Abstract
Paced 0.1 Hz breathing causes high amplitude HR oscillation, triggering resonance in the cardiovascular system (CVS). This oscillation is considered to be a primary therapeutic factor in HRV biofeedback treatments. This study examined whether rhythmical skeletal muscle tension (RSMT) can also cause 0.1 Hz resonance in the CVS, and compared oscillatory reactivity in CVS functions caused by RSMT and paced breathing (PB). Sixteen young healthy participants completed five tasks: baseline, three RSMT tasks at frequencies of 0.05, 0.1, and 0.2 Hz, and a 0.1 Hz PB task. ECG, respiration, finger pulse, and skin conductance data were collected. Results showed that 0.1 Hz RSMT as well as 0.1 Hz PB triggered resonance in the CVS and caused equivalent oscillations in all measured CVS functions, although in women, RSMT compared to PB caused lower HR oscillation. Clinical application of 0.1 Hz RSMT is discussed.
Resonance in the Cardiovascular System Caused by Rhythmical Muscle Tension
Resonance is a phenomenon characterized by the appearance of oscillation in a system at a specific frequency (resonance frequency) in response to perturbation. Such a system is called a resonance system or a system with a resonance property. The cardiovascular system (CVS) reveals resonance properties at frequencies of about 0.1 and 0.03 Hz due to the arterial baroreflex (Vaschillo, et al., 2002; van de Vooren, et al., 2007; Hammer and Saul, 2005). The 0.1 Hz resonance property of the CVS has demonstrated clinical utility in the treatment of disorders that involve autonomic nervous system dysregulation because patients may be trained to voluntarily produce high-amplitude oscillations in cardiovascular functions. Paced breathing at a rate of 0.1 Hz has been used effectively in heart rate variability (HRV) biofeedback (Lehrer, Vaschillo, and Vaschillo, 2000) to improve symptoms of physical and mental disorders including asthma (Lehrer et al., 2004), hypertension (McCraty et al., 2003; Yucha et al., 2005), coronary heart disease (Cowan, Pike, & Budzynski, 2001; Del Pozo et al., 2004; Nolan et al., 2005), major depression (Karavidas et al., 2007), and fibromyalgia (Hassett et al., 2007). The therapeutic effects of HRV biofeedback are thought to be due to the induction of high-amplitude oscillations in HR, BP, and VT which exercise and activate homeostatic reflexes (e.g., the baroreflex), retrain them (Chernigovskaya et al., 1990; Lehrer et al., 2003, 2004), and initiate, through the baroreceptors, a cascade of neurobiological events that produces a generalized inhibitory effect on the brain (Dworkin et al., 1994; Elbert et al., 1992; Nyklicek et al., 2005; Rau et al., 1993; Yasumasu et al., 2006). France et al., 2006 further showed that rhythmical skeletal muscle tension at a rate of 0.1 Hz increases brain oxygenation and effectively prevents the vasovagal reaction in people with a history of neurocardiogenic syncope, yet did not link these effects to resonance in the CVS. If rhythmical skeletal muscle tension at a rate of 0.1 Hz also induces high-amplitude oscillations in cardiovascular functions, it could lead to new clinical applications in the areas rehabilitation and sports medicine. The present study examined whether 0.1 Hz rhythmical skeletal muscle tension triggers resonance in the CVS, rather than forced oscillations, by comparing the amplitude of oscillation at other muscle tension frequencies, as well as to oscillations triggered by 0.1 Hz breathing.
The Baroreflex Provides Resonances in the CVS
The arterial baroreflex is believed to be the mechanism that provides for resonance in the CVS. The baroreflex links at least three cardiovascular functions. Blood pressure (BP) interrelates with heart rate (HR) in the HR baroreflex branch and with vascular tone (VT) in the VT baroreflex branch. Both branches of the baroreflex have been conceptualized as closed-loop control systems with negative feedback that effectively buffers incidental blood pressure fluctuations (Just et al., 1994; Christou, Jones, & Seals, 2003; Jones, Christou, Jordan, & Seals, 2003; Jordan et al., 2002). Because the baroreflex closed-loop has an inherent delay, a shift in BP is not corrected immediately. Rather, a BP shift triggers the baroreflex which eliminates the shift, but only after the delay. Then, elimination of the BP shift again triggers the baroreflex which in turn, after a delay, causes a new BP shift, but with a lower magnitude. This process continues until the new shift magnitude reaches ‘0’. Thus, any BP shift will evoke a faded BP oscillation at a resonance frequency that depends on the time of the delay.
In the HR baroreflex branch, BP response to HR change is delayed for 5–6 heart beats. Thus, the delay of BP response to HR changes in the HR baroreflex branch lasts for about 5 s in humans, about 1.7 s in rabbits, and about 1.25 s in rats. Accordingly, HR and BP oscillation occurs at a frequency of about 0.1 Hz (10 s oscillation period) in humans (De Boer, Karemaker, & Strackee, 1987; Saul et al., 1991; Vaschillo et al., 2002), 0.3 Hz (3.3 s oscillation period) in rabbits (Janssen et al., 1997; Malpas & Burgess, 2000), and 0.4 Hz (2.5 s oscillation period) in rats (Bertram et al., 1998; Burgess, et al., 1997; Madwed et al., 1989; Bertram et al., 2000). Due to the delay in the closed-loop, a stabilizing negative-feedback baroreflex system becomes a resonance system that oscillates at a frequency whose period equals twice the magnitude of the delay (Grodins, 1963; Hammer & Saul, 2005). The same conversion of a stabilizing system into resonance system may be observed in the VT baroreflex branch. The VT baroreflex closed loop has resonance properties at a frequency of about 0.03 Hz due to a 12–15 s delay of the VT reaction to BP change (Vaschillo et al., 2002; Magosso, Biavati, & Ursino, 2001).
Rhythmical Stimulation of the CVS May Elicit Oscillation in Its Functions
A single internal or external impact on the baroreflex system causes faded oscillations in the cardiovascular functions at resonance frequencies. Rhythmical internal or external stimulation at resonance frequencies produces stable high-amplitude oscillations in these functions (Vaschillo et al., 2010). Breathing continuously affects HR through the mechanism of respiratory sinus arrhythmia (RSA), eliciting oscillation in HR and BP at the respiratory frequency. Due to resonance, breathing at a frequency of about 0.1 Hz elicits HR oscillation with significantly higher amplitude than breathing at other frequencies (Angelone & Coulter, 1964; De Boer, Karemaker, & Strackee, 1987; Vaschillo and Vaschillo, 2009; Vaschillo et al., 2010). Although breathing has been most studied, there is some evidence that other forms of rhythmical stimulation at 0.1 Hz also elicit high-amplitude oscillations in HR at a frequency of 0.1 Hz. We previously showed that high-amplitude 0.1 Hz HR oscillations were caused by emotional picture cue stimulation at a rate of 0.1 Hz (5 s picture on, 5 s picture off) (Vaschillo et al., 2008). Effects of rhythmical physical load on CVS functions also have been investigated (Wigertz, 1971; Tiedt, Wohlgemuth, & Wohlgemuth, 1975; Lehrer et al., 2009). The most comprehensive study (Wigertz, 1971) found that rhythmical physical loads varying from 250 to 1050 kpm/min at discrete frequencies in a range of 0.022 – 0.001 Hz (period range 0.75–16.67 min) caused sine-wave oscillations in BP, HR, VT, partial O2 pressure in blood (PO2), respiration rate, and tidal volume. Transfer functions of HR, BP, PO2, and respiration control systems revealed resonance properties in BP and PO2 control systems at frequencies of ~0.0023 Hz (7.25 min period) and ~0.0055 Hz (3 min period), respectively. Wigertz’s study could not reveal CVS resonance at 0.1 Hz, however, because the frequencies tested were significantly lower than 0.1 Hz.
More recent study (France et al., 2006; Lehrer et al., 2009) showed that rhythmical muscle tension stimulation at 0.1 Hz did cause high-amplitude oscillation in HR, BP, and VT. France et al., (2006) developed a 0.1 Hz rhythmical skeletal muscle tension (0.1 Hz RSMT) technique that effectively prevented the vasovagal reaction by significantly increasing blood pressure and brain oxygenation. The technique consisted of paced skeletal muscle tense-release cycles at a frequency of 0.1 Hz wherein the patient continuously repeated cycles of 5 s voluntary muscle tension and 5 s muscle relaxation. We hypothesize that high-amplitude oscillations elicited by RSMT improve autonomic regulation and inhibitory modulation of the brain through the baroreceptops, as do oscillations elicited by 0.1 Hz paced breathing (Dworkin et al., 1994; Nyklicek et al., 2005; Yasumasu et al., 2006), and by doing so, may actively participate in increasing brain blood flow, providing a mechanism which averts vasovagal reaction (France et al., 2006). Human’s ability to voluntarily produce high amplitude oscillations in CVS through RSMT may thus be able to be used more widely in clinical practice.
In summary, the previous literature demonstrates that high-amplitude 0.1 Hz oscillation in the CVS can be elicited by paced breathing, visual stimulation, and muscle tension. Yet, only paced breathing at a frequency of ~0.1 Hz has been clearly shown to elicit considerably higher oscillation in cardiovascular functions than at other frequencies (De Boer, Karemaker, & Strackee, 1987; Cooke, et al., 1998; Vaschillo and Vaschillo, 2009; Vaschillo et al., 2010), indicating that 0.1 Hz stimulation elicits resonance, not forced oscillation in the CVS. Although it is likely that high-amplitude oscillations induced by paced muscle tension also are related to the 0.1 Hz resonance in the CVS, this has not been empirically demonstrated by comparing the amplitude of oscillation at other frequencies close to 0.1 Hz.
Goals of the Study
This study extended the Wigertz (1971) and Lehrer et al. (2009) studies by testing cardiovascular reactions to rhythmical muscle load in a frequency range of 0.05 – 0.2 Hz, to determine whether 0.1 Hz rhythmical physical load can trigger resonance in the CVS. We compared the effects of RSMT at 0.05, 0.1, and 0.2 Hz on HR, pulse transit time (PTT), finger pulse amplitude (FPA), skin conductance (SC), and respiration volume (RV). The 0.1 Hz testing frequency corresponded to the resonance frequency of the CVS; the other two testing frequencies were selected to contrast with the resonance frequency. They included 0.05 Hz, which is in the very low frequency range of the spectrum (0.005 – 0.05 Hz), but above the vascular tone baroreflex resonance frequency (0.03 Hz) (Vaschillo et al., 2002), and 0.2 Hz in the high frequency (respiratory) range of the spectrum (0.15 – 0.5 Hz) (Task Force, 1996). HR and PTT are functions that directly contribute to 0.1 Hz and 0.03 Hz resonance origination, respectively. FPA was studied as a component function of the CVS that does not directly participate in the mechanism of resonance. SC was included as an autonomic function that is not directly mediated by the CVS, however we propose that it can reveal spreading of resonance oscillation in the CVS through the autonomic nervous system (ANS). Respiration is considered as a continuous stimulator of the CVS. Cardiovascular effects caused by 0.1 Hz-rhythmical skeletal muscle tension and 0.1 Hz paced breathing were compared to evaluate the potential of further application of RSMT stimulation in clinical practice.
METHOD
Participants
Sixteen healthy young adults (9 females and 7 males) with a mean age of 21.6 years (S.D. = 3.8) participated in the study. We chose young and healthy volunteers to study the intact mechanism of the baroreflex resonance. Participants were asked to abstain from alcohol, caffeine, and strenuous exercise on the day of testing. The project was approved by the Rutgers University Institutional Review Board for the Protection of Human Subjects Involved in Research. Written informed consent was obtained from all participants.
Procedures
Participants were seated in a comfortable armchair in front of a computer screen with their legs extended and supported parallel to the floor. Electrodes and sensors to record physiological signals were attached. Participants performed a 5-min baseline task, then, after 1-min of instruction and 3–4 min of RSMT training, three RSMT tasks at frequencies of 0.05, 0.1, and 0.2 Hz were completed in random order. Each task lasted for 3.5-min, with a 30-s inter-task interval. Next, after a 1-min rest period, they completed a 5-min paced breathing task at a frequency of 0.1 Hz (6 breaths per minute). This task was always conducted last because 5-min of 0.1 Hz breathing elicits a strong effect on cardiovascular regulation (Lehrer et al., 2003) which may have interfered with interpretation of the primary RMST comparisons. Participants were instructed and trained in slow breathing during a 3 – 4 min rest period before the paced breathing task. Each person was tested individually.
Electrocardiogram (ECG), respiration, finger pulse, and SC were recorded continuously across all tasks. To collect ECG data, a negative electrode was attached to the upper right arm, a positive electrode to the lower left leg, and a ground electrode to the upper left arm. To record respiration data, a belt with a sensor was placed around the chest. Skin preparation for recording SC consisted of washing the hands in soapy water, rinsing, and thorough drying. No conductive paste was used for the skin area under the electrodes. SC electrodes (metallic 25 × 25 mm squares) were attached to the edges of the palm (to eminentia thenar and eminentia hypothenar) of the right hand and secured with surgical tape. The SC signal was set to 0 prior to recording. A piezoelectric finger pulse sensor was attached to the palm of the right index finger and fixed with a cuff. Participants were asked to keep their right hand still during the experiment.
Experimental Tasks
The baseline “plain vanilla” task (BL task) was a low cognitive demand task used to equate mental activity across individuals (Jennings et al., 1992). Participants viewed a colored computer screen, which sequentially changed color every 10 s. They were asked to silently count the number of blue screens.
In the RSMT tasks, participants performed repeated “muscle tension – muscle relaxation” sequences that were temporally paced according to a change in color (red/green) on the computer screen. They contracted muscles when the screen was red and relaxed their muscles when the screen became green. Specifically, participants were instructed: “You will need to tense all your muscles from waist down including muscles of abdomen, buttocks, and legs and all muscles in your left arm, as soon as you see a red computer screen, and quickly relax them when the color of the screen becomes green.” Each participant completed three RSMT tasks at different testing frequencies: 0.2 Hz (0.2 Hz RSMT task), 0.1 Hz (0.1 Hz RSMT task), and 0.05 Hz (0.05 Hz RSMT task) wherein the screen changed its color every 2.5 s, 5 s, and 10 s, respectively. Participants were trained in the RSMT technique at different frequencies during 2–3 minutes after they finished the baseline task, just before starting the RSMT tasks.
In the six times per minute paced breathing task (0.1 Hz PB task), participants breathed following a visual 0.1 Hz pacer-bar on the computer screen wherein they inhaled along with the pacer for 5 s as it moved up on the screen and exhaled along with the pacer for 5 s as it moved down. They were asked not to breathe too deeply in order to avoid hyperventilation.
Apparatus and Software
A PowerLab Acquisition System (ADInstruments, Colorado Springs, CO) was used to collect ECG, respiration, finger pulse, skin conductance, and the time marker signal used to select experimental tasks for data analysis. The data sampling rate for each channel was 1000 times per second. To collect SC data, a Powerlab Galvanic Skin Response (GSR) amplifier was used. The GSR used constant-voltage AC excitation (22 mV rms @ 75 Hz). The current density was < 0.5 µA cm2.
WinCPRS software (Absolut Aliens Oy, Turku, Finland) was used to analyze physiological data. A 75-cm LCD TV screen (View Sonic N3000W) was used to present the muscle tension rhythm to participants. The pacer rhythm was programmed using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) with an accuracy of ±1 ms.
Psychophysiological Data Analysis
Analysis was performed separately for each of the tasks, including the baseline task and four stimulation tasks: the 3 RSMT tasks and the paced breathing task. WinCPRS software was used to measure the sequences of beat-to-beat RR intervals (RRI) of ECG, beat-to-beat pulse transit time (PTT), and beat-to-beat finger pulse amplitudes (FPA). PTT was measured as the time between the R-spike of the ECG wave and the apex of the finger pulse wave. PTT served as an estimate of the vascular tone (VT), with shorter PTT corresponding to higher VT (Naschitz et al., 2004; Naka et al., 2006). Artifacts and missed or irregular beats were manually modified by interpolation prior to analysis. Spectral analysis of RRI, PTT, and FPA data was conducted as described by Cooke et al. (1999) and Taylor et al. (1998) using WinCPRS software. Cubic interpolation of the non-equidistant waveforms of the RRI, PTT, and FPA sequences was completed, and measurements were resampled at 4 Hz.
To estimate the magnitude of the functions’ oscillatory response (amplitudes of the functions’ oscillation) to rhythmical stimulation, spectral power indices were measured as the power of the RRI, PTT, FPA, RV, and SC spectra at each tested frequency (i.e., 0.05, 0.1, and 0.2 Hz) for each of the four stimulation tasks.
Statistical Analysis
To estimate the significance of differences between the magnitudes of oscillatory responses to 0.05, 0.1, and 0.2 Hz RSMT stimulation, a 2 × 3 multivariate analyses of variance (MANOVA) with repeated measures was used to examine the effect of RSMT on spectral power indices of the five physiological functions (RRI, PTT, FPA, RV, and SC) within a mixed between-subjects (gender group: M and F) and within-subjects (stimulus frequency tasks) design. Univariate contrasts were used to probe significant omnibus effects. The results of these analyses determine the frequency at which RSMT stimulation caused the strongest effect. A similar 2 × 2 repeated measures MANOVA was used to examine differences in spectral power indices between the 0.1 Hz RSMT and the 0.1 Hz paced breathing conditions. The comparison of 0.1 Hz rhythmical muscle tension and breathing is informative for understanding whether RMST may be useful in clinical applications where 0.1 Hz breathing biofeedback has shown therapeutic effects (Lehrer at al., 2004, Karavidas et al., 2007; Hassett at al., 2007; McCraty et al., 2003; Yucha et al., 2005; Del Pozo et al., 2004;, Nolan et al., 2005).
In addition to these analyses of spectral power indices which represent oscillatory magnitude, mean values of the functions during rhythmical stimulation were calculated in order to better understand the dynamics of their oscillatory activity. Oscillatory RRI activity closely links to the mean values of the RRI. Thus, healthy people with higher mean RRI usually have higher heart rate variability (HRV) and HRV in an individual usually goes down when the mean RRI decreases. A 2 × 5 repeated measures MANOVA was used to examine differences in the mean values of the physiological functions within a mixed between-subjects (gender group: M, F) and within-subjects (BL task, 0.1 Hz PB task, 0.05 Hz RSMT task, 0.1 Hz RSMT task, and 0.2 Hz RSMT task) design. Analyses were performed using SAS 9.1. p<0.05 was considered statistically significant. All indices were log transformed prior to analysis to provide normal distributions.
RESULTS
An example of one participant’s oscillation in RV, RRI, PTT, FPA, and SC during the 0.1 RSMT is presented in Figure 1.
Figure 1.
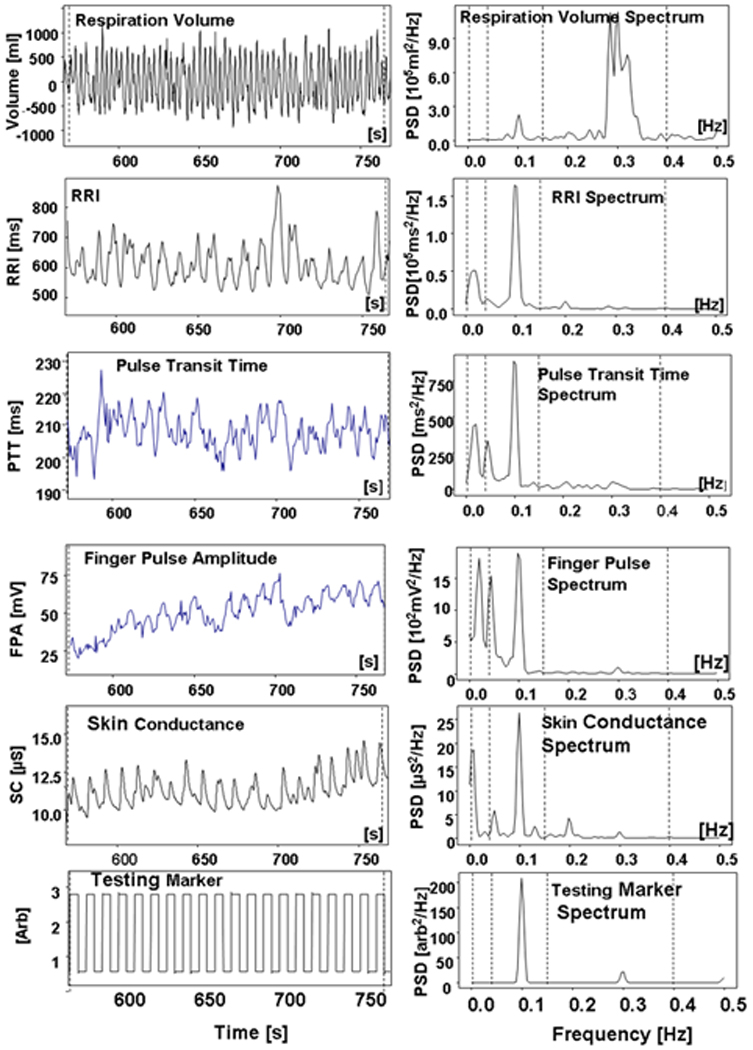
An example of oscillatory response of autonomic nervous system functions to the 0.1 Hz RSMT procedure.
Frequency Dependence of Functions’ Oscillatory Reactions to RSMT
The amplitude of the oscillations in all functions assessed by spectral power indices depended on the frequency of the RSMT procedure. The omnibus tests of the repeated measures MANOVA (see Table 1) of the spectral power indices showed statistically significant differences in the amplitudes of oscillatory reactions caused by stimulation at the three frequencies for RRI, PTT, FPA, and SC, but not RV.
Table 1.
Repeated-measures MANOVA and univariate contrasts of physiological reactions to three frequencies of RSMT.
| Overall Effect | RRI | PTT | FPA | SC | RV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | df | F or t | p | df | F or t | p | df | F or t | p | df | F or t | p | df | F or t | P |
| Within-subjects effects | |||||||||||||||
| Tasks omnibus test | (2, 28) | 23.73 | <.0001 | (2, 28) | 17.61 | <.0001 | (2, 26) | 10.94 | .0004 | (2, 28) | 7.03 | .0039 | (2,28) | 3 18 | .0663 |
| 0.1 Hz vs. 0.05 Hz | (1, 14) | 31.35 | <.0001 | (1, 14) | 9.49 | .0088 | (1, 13) | 5.43 | .0366 | (1, 14) | 0.56 | .467 | (1, 14) | 5.42 | .0354 |
| 0.1 Hz vs. 0.2 Hz | (1, 14) | 96.60 | <.0001 | (1, 14) | 41.10 | <.0001 | (1, 13) | 6.47 | .0245 | (1, 14) | 6.62 | .022 | (1, 14) | 4.25 | .0583 |
| 0.05 Hz vs. 0.2 Hz | (1, 14) | 0.10 | 0.76 | (1, 14) | 7.92 | .0146 | (1, 13) | 18.73 | .0008 | (1, 14) | 15.36 | .0015 | (1, 14) | 0.64 | .4375 |
| Within-subjects by Between-subjects Interaction Effects | |||||||||||||||
| Tasks × Gender omnibus test | (2,28) | 0.71 | .45 | (2, 2) | 2.62 | .1084 | (2, 26) | 0.89 | .886 | (2, 28) | 0.47 | .466 | (2, 28) | 0.11 | .1194 |
| Between-subjects Effects | |||||||||||||||
| Gender omnibus test | (1, 14) | 7.06 | .0188 | (1, 14) | 0.34 | .5715 | (1, 13) | 5.36 | .0377 | (1, 14) | 0.34 | .5706 | (1, 14) | 0.38 | .5 |
| Between-subjects Effects per Each Stimulus Type | |||||||||||||||
| 0.1 Hz: F vs. M | (1, 14) | −2.30 | .0373 | (1, 14) | −1.00 | .3368 | (1, 13) | −1.83 | .0897 | (1, 14) | 0.76 | .4581 | (1, 14) | −0.64 | .5357 |
| 0.2 Hz: F vs. M | (1, 14) | −2.44 | .0284 | (1, 14) | 0.75 | .4656 | (1, 13) | −2.14 | .0521 | (1, 14) | 0.76 | .4588 | (1, 14) | 0.08 | .9402 |
| 0.05 Hz: F vs. M | (1, 14) | −1.98 | .0674 | (1, 14) | −1.52 | .1519 | (1, 13) | −2.05 | .0612 | (1, 14) | −0.08 | .9377 | (1, 14) | −1.42 | .1783 |
Notes. The physiological reactions are estimated by Spectral power indices that were measured as the power of RRI, PTT, FPA, SC, and RV spectra for each of the three (0.05 Hz, 0.1 Hz, and 0.2 Hz) Rhythmical Skeletal Muscles Tension (RSMT) stimulation tasks. RRI – R-R interval of ECG; PTT – Pulse Transit Time as time between R wave of ECG and apex of finger pulse; FPA – Finger Pulse Amplitude; SC – Skin Conduction; RV – Respiration Volume.
All indices were log transformed. All analyses met the assumption of sphericity. 0.05 Hz = 0.05 Hz RSMT task; 0.1 Hz = 0.1 Hz RSMT task; 0.2 Hz: = 0.2 Hz RSMT task.
As shown by the 0.1 Hz RRI spectral power indices, participants’ average magnitude of RRI oscillatory response to 0.1 Hz RSMT stimulation was significantly higher than to either 0.05 Hz or 0.2 Hz RSMT stimulation (see Figure 2C). Similarly, participants’ average magnitude of PTT oscillatory response to 0.1 Hz RSMT stimulation was significantly higher than to either 0.05 Hz or 0.2 Hz (see Figure 2D). The results thus confirm the hypothesized higher RRI and PTT responses to RSMT stimulation at 0.1 Hz than at 0.05 and 0.2 Hz, although there was more variability in the 0.1Hz PTT index. Data plotted from each participant, shown in Figures 2A and 2B, suggest that these significant differences in the sample mean well-represented individual level responses.
Figure 2.
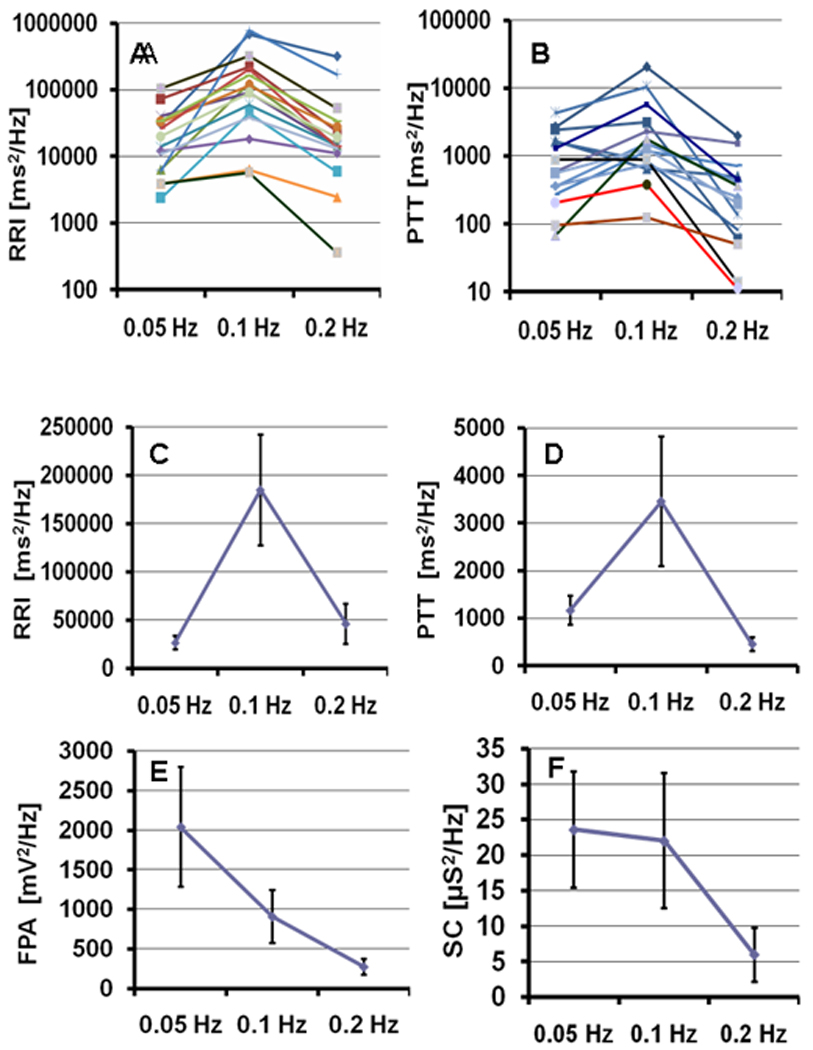
Spectral estimates of the function oscillatory response to 0.05 Hz, 0.1 Hz, and 0.2 Hz RSMT tasks (Spectral power indices that were measured as the power of RRI, PTT, FPA, SC, and RV spectra for each of the three (0.05 Hz, 0.1 Hz, and 0.2 Hz) Rhythmical Skeletal Muscles Tension (RSMT) stimulation tasks. RRI – R-R interval of ECG; PTT – Pulse Transit Time as time between R wave of ECG and apex of finger pulse; FPA – Finger Pulse Amplitude; SC – Skin Conductance) . A and B - Individual responses of RRI and PTT for each participant. (Note: The Y-axis in A and B utilizes a logarithmic scale). C, D, E, and F – Participants’ averaged RRI, PTT, FPA, and SC responses, respectively. Error bars represent 1.96 standard errors.
Participants’ average magnitude of FPA and SC oscillatory responses to RSMT stimulation also significantly varied at the three tested frequencies, but the pattern was different than for RRI and PTT. The oscillatory response to 0.1 Hz RSMT was not dominant for these functions, rather the amplitudes of their imposed oscillations tended to be the higher as the RSMT frequency was lower (see Figures 2E and 2F).
Interaction effects between gender and tasks were not statistically significant (Table 1). The omnibus gender test indicated significant differences between men and women only in RRI and FPA oscillatory response. Men had significantly higher RRI spectral power indices in response to 0.1 Hz and 0.2 Hz RSMT stimulations than did women. Gender differences in FPA were in the same direction, but not statistically significant (p>.05).
Comparison of Oscillatory Reactions to 0.1 Hz RSMT and 0.1 Hz Paced Breathing
Results of the comparison of 0.1 Hz oscillations caused by 0.1 Hz RSMT and 0.1 Hz paced breathing are presented in Table 2. RRI and RV 0.1 Hz oscillations elicited by paced breathing were significantly higher than those elicited by RSMT stimulation. Participants’ mean (std) log transformed 0.1 Hz RRI spectral power index was 13.02(0.88) vs. 11.37(1.43) [log(ms2/Hz)], and mean (std) log transformed 0.1 Hz RV spectral power index was 7.91(1.71) vs. 4.92(2.46) [log(ml2/Hz)] for paced breathing and muscle tension, respectively. No main gender effects were statistically significant, although there was one significant task by gender interaction effect. Women’s RRI oscillatory reaction to 0.1 Hz RSMT, but not to 0.1 Hz breathing, was significantly lower than men’s (see Figure 3).
Table 2.
Repeated-measures MANOVA and univariate contrasts for comparison of physiological reactions to 0.1 Hz RSMT and 0.1 Hz breathing.
| Overall Effect | RRI | PTT | FPA | SC | RV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | df | F or t | p | df | F or t | p | df | F or t | p | df | F or t | p | df | F or t | P |
| Within-subjects Effects | |||||||||||||||
| Task test | (1, 14) | 22.80 | <.0003 | (1, 13) | 0.19 | .674 | (1,13) | 0.20 | .664 | (1, 14) | 0.47 | .504 | (1,14) | 24.99 | .0002 |
| Task × Gender test | (1,14) | 12.65 | .0032 | (1, 14) | 0.77 | .394 | (1, 13) | 0.10 | .762 | (1,14) | 0.59 | .455 | (1,14) | 0.11 | .1194 |
| Between-subjects Effects | |||||||||||||||
| Gender test | (1, 14) | 0.61 | .447 | (1, 14) | 0.18 | .681 | (1, 13) | 3.67 | .077 | (1, 14) | 1.94 | .185 | (1,14) | 0.09 | .773 |
| Between-subjects Effects per Each Stimulus Type | |||||||||||||||
| 0.1 Hz RSMT: F vs. M | (1, 14) | −2.30 | .037 | (1, 14) | −1.00 | .337 | (1, 13) | −1.83 | .089 | (1, 14) | 0.76 | .458 | (1, 14) | −0.64 | .536 |
| 0.1 Hz PB: F vs. M | (1, 14) | 1.94 | .073 | (1, 14) | 0.57 | .578 | (1, 13) | −1.74 | .105 | (1, 14) | 1.79 | .096 | (1, 14) | 0.30 | .772 |
Notes. The physiological reactions are estimated by Spectral power indices that were measured as the power of RRI, PTT, FPA, SC, and RV spectra for 0.1 Hz Rhythmical Skeletal Muscles Tension (RSMT) and 0.1 Hz PB (Paced Breathing) stimulation tasks. RRI – R-R interval of ECG; PTT – Pulse Transit Time as time between R wave of ECG and apex of finger pulse; FPA – Finger Pulse Amplitude; SC – Skin Conduction; RV – Respiration Volume.
All indices were log transformed. All analyses met the assumption of sphericity.
Figure 3.
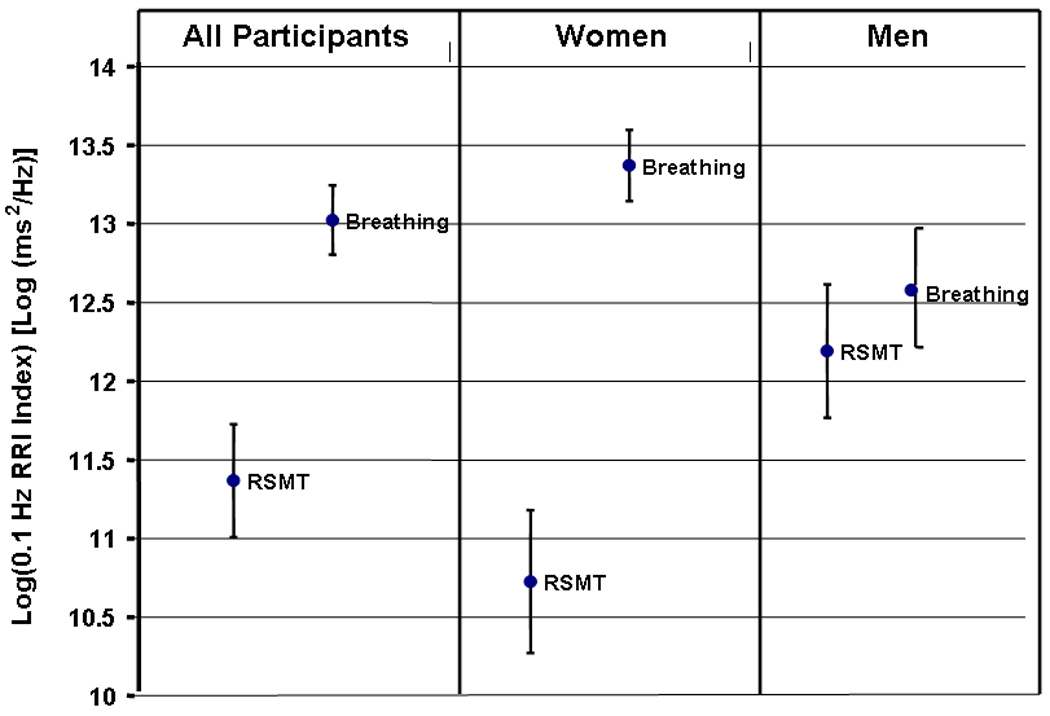
Averaged 0.1 Hz RRI indices (Spectral power indices that were measured as the power of RRI spectra at 0.1 Hz) in 0.1 Hz RSMT and 0.1 Hz paced breathing tasks for women, men, and all participants. Error bars represent 1.96 standard errors.
Changes in Mean Values of CVS Functions in Response to RSMT and 0.1 Hz Breathing
The omnibus tests of the repeated measures MANOVA of mean function values in the five tasks (baseline, 0.1 Hz PB, and the three RSMT) showed statistically significant differences for RRI F(4,56) = 13.8, p = <0.0001; FPA F(4,56) = 2.58, p = 0.047; and SC F(4,56) = 16.88, p = <0.0001, but not PTT. The omnibus tests of Gender and the Task × Gender interaction were not significant.
Table 3 shows mean (standard deviation) values for each task. Mean RRI decreased in response to RSMT stimulation at all frequencies and tended to be lower, the higher the stimulation frequency. The mean RRI was significantly lower during all RSMT stimulation tasks, compared to baseline and the PB task. The mean RRI in the PB task was virtually the same as at baseline, suggesting that participants’ cardiovascular state at the start of the PB task had recovered from the physical load of the RSMT tasks. Rhythmical stimulations significantly increased the mean value of SC compared to the baseline. The mean FPA did not react to RSMT stimulations, but decreased significantly in response to 0.1 Hz breathing.
Table 3.
Participant’s averaged across tasks mean values of the physiological functions.
| Function | RRIMean m (std) |
PTTMean m (std) |
FPAMean m (std) |
SCMean m (std) |
|
|---|---|---|---|---|---|
| Task | |||||
| [ms] | [ms] | [arb] | [µS] | ||
|
Baseline task |
a | 827.12 (106.45) c**,d**,e** | 249.27 (24.56) | 44.24 (36.61) b** | −1.88(5.48) b**, c**,d**,e** |
|
PB 6 b/min task |
b | 824.87 (79.44) c**,d**,e** | 248.23 (22.64) | 35.26 (37.21) a**,c*,d*,e* | 10.47(8.27) a** |
|
RSMT 0.05 Hz task |
c | 769.81 (92.92) a**,b**,e** | 247.36 (35.02) | 43.37 (42.06) b* | 8.88 (9.76) a** |
|
RSMT 0.1 Hz task |
d | 758.37 (90.12) a**,b ** | 245.68 (45.45) | 44.33 (44.18) b** | 9.65 (10.15) a** |
|
RSMT 0.2 Hz task |
e | 744.37 (88.74) a**,b**,c ** | 246.26 (43.32) | 42.74 (40.80) b* | 9.58 (8.66) a** |
Note: RRI – R-R interval of ECG; PTT – Pulse Transit Time as time between R wave of ECG and apex of finger pulse; FPA – Finger Pulse Amplitude; Baseline - the plain vanilla task; PB – six times per minute breathing task; Rhythmical Skeletal Muscles Tension (RSMT) at 0.05 Hz, 0.1 Hz, and 0.2 Hz - tasks.
p < 0.01,
p< 0.001.
DISCUSSION
CVS Oscillatory Reactions to RSMT Stimulation at Different Frequencies
The present results indicate that RSMT stimulation elicits oscillations in RRI, PTT, FPA, SC, and RV at all tested frequencies. The amplitude of the oscillations in these functions (spectral power indices), except for RV, significantly depended on the stimulation frequency. RRI and PTT revealed the strongest reaction to 0.1 Hz RSMT stimulation, while FPA and SC response to RSMT was higher for the lower stimulation frequency (Figures 2E and 2F).The strongest RRI reaction was in response to 0.1 Hz stimulation, however large individual differences in RRI reactions to various frequency stimulations were observed (Figure. 2A), likely reflecting individual differences in baroreflex activity (in the baroreflex gain). Significantly higher RRI response to RSMT at 0.1 Hz than to RSMT at 0.2 or 0.05 Hz appears to be an indicant of the 0.1 Hz resonance in the CVS that is produced by HR - BP interaction in the HR baroreflex closed-loop. This suggests that 0.1 Hz RSMT stimulation triggered resonance in the HR baroreflex closed-loop. Although BP was not assessed in this study, it has previously been shown that 0.1 Hz RSMT elicits high amplitude oscillation in BP, as well as in HR (Lehrer et al., 2009).
The dynamics of the PTT oscillations were close to the dynamics of the RRI oscillations, presumably because the same BP oscillation was circulating in both the HR and VT baroreflex closed-loops. We assume that BP oscillation caused forced PTT oscillation in the VT baroreflex closed-loop. FPA, SC, and RV did not react to 0.1 Hz RSMT stimulation stronger than to the other frequencies of RSMT stimulation, likely because they do not relate directly to the HR baroreflex closed-loop. RRI and PTT reflect the dynamics of central cardiovascular processes, whereas FPA and SC are more related to peripheral hemodynamics (Zahedy et al., 2008). We propose that RSMT, as a rhythmical physical load, induced forced oscillation in FPA, SC and RV mechanically and through reflexes, controlling these functions. It is possible that closed-loops of these reflexes also have resonance properties that amplify FPA, SC, and RV oscillations, however their resonance frequencies would be considerably lower or higher than the frequencies tested in the present study.
Gender differences were found only in RRI and FPA reactions. RRI and FPA oscillatory responses to RSMT were significantly higher in men than in women, while PTT, SC, and RV oscillatory responses did not differ between genders (Table 1). It is known that in a certain loading range, HR and BP increase proportionally to physical load increase. Future research should measure level of muscle tension during RSMT to determine whether men showed higher RRI oscillatory responses to RSMT because they produced stronger muscle contractions than did women. Gender differences in FPA oscillatory response to RSMT may reflect differences in BP oscillation amplitude in men and women.
CVS Oscillatory Reactions to 0.1 Hz RSMT Versus 0.1 Hz Paced Breathing
We found that in most cases both RSMT and paced breathing procedures caused 0.1 Hz oscillations in all studied functions (RRI, PTT, FPA, SC, and RV). These oscillations were considerably amplified in RRI and PTT due to the resonance in the HR baroreflex closed-loop. The amplitudes of the 0.1 Hz oscillations elicited by RSMT stimulation were almost the same in value as the amplitudes of the 0.1 Hz oscillations elicited by paced breathing in PTT, FPA, and SC, but significantly different in RRI and RV (see Table 2).
Paced breathing caused significantly higher 0.1 Hz RRI and RV oscillations than did the RSMT procedure, however, analysis of gender differences (see Table 2 and Figure 3) showed that this applied only to women. We assume that the amplitude of 0.1 Hz RRI oscillation depended on two conditions: the depth of breathing or the strength of muscle tension, and the power of resonance in the HR baroreflex closed-loop. An increase in rhythmical muscle tension increases the amplitude of 0.1 Hz RRI oscillation. Humans can voluntarily control muscle tension, and consequently, the amplitude of 0.1 Hz RRI oscillation elicited by RSMT. Further research is needed to determine if men and women who perform the RSMT procedure at the same intensity will produce equivalent 0.1 Hz RRI oscillation amplitudes in RSMT tasks, and if these amplitudes are of the same value as generated by paced breathing. It is possible that the amplitude of 0.1 Hz RRI oscillation elicited by paced breathing and the maximal amplitude of 0.1 Hz RRI oscillation elicited by RSMT could be equal for the same person.
0.1 Hz RV (i.e., tidal volume) oscillations in the paced breathing task were significantly higher than in RSMT task in both men and women. The difference in RV oscillatory reactions to 0.1 Hz RSMT and 0.1 Hz PB may be related to differences in minute volume ventilation during muscle tension versus paced breathing. Tidal volume may vary in both tasks, while rate (0.1 Hz frequency) of respiration is necessarily constant in paced breathing, yet may vary in RMST. In order to support the necessary minute volume ventilation in the 0.1 Hz paced breathing task, participants need to breathe deeply, whereas in the RSMT task, physical load increases the participant’s respiration rate, but not necessarily tidal volume. Respiratory effort to produce RRI oscillation in the PB task is limited in two ways: breathing too deeply at a rate of 0.1 Hz causes hyperventilation, whereas shallow breathing at this rate cannot provide the needed minute volume ventilation. When participants find a comfortable breathing depth between these two extremes, respiratory tidal volume becomes automatically set. Thus, the amplitude of 0.1 Hz RRI oscillation elicited by paced breathing cannot be voluntarily controlled. In contrast, humans can voluntarily control muscle tension, and consequently, the amplitude of 0.1 Hz RRI oscillation elicited by RSMT.
High-amplitude 0.1 Hz oscillation in the CVS elicited by 0.1 Hz PB and RSMT procedures appears to have worthwhile clinical application. Therapeutic effects from rhythmical stimulation at 0.1 Hz have been explained in terms of high-amplitude oscillation in autonomic functions which trained homeostatic reflexes and provided inhibitory modulation of the brain via the baroreceptors (Chernigovskaya et al., 1990; Lehrer et al., 2003, 2004, 2009). Comparison of RMST versus breathing effects on cardiovascular functions may be useful for further development of clinical applications. It is known that paced breathing at 0.1 Hz synchronizes respiration with HR oscillation. Synchronization improves the blood gas exchange since during the inhalation, when lungs are fully oxygenated, HR increases and intensifies oxygen utilization in blood (Yasuma, & Hayano, 2004; Giardino, Chan, & Borson, 2004). Paced breathing at 0.1 Hz increases baroreflex gain during the procedure (Lehrer et al., 2003; Chacko et al., 2005). Systematic use of the 0.1 Hz paced breathing procedure cumulatively increases baroreflex gain and peak expiratory flow over time in healthy people via training autonomic reflexes and restoring autonomic balance (Lehrer et al., 2003). RSMT at 0.1 Hz does not synchronize respiration with HR oscillation, but significantly increases cerebral oxygenation (France, France, & Patterson, 2006). Physical load decreases parasympathetic and increases sympathetic activity, considerably increases mean HR and relatively suppresses HRV during the procedure. Although RSMT elicits resonance oscillation in HR, total HRV remains at baseline levels, while total BP variability increases (Lehrer et al., 2009). As a result, HR baroreflex gain does not increase during the procedure. However, beneficial effects can still be expected due to high-amplitude oscillations in cardiovascular functions and increased blood flow. Also it is known that baroreflex gain decreases during physical load (exercise), but increases to higher than baseline levels after the load, resulting in a cumulative increase in baroreflex gain over time (Cottin et al., 2008; Potts et al., 1993; Pagani et al., 1988).
SC Oscillatory Reactions to RSMT and 0.1 Hz Paced Breathing Stimulation
The main SC focus in this study was on oscillations elicited by RSMT and PB stimulation. Although the mean value of SC was of secondary interest, we did find that RSMT and PB stimulation significantly increased mean SC compared to baseline (see Table 3). We acknowledge that spectral analysis is not the typical method for analyzing SC data, however this approach is consistent with the design of our study, and our results suggest that this approach may offer additional insight into the interconnected control of autonomic functions.
We found that PB and RSMT at all stimulation frequencies caused SC oscillations manifested by the peaks in SC spectra at corresponding frequencies. SC has been most studied as a function that relates to emotion regulation and does not react directly to peripheral muscle tension or blood vessel activity. Lader and Montagu (1962), for example, showed that pharmacological blockage of peripheral vessel regulation did not suppress SC reaction to emotional stimuli. The present study did not involve emotional stimulation, but rather likely reflected SC as a thermal and perspiration regulatory function. RSMT and 0.1 Hz breathing modulated ANS activity by eliciting high amplitude oscillation in the CVS. Accordingly, we propose that modulation of sympathetic nervous system activity caused by RSMT or 0.1 Hz breathing can impose oscillation in various autonomic functions including SC.
In spite of the fact that SC response to a stimulus is a slow process that sometimes lasts for about 60 s, SC oscillations in our study were caused by 0.1 Hz paced breathing and 0.2, 0.1, or 0.05 Hz RSMT stimulations (i.e., when stimulation was applied every 5, 10, or 20 s). The ability to impose SC oscillations in response to rhythmical stimulation in a range of 0.2 – 0.05 Hz is supported by Edelberg’s (1970, 1972) findings. He showed that the duration of SC response to a stimulus is determined by the shape of the recovery limb of the response. Recovery rates do not depend on the response amplitude, but they are different in response to an alerting signal versus an execution signal for a task. SC responses to execution task signals have significantly faster recovery rates and slower habituation than SC responses to alerting signals. Consequently, the task signals in our study could cause relatively short SC responses and slow habituation, the two conditions needed to impose SC oscillatory response.
The present study results support this idea given that the amplitude of imposed SC oscillations significantly depended on the frequency of stimulation. The 0.1 and 0.05 Hz RSMT elicited almost equivalent amplitude oscillations in SC, while the relatively faster 0.2 Hz RSMT elicited significantly lower SC oscillatory responses (see Fig 2 and Table 1). These findings suggest that 0.1 Hz oscillation in the CVS elicited by RSMT can spread to other autonomic functions, potentially activating and training reflexes that control these functions.
Limitations and Future Directions
Despite the statistical significance of the results, the small sample size of the present study may limit the generalizability of our findings, especially in regard to gender differences. Another limiting factor is the narrow age range of the participants. In line with our aim of investigating the intact mechanism of baroreflex resonance, only young and healthy participants were selected. Further research is needed to examine the influence of gender, age and other salient individual difference characteristics on the RSMT procedure for eliciting high-amplitude CVS oscillation. In addition, future studies should quantify values of muscle tension to evaluate task performance, especially in regard to gender differences. In the current study, we know that participants were able to spare the muscles of the right arm during RSMT, as instructed, because the physiological records from the finger pulse sensor and SC electrode attached to the right hand were artifact-free. However, we did not measure individual differences in muscle tension. Future research should also assess beat-to-beat BP to more fully characterize the involvement of the baroreflex mechanism. Finally, in line with our primary goal to evaluate resonance due to muscle tension, the paced breathing condition was always administered after the randomized RSMT tasks were completed because of the known effects of PB on cardiovascular functions. Thus, we cannot describe how participants’ completion of the muscle tension tasks may have affected the magnitude of oscillation due to resonance paced breathing.
Potential Clinical Application of Rrhythmical Muscle Tension Techniques
Physical activity strongly affects the CVS. We found that RSMT procedures may trigger 0.1 Hz resonance in the CVS. Human’s ability to voluntarily produce high amplitude oscillations in the CVS at resonance frequencies makes rhythmical muscle tension techniques potentially valuable for developing clinical applications. Previously, RSMT procedures at 0.1 Hz were effectively used to avert vasovagal reaction (France et al., 2006). France’s RSMT technique was a single session intervention for use immediately prior to an event that could induce syncope. We suggest that other kinds of 0.1 Hz RSMT procedures can be exploited by researchers and clinicians for the development of novel approaches to correct abnormal autonomic regulation and enhance health. For example, systematic daily use of 0.1 Hz paced RSMT may produce a cumulative and long lasting therapeutic effect in the same way as paced breathing does in HRV biofeedback (Lehrer et al., 2003, 2004; Cowan, Pike, & Budzynski, 2001; Del Pozo et al., 2004;, Nolan et al., 2005; Karavidas et al., 2007; Hassett et al., 2007). The contribution of physical exercise to health promotion is well known. It is possible that including rhythmical elements at the HR resonance frequency in exercise may enhance its beneficial cardiac effects via training autonomic reflexes and increasing brain oxygenation. Such procedures could conceivably improve sports performance and prove especially useful in the rehabilitation process of patients following a cerebral stroke or myocardial infarction, where light physical exercises are prescribed (Buch, Coote, & Townend, 2002) because exercise effects may be enhanced by high-amplitude oscillations in the CVS.
CONCLUSION
We found that the 0.1 Hz RSMT procedure triggers resonance in the CVS. RSMT caused evident oscillations in RRI, PTT, FPA and often in SC, and RV at all tested frequencies (0.05, 0.1, and 0.2 Hz). However, only RRI oscillation in response to 0.1 Hz RSMT stimulation can be conceptualized as a resonance oscillation that originated in the HR baroreflex closed loop. Oscillations at tested frequencies in PTT, FPA, SC, and RV were forced oscillations. Oscillatory reaction in PTT, FPA, and SC functions to the 0.1 Hz RSMT and to 0.1 Hz paced breathing stimulations was nearly equivalent, while for RRI and RV it differed significantly. 0.1 Hz RSMT stimulation elicited lower RRI oscillation than 0.1 Hz paced breathing only in women. The findings suggest that resonance caused by 0.1 Hz RSMT stimulation may be used to produce therapeutic effects similar to 0.1 Hz paced breathing, and thus may be useful in developing new behavioral therapeutic applications.
Acknowledgments
This research was supported by grants and contracts from the National Institute of Alcohol Abuse and Alcoholism (HHSN275201000003C, R01 AA015248, and K02 AA00325) and the National Institute of Drug Abuse (P20 DA017552). The authors thank students Alex Vaschillo and Anton Manyak for their assistance in testing participants and post processing the physiological data. Preliminary results were presented at the Annual 2009 Meetings of the Society for Psychophysiological Research and the Association for Applied Psychophysiology and Biofeedback.
REFERENCES
- Angelone A, Coulter NA., Jr Respiratory sinus arrhythmia: A frequency depended phenomenon. Journal of Applied Physiology. 1964;19:479–482. doi: 10.1152/jappl.1964.19.3.479. [DOI] [PubMed] [Google Scholar]
- Bertram D, Barres C, Cheng Y, Julien C. Norepinephrine reuptake, baroreflex dynamics, and arterial pressure variability in rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2000;279:1257–1267. doi: 10.1152/ajpregu.2000.279.4.R1257. [DOI] [PubMed] [Google Scholar]
- Bertram D, Barres C, Cuisinaud G, Julien C. The arterial baroreceptor reflex of the rat exhibits positive feedback properties at the frequency of Mayer waves. The Journal of Physiology. 1998;513:251–261. doi: 10.1111/j.1469-7793.1998.251by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Brown LV, Patwardhan A, Randall DC. Sympathetic activity and blood pressure are tightly coupled at 0.4 Hz in conscious rats. The American Journal of Physiology. 1994;267:1378–1384. doi: 10.1152/ajpregu.1994.267.5.R1378. [DOI] [PubMed] [Google Scholar]
- Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training—what’s the link? Experimental Physiology. 2002;87:423–435. doi: 10.1111/j.1469-445x.2002.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Burgess DE, Hundley JC, Li SG, Randall DC, Brown DR. First-order differential-delay equation for the baroreflex predicts the 0.4-Hz blood pressure rhythm in rats. The American Journal of Physiology. 1997;273:1878–1884. doi: 10.1152/ajpregu.1997.273.6.R1878. [DOI] [PubMed] [Google Scholar]
- Chacko NJ, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi R, Bernardi L. Slow Breathing Improves Arterial Baroreflex Sensitivity and Decreases Blood Pressure in Essential Hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- Chernigovskaya NV, Vaschillo EG, Petrash VV, Rusanovsky VV. Voluntary regulation of the heart rate as a method of functional condition correction in neurotics. Human Physiology. 1990;16:58–64. [Google Scholar]
- Christou DD, Jones PP, Seals DR. Baroreflex buffering in sedentary and endurance exercise-trained healthy men. Hypertension. 2003;41:1219–1222. doi: 10.1161/01.HYP.0000072011.17095.AE. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Cox JP, Diedrich AM, Taylor JA, Beightol LA, Ames IY, J E, et al. Controlled breathing protocols probe human autonomic cardiovascular rhythms. American Journal of Physiology. 1998;274:H709–H718. doi: 10.1152/ajpheart.1998.274.2.h709. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg D. Human responses to upright tilt: a window on central autonomic integration. The Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottin F, Medigue C, Papelier Y. Effect of heavy exercise on spectral baroreflex sensitivity, heart rate, and blood pressure variability in well-trained humans. American Journal of Physiology: Heart and Circulatory Physiology. 2008;295:H1150–H1155. doi: 10.1152/ajpheart.00003.2008. [DOI] [PubMed] [Google Scholar]
- Cowan MJ, Pike KC, Budzynski HK. Psychosocial nursing therapy following sudden cardiac arrest: Impact on two-year survival. Nursing Research. 2001;50:68–76. doi: 10.1097/00006199-200103000-00002. [DOI] [PubMed] [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. The American Journal of Physiology. 1987;253:H680–H689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. American Heart Journal. 2004;147:G1–G6. doi: 10.1016/j.ahj.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, Brunia CH. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proceedings of the National Academy of Science of the USA. 1994;91:6329–6333. doi: 10.1073/pnas.91.14.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelberg R. The information content of the recovery limb of the electrodermal response. Psychophysiology. 1970;6:527–539. doi: 10.1111/j.1469-8986.1970.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Edelberg R. Electrodermal Recovery Rate, Goal-Orientation, and Aversion. Psychophysiology. 1972;9:512–520. doi: 10.1111/j.1469-8986.1972.tb01805.x. [DOI] [PubMed] [Google Scholar]
- European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- France CR, France JL, Patterson SM. Blood pressure and cerebral oxygenation responses to skeletal muscle tension: a comparison of two physical maneuvers to prevent vasovagal reactions. Clinical Physiology and Functional Imaging. 2006;26:21–25. doi: 10.1111/j.1475-097X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Giardino N, Chan L, Borson S. Combined heart rate variability and pulse oximetry biofeedback for chronic obstructive pulmonary disease: a feasibility study. Applied Psychophysiology and Biofeedback. 2004;29:121–133. doi: 10.1023/b:apbi.0000026638.64386.89. [DOI] [PubMed] [Google Scholar]
- Grodins FS. Control theory and biological systems. New York: Columbia University Press; 1963. [Google Scholar]
- Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: Arterial blood pressure waves accompanying postural stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2005;288:1637–1648. doi: 10.1152/ajpregu.00050.2004. [DOI] [PubMed] [Google Scholar]
- Hassett AL, Radvanski DC, Vaschillo EG, Vaschillo B, Sigal LH, Karavidas MK, Buyske S, Lehrer PM. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Applied Psychophysiology and Biofeedback. 2007;32:1–10. doi: 10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- Janssen BJ, Malpas SC, Burke SL, Head GA. Frequency-dependent modulation of renal blood flow by renal nerve activity in conscious rabbits. The American Journal of Physiology. 1997;273:597–608. doi: 10.1152/ajpregu.1997.273.2.R597. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology. 1992;29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- Jordan J, Tank J, Shannon JR, Diedrich A, Lipp A, Schroder C, Arnold G, Sharma AM, Biaggioni I, Robertson D, Luft FC. Baroreflex buffering and susceptibility to vasoactive drugs. Circulation. 2002;105:1459–1464. doi: 10.1161/01.cir.0000012126.56352.fd. [DOI] [PubMed] [Google Scholar]
- Just A, Wittmann U, Nafz B, Wagner CD, Ehmke H, Kirchheim HR, Persson PB. The blood pressure buffering capacity of nitric oxide by comparison to the baroreceptor reflex. The American Journal of Physiology. 1994;267:H521–H527. doi: 10.1152/ajpheart.1994.267.2.H521. [DOI] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, Malinovsky I, Radvanski D, Hassett A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Lader MH, Montagu JD. The psycho-galvanic reflex: A pharmacologic study of the peripheral mechanism. Journal of Neurