatory cytokines (25). Such DCs secrete high levels of IL-10 and induce tolerogenic response (25). In addition, a broad range of microbial stimuli can program DCs to acquire tolerogenic properties (6), and these are discussed in detail in the following section.
DC subsets
DCs can be classified into distinct subsets, based on their phenotype, microenvironmental localizations and functions (7, 17, 18). A detailed discussion of DC subsets and their influence on adaptive immunity, is outside the scope of the present review, and the reader is referred elsewhere(18). In the present section, we will summarize what is known about the role of particular DC subsets in inducing T cell tolerance. Under so called “steady-state conditions,” (i.e. in the absence of any detectable infection or overt inflammation), specific subsets of DCs in the periphery or in the lymphoid tissues seem to be efficient at inducing T-cell tolerance (Table 1). For example, pDCs, which in their steady state are immature since they express low levels of MHC class II and costimulatory molecules (26), have been shown to potently induce T-regulatory cells (27), mediate allo-antigen-specific T-cell tolerance (28), and mediate oral tolerance (29). In addition, a subset of human antigen-presenting cells that express indoleamine-2,3-dioxygenase (IDO) and inhibit T-cell proliferation in vitro was described (30, 31). IDO-positive APCs constituted a discrete subset identified by co-expression of the cell-surface markers CD123 and CCR6. These cells included mature and immature CD123+ DCs (30, 31). IDO+ DCs could also be readily detected in vivo, which suggests that these cells may represent a regulatory subset of antigen-presenting cells, including DCs, in humans.
Furthermore, in mice the splenic CD11c+DEC205+CD11b-CD8α+ DCs in the resting state have been shown to induce sub-optimal T-cell priming in vitro, relative to the CD11c+ DEC205− CD11b+CD8α− DCs (32–34). In vivo, the CD11c+DEC205+CD11b−CD8α+ DCs have been shown to be responsible for inducing peripheral T-cell tolerance to tissue-associated antigens (35). Consistent with these observations, targeting antigens in vivo to CD8α+ DCs induced Foxp3+ T-regulatory cells more efficiently than targeting to CD8α− DCs (36). In contrast to these studies, adoptive transfer of ex vivo isolated antigen pulsed splenic CD8α+ DCs into mice induces potent Th1 responses (37, 38), and targeting antigens to CD8α+ DCs in the presence of an adjuvant also induces robust Th1 immunity (39). These observations suggest that in the resting steady state certain DC subsets have a propensity to induce tolerogenic T cells but that activation resulting from the isolation process per se or caused by microbial stimuli can reprogram DCs to an immunogenic state.
Environment
At mucosal surfaces, the immune system has a particularly challenging task of maintaining tolerance to self-antigens and commensals, while launching robust immunity to pathogens. Tolerogenic antigen-presenting cells in the mucosal compartment prevent excessive inflammation and immunity against commensals and food or environmental antigens. For example, in the intestine, CD11chigh DCs that express CD103(40–42) have been shown to efficiently induce Foxp3+ T-regulatory cells in vitro, via a mechanism dependent on retinoic acid (40, 42). However, the tolerogenic properties of these cells appear to be rather ‘plastic’, as CD103+ DCs taken from colitic mice are impaired in their ability to induce Foxp3+ T-regulatory and instead favor the emergence of IFN-γ-producing CD4+ T cells compared with their steady-state counterparts (43). Consistent with this plasticity, our recent work demonstrates that the ability of intestinal DCs to induce T-regulatory cells is dependent on the ratio of DCs: T cells, with there being much more efficient T-regulatory cell induction, at the lower DC: T-cell ratios (Denning et al., manuscript submitted). Moreover, we have recently identified at least 2 different CD11chigh intestinal DC subsets that express CD103: the CD11chighCD11b−CD103+ DCs and CD11chighCD11b+CD103+ DCs. Interestingly, in the ‘Th17 prone’ conventionally raised C57BL/6 strain of mice from Charles River but not in the same strain of mice from Jackson Laboratories [in which the frequencies of Th17 cells are much lower (44)], the CD11chighCD11b+CD103+ DCs induced greater Th17 responses than the CD11chighCD11b+CD103− DCs. In addition to DCs, CD11cdull CD11b+ lamina propria macrophages are hyporesponsive to various Toll-like receptor (TLR) ligands and have the ability to spontaneously secrete IL-10 and efficiently suppress inflammatory DC function (45). IL-10 production by these lamina propria macrophages is responsible for maintaining Foxp3 expression in T-regulatory cells during intestinal inflammation (46).
Emerging studies show that like intestinal DCs, lung DCs also play important regulatory roles in response to inhaled inert antigens. Similar to the intestine, lung CD103+ DCs also have the ability to promote the induction of T-regulatory cells; in comparison lung CD103− DCs represent the major producers of proinflammatory cytokines in response to airborne allergens or TLR ligands (47–49). However, in the skin, CD103−CD11b+ migratory dermal DCs are much more potent in inducing Foxp3+ T-regulatory cells as compared to CD103+CD11b+ DCs (50).
Like the intestine and the lungs, the skin is continuously exposed to pathogens. Skin-derived DCs include Langerhans cells, classical dermal DCs, and a langerin+CD103+ dermal subset (51). The relative importance of these DCs in inducing immunity versus tolerance is not clear, but in a transgenic mouse model in which keratinocytes were forced to express membrane bound ovalbumin, presentation of this surrogate self-antigen was confined to skin-derived migrating DCs (52). Steady state presentation resulted in deletional T-cell tolerance despite these DCs expressing a relatively mature phenotype as measured by traditional markers such as the level of MHC class II and CD86 expression (52). Thus, self-antigen can be carried to the draining lymph nodes by skin-derived DCs and there presented by these same cells for tolerization of the circulating T-cell pool. The relative roles of Langerhans cells versus the DC subsets in the dermis in this process needs to be more precisely defined, but ablation of Langerhans cells has been shown to lead to exacerbation of contact sensitization in some (53) but not other (54, 55) transgenic mouse systems, and individuals with psoriasis have been found to have defects in Langerhans cell migration (56), suggesting that these cells may under some circumstances play a regulatory role dampening immune responses. However, skin-derived DCs have also been linked to certain types of antiviral immunity (57), and dermal DCs are known to present antigens during Th2 responses to cysteine proteases (58). Thus, although skin-derived DCs may induce tolerogenic responses in the steady state, they exhibit functional plasticity, in response to inflammatory stimuli.
In the liver, under steady-state conditions, myeloid DCs, and plasmacytoid DCs induce tolerogenic T-cell responses (59). Liver myeloid DCs have a mature phenotype but produce high levels of various anti-inflammatory factors such as prostaglandin E2 (60), IL-10, retinoic acid, tumor growth factor-β, IL- 27(60–64). In contrast, liver pDCs have an immature phenotype with low expression of MHC and costimulatory molecules but produces high levels of IL-10(29, 65, 66).
In the immunologically privileged sites, like the eye, specific subsets of immature DCs that expresses low levels of MHC II costimulatory molecules induce tolerogenic responses (23). Furthermore, mature DCs, macrophages, and F4/80+ microglia in the retina promote tolerance to self-antigens (67, 68). In the central nervous system (CNS), unlike in the eye, DCs are fewer and are mostly localized in the vascular rich areas. The DCs and microglia that are present in the CNS are mostly tolerogenic (69). Thus, distinct DCs in various tissues induce tolerogenic responses. In addition to specific subsets of tissue resident DCs, under steady state DCs constantly migrate to the regional lymph node carrying tissue antigens or apoptotic cells (70–72). These migrating DCs promote tolerance to self-antigens in various tissues.
Exposure to anti-inflammatory mediators
Several reports demonstrate that exposure to anti-inflammatory cytokines and immunosuppressive agents can condition DCs to a tolerogenic state (7, 17, 73). For example DCs generated in vitro, in the presence anti-inflammatory factors such as vitamin A, vitamin D3, prostaglandin E2, IDO, IL10, TGF-β, retinoids, hepatocyte growth factor, and vasoactive intestinal peptide exhibit tolerogenic functions (49, 59, 74–80). These tolerogenic DCs induce T-cell tolerance and can suppress disease in experimental models of autoimmunity such as type 1 diabetes and multiple sclerosis. Furthermore, genetic manipulation of DCs by overexpressing immune regulatory molecules or inhibiting or silencing immune stimulatory molecules promotes tolerogenic function (81). For example, silencing costimulatory molecules such CD40, CD80, CD86 or overexpression of IL-10 or IL-4 or CTLA-4 in DCs renders them tolerogenic, and these DCs can promote graft survival or suppress autoimmune diseases (82–90). Activation of DCs by tumor-derived factors modulates their function to promote induction of T-regulatory cells (91).
DC receptors that mediate tolerogenic responses
The innate immune system relies on a diverse array of receptors that can sense components of pathogens (92, 93). These pattern recognition receptors include TLRs, C-type lectin receptors (CLRs), RIG-I like receptors (RLRs), and NOD-like receptors (NLRs), which recognize a wide range of microbial structures and activate DCs (93–95). It was originally postulated that the TLRs represent a family of receptors that recognize pathogen-associated molecular patterns (PAMPs) and promote the activation of the adaptive immune system (96). However, it has become clear that in addition to recognizing a wide range of microbial products, innate receptors also recognize so called endogenous ligands from host cellular debris from injured or dying tissue, termed DAMPs (damage-associated molecular patterns)(97–100) (Fig. 1). Thus, TLRs can sense wide range of self-antigens that are released during tissue damage, inflammation, or necrosis leading to inappropriate activation resulting in sterile inflammation or autoimmune responses (97–99).
Fig. 1. Environmental stimuli and their receptors that program tolerogenic DCs.
A broad range of stimuli including microbial components, tissue antigens (mannosylated antigens, glycoconjugate), and apoptotic cells interact with specific receptors on DCsand program DCs to a tolerogenic state. Various anti-inflammatory cytokines and immunosuppressive agents also programDCs to acquire tolerogenic properties and promotethe induction of IL-10, IDO, and TGF-β that are critical for promoting T-regulatory response, orinducethe expression cell surface molecule such as ILT3/4, PDL1/2, ICOS-L, B7.H, CD95L that promotes T-cell anergy or deletion.
TLRs
In addition to promoting immunity against pathogens, TLRs have been implicated in inducing tolerogenic responses. Some TLRs (TLR-1, TLR-2, TLR-4, TLR-5, TLR-6, TLR-10) are expressed on the cell surface (while some are located within endosomal compartments (TLR-3, TLR-7, TLR-8, TLR-9) on different cell types (101). Activation of most TLRs promotes Th1 responses(93). In contrast, certain microbial products that activate TLR2 in DCs promote Th2 or tolerogenic responses (102–106) (Fig. 1). For example, yeast zymosan (104, 107–109) or Y. pestis virulence factor Lcr (110) that signals through TLR2-TLR6 in DCs induce regulatory T cells. Similarly, phosphatidylserine and lysophosphatidylserines from S. mansoni (111) and filamentous hemagglutinin (FHA) from the bacteria Bordetella pertussis (112) can program DCs to induce regulatory T cells (Fig. 1).
In general, TLR-9-activation of DCs promotes Th1 responses (113). However, some reports suggest that in pDCs, TLR-9 activation induces IDO, which promotes differentiation of regulatory T cells and suppresses T-cell responses (114, 115). Like TLR-9, thymosin α1, an immunomodulatory peptide produced in small amounts by several peripheral lymphoid and non-lymphoid tissues, can induce IDO in pDCs through TLR-9(116). Consistent with this thymosin α1-conditioned DCs induced FoxP3+ T-regulatory cells through the induction of IDO, as IDO blockade abolished the induction of regulatory T cells (116, 117). Importantly, thymosin α1 also programmed DCs to prime Th1 cells, suggesting that the instructive signals imparted to DCs allow for a balanced inflammatory and tolerogenic responses. However, the mechanism(s) by which thymosin α1 induces IDO expression in DCs is currently unknown.
CLRs
CLRs belong to a large superfamily of transmembrane and soluble proteins that sense carbohydrate components of several pathogens as well as self-glycoproteins. Like TLRs, recent studies have shown that CLRs can activate various signaling pathways that can induce DCs to stimulate Th1, Th2, or Th17 cell responses (118). In addition, CLRs can recognize gylcosylated tissue antigens that is critical immune homeostasis (119). Similarly, in vivo targeting of antigen into DCs via the endocytic receptor DEC-205 (22, 120), which is expressed on DCs, resulted in induction of Foxp3-expressing T-regulatory cells (121). DC-SIGN is an adhesion receptor that interacts with intercellular adhesion molecule 2 (ICAM2) and ICAM3 and mediates DC migration and its interaction with other cell types. DC-SIGN was shown to recognize several pathogens such as bacteria, fungi, and viruses. Activation of DC-SIGN in DCs by cell surface compounds of Lactobacillus reuteri and L. casei promotes T-regulatory cell responses (122) (Fig. 1). Furthermore, ManLAM of Mycobacterium (123), Lewis antigens on LPS from Helicobacter pylori (124), and lpA of L. acidophilus NCFM (125) can bind to DC-SIGN to induce IL-10 production and suppress T-cell effector responses, although whether DCs exposed to such stimuli induce regulatory or anergic T cells remains to be determined. In contrast, several studies have shown that DC-SIGN activation promotes effector responses (126). The question of how signals mediated through the same receptor can promote tolerogenic response versus effector response to different pathogens is yet to be answered.
Surface C-type lectin, MGL1/CD301a specifically recognizes monosaccharides Gal/GalNAc, is highly expressed on immature DCs (127, 128) and mediates efficient uptake of antigens (128). MGL1 can recognize the intestinal commensal bacteria Streptococcus sp. and Lactobacillus sp and induce high levels of IL10 production in intestinal lamina propria macrophages (129), suggesting a regulatory role for this receptor in intestine. Consistent with this observation, MGL1-deficient lamina propria macrophages produced lower levels of IL-10 and MGL-1-deficient mice are more susceptible DSS-induced colitis (129). A similar regulatory role was observed with SIGNR-1/CD209b, a DC-SIGN homolog in mice that recognizes mannosylated antigens, in the intestine in response to food induced anaphylaxis (130). Activation of SIGNR-1 in lamina propria DCs selectively induced IL-10 expression and promoted the induction of Tr1 regulatory cells (130). However, the precise signaling pathways by which MGL1 and SIGNR-1 program DCs to a tolerogenic state is not known. Collectively, these observations show that MGL1 and SIGNR1 play critical role in intestinal homeostasis.
Recent data suggest that CLRs are highly expressed on immature DCs in peripheral tissues and might play an important role in the clearance of self-antigen and induction of immunological tolerance Immature DCs in the placenta expresses high levels of DC-SIGN and MR are believed to play critical role in tolerance (131). In contrast, CLRs like the dendritic cell immunoreceptor (DCIR) in DCs promotes immune tolerance and homeostasis by negatively regulating the DC expansion and function (132). DCIR deficiency in mice causes development of autoimmune diseases in mice due to excess expansion of DCs (104). DCIR knockout mice develop autoantibodies and are display enhanced susceptibility to collagen induced arthritis (132). Additionally, aged DCIR knockout mice showed increased levels of DCs and developed joint abnormalities. Consistent with this observation, bone marrow cells from DCIR knockout mice differentiated into DCs and proliferated more in response to granulocyte-macrophage colony-stimulating factor (GM-CSF) than bone marrow cells from wildtype mice (132). Furthermore, activation of DCIR in human pDCs inhibits TLR9-mediated IFN-α production (133, 134). Collectively, these studies show that in addition to antigen uptake, CLR expression on DCs plays critical role in immune tolerance and homeostasis.
Recognition and engulfment of apoptotic cells by DC and macrophages also promote tolerogenic responses to self-antigen. In addition to mediating tolerogenic signals, some CLRs such as Clec9A (also known as DNGR-1) and mincle specifically recognize necrotic cells and are critical for uptake of necrotic cells by DCs and macrophages, and promote cross-presentation of self-antigens and tissue homeostasis(135, 136) (Fig. 1). Injection of small amounts of antigen-coupled anti-DNGR-1 monoclonal antibody into mice promoted MHC class II antigen presentation selectively by CD8α+ DCs, and in the steady state, this was sufficient to drive the differentiation of naive CD4+ T cells into Foxp3+ regulatory cells (137). Co-administration of adjuvants prevented this induction of tolerance and promoted immunity.
Galectins (S-type lectins)
Like CLRs, galectins, glycan binding proteins, regulate both innate and adaptive immune responses (138). Mammals express as many as 15 different galectins that interact with specific cell surface glycoconjugates (139) and have diverse biological roles. In addition to their well-established role in regulating T cell and B cell functions, recent studies have shown galectins can regulate DC function. A recent study has shown that in DCs, activation of galectin-1 results in high levels of IL-27 and IL-10 production and promotes T cells tolerance by suppressing Th1/Th17 cell differentiation and inducing T-regulatory response (140) (Fig. 1). Consistent with this observation, galectin-1-deficient mice show increased number of Th1 and Th17 cells and are more susceptible to autoimmune diseases (140, 141). Furthermore, galectin-1 also plays a critical role in maternal-fetal tolerance (142). Galectin-1-mediated signals promote tolerance in DCs by inducing the expression of several regulatory molecules like STAT3, SOCS1, and HDAC11. Like cell surface galectins, secreted galectins (143) and endogenous galectins (143, 144) can also modulate immune responses by promoting tolerance. In contrast to galectin-1, interaction of galectin-9 with its ligand Tim-3 on activated T cells induces apoptosis of Th1 cells (145, 146). Furthermore, galectin-9 was shown to promote T-regulatory cell differentiation and suppresses Th17 cell differentiation (147). Consistent with this, galectin-9 deficient mice display enhanced susceptibility to autoimmune arthritis (147).
TAM receptors
The Tyro3, Axl, and MerTK (TAM) family of receptor tyrosine kinases (RTKs) has been reported to regulate homeostatic activation of macrophages and DCs. Recent studies have shown that Mer tyrosine kinase (MerTK) plays a critical role in sensing and phagocytosing apoptotic cells by DCs and macrophages (148, 149)(Fig. 1). MerTK specifically recognizes Gas6 and Protein S that are present on the apoptotic cell membranes. Furthermore, signaling through MerTK in DCs inhibits DC activation and maturation. Consistent with this observation, pretreatment of DCs from NOD mice with apoptotic cells blocked the secretion of pro-inflammatory cytokines and prevented the autoimmune T-cell activation, whereas NOD mice that are deficient in MerTK displayed exacerbated diabetes (150). In addition to its well established role in pheripheral tolerance, a recent study has shown that MerTK plays a crucial role in central tolerance (151).
Fc receptors
Fcγ receptors (FcγRs) play an essential role in regulating immune responses (152). Activating/inhibiting FcγRs are expressed on DCs and regulate DC maturation and immunogenicity. Activating FcγRIII (CD16) receptor, which contains a cytoplasmic ITAM domain, enhances DC maturation and promotes immunity. In contrast, recent studies have shown that activation of the inhibitory FcγRIIb (CD32) receptor on DCs regulates peripheral CD4+ and CD8+ T-cell tolerance (153, 154). Consistent with this observation, FcγRIIB activation on DCs promotes antigen-specific T-regulatory cell induction and peripheral tolerance by modulating the DC activation status and IL-10 production (153, 154). In autoimmune models of diabetes, mice lacking FcγRIIB receptors in DCs are more susceptible to disease. Furthermore, FcγRIIB receptor signaling promote peripheral tolerance activation by inhibiting DC activation alone or by also limiting processing of exogenously acquired antigen of FcγIIb receptor in DCs dampens TLR4-mediated proinflammatory responses through PI3K/AKT pathway(155) (Fig. 1).
ILT3 and ILT4 receptors
Immunoglobulin-like transcript 3 (ILT3) and ILT4 receptor-mediated signals in DCs inhibit the expression of costimulation molecules and program them to tolerogenic state. In humans, tolerogenic DCs, which express high levels of inhibitory receptors ILT3 and ILT4 (156), promote antigen-specific unresponsiveness in CD4+ Th cells (157) and promote the differentiation of CD4+ and CD8+ regulatory T cells (158–161).
Signaling networks that program DCs to a tolerogenic state
ERK-RALDH pathway
Despite the plethora of stimuli that stimulate DCs to induce tolerogenic responses, relatively little is known about the signaling mechanisms involved. We have shown signaling via TLR2 conditions DCs to express enhanced and sustained levels of phosphorylated extracellular signal regulated kinase (ERK) mutagen-associated protein kinase (MAPK), which suppresses IL-12p70 production and promotes IL-10 (102–104, 162, 163) (Fig. 2A). In particular, the yeast cell wall extract zymosan, which signals through the TLR2/6 heterodimer as well as the C-type lectin dectin-1 (164), stimulates DCs to produce robust levels of IL-10 and induce tolerogenic T-cell responses, via a TLR2-dependent mechanism (104). Furthermore, our more recent work also demonstrates that TLR2/6 signaling by zymosan also promotes induction of the retinoic acid metabolizing enzymes RALDH1 and RALDH2 in DCs, via an ERK-dependent mechanism (99) (Fig. 2A). Accumulating evidence show that retinoic acid directly influences the development and functions of various immune cell types. For example, retinoic acid promotes the differentiation of T-regulatory cells and suppresses the differentiation of Th1/Th17 cells (165). Recent studies have identified DCs as major immune cells capable of metabolizing vitamin A to retinoic acid and a critical player in vitamin A mediated immune functions. Retinoic acid synthesis is a tightly regulated process that includes several key enzymes involved in oxidation of vitamin A (retinol). Vitamin A is oxidized to retinaldehyde by alcohol dehydrogenases (ADH-1,-4,-5) and then to retinoic acid by retinal dehydrogenases (RALDH). All DC subsets express ADH in various tissues, whereas only specific subset of DCs and macrophages express RALDH and is generally believed to be the critical and rate limiting step in the biosynthesis of RA. CD103+ DCs in the intestine lamina propria and mesenteric lymph nodes express high levels of RALDH and have the ability to produce retinoic acid and can specifically induce Foxp3+ T-regulatory cells from naive CD4+ T cells (40, 41). Similarly, we have shown that lamina propria macrophages also express RALDH enzyme constitutively and can efficiently promote regulatory T-cell differentiation (45).
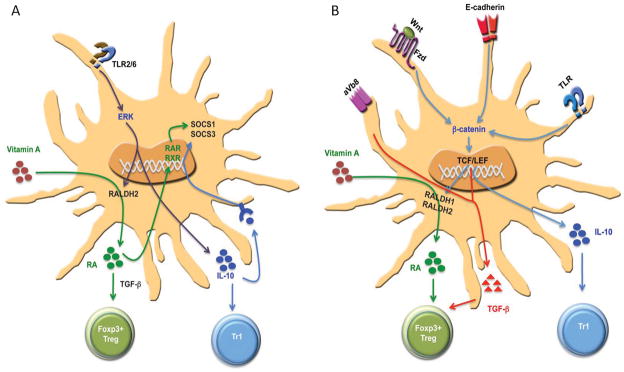
Fig. 2. Signaling networks that program DCs to induce T-regulatory responses.
(A) TLR-ERK-mediated induction of retinoic acid metabolizing enzymes and IL-10. Activation of DCs through TLR2/6 ligands leads to ERK activation, which mediates induction of Raldh2. Thisresults in the conversion of retinal to retinoic acid (RA), which then exerts an autocrine effect on DCsvia RAR or RXR to induce SOSC3. This thensuppresses activation of p38 MAPK and pro-inflammatory cytokines. Furthermore, IL-10 and RA programDCs to induce T-regulatory cell responses and limit theinflammatory responses. (B) β-catenin signaling pathways in programming regulatory DCs. Activation of β-catenin pathway by E-cadherine, TLRs, or wnt-ligands in DCs promotes induction of anti-inflammatory factors such vitamin A, IL-10, and TGF-β that are critical for promoting T-regulatory cell response and limiting inflammatory responses.
Consistent with this observation, splenic DCs from mice that are deficient in TLR2, TLR6, or ERK1 showed significantly reduced levels of RALDH in response to zymosan. Retinoic acid is also an autocrine factor that promotes RALDH expression in DCs via RAR/RXR-mediated signaling. DCs express several isoforms of retinoid receptors such as RAR-α, RAR-β, RAR-γ, and RXR-α. Thus, addition of exogenous RA to DCs increases RALDH expression, whereas blocking RAR/RXR signaling in DCs decreased the RALDH expression (166). Like other cell types (166–168), retinoic acid regulates its own synthesis through RAR-mediated signaling and RALDH expression in DCs (166). Collectively, these studies suggest microbial stimuli can induce the expression of RALDH enzymes in DCs via a TLR-ERK dependent signaling pathway and program them to a tolerogenic state.
In addition, a recent study has shown GM-CSF and IL-4 act synergistically in promoting RA-producing enzyme (RALDH2) in intestinal DCs. These conditioned DCs induced α4β7 gut-homing receptors and Foxp3 in T cells. Consistent with this observation, intestinal DCs isolated mice deficient in GM-CSF receptor showed reduced RALDH expression and ALDH activity. GM-CSF treatment was shown to prevent several autoimmune diseases in mice through the induction of tolerogenic DCs and regulatory T cells (169–171). However, it is not known whether GM-CSF mediated induction of tolerogenic DCs and regulatory T cells is dependent on retinoic acid.
Wnt-β-catenin signaling pathway
A second signaling pathway in intestinal DCs that induces retinoic acid-producing enzymes involve Wnt-β-catenin pathway. Wnt-β-catenin signaling pathway plays an important role in the development of various organs as well as hematopoietic stem cells (HSCs)(172). Further, wnt-β-catenin signaling pathway has been implicated in the differentiation of myeloid DCs and pDC differentiation from HSCs(172). Wnt ligands are secreted glycoprotein that binds their seven-pass transmembrane protein Frizzled (Fz) family receptors leading to accumulation of β-catenin in the cytoplasm and its translocation to the nucleus, where it interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF) family members and regulates transcription of several target gene s(173). In the absence of Wnt signaling, glycogen synthase kinase 3β (GSK-3β) phosphorylates β-catenin resulting in its ubiquitination by β-transducin repeat containing (β-TrCP) protein, leading to proteosomal degradation (172, 173). In addition, Wnt can activate other non-canonical signaling pathways that are independent of β-catenin (172, 173).
Our recent work shows that unlike in splenic DCs, β-catenin signaling is constitutively active in intestinal DCs and macrophages (174). DC-specific deletion of β-catenin in mice lead to strikingly reduced Foxp3+ regulatory T cells and enhanced frequencies of Th1 and Th17 cells, in the intestine but not in the spleen. Consistently, intestinal DCs deficient in β-catenin displayed reduced levels of RALDH enzymes and IL-10 production and promoted inflammatory T cells responses in the steady state (Fig. 2B). Furthermore, mice the specifically lack β-catenin in DCs are more susceptible to DSS-induced colitis (174). However, the role of Wnt-β-catenin pathway in the RALDH enzymes in skin and lung DCs is currently not known. Collectively, these data illustrate the emerging role of β-catenin signaling in programming DCs to promote intestinal homeostasis and tolerance. In the periphery, disruption of E-cadherin-E-cadherin interactions in DCs activates β-catenin signaling, which in turn programs DCs to tolerogenic state (25). Importantly, immunization of these tolerogenic DCs can protect mice against experiment autoimmune encephalomyelitis (25).
MerTK signaling
Recent studies have shown receptor tyrosine kinase Mer (MerTK)binding of apoptotic cells lead to the activation of PLC-γ2, PI3K-AKT, and STAT3(175), and inhibition of NF-κB activation. Consistent with this, monocyte-derived DCs expressing kinase deficient MerTK or blocking of PI3K-AKT pathway leads to activation of NF-κB and enhanced secretion of proinflammatory cytokines upon apoptotic cell binding. In addition, TAM receptor (MerTK, Tyro3, Axl) activation leads to the upregulation of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 through IFNR/STAT1 pathway, thereby suppressing the TLR-mediated signaling and secretion of pro-inflammatory cytokines.
ILT3 and ILT4 signaling
In humans, tolerogenic DCs express high levels of the inhibitory Ig-like receptors ILT3 and ILT4. Recent studies have shown various factors such as IDO, IL-10, Cox1/2 inhibitors, vitamin D3, IL-10 can induce the expression of ILT3 and ILT4 on DCs and promote tolerogenic responses(156, 176, 177). Activation of ILT3 receptors on DCs leads to the recruitment of protein phosphatase SHP-1(178) and SHIP-1(179) to the ITIM motif resulting in the inhibition of NFκB and MAPK p38 pathways that are critical for inflammatory responses (179). Furthermore, ILT3 and ILT4 mediated signal can antagonize TLR-mediated signals and suppress their ability to induce inflammatory cytokines(179). Consistent with this, knock-down of ILT3 in DCs results in enhanced production pro inflammatory cytokines in response to various TLR-ligands.
Cell-cell co-operation in orchestrating tolerogenic responses
DC-macrophage collaboration
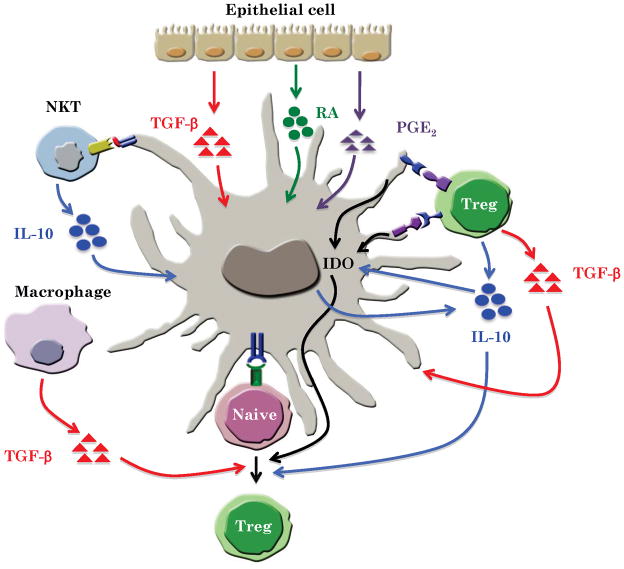
It is increasingly clear that the induction of tolerogenic responses requires the interaction of several cell types, including DCs (6, 18) (Fig. 3). Our earlier work demonstrates that injection of antigen plus the yeast cell wall zymosan into mice induces antigen-specific tolerance, via a mechanism dependent on the concerted action of DCs and macrophages (104). Zymosan induced robust IL-10 production in splenic DCs via a TLR2-dependent mechanism, and induced biologically active TGF-β in splenic macrophages, and the concerted action of IL-10 and TGF-β was necessary to induce tolerogenic responses (104).
Fig. 3.
Cell-cell cooperation in programming tolerogenic DCs.
In the intestine, IL-10 plays critical role in suppressing inflammation such as colitis. A subset of macrophage in the lamina propria constitutively produces IL-10 and induces Foxp3+ T-regulatory cells (45). Interestingly, IL-10 secretion by this macrophage subset was shown to be critical responsible for maintaining Foxp3 expression in T-regulatory cells during intestinal inflammation (46). Similar to the aforementioned concerted action of DCs and macrophages in mediating zymosan-induced tolerogenic responses (104), it is possible that tolerogenic responses in the intestine are maintained through the concerted action of IL-10-secreting macrophages and DCs (Fig. 3).
Reciprocal DC-T-regulatory cell interactions
Another emerging theme that highlights cell-cell cooperation is the reciprocal interactions between DCs and T-regulatory cells (Fig. 3). Although DCs are known to induce T-regulatory cells, more recent evidence points to T-regulatory cells in programming DCs (as well as macrophages) to a tolerogenic state. For example, in tissue culture, T-regulatory cells have been known to inhibit DC maturation and suppress the induction of pro-inflammatory cytokines (180–184). Furthermore, DCs and macrophages present at the maternal-fetal interface express high levels of IDO(185), and the induction of IDO is dependent on CD4+CD25+ T-regulatory cells through a mechanism involving the interaction of CTLA-4 on T-regulatory cells with B7 on DCs and macrophages (186, 187). This T-regulatory-DC interaction is critical for the induction of maternal–fetal tolerance (185). In addition, IL-10 produced by T-regulatory cells is also critical for sustaining IDO expression in DCs (30). In addition to B7/CTLA-4 interaction, a recent study has shown that CCL22/CCR4 interaction between DCs and T-regulatory cells is critical for induction of IDO in MLN DCs (188). Consistent with the effects of T-regulatory cells in programming DCs, acute in vivo ablation of T-regulatory cells resulted in enhanced DC numbers of activated DCs (189, 190). Importantly, recent work suggests the existence of a feedback regulatory loop between DCs and T-regulatory cells. Loss of DCs lead to a loss of T-regulatory cells, and the remaining T-regulatory cells exhibited decreased Foxp3 expression. The DC-dependent loss in T-regulatory cells leads to an increase in the number of T cells producing inflammatory cytokines, such as IFN-γ and IL-17. Conversely, increasing the number of DCs lead to increased T-regulatory cell division and accumulation by a mechanism that requires MHC class II expression on DCs(191).
DC-NK-T-cell cooperation
Invariant natural killer T cells (iNKT) play an important role in peripheral tolerance towards self -antigens and prevent - autoimmune diseases including multiple sclerosis, type 1 diabetes, rheumatoid arthritis, and systemic lupus erythematosus (192). A recent study has shown that iNKT-DC interactions through CD1d receptor induces mature-tolerogenic DCs that can promote T-regulatory and prevent autoimmune diseases (193). Induction of tolerogenic DCs is dependent on ERK signaling pathway. These DCs secrete high levels of IL-10 and very low levels of pro-inflammatory cytokines (193).
DC-non-hematopoietic cell cooperation
A typical example of cooperation between non-hematopoietic cells and DCs in the induction of T-regulatory cells is observed in intestine and liver. Recent studies using human and mouse intestinal epithelial cells (IECs) have shown that IECs play an important role in conditioning the intestinal DCs to tolerogenic state. Factors secreted by IECs can program DCs to T-regulatory-polarizing DCs. Anti-inflammatory mediators such as TGF-β and retinoic acid or GM-CSF secreted by IEC plays an important role in conditioning DCs to T-regulatory-polarizing DCs in the intestine. Consistent with this, addition of exogenous retinoic acid or GM-CSF + IL4 or supernatants from cultured IECs can program inflammatory bone marrow-derived DCs or monocyte-derived DCs to T-regulatory cell-promoting tolerogenic DCs (194–196). In addition, IEC supernatant can also promote development of CD103+ tolerogenic DCs from CD103− DCs and these EC-conditions DCs inhibited the development of Th1 and Th17 cells (195, 196). DC interactions with non hematopoietic cells also program thymic DCs to induce T-regulatory cells. As mentioned above, in humans it was recently shown that Hassall’s corpuscles express thymic stromal lymphopoeitin (TSLP) and instruct thymic CD11c+ DCs to induce Foxp3+ T-regulatory cells (14).
In addition to epithelial cells, stromal cells also play a critical in conditioning DCs to regulatory or tolerogenic state in various organs such as the liver, intestine, gut-associated lymphoid tissues (GALT), and spleen (197–201). For example, liver stromal and epithelial cells can drive the differentiation of tolerogenic DCs from monocyte or hematopoietic progenitor cells. Similarly, stromal cells in mesenteric lymph nodes express RALDH and might contribute to conditioning of DCs to tolerogenic state in an retinoic acid-dependent manner (200, 201). Like mesenteric lymph node stromal cells, lung stromal cells promote regulatory DCs via a TGF-β dependent mechanism (202). Finally, myeloid-derived suppressor cells are a heterogeneous mix of cells that expand during cancer, inflammation and infection and potently suppress T-cell responses. These cells may augment the immunosuppressive effects of tolerogenic DCs (203).
Inductive signals from the local microenvironment (Fig. 4)
Fig. 4. Tissue microenvironments condition DCs to induce T-regulatory responses.
In the liver, hepatocytes, stellate cells, and Kupffer cells produce various anti-inflammatory factors such IL-10, RA, TGF-β, and PGE2 that condition DCs to a tolerogenic state. Furthermore, these DCs produce IL-10 and TGF-β to induceT-regulatory cells or produce IL-27 and PGE2 to induce T-cell anergy or apoptosis.
The observations that DC subsets in specific microenvironments, such as those in the gut, lung, eye, and placenta, display tolerogenic propensities suggests that the local environment impart inductive signals to program tolerogenic DCs in situ. In the intestine, various factors produced by the epithelial cells and stromal cells such as TGF-β (204), TSLP (196, 205), retinoic acid (206, 207) shape the functions of tolerogenic DCs.
In addition to various cell types, intestinal commensal also play critical role in shaping the DCs function and promoting tolerance (208–210). Thus, commensal bacteria promote the expression the TSLP and TGF-β in the intestinal epithelial cells (211). Interestingly however, some commensals and their components seem to promote T-regulatory cells, while others seem to suppress T-regulatory cells and promote Th17 responses. For example, DCs cultured in the presence intestinal epithelial cells and Gram-positive commensal bacteria differentiate into IL-10-producing tolerogenic DCs (211). Similarly, colonization of the human commensal Bacteroides fragilis in germ-free mice induces the development of Foxp3+ T-regulatory cells with a unique ‘inducible’ genetic signature (212). Furthermore, it was shown that the immunomodulatory molecule polysaccharide A (PSA) of B. fragilis mediated the conversion of CD4+ T cells into Foxp3+ T-regulatory cells that produce IL-10 during commensal colonization. Functional Foxp3+ T-regulatory cells were also produced by PSA during intestinal inflammation, and TLR2 signaling was required for both T-regulatory cell induction and IL-10 expression (212), consistent with earlier studies that TLR2 signaling promotes IL-10 and tolerogenic responses (104, 107, 110).
In addition to the commensal bacteria, intestinal helminths can also promote T-regulatory cell differentiation by activating TGF-βR on the antigen-presenting cells (213). Absence of commensals does affect the natural regulatory T-cell development and its functions (214). However, deletion of T-regulatory cells promotes severe systemic inflammatory responses even in the absence of commensal microbiota, suggesting the regulatory T cells play critical role in regulating systemic mediated immune responses (214). In contrast to the T-regulatory cell promoting effect of some commensals, others are known to promote Th17 responses. Thus, colonization of the small intestine of mice with a single commensal microbe, segmented filamentous bacterium (SFB), is sufficient to induce the appearance of CD4+ T-helper cells that produce IL-17 and IL-22 (Th17 cells) in the lamina propria (44). Furthermore, adenosine 5′-triphosphate (ATP) that can be derived from commensal bacteria activates a unique subset of lamina propria cells, CD70+CD11clow cells, to promote the differentiation of Th17 cells (215). Germ-free mice exhibit much lower concentrations of luminal ATP, accompanied by fewer lamina propria Th17 cells, compared to specific pathogen-free mice. Systemic or rectal administration of ATP into these germ-free mice results in a marked increase in the number of lamina propria Th17 cells. A CD70+CD11clow subset of the lamina propria cells expressed Th17-inducing molecules, such as IL-6, IL-23p19, and TGF-β-activating integrin-αV and -β8, in response to ATP stimulation, and preferentially induces Th17 differentiation of co-cultured naive CD4+ T cells (215). Furthermore, Belkaid et al. (216) have demonstrated that gut flora DNA signals via TLR9 to suppress T-regulatory cells and promote effector T-cell differentiation. Taken together, these data suggest that commensals exert complex pleiotropic effects on programming DCs to induce the differentiation of regulatory versus effector T cells. Future work should thus be aimed at understanding the contexts and mechanisms that govern whether a commensal or its product induces regulatory or effector T-cell differentiation.
The liver is another unique microantomical and immunological environment where gut-derived nutrients and microbial products are cleared from the blood (217). In the liver, stromal cells, epithelial cells, Kupffer cells, and stellate cells secrete several anti-inflammatory factors such PGE2, IL-10, TGF-β, and retinoic acid and condition liver DCs to express IDO, IL-27, and retinoic acid and programs them to induce T-regulatory cells (217) (Fig. 4).
Like the intestinal lamina propria, the skin microenvironment plays critical role in the development and conditioning DCs to a tolerogenic state. As indicated above, under the so-called steady state condition, migratory DCs in the skin ferry skin-derived antigen and induce T-cell tolerance in the lymph node (52). In the steady-state condition, TGF-β1 present in the skin microenvironment plays a critical role in the development of Langerhans cells, as mice deficient in TGF-β or Id2, the downstream signaling molecule of TGF-βR lack Langerhans cells in the dermis (218–220). A recent study using conditional of deletion of TGF-βR in DCs has shown that TGF-β is required to maintain a pool of Langerhans cells in an immature state (221). Consistent with this observation, TGF-βR-deficient Langerhans cells showed spontaneous maturation with enhanced surface expression of MHC class II, costimulatory molecules, and CCR7, and downregulation of E-cadherin (221). Furthermore, a recent study has shown the TGF-β promotes the expression of E-cadherin in Langerhans cells and prevents their hyperactivation (222). Collectively, this shows TGF-β as a critical factor in the development as well as in controlling Langerhans cell homeostasis and function in the steady state.
In the immunologically privileged sites such as the eye and brain, the local microenvironments promote immune tolerance by conditioning antigen presenting cells to a tolerogenic state, through several immunosuppressive molecules. Ocular immune privilege is mediated by the presence of high levels of various immunomodulators in the aqueous humor such as TGF-β2, a-melanocyte stimulating hormone, vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), somatostatin and thombospondin (TSP-1)(23). DCs conditioned in this environment secrete high levels of IL-10 and TGF-β and promote tolerance (23).
Innate control of breakdown of tolerance and autoimmunity
Loss of both central and peripheral tolerance results in several autoimmune diseases. DCs play a critical in promoting tolerogenic responses, as depletion of DCs in mice leads to severe autoimmune disease under steady-state conditions (15). Furthermore, DCs have been implicated in a variety of autoimmune diseases. Several observations in mouse and humans show that DCs can exert both pathogenic and protective roles in several autoimmune diseases including inflammatory bowel disease and celiac diseases(208), rheumatoid arthritis(223), type 1 diabetes (224, 225), autoimmune pancreatitis (226), and multiple sclerosis (226). Multiple factors contribute to a break-down in the tolerogenic potential in DCs, resulting in inflammatory diseases.
Systemic lupus erythematosus
In addition to promoting tolerance, TLR activation on DCs has been implicated in instigating autoimmunity. For example, activation of intracellular TLR7, TLR8, and TLR9 on pDCs has been implicated in the induction of autoreactive B cells and pathogenesis of systemic lupus erythematosus (SLE) (227, 228). In human SLE patients, pDCs are known to be hyperactivated and display increased production of IFN-α in response to DNA- or RNA bound to specific auto-antibodies (229, 230). Furthermore, serum from lupus patients could induce differentiation of monocytes into DCs, and this process was dependent on the levels of IFN-α present in the serum(231). Such DCs could capture antigens from dying cells and present them to CD4+ T cells. Thus, unabated induction of DCs by IFN-α may drive the autoimmune response in SLE. In addition, in vitro studies revealed that self DNA or RNA immune complexes are detected by endosomal expression of either TLR9 or TLR7 in pDCs upon internalization and this process has been implicated in the pathogenesis of SLE (232). Presently, inhibitors that block the activation of TLR 7, 8, 9 are being tested in preclinical trials to prevent the induction and progression of SLE (233, 234).
Inflammatory bowel diseases
Inflammatory bowel diseases (IBDs) such as ulcerative colitis and Crohn’s disease are characterized by chronic intestinal inflammation induced by the dysfunctional relationship between the host immune system and the benign commensal flora. An important of role of DCs in preventing or promoting colitis was shown in a mouse model of colitis using oral administration of DSS, which promotes both acute as well as chronic inflammation. Ablation of DCs before DSS administration exacerbates colitis (235), whereas ablation of DCs during DSS treatment ameliorates colitis(236). This finding suggests that DCs may play protective role in the initial phase in part, where as might be pathogenic during inflammatory phase. Moreover, as outlined above, numerous subsets of DCs exist in the intestinal microenvironments, and their precise roles in promoting tolerogenic versus inflammatory responses need to be ascertained.
In the intestine, anti-inflammatory cytokines such IL-10, TGF-β, and retinoic acid produced by DCs and macrophages are critical of for maintaining tolerance and have been critically linked to the prevention of colitis. Mice deficient in IL-10 or IL-10R develop spontaneous inflammation of the large intestine in response to commensal bacteria (237). TGF-β is another molecule expressed in the intestine, which is critical in promoting tolerogenic responses. Thus, loss of integrin αvβ8, TGF-β activating factor, on DCs results in autoimmunity and severe colitis in mice (238). Loss of both IL-10 and TGF-β promotes much more severe colitis in mice than loss of either molecule alone (239).
Multiple sclerosis
Multiple sclerosis (MS) is a demyelinating neurological disease of the CNS that develops in young adults resulting in debilitating motor and sensory dysfunction. The diseases are characterized by the activation of encephalitogenic myelin-reactive CD4+ T cells that produce either IFN-γ or IL-17 (240). The immune phenotype of MS is best mimicked in the EAE model in mice where the injection of myelin components into susceptible animals leads to a CD4+-mediated autoimmune disease that shares striking similarities with MS (241) The crucial early events in CNS are mediated by the inflammatory resident CNS innate cells such as microglia and astrocytes (240). Even though DCs are present in very low numbers, their numbers increases in the brain and spinal cord during autoimmunity, infection, or trauma (242–244). In addition to a pathological role, recent studies have shown that DCs might play regulatory roles in limiting the inflammation during the autoimmunity (245, 246). Depletion of pDCs during the acute or relapse phase of EAE exacerbates the disease. pDCs isolated from CNS can actively suppress the ability of myeloid DCs to promote Th1 or Th17 cell differentiation (247). The suppressive effect of pDCs is independent of anti-inflammatory cytokines but dependent on IFN-β, a known suppressor of IFNγ and IL-17 production. In addition to their suppressive effect, pDCs present antigen, which is also critical for its protective function against autoimmune diseases. Conditional deletion of MHC class II expression in pDCs in mice results in exacerbated EAE (248). Consistently, impaired maturation and altered functional properties of pDCs have been observed in multiple sclerosis patients (249, 250). pDCs isolated form MS patients contain two different subsets based on the expression CD123 and CD58 (251). Plasmacytoid DC1 (CD123highCD58low) promoted the induction of IL-10 production Tr1 cells, whereas plasmacytoid DC2 (CD123lowCD58high) promoted the differentiation of IL-17 production. Under steady-state conditions, plasmacytoid DC1 are present in higher number, where under pathological conditions plasmacytoid DC2 are more abundant (251). An imbalance in the ratio of these two subsets is observed in MS patients and IFN-β treatment induces the plasmacytoid DC1 phenotype (251).
The importance of innate receptors is well characterized in the mouse model of EAE (252). The adapter molecule myeloid differentiation primary response gene 88 (MyD88) is both essential for the initiation of EAE, and for the subsequent differentiation of pro-inflammatory autoreactive CD4+ T cells. Even though it is known that the process of diseases initiation is dependent on TLR signaling, little is known about the specific role of TLRs in different cell subtypes. To judge to what extent the absence of TLRs and its signaling molecule MyD88 affects the course of a sterile autoimmune CNS disease, EAE was induced in mice deficient for TLR2, TLR9, and MyD88. After immunization with myelin oligodendrocyte glycoprotein (MOG) peptide emulsified in CFA, all wildtype and TLR2−/− mice developed EAE with an incidence of 100% and a similar mean disease onset and severity. These results clearly demonstrate that TLR2 is dispensable for the induction and progression of disease. In contrast, MyD88−/− mice were completely EAE resistant, and TLR9−/− mice developed EAE with a significant delay in disease onset (253).
Tolerogenic DCs and cancer
DCs present tumor antigens to T cells and initiate a robust anti-tumor T-cell response. However, excessive production of anti-inflammatory factors such as IL-10, TGF-β, VEGF, and PGE2 by tumors cells can condition DCs to a tolerogenic state, and anergize T cells. Tolerogenic DCs in the tumor microenvironment express low levels of MHC and costimulatory molecules (CD40, CD80, CD86) and produce low levels of proinflammatory cytokines (254–256), as well as TGF-β and IL-10, and induce regulatory T cells and anergize antigen specific T cells (257–261). These DCs are resistant to maturation stimuli, whereas blocking IL-10 enables their maturation (262–264). Consistent with this observation, IL-10-deficient mice are resistant to ultraviolet (UV)-induced skin carcinogenesis (265) and immunogenic transplantable tumors. In contrast, IL-10 transgenic mice show impaired immune response to tumors (266). Further studies showed the IL-10 deficient DCs are more immunogenic and can mount more robust anti-tumor Th1 and CTL responses (267–269). In addition to DCs, MDSCs in the tumor microenvironment produce high levels of anti-inflammatory cytokines.
Tolerogenic DCs in chronic infections
It is likely that tolerogenic DCs play crucial roles in the pathogenesis of chronic infections. Several pathogens can modulate DC function as a mechanism to evade host immune response resulting in the establishment of chronic infection (270). Much of the knowledge on immunological mechanisms during persistent viral infections is drawn from the mouse model of the lymphocytic choriomeningitisvirus (LCMV). In mice, infection with the LCMV clone 13 strain results in a generalized immune suppression resulting in persistent infection (271, 272). Splenic DCs infected with LCMV clone 13 showed markedly reduced expression levels of surface MHC class I and II and costimulatory molecules such as CD40, CD80, CD86 (273). Furthermore, anti-inflammatory cytokines such IL-10 produced by DCs play a critical role in establishing chronic infection (274). Recent studies have shown IL-10 production increases during the chronic of phase of LCMV clone 13 infection and that blockade of IL-10 results in enhanced immunity and clearance of virus (275, 276). DCs are a major source for IL-10 production during chronic infection (275, 276). In addition, a recent study has shown that IL-10 directly suppresses CD4 effector and memory T-cell response during acute LCMV infection (277). Similarly, DCs isolated from patients with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) produce high levels of IL-10 (278, 279) and are less potent in stimulating allogeneic T cells (279–283). Furthermore, neutralizing IL-10 in culture could induce robust allogeneic T-cell response, suggesting a critical role for IL-10 in chronic infection.
In addition to IL-10, recent studies have shown that expression of PD-1 on T cells and PD-L1 on antigen presenting cells is critical for the establishment of chronic infection in LCMV (284) and in other chronic infections such as such HIV, HCV, HBV, and simian immunodeficiency virus (SIV) (285–293). Consistent with this, blocking PD-1 interaction with its ligand in mice with LCMV clone 13 infection enhances T-cell immune response and clearance of persistent virus (294). Similarly, blocking the interaction between PD-1 and its ligands in vitro partially restored effector function of CD8+ T cells in other chronic infections such HIV, HCV, HBV and SIV (290–293, 295). A recent study has shown PD-L1 expression on DCs is also critical for inducing and sustaining regulatory T cells (296). Collectively, these studies shown viruses can render DCs to tolerogenic state, which is critical for establishment of chronic infections.
Summary: hierarchies of organization within innate immune system
Although DCs were originally recognized as the most potent stimulators of adaptive immunity, research over the past decade has revealed a fundamental role for these cells in suppressing immune responses. It is now clear that tolerogenic responses are controlled by multiple parameters of the innate immune system: (i) the innate receptors on DCs that sense microbial and nonmicrobial stimuli, (ii) the intracellular signaling pathways that program DCs to a tolerogenic state, (iii) the intercellular interactions between DCs and multiple cell types, and (iv) inductive signals from the local microenvironment. As we have noted elsewhere (6), these parameters can be considered to represent different hierarchies of organization in the innate immune system. Thus, the cell (in this case, the DC) can be considered the ‘ground level’ of this hierarchy, and more detailed levels resolution can be obtained by studying the innate receptors (hierarchy level −1) and signaling networks and transcription factors (hierarchy level −2). In contrast, more global views can be obtained by of multicellular cooperation (for example, between DCs and macrophages or between DCs and T-regulatory cells—hierarchy level +1) and the influence of local microenvironments (for example, intestine versus lung—hierarchy level +2) (6). Much of our understanding of DC biology has been obtained by studies that focus on a single level of this hierarchy (e.g. describing DC subsets that are involved in tolerance induction or describing a role for a given innate immune receptor in this process), but such insights offer only a limited view of events that orchestrate tolerogenic responses. Therefore, future research should seek to acquire and integrate data from multiple levels of this hierarchy (i.e. DC signaling pathways, innate receptors, intercellular interactions, and the impact of the microenvironment). It is clear that a unified theory of tolerogenic responses will only be possible by integrating information obtained at each of these hierarchical levels. Such understanding will be invaluable in the rational design of drugs and therapeutics that can reprogram the balance between immunity and tolerance in the control of autoimmune diseases, cancer and chronic infections.
Acknowledgments
We gratefully acknowledge the generous support of the National Institutes of Health and the Bill and Melinda Gates Foundation in our work.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Burnet FM. A modification of Jerne’s theory of antibody production using the concept of clonal selection. CA Cancer J Clin. 1976;26:119–21. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- 2.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–39. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 3.Miller JF, Morahan G. Peripheral T cell tolerance. Annu Rev Immunol. 1992;10:51–69. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–55. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–7. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 10.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–50. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–84. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 13.Proietto AI, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008;105:19869–74. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 15.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–59. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birnberg T, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloidproliferative syndrome. Immunity. 2008;29:986–97. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 18.Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008;20:61–7. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 22.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak N, Siepmann K, Zierhut M, Bieber T. The good, the bad and the ugly--APCs of the eye. Trends Immunol. 2003;24:570–4. doi: 10.1016/j.it.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 234:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–24. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 27.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 29.Goubier A, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munn DH, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 31.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 32.Kronin V, et al. A subclass of dendritic cells regulates the response of naive CD8 T cells by limiting their IL-2 production. J Immunol. 1996;157:3819–27. [PubMed] [Google Scholar]
- 33.Rizzitelli A, Hawkins E, Todd H, Hodgkin PD, Shortman K. The proliferative response of CD4 T cells to steady-state CD8+ dendritic cells is restricted by post-activation death. Int Immunol. 2006;18:415–23. doi: 10.1093/intimm/dxh382. [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 35.Belz GT, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamazaki S, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–33. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulendran B, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maldonado-Lopez R, et al. CD8alpha+ and CD8alpha-subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonifaz LC, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coombes JL, et al. A functionally specialized populationof mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siddiqui KR, Powrie F. CD103+ GALT DCs promote Foxp3+ regulatory T cells. Mucosal Immunol. 2008;1 (Suppl 1):S34–8. doi: 10.1038/mi.2008.43. [DOI] [PubMed] [Google Scholar]
- 43.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877–83. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 46.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 48.Buenafe AC, Bourdette DN. Lipopolysaccharide pretreatment modulates the disease course in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;182:32–40. doi: 10.1016/j.jneuroim.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 50.Guilliams M, et al. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 115:1958–68. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 51.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 52.Waithman J, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol. 2007;179:4535–41. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–76. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cumberbatch M, Singh M, Dearman RJ, Young HS, Kimber I, Griffiths CE. Impaired Langerhans cell migration in psoriasis. J Exp Med. 2006;203:953–60. doi: 10.1084/jem.20052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–56. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 60.Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112:3175–85. doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bamboat ZM, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901–11. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–69. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jinushi M, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, et al. Distinct response of liver myeloid dendritic cells to endotoxin is mediated by IL-27. J Hepatol. 2009;51:510–9. doi: 10.1016/j.jhep.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–32. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–28. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 67.Gregerson DS, Heuss ND, Lehmann U, McPherson SW. Peripheral induction of tolerance by retinal antigen expression. J Immunol. 2009;183:814–22. doi: 10.4049/jimmunol.0803748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein-Streilein J, Watte C. Cross talk among cells promoting anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:115–30. doi: 10.1159/000099262. [DOI] [PubMed] [Google Scholar]
- 69.Zozulya AL, Clarkson BD, Ortler S, Fabry Z, Wiendl H. The role of dendritic cellsin CNS autoimmunity. J Mol Med. 88:535–44. doi: 10.1007/s00109-010-0607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mishima Y. Melanosomes in phagocytic vacuoles in Langerhans cells. Electron microscopy of keratin-stripped human epidermis. J Cell Biol. 1966;30:417–23. doi: 10.1083/jcb.30.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hemmi H, et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int Immunol. 2001;13:695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- 72.Inaba K, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–73. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 74.Penna G, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–53. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 75.Sato K, Yamashita N, Matsuyama T. Human peripheral blood monocyte-derived interleukin-10-induced semi-mature dendritic cells induce anergic CD4(+) and CD8(+) T cells via presentation of the internalized soluble antigen and cross-presentation of the phagocytosed necrotic cellular fragments. Cell Immunol. 2002;215:186–94. doi: 10.1016/s0008-8749(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 76.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells inducedby interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 77.Geissmann F, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 78.Geissmann F, et al. Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med. 2003;198:623–34. doi: 10.1084/jem.20030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rutella S, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–27. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107:3632–8. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol. 29:156–83. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 82.Liang X, et al. Administration of dendritic cells transduced with antisense oligodeoxyribonucleotides targeting CD80 or CD86 prolongs allograft survival. Transplantation. 2003;76:721–9. doi: 10.1097/01.TP.0000076470.35404.49. [DOI] [PubMed] [Google Scholar]
- 83.Xiang J, Gu X, Zhou Y, Gong X, Qian S, Chen Z. Administration of dendritic cells modified by RNA interference prolongs cardiac allograft survival. Microsurgery. 2007;27:320–3. doi: 10.1002/micr.20364. [DOI] [PubMed] [Google Scholar]
- 84.Zheng X, et al. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood. 2009;113:2646–54. doi: 10.1182/blood-2008-04-151191. [DOI] [PubMed] [Google Scholar]
- 85.Zheng X, et al. Treatment of autoimmune arthritis using RNA interference-modulated dendritic cells. J Immunol. 184:6457–64. doi: 10.4049/jimmunol.0901717. [DOI] [PubMed] [Google Scholar]
- 86.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol. 2004;173:4331–41. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 87.Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166:3499–505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- 88.Coates PT, Krishnan R, Kireta S, Johnston J, Russ GR. Human myeloid dendritic cells transduced with an adenoviral interleukin-10 gene construct inhibit human skin graft rejection in humanized NOD-scid chimeric mice. Gene Ther. 2001;8:1224–33. doi: 10.1038/sj.gt.3301513. [DOI] [PubMed] [Google Scholar]
- 89.Lu L, et al. Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther. 1999;6:554–63. doi: 10.1038/sj.gt.3300862. [DOI] [PubMed] [Google Scholar]
- 90.Tan PH, et al. Creation of tolerogenic human dendritic cells via intracellular CTLA4: a novel strategy with potential in clinical immunosuppression. Blood. 2005;106:2936–43. doi: 10.1182/blood-2005-05-1826. [DOI] [PubMed] [Google Scholar]
- 91.Gerner MY, Mescher MF. Antigen processing and MHC-II presentation by dermal and tumor-infiltrating dendritic cells. J Immunol. 2009;182:2726–37. doi: 10.4049/jimmunol.0803479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 93.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330:1061–4. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- 96.Medzhitov R, Janeway CA., Jr Innateimmunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 97.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 98.Wagner H. Endogenous TLR ligands and autoimmunity. Adv Immunol. 2006;91:159–73. doi: 10.1016/S0065-2776(06)91004-9. [DOI] [PubMed] [Google Scholar]
- 99.Manfredi AA, Capobianco A, Bianchi ME, Rovere-Querini P. Regulation of dendritic-and T-cell fate by injury-associated endogenous signals. Crit Rev Immunol. 2009;29:69–86. doi: 10.1615/critrevimmunol.v29.i1.30. [DOI] [PubMed] [Google Scholar]
- 100.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 102.Agrawal S, et al. Cutting edge: different Toll-like receptoragonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 103.Dillon S, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–43. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 104.Dillon S, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–28. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wenink MH, et al. TLR2 promotes Th2/Th17 responses via TLR4 and TLR7/8 by abrogating the type I IFN amplification loop. J Immunol. 2009;183:6960–70. doi: 10.4049/jimmunol.0900713. [DOI] [PubMed] [Google Scholar]
- 106.Redecke V, et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–43. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 107.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–9. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J Immunol. 2008;181:8323–34. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burton OT, et al. Roles for TGF-beta and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. J Immunol. 2010;185:2754–62. doi: 10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 110.Depaolo RW, et al. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–61. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Kleij D, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 112.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 114.Orabona C, et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107:2846–54. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 115.Moseman EA, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 116.Romani L, et al. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265–74. doi: 10.1182/blood-2006-02-004762. [DOI] [PubMed] [Google Scholar]
- 117.Pierluigi B, et al. Thymosin alpha1: the regulator of regulators? Ann N Y Acad Sci. 1194:1–5. doi: 10.1111/j.1749-6632.2010.05465.x. [DOI] [PubMed] [Google Scholar]
- 118.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garcia-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev. 2009;230:22–37. doi: 10.1111/j.1600-065X.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- 120.Bonifazi P, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–74. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 121.Bruder D, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- 122.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 123.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergman MP, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979–90. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Konstantinov SR, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci U S A. 2008;105:19474–9. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steeghs L, et al. Neisseria meningitidis expressing lgtB lipopolysaccharide targets DC-SIGN and modulates dendritic cell function. Cell Microbiol. 2006;8:316–25. doi: 10.1111/j.1462-5822.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 127.Denda-Nagai K, et al. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem. 2010;285:19193–204. doi: 10.1074/jbc.M110.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Higashi N, et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 2002;277:20686–93. doi: 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- 129.Saba K, Denda-Nagai K, Irimura T. A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. Am J Pathol. 2009;174:144–52. doi: 10.2353/ajpath.2009.080235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou Y, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 16:1128–33. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kammerer U, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN(CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–96. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fujikado N, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14:176–80. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 133.Meyer-Wentrup F, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–53. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 134.Jahn PS, Zanker KS, Schmitz J, Dzionek A. BDCA-2 signaling inhibits TLR-9-agonist-induced plasmacytoid dendritic cell activation and antigen presentation. Cell Immunol. 2010;265:15–22. doi: 10.1016/j.cellimm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 135.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–88. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 137.Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol. 2010;40:1255–65. doi: 10.1002/eji.201040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 139.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12:616–23. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 140.Ilarregui JM, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–91. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 141.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 142.Blois SM, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–7. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 143.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 144.Kubach J, et al. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–8. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 145.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 146.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interactionin viral-induced immunopathology: shifting the balance toward regulators. J Immunol. 2009;182:3191–201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seki M, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 148.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 149.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 150.Wallet MA, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–32. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wallet MA, et al. MerTK regulates thymic selection of autoreactive T cells. Proc Natl Acad Sci U S A. 2009;106:4810–5. doi: 10.1073/pnas.0900683106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 153.Samsom JN, et al. Fc gamma RIIB regulates nasal and oral tolerance: a role for dendritic cells. J Immunol. 2005;174:5279–87. doi: 10.4049/jimmunol.174.9.5279. [DOI] [PubMed] [Google Scholar]
- 154.Desai DD, et al. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J Immunol. 2007;178:6217–26. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- 155.Wenink MH, et al. The inhibitory Fc gamma IIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J Immunol. 2009;183:4509–20. doi: 10.4049/jimmunol.0900153. [DOI] [PubMed] [Google Scholar]
- 156.Vlad G, Chang CC, Colovai AI, Vasilescu ER, Cortesini R, Suciu-Foca N. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int Rev Immunol. 29:119–32. doi: 10.3109/08830180903281185. [DOI] [PubMed] [Google Scholar]
- 157.Chang CC, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 158.Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907–14. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 159.Gregori S, et al. Differentiation of type 1 T-regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–44. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 160.Manavalan JS, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–58. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 161.Brenk M, et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T-regulatory cells. J Immunol. 2009;183:145–54. doi: 10.4049/jimmunol.0803277. [DOI] [PubMed] [Google Scholar]
- 162.Rogers NC, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–17. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 163.Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1-/-mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:5788–96. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 164.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 166.Szatmari I, et al. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–62. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duester G, Shean ML, McBride MS, Stewart MJ. Retinoic acid response element in the human alcohol dehydrogenase gene ADH3: implications for regulation of retinoic acid synthesis. Mol Cell Biol. 1991;11:1638–46. doi: 10.1128/mcb.11.3.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leroy P, Nakshatri H, Chambon P. Mouse retinoic acid receptor alpha 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc Natl Acad Sci U S A. 1991;88:10138–42. doi: 10.1073/pnas.88.22.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zaheer A, Zaheer S, Sahu SK, Yang B, Lim R. Reduced severity of experimental autoimmune encephalomyelitis in GMF-deficient mice. Neurochem Res. 2007;32:39–47. doi: 10.1007/s11064-006-9220-x. [DOI] [PubMed] [Google Scholar]
- 170.Xiao BG, Zhu WH, Lu CZ. The presence of GM-CSF and IL-4 interferes with effect of TGF-beta1 on antigen presenting cells in patients with multiple sclerosis and in rats with experimental autoimmune encephalomyelitis. Cell Immunol. 2007;249:30–6. doi: 10.1016/j.cellimm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 171.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–82. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–93. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 173.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 174.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–53. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yi Z, Li L, Matsushima GK, Earp HS, Wang B, Tisch R. A novelrole for c-Src and STAT3 in apoptotic cell-mediated MerTK-dependent immunoregulation of dendritic cells. Blood. 2009;114:3191–8. doi: 10.1182/blood-2009-03-207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cortesini NS, et al. Role of regulatory and suppressor T-cells in the induction of ILT3+ ILT4+ tolerogenic endothelial cells in organ allografts. Transpl Immunol. 2004;13:73–82. doi: 10.1016/j.trim.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 177.Svajger U, Vidmar A, Jeras M. Niflumic acid renders dendritic cells tolerogenic and up-regulates inhibitory molecules ILT3 and ILT4. Int Immunopharmacol. 2008;8:997–1005. doi: 10.1016/j.intimp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 178.Cella M, et al. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med. 1997;185:1743–51. doi: 10.1084/jem.185.10.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chang CC, et al. Ig-like transcript 3 regulates expression of proinflammatory cytokines and migration of activated T cells. J Immunol. 2009;182:5208–16. doi: 10.4049/jimmunol.0804048. [DOI] [PubMed] [Google Scholar]
- 180.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 181.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–8. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 182.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ Tcells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 183.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–10. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 184.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–9. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miwa N, et al. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. 2005;11:865–70. doi: 10.1093/molehr/gah246. [DOI] [PubMed] [Google Scholar]
- 186.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 187.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 188.Onodera T, et al. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J Immunol. 2009;183:5608–14. doi: 10.4049/jimmunol.0804116. [DOI] [PubMed] [Google Scholar]
- 189.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 190.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–64. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Darrasse-Jeze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–62. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Caielli S, Sorini C, Falcone M. The dangerous liaison between iNKT cells and dendritic cells: Does it prevent or promote autoimmune diseases? Autoimmunity. 2010 doi: 10.3109/08916931003782130. [DOI] [PubMed] [Google Scholar]
- 193.Caielli S, et al. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J Immunol. 2010;185:7317–29. doi: 10.4049/jimmunol.1000400. [DOI] [PubMed] [Google Scholar]
- 194.Yokota A, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–77. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenicdendritic cells. Gut. 2009;58:1481–9. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 196.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–50. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 197.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang M, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–33. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 199.Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–16. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 200.Molenaar R, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 201.Hammerschmidt SI, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol. 2008;38:2751–61. doi: 10.1002/eji.200838542. [DOI] [PubMed] [Google Scholar]
- 203.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators ofthe immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 206.Mucida D, Park Y, Cheroutre H. From the diet to the nucleus: vitamin A and TGF-beta join efforts at the mucosal interface of the intestine. Semin Immunol. 2009;21:14–21. doi: 10.1016/j.smim.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol. 2009;21:22–7. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119:2441–50. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–67. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Grainger JR, Hall JA, Bouladoux N, Oldenhove G, Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol Rev. 2010;234:305–16. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Grainger JR, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 207:2331–41. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Francioni S, Pastore M, Castellaro E. Tumor markers and chemotherapy. J Nucl Med Allied Sci. 1990;34:251–9. [PubMed] [Google Scholar]
- 215.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 216.Hall JA, et al. Commensal DNA limits regulatory Tcell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–49. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 10:753–66. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 218.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–52. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hacker C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 221.Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol. 185:3248–55. doi: 10.4049/jimmunol.1000981. [DOI] [PubMed] [Google Scholar]
- 222.Ohtani T, et al. TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation. Immunology. 2009;126:485–99. doi: 10.1111/j.1365-2567.2008.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wenink MH, Han W, Toes RE, Radstake TR. Dendritic cells and their potential implication in pathology and treatment of rheumatoid arthritis. Handb Exp Pharmacol. 2009:81–98. doi: 10.1007/978-3-540-71029-5_4. [DOI] [PubMed] [Google Scholar]
- 224.Pechhold K, Koczwara K. Immunomodulation of autoimmune diabetes by dendritic cells. Curr Diab Rep. 2008;8:107–13. doi: 10.1007/s11892-008-0020-3. [DOI] [PubMed] [Google Scholar]
- 225.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boomershine CS, et al. Autoimmune pancreatitis results from loss of TGFbeta signalling in S100A4-positive dendritic cells. Gut. 2009;58:1267–74. doi: 10.1136/gut.2008.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–61. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barrat FJ, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–92. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 230.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 231.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 232.Means TK, Luster AD. Toll-like receptor activation in the pathogenesis of systemic lupus erythematosus. Ann N Y Acad Sci. 2005;1062:242–51. doi: 10.1196/annals.1358.027. [DOI] [PubMed] [Google Scholar]
- 233.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 234.Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm Allergy Drug Targets. 2007;6:223–35. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- 235.Qualls JE, Tuna H, Kaplan AM, Cohen DA. Suppression of experimental colitis in mice by CD11c+ dendritic cells. Inflamm Bowel Dis. 2009;15:236–47. doi: 10.1002/ibd.20733. [DOI] [PubMed] [Google Scholar]
- 236.Abe K, et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci U S A. 2007;104:17022–7. doi: 10.1073/pnas.0708469104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sellon RK, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kang SS, et al. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Med. 2008;5:e41. doi: 10.1371/journal.pmed.0050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann N Y Acad Sci. 2008;1143:21–34. doi: 10.1196/annals.1443.012. [DOI] [PubMed] [Google Scholar]
- 241.Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–23. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 242.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–62. [PubMed] [Google Scholar]
- 243.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 244.Lande R, et al. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008;67:388–401. doi: 10.1097/NEN.0b013e31816fc975. [DOI] [PubMed] [Google Scholar]
- 245.Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26:149–88. doi: 10.1615/critrevimmunol.v26.i2.40. [DOI] [PubMed] [Google Scholar]
- 246.Deshpande P, King IL, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. J Immunol. 2007;178:6695–9. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- 247.Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180:6457–61. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Irla M, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 207:1891–905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stasiolek M, et al. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain. 2006;129:1293–305. doi: 10.1093/brain/awl043. [DOI] [PubMed] [Google Scholar]
- 250.Bayas A, Stasiolek M, Kruse N, Toyka KV, Selmaj K, Gold R. Altered innate immune response of plasmacytoid dendritic cells in multiple sclerosis. Clin Exp Immunol. 2009;157:332–42. doi: 10.1111/j.1365-2249.2009.03964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schwab N, Zozulya AL, Kieseier BC, Toyka KV, Wiendl H. An imbalance of two functionally and phenotypically different subsets of plasmacytoid dendritic cells characterizes the dysfunctional immune regulation in multiple sclerosis. J Immunol. 184:5368–74. doi: 10.4049/jimmunol.0903662. [DOI] [PubMed] [Google Scholar]
- 252.Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 42:506–18. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 253.Webb CP, Greenfield SA. Non-cholinergic effects of acetylcholinesterase in the substantia nigra: a possible role for an ATP-sensitive potassium channel. Exp Brain Res. 1992;89:49–58. doi: 10.1007/BF00229000. [DOI] [PubMed] [Google Scholar]
- 254.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang X, et al. CD4-8-dendritic cells prime CD4+ T regulatory 1 cells to suppress antitumor immunity. J Immunol. 2005;175:2931–7. doi: 10.4049/jimmunol.175.5.2931. [DOI] [PubMed] [Google Scholar]
- 258.Roncarolo MG, Levings MK, Traversari C. Differentiation of T-regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ghiringhelli F, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu VC, et al. Tumor evasion of the immune system by converting CD4+CD25-T cells into CD4+CD25+ T-regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 261.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182:2795–807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 262.Salazar-Onfray F. Interleukin-10: a cytokine used by tumors to escape immunosurveillance. Med Oncol. 1999;16:86–94. doi: 10.1007/BF02785841. [DOI] [PubMed] [Google Scholar]
- 263.Huang LY, Reis e Sousa C, Itoh Y, Inman J, Scott DE. IL-12 induction by a TH1-inducing adjuvant in vivo: dendritic cell subsets and regulation by IL-10. J Immunol. 2001;167:1423–30. doi: 10.4049/jimmunol.167.3.1423. [DOI] [PubMed] [Google Scholar]
- 264.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–7. [PubMed] [Google Scholar]
- 265.Loser K, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–71. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 266.Hagenbaugh A, et al. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–10. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen YX, et al. A crucial role for dendritic cell (DC) IL-10 in inhibiting successful DC-based immunotherapy: superior antitumor immunity against hepatocellular carcinoma evoked by DC devoid of IL-10. J Immunol. 2007;179:6009–15. doi: 10.4049/jimmunol.179.9.6009. [DOI] [PubMed] [Google Scholar]
- 268.He Q, et al. Molecular basis for the potency of IL-10-deficient dendritic cells as a highly efficient APC system for activating Th1 response. J Immunol. 2005;174:4860–9. doi: 10.4049/jimmunol.174.8.4860. [DOI] [PubMed] [Google Scholar]
- 269.Igietseme JU, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164:4212–9. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 270.Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. A look behind closed doors: interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8:350–60. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–40. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–70. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–45. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 275.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–72. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107:3018–23. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturationbut can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004;101:7669–74. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dolganiuc A, Paek E, Kodys K, Thomas J, Szabo G. Myeloid dendritic cells of patients with chronic HCV infection induce proliferation of regulatory T lymphocytes. Gastroenterology. 2008;135:2119–27. doi: 10.1053/j.gastro.2008.07.082. [DOI] [PubMed] [Google Scholar]
- 280.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 281.Chehimi J, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 283.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 284.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 285.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang JY, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–8. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 287.Penna A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 288.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 289.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 290.Radziewicz H, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Petrovas C, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–36. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Velu V, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–28. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronicinfection. J Exp Med. 2008;205:543–55. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Urbani S, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]