Abstract
α7 nicotinic acetylcholine receptors (α7nAchRs) modulate immune activation by suppressing production of pro-inflammatory cytokines in peripheral immune cells. α7nAchRs also modulate inhibitory output in the hippocampus, which provides input to key circuits of the HPA axis. Therefore the α7 nicotinic acetylcholine receptor gene (CHRNA7) may be associated with cortisol stress response. Polymorphisms in the CHRNA7 promoter decrease its expression and may dampen the cholinergic response, leading to an increase in inflammation. Increased inflammation may change the intrauterine environment, altering neuroendocrine development in the offspring. Maternal CHRNA7 genotype could affect an offspring’s HPA regulation via reprogramming in utero. Patients with allergic disorders have a differential cortisol response to stress. This study utilized samples collected from a cohort of 198 adolescents in a previous study of atopic disorders, who demonstrated a disturbance in HPA response associated with atopy. Salivary cortisol samples collected from the adolescents after a series of laboratory procedures and DNA samples collected from the adolescents and their parents were used for further analysis. DNA samples were genotyped for allelic variation in the CHRNA7 promoter. Genetic association analyses with the cortisol levels were performed in the adolescents. Maternal genotype influences were investigated for the CHRNA7 gene. We also included maternal and child atopy diagnosis as covariates in determining cortisol levels and tested for association of CHRNA7 to atopy. Polymorphisms in the CHRNA7 promoter were associated with lower cortisol levels after a small laboratory stress. Our findings also show that although the child’s CHRNA7 genotype affects stress response, the maternal genotype has a stronger influence on cortisol release after stress in male offspring. These effects were independent of atopy status.
Keywords: Stress, Cortisol, Genetics, Association Study, Adolescents
1. Introduction
The early developmental environment, influenced by genetic background in the mother, may selectively alter phenotypic traits. For example, recent studies have shown that maternal rearing behaviors in rats are associated with changes in hypothalamic-pituitary axis (HPA) regulation in offspring and that these changes result from the epigenetic reprogramming of gene expression [1, 2]. Effects during development extend to the intrauterine environment. Maternal genotype for the SGK1 glucocorticoid inducible kinase in mice regulates fetal programming of blood pressure [3]. Both human epidemiological studies [4] and animal models [5] indicate that immune activation during pregnancy increases risk for neurodevelopmental disorders such as schizophrenia in the offspring. Maternal genetic vulnerability to stress may represent a secondary risk factor during fetal neurodevelopment [6]. The α7 neuronal nicotinic acetylcholine receptor gene (CHRNA7) is involved in regulation of immune activation [7] and is associated with schizophrenia [8], suggesting that maternal CHRNA7 genetic background may alter maternal response to immunological stress and change the course of neural development in the fetus. The current study examines the relationship of genetic mutations in CHRNA7, in both mother and child, to HPA stress response in the child.
α7 nicotinic acetylcholine receptors (α7nAchRs) modulate immune activation by suppressing production of pro-inflammatory cytokines in peripheral immune cells [7]. CHRNA7 is also widely expressed in the central and peripheral nervous systems [9–11]. There are multiple functional single nucleotide polymorphisms (SNPs) in the promoter region of this gene that decrease its expression [12]. Polymorphisms in the promoter of CHRNA7 may dampen the cholinergic anti-inflammatory response, leading to an increase in inflammation. Decreased expression of α7nAchRs may change the intrauterine environment via increased inflammation, altering neuroendocrine development in the offspring. Maternal CHRNA7 genotype could, therefore, affect an offspring’s HPA regulation via reprogramming in utero.
The α7nAchR may also be associated with HPA stress response through its role in the central nervous system. α7nAchRs are involved in inhibitory processes in the brain, and CHRNA7 promoter polymorphisms are associated with the loss of these processes [12]. The α7nAchR is highly expressed in the hippocampus and the suprachiasmatic nucleus, both of which provide important input into HPA regulation. Thus, CHRNA7 mutations may have a dual role as mediators of CNS inhibition and peripheral stress responses. Both child and maternal CHRNA7 genotype could be associated with HPA regulation.
The current study is a preliminary study performed to explore these hypotheses. We performed a supplementary analysis of a cohort from a previous study [13]. This cohort consisted of 198 adolescents recruited from the Colorado Twin Study for a study of atopic disorders. During the study, salivary cortisol levels were collected at baseline and following three different laboratory challenges: a blood draw, skin tests for allergies, and a semistructured psychiatric interview. Approximately 71% of the cohort was atopic. Atopic adolescents in the study had an attenuated cortisol response to the blood draw compared to controls or those with positive skin tests without atopic illness similar to the observations of Buske-Kirschbaum et al. [14].
These adolescents have a known disturbance in HPA stress response associated with an inflammatory condition. They are an interesting cohort in which to look for genetic contributions to changes in the HPA that are mediated by immune activation. In the previous study, blood samples were taken from subjects and both parents for DNA analysis. These DNA samples were genotyped for allelic variation in the CHRNA7 promoter. Exploratory genetic association analyses with cortisol levels during the challenges were performed in the adolescents. In addition, maternal genotype influences were investigated for the CHRNA7 gene. Because HPA disturbances in these subjects were associated with atopy, we also included the child’s atopy diagnosis as a covariate in determining cortisol levels and tested for association of CHRNA7 to atopy. In addition, because maternal atopy may increase the amount of inflammation experienced by the offspring in utero, maternal atopy diagnosis was also included in the analysis.
Our findings show that although the child’s CHRNA7 genotype does affect stress response, the maternal genotype has a stronger influence on cortisol release after stress in male offspring. These effects were independent of atopy status in the mother or child.
2. Materials and methods
2.1. Subjects
Subjects were 198 adolescents 12 to 19 years of age (mean age, 16.2 ± 1.96 years) recruited in a previous study. Ninety percent of the sample was white and 52% was male. The analyzed sample contained 99 nuclear families with two parents and a set of twins. 52% of the twin pairs were monozygotic; 31% were dizygotic, same sex twins, and 17% were dizygotic, opposite sex twins. 70.7% of the twins were atopic.
The salivary cortisol levels from the previous study [13] were used to determine genetic association. In that study, half the participants were given appointments beginning at 8 AM, and half beginning at 1 PM. These cortisol levels could not be compared directly due to diurnal variation. The morning and afternoon groups were analyzed separately. There were no significant differences between the AM and PM groups in age (Student’s t = 0.49, p = 0.63), sex ratio (χ2 = .74, 1 df, p = 0.39), atopy status (χ2 = .72, 1 df, p = 0.40), or twin zygosity (χ2 = 5.39, 2 df, p = 0.07).
2.2. Genotyping
DNA samples obtained during the Wamboldt et al. (2003) study were used for analysis. Genotyping was done in both parents and the children. Primers for amplification of the proximal promoter region of the CHRNA7 gene were ordered from Integrated DNA Technologies, Skokie, IL (sense: 5′ AGTACCTCCCGCTCACACCTCG 3′ and antisense: 3′ GACGTCGAGGCCCTGAGTTGTA 5′). Utilizing the following program, a PCR product of 271bp was generated: 95°C 3 min, (95°C 30s, 62°C (−0.5°C decrease each cycle to 55°C), 72°C 45 s) X 14 cycles, (95°C 30 s, 55°C 30 s, 72°C 45s) X 24 cycles, 72°C 7 min, hold 4°C. The PCR product was analyzed by DHPLC on a Transgenomic WAVETM (Transgenomic Inc., San Jose, CA) as described [12]. Samples were “spiked” with control DNA to ensure that a homozygous variant that might migrate as a single peak would be detected. Unusual WAVETM patterns were resolved by automated DNA sequencing on an Applied Biosystems 2100 Avant DNA Sequencer (Applied Biosystems, Foster City, CA).
2.3. Cortisol radioimmunoassay
Cortisol levels were measured in the previous study with a commercially available radioimmunoassay kit (Diagnostic Products Company) as previously described [13]. Inter- and intra-coefficients of variation were less than 6.5% with a detection limit of 0.01 ug/dL.
2.4. Statistical analysis
2.4.1. Genetic analysis of CHRNA7
There are currently 21 known SNPs in the 230bp core promoter fragment upstream of exon 1 in the CHRNA7 gene. Most of these mutations decrease transcription thus would produce the same finding [12]. Individually these SNPs are too rare for statistical analysis. By convention all functional SNPs were pooled into a single allele for analysis [12]. The wild type promoter was designated as allele 1, and the presence of any functional proximal promoter polymorphism was designated allele 2. Genotypes were then derived from combinations of these two alleles. An individual with two wild-type promoters was designated genotype 1/1, an individual carrying one CHRNA7 copy with a wild-type promoter and one containing a proximal promoter polymorphism, 1/2. Any individual with two promoter polymorphisms was designated genotype 2/2. The two mutations are always on different alleles [12].
2.4.2. Association analysis
Maternal and child genotype effects on stress response in the child were explored by testing for mean differences in salivary cortisol levels between genotypic groups, defined by maternal and child genotype combinations at each time point. The procedure utilized a variance components approach implemented in SAS PROC NLMIXED (SAS Institute, Inc., Cary, NC). This approach allows the introduction of variance components that adjust for the correlations that arise due to family of origin and in monozygotic twins. In order to determine whether this variance components approach adjusted appropriately for monozygotic twins, the analysis was also run after deleting one twin from each monozygotic twin pair. This did not substantively change the results of the analysis (data not shown).
Due to the low number of individuals with two CHRNA7 promoter polymorphisms, testing was done assuming a dominant model for having at least one promoter polymorphism. Interaction between maternal and child genotypes was tested via a cross-product term between the two dominant model genotypic terms. The effect of sex and baseline cortisol on stress-induced cortisol levels has been repeatedly demonstrated in the literature [15–18]. In order to address this, we controlled for the child’s sex and baseline cortisol level by including them as independent explanatory variables.
Since CHRNA7 modulates inflammation and atopy is an inflammatory illness, CHRNA7 could be associated with atopy. Tests of linkage and association of CHRNA7 to atopy were carried out using a pedigree disequilibrium test implemented in the UNPHASED software package [19]. Atopy has a significant effect on cortisol response in this cohort. Atopy diagnosis was included as a covariate in the variance components association analysis of CHRNA7 to cortisol levels. Since maternal atopy might also affect levels of inflammation experienced in utero by the offspring, maternal atopy status was also included as a covariate in the analysis of child cortisol levels.
3. Results
3.1. Allele frequencies
The frequency of the wild-type allele (allele 1) was 0.87 and 0.13 for a proximal promoter polymorphism (allele 2). Alleles were in Hardy-Weinberg equilibrium (χ2HWE = 0.34). The most common polymorphisms were a −86 C/T SNP and a −194 G/C SNP with minor allele frequencies of 0.054 and 0.055 respectively comprising 85% of the polymorphisms detected. These are common functional polymorphisms that decrease gene activity by 22% and 26% respectively in a luciferase reporter gene assay [12].
3.2 Salivary cortisol levels
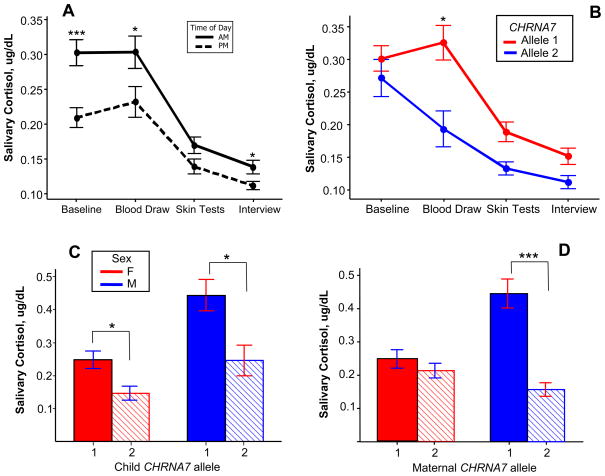
Cortisol levels decreased during the test period in both the AM and PM Groups (Figure 1A). PM levels were significantly lower compared to the AM group at baseline (p < 0.0001, 2 sample T-test), after the blood draw, and after the interview (p < 0.05).
Fig. 1.
Effect of maternal child CHRNA7 polymorphisms on cortisol levels after the blood draw stressor. A. Pattern of cortisol levels during the test day, AM and PM groups. Average cortisol levels decreased at each time point, except at the blood draw time point. PM levels were significantly lower compared to the AM group at baseline, after the blood draw, and after the interview (2 sample T-test). AM group: N = 95; PM group: N = 87 *p < 0.05 ***p < 0.001. B. Cortisol levels during the test day stratified by child genotype (AM group only). On average, adolescents with a normal CHRNA7 promoter (allele 1, red) had a rise in cortisol following the blood draw stressor while those who carried a promoter polymorphism (Allele 2, blue) did not. *p = 0.023. C. Effect of child CHRNA7 polymorphisms on cortisol levels after the blood draw stressor (AM Group). Male and female adolescents with allele 1 had significantly higher salivary cortisol levels after the blood draw compared with those with allele 2. Allele 1, N = 40 females and 32 males; Allele 2, N = 12 females and 11 males. *p < 0.05. D. Effect of maternal CHRNA7 polymorphisms on child cortisol levels after the blood draw (AM Group). Males whose mothers carried a polymorphism had significantly lower cortisol levels compared to those whose mothers had normal promoters. Females were not affected by maternal genotype. Maternal Allele 1, N = 37 females and 35 males; Allele 2, N = 15 females and 8 males. ***p < 0.001. Error bars are one standard error from the mean.
3.3. Child CHRNA7 mutations affect cortisol levels after a stressor
The AM and PM groups were analyzed separately. In the AM group, the child’s genotype was significantly associated with cortisol levels in the child after the blood draw (Table 1). A CHRNA7 polymorphism in the child was associated with stressor induced cortisol levels that were 15.01% lower on average (p = 0.023 by variance components analysis). The overall effect of child CHRNA7 genotype on salivary cortisol levels throughout the test period in the AM group is illustrated in Figure 1B. Subjects with normal CHRNA7 promoters (allele 1) had significantly higher salivary cortisol levels after the blood draw compared to those who carried a promoter polymorphism (allele 2). There were no significant differences between groups at the other three time points. CHRNA7 genotype did not have a significant effect in the PM group.
Table 1. Overall effect of maternal and child CHRNA7 genotype on salivary cortisol levels after a stress.
In the AM Group, the presence of a promoter polymorphism in the child decreased cortisol levels by 15.01%. There was a significant interaction between maternal genotype and sex: Maternal promoter polymorphisms deceased cortisol levels by 37.6% but female offspring of women carrying a polymorphism had 44.72% higher cortisol levels compared to males with the same maternal genotype. In female children, higher baseline cortisol levels were associated with higher levels after the blood draw. This did not occur in males.
In the PM Group, the only parameter associated with cortisol levels after the blood draw was baseline cortisol. There were no significant interactions between any of the variables in this group. CHRNA7 = effect of a CHRNA7 promoter polymorphism in the child (after adjusting for all the other variables). MatCHRNA7 = effect of a CHRNA7 promoter polymorphism in the mother; Sex = effect of female sex; Baseline = effect of baseline cortisol levels on levels after the stress. The effect size indicates that each 1 ug/dL increase in baseline cortisol was associated with a 0.1219 ug/dL increase in cortisol level after the stress. Sex*MatCHRNA7 = the additional effect seen when the child’s sex is female and the mother carries a polymorphism; Baseline* Sex = the additional effect of baseline cortisol levels observed when the child’s sex is female. Child Atopy = presence of an atopic disorder in the child. Maternal Atopy = presence of an atopic disorder in the mother
| AM Group |
PM Group |
||||
|---|---|---|---|---|---|
| Parameter | Effect Size Estimate | P value | Parameter | Effect Size Estimate | P value |
| CHRNA7 | −15.01% | 0.0234 | CHRNA7 | −9.01% | 0.3641 |
| MatCHRNA7 | −37.60% | 6.6x10−4 | MatCHRNA7 | 6.49% | 0.3706 |
| Sex*MatCHRNA7 | 44.72% | 7.0x10−4 | Sex (female) | −10.27% | 0.1539 |
| Sex (female) | 7.32% | 0.4983 | Baseline | 44.67% | 2.6x10−4 |
| Baseline | 12.19% | 0.3969 | Child Atopy | 6.74% | 0.4921 |
| Baseline*Sex | 62.05% | 0.0010 | Maternal Atopy | 6.68% | 0.4676 |
| Child Atopy | −7.10% | 0.1789 | |||
| Maternal Atopy | −6.70% | 0.2365 | |||
3.4 Maternal CHRNA7 genotype affects stress response in male offspring
Maternal genotype was significantly associated with stress induced cortisol levels in the child. Maternal CHRNA7 polymorphisms were associated with 37.6% lower cortisol levels in the child after the blood draw (p = 6.6 × 10−4) (Table 1). There was also a significant interaction between the child’s sex and the maternal genotype indicating the effect of maternal genotype is different in males and females. Due to the interaction, females whose mother carried a CHRNA7 polymorphism had cortisol levels after the stressor that were 44.72% higher than would be expected given the maternal genotype effect (−37.6%) alone (p = 7 × 10−4) (Table 1). Female sex alone was not significantly associated with cortisol levels after the stressor (p = 0.4983).
Overall, baseline cortisol levels were not significantly associated with levels after the stress (p = 0.3969) (Table 1). However, there was a significant interaction between baseline cortisol level and the child’s sex (p = .001). In females, for each 1 ug/dL increase in baseline cortisol there was an additional 0.6205 ug/dL increase in cortisol level after the stressor (p = .001).
There were no significant associations for maternal or child CHRNA7 genotype in the AM group at any of the other three time points (baseline, skin testing or psychiatric interview). Only sex was significantly associated with cortisol levels at these times, with females having lower levels. There was no interaction between sex and baseline cortisol levels at these time points.
In the PM Group there were no significant associations to stressor induced cortisol levels for either child or maternal CHRNA7. In this group, only baseline cortisol levels were associated with stressor induced cortisol levels. The association was significant in both males and females (p = 2.6 × 10−4) (Table 1).
Table 2 contains a summation of the differences between male and female offspring in stress induced cortisol levels in the AM group. In males maternal CHRNA7 polymorphisms were associated with 37.6% lower cortisol levels after the blood draw (p = 6.6 × 10−4), accounting for the maternal genotype effect size shown in Table 1. In female offspring, Maternal CHRNA7 polymorphisms were associated with a nonsignificant 7.12% increase in cortisol levels.
Table 2. Differential effect of CHRNA7 polymorphisms, baseline cortisol levels and atopy on stress induced cortisol levels in male vs. female adolescents (AM group).
CHRNA7 polymorphisms in the child were associated with significantly lower cortisol levels after a stress in both males and females. Maternal CHRNA7 polymorphisms were associated with lower stress induced cortisol levels in males but not females. Higher baseline cortisol levels were associated with higher stress induced cortisol levels, but only in females: for each 1 ug/dL increase in baseline cortisol, there was on average a 0.7424 ug/dL increase in stress induced cortisol.
CHRNA7 = Effect of a CHRNA7 Polymorphism in the child on stress induced cortisol levels; MatCHRNA7 = Effect of a maternal CHRNA7 polymorphism on stress induced cortisol levels in the child; Baseline = effect of baseline cortisol levels on levels after the stress. Child Atopy = presence of an atopic disorder in the child. Maternal Atopy = presence of an atopic disorder in the mother.
| Parameter | Male | P Value | Female | P value |
|---|---|---|---|---|
| CHRNA7 | −15.01% | 0.0234 | −15.01% | 0.0234 |
| MatCHRNA7 | −37.60% | 6.6x10−4 | 7.12% | 0.3637 |
| Baseline | 12.19% | 0.3969 | 74.24% | 4.14x10−8 |
| Child Atopy | −7.10% | 0.1789 | −7.10% | 0.1789 |
| Maternal Atopy | −6.70% | 0.2365 | −6.70% | 0.2365 |
In summary, the data suggests that in the AM group, CHRNA7 polymorphisms in the child were significantly associated with decreased cortisol levels after the blood draw in both males and females (Figure 1C). Maternal CHRNA7 polymorphisms only affected male offspring, but had a larger effect size than the child’s genotype (Figure 1D).
3.5 CHRNA7 genotype is not associated with atopy
The Pedigree Disequilibrium Test indicated no significant association of CHRNA7 to atopy diagnosis (p = 0.202). Maternal and child atopy status were included as covariates in determining child cortisol levels in the variance components analysis (Table 1). After adjusting for the other variables (i.e. maternal and child genotype, baseline cortisol levels and sex), the data suggest that the presence of an atopy diagnosis in the child had an attenuating effect on cortisol levels after the blood draw, decreasing them by 7.1% on average; however this effect did not attain significance (p = 0.1789), as was found in the previous study (p < 0.01). Maternal atopy status was not significantly associated with cortisol levels in the offspring (p = 0.2365) (Table 1). There were no significant interactions between maternal or child atopy and other variables.
4. Discussion
Significant associations between polymorphisms in the CHRNA7 proximal promoter and stressor induced cortisol levels were found in the study population. Maternal CHRNA7 genotype was a key factor in determining the stress response, but CHRNA7 effects were different depending on both the child’s sex and the time of day. Effects on the stress response were seen only in the morning, and maternal genotype only affected male offspring.
Maternal and child CHRNA7 polymorphisms were both associated with lower cortisol levels after a stressor, but the pattern of these effects differed by the sex of the child. CHRNA7 polymorphisms in the child significantly decreased cortisol levels in both males and females (Figure 1C). Maternal CHRNA7 polymorphisms only affected male offspring, but had a larger effect size (Figure 1D). Previous work has demonstrated that differences in maternal cortisol level as well as prenatal stress have dimorphic effects on HPA axis activation and behavioral phenotype in male vs. female offspring in both humans and animals [20–23]. Fetal sex steroid production and differential vulnerability during neural development may play a role differentiating outcomes between males and females [18, 24].
α7nAchRs are likely to affect HPA response due to their location in CNS circuits that are important for cortisol release. α7nAchRs are highly expressed in the hippocampal subiculum and the suprachiasmatic nucleus (SCN) (Breese et al., 1997). Both of these regions send projections to the periventricular nucleus (PVN), the site of corticotrophin-releasing hormone (CRH) secretion. Activation of α7nAchRs modulates pathways within SCN and subiculum that inhibit their normal negative regulatory output to the PVN, permitting release of CRH [9, 25–29]. Thus in individuals with fewer α7nAchRs, inhibitory output to the PVN would increase, limiting the release of CRH and of cortisol. High corticosteroid levels decrease the number of functional α7nAchRs in the hippocampus and hypothalamus [30], suggesting that a feedback mechanism for this system exists.
Effects on stressor induced cortisol were only seen in the morning. The SCN is closely tied to diurnal rhythm, and its regulatory output of cortisol is phasic in nature. The inhibitory output of the SCN rises throughout the day, causing a decrease in cortisol levels. It is possible that the circuits within the SCN that are modulated by α7nAchRs decrease in activity in the afternoon to allow increased output from the SCN. This may in part explain why association to CHRNA7 was not significant in the afternoon, when the output of the SCN is nearing its apex.
Decreased CHRNA7 activity may lead to chronically upregulated inflammatory processes in response to stressors or infection, with increased cytokine output from peripheral immune cells. In 2000, Borovikova et al. described a previously unrecognized parasympathetic pathway by which the brain, via the vagus nerve, decreases systemic inflammatory response [31]. The principal receptor in this cholinergic anti-inflammatory pathway is the α7nAchR. Stimulation of α7nAchRs decreases the production of inflammatory cytokines, including TNFα, interleukin-6, and interleukin-1β [7]. Lower levels of α7nAchR expression such as those seen in individuals with CHRNA7 promoter polymorphisms would result in higher levels of these cytokines following a stressor. In Chrna7 knockout mice, there was no dampening of cytokine release following endotoxin administration. This resulted in increased morbidity and death [7]. Additionally, neutrophils and CD3+ T cells also express α7nAchRs. Stimulation of these receptors inhibits recruitment of these cells in inflammation [32–34]. All of these anti-inflammatory effects would presumably be down regulated in the presence of low CHRNA7 expression, and this could lead to chronic inflammatory states. Chronic inflammation is associated with poor activation of the HPA axis after acute stressors in both animal models [35] and in human inflammatory disease [36, 37]. Thus chronically activated inflammatory pathways, induced by a down regulated CHRNA7 gene, may partially explain the decreased cortisol responses in the current study.
We did not find an association of CHRNA7 genotype with atopy. CHRNA7 may not be a causative factor in atopy. There is no evidence that CHRNA7 is involved in initiating inflammatory responses. Instead it may modulate the strength of the inflammatory reaction that occurs in allergy and other inflammatory disorders. This would lead to a phenotype of more severe symptoms in these illnesses. However, the limited number of informative families for this marker in the sample may have reduced power to detect an association. As in the previous study, the data suggests that atopy may be associated with lower cortisol levels after the blood draw, although this effect did not reach significance in this analysis. This may be due to the fact that we were adjusting for several variables in a relatively small sample. A larger sample size may improve power to detect this effect, which was seen in the previous study.
There were some characteristics of the Wamboldt study that were not ideal for the purposes of the current analysis. Cortisol was sampled in the morning for half the cohort and in the afternoon for the rest, and the stressors in this study are not conventionally used to examine stress response. A more standardized stress protocol would provide a more informative interpretation of this pilot data. Because the subjects were separated into AM and PM groups, there may be spurious effects due to the fact that different groups were tested at different times of day. Further experiments based on this pilot study are needed, and a cross over design where the same subjects are tested both in the morning and in the afternoon might address this question.
The inclusion of identical twins in the analysis while potentially interesting also served as a potential confounding factor. However, deleting one twin from each monozygotic twin pair did not substantively change the results of the analysis suggesting that we were able to adequately adjust for this in the statistical methods. However, we note that we have a small number of mother-offspring pairs in some of the genotype groups, and that power to test for interactions is low in some cases
The most important and interesting finding in the study was the effect of maternal genotype on stress response in the child. The presence of maternal CHRNA7 promoter polymorphisms could result in both suppression of cortisol production and excessive production of pro-inflammatory cytokines, leading to a physiological “double hit” that produces exaggerated maternal inflammatory responses and affects the offspring in utero. Immune activation during pregnancy has effects on neural development in the fetus [4, 5]. This is true of schizophrenia, a disease with which CHRNA7 is known to be associated [8, 38–42]. These findings suggest that a mother’s contribution is more complex than the simple transmission of her genes. The prenatal environment she provides, modulated by her own genetic make-up, may enact permanent changes in her offspring’s capacity to respond to stress. This has implications for how we view the genetics of developmental gene polymorphism in complex disorders. These data emphasize the potential importance of considering both maternal and offspring genotype in studies of developmentally influenced traits.
Acknowledgments
T32 MH15442 to MLS; AA013973 to MLL; NIDA DA09457, NIMH MH81177, and The Veterans Affairs Medical Research Service to SL.
Reference List
- 1.Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 2.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 3.Rexhepaj R, Boini KM, Huang DY, Amann K, Artunc F, Wang K, Brosens JJ, Kuhl D, Lang F. Role of maternal glucocorticoid inducible kinase SGK1 in fetal programming of blood pressure in response to prenatal diet. Am J Physiol Regul Integr Comp Physiol. 2008;294:R2008–R2013. doi: 10.1152/ajpregu.00737.2007. [DOI] [PubMed] [Google Scholar]
- 4.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 5.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin B, Klei L, Myles-Worsley M, Tiobech J, Otto C, Byerley W, Roeder K. Genetic liability to schizophrenia in Oceanic Palau: a search in the affected and maternal generation. Hum Genet. 2007;121:675–684. doi: 10.1007/s00439-007-0358-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang HC, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha 7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 8.Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, Tsuang MT, Farone SV, Malaspina D, Svrakic DM, Sanders A, Gejman P. Linkage disequilibrium for schizophrenia at the chromosome 15q13-14 locus of the alpha 7-nicotinic acetylcholine receptor subunit gene (CHRNA7) American Journal of Medical Genetics. 2001;105:20–22. [PubMed] [Google Scholar]
- 9.Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, Sullivan B, DeMasters BK, Freedman R, Leonard S. Comparison of the regional expression of nicotinic acetylcholine receptor α7 mRNA and [125I]-α-bungarotoxin binding in human postmortem brain. J Comp Neuro. 1997;387:385–398. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 11.Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine & Tobacco Research. 2001;3:203–223. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- 12.Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Association of promoter variants in the alpha 7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 13.Wamboldt MZ, Laudenslager M, Wamboldt FS, Kelsay K, Hewitt J. Adolescents with atopic disorders have an attenuated cortisol response to laboratory stress. J Allergy Clin Immunol. 2003;111:509–514. doi: 10.1067/mai.2003.140. [DOI] [PubMed] [Google Scholar]
- 14.Buske-Kirschbaum A, Geiben A, Hollig H, Morschhauser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. 2002;87:4245–4251. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- 15.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- 20.Butkevich I, Mikhailenko V, Semionov P, Bagaeva T, Otellin V, Aloisi AM. Effects of maternal corticosterone and stress on behavioral and hormonal indices of formalin pain in male and female offspring of different ages. Hormones and Behavior. 2009;55:149–157. doi: 10.1016/j.yhbeh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually Dimorphic Effects of Prenatal Stress on Cognition, Hormonal Responses, and Central Neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 23.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood CE, Saoud CJ, Stoner TA, Keller-Wood M. Estrogen and androgen influence hypothalamic AVP and CRF concentrations in fetal and adult sheep. Regulatory Peptides. 2001;98:63–68. doi: 10.1016/s0167-0115(00)00231-7. [DOI] [PubMed] [Google Scholar]
- 25.Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580:62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- 26.Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–R1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- 27.Buijs RM, Hou YX, Shinn S, Renaud LP. Ultrastructural evidence for intra-and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J Comp Neurol. 1994;340:381–391. doi: 10.1002/cne.903400308. [DOI] [PubMed] [Google Scholar]
- 28.O’Hara BF, Edgar DM, Cao VH, Wiler SW, Heller HC, Kilduff TS, Miller JD. Nicotine and nicotinic receptors in the circadian system. Psychoneuroendocrinology. 1998;23:161–173. doi: 10.1016/s0306-4530(97)00077-2. [DOI] [PubMed] [Google Scholar]
- 29.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 30.Stitzel JA, Farnham DA, Collins AC. Chronic corticosterone treatment elicits dose-dependent changes in mouse brain alpha-bungarotoxin binding. Neuroscience. 1996;72:791–799. doi: 10.1016/0306-4522(95)00584-6. [DOI] [PubMed] [Google Scholar]
- 31.Borovikova LV, Ivanova S, Zhang MH, Yang H, Botchkina GI, Watkins LR, Wang HC, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 32.Giebelen IA, van Westerloo DJ, Larosa GJ, de Vos AF, van der PT. Stimulation of alpha 7 cholinergic receptors inhibits lipopolysaccharide-induced neutrophil recruitment by a tumor necrosis factor alpha-independent mechanism. Shock. 2007;27:443–447. doi: 10.1097/01.shk.0000245016.78493.bb. [DOI] [PubMed] [Google Scholar]
- 33.Razani-Boroujerdi S, Boyd RT, vila-Garcia MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol. 2007;179:2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- 34.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol. 2001;13:905–911. doi: 10.1046/j.1365-2826.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- 36.Cutolo M, Foppiani L, Minuto F. Hypothalamic-pituitary-adrenal axis impairment in the pathogenesis of rheumatoid arthritis and polymyalgia rheumatica. J Endocrinol Invest. 2002;25:19–23. [PubMed] [Google Scholar]
- 37.Crofford LJ. The hypothalamic-pituitary-adrenal axis in the pathogenesis of rheumatic diseases. Endocrinol Metab Clin North Am. 2002;31:1–13. doi: 10.1016/s0889-8529(01)00004-4. [DOI] [PubMed] [Google Scholar]
- 38.Stephens SH, Logel J, Barton A, Franks A, Schultz J, Short M, Dickenson J, James B, Fingerlin TE, Wagner B, Hodgkinson C, Graw S, Ross RG, Freedman R, Leonard S. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109:102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu JZ, Pato MT, Dalla Torre C, Medeiros H, Carvalho C, Basile VS, Bauer A, Dourado A, Valente J, Soares MJ, Macedo AA, Coelho I, Ferreira CP, Azevedo MH, Macciardi F, Kennedy JL, Pato CN. Evidence for linkage disequilibrium between the alpha 7- nicotinic receptor gene (CHRNA7) locus and schizophrenia in Azorean families. Am J Med Gen. 2001;105:669–674. doi: 10.1002/ajmg.1549. [DOI] [PubMed] [Google Scholar]
- 40.Leonard S, Gault J, Moore T, Hopkins J, Robinson M, Olincy A, Adler LE, Cloninger CR, Kaufmann CA, Tsuang MT, Faraone SV, Malaspina D, Svrakic DM, Freedman R. Further investigation of a chromosome 15 locus in schizophrenia: analysis of affected sibpairs from the NIMH Genetics Initiative. Am J Med Genet. 1998;81:308–312. doi: 10.1002/(sici)1096-8628(19980710)81:4<308::aid-ajmg6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 41.Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Drebing C, Short M, Walton K, Berger R, Ross R, Olincy A, Adler L, Freedman R. DNA variants in the alpha 7 nicotinic receptor gene promoter are associated with schizophrenia. Biological Psychiatry. 2001;49:571. [Google Scholar]
- 42.Tsuang DW, Skol AD, Faraone SV, Bingham S, Young KA, Prabhudesai S, Haverstock SL, Mena F, Menon AS, Bisset D, Pepple J, Sauter F, Baldwin C, Weiss D, Collins J, Boehnke M, Schellenberg GD, Tsuang MT. Examination of genetic linkage of chromosome 15 to schizophrenia in a large veterans affairs cooperative study sample. Am J Med Gen. 2001;105:662–668. [PubMed] [Google Scholar]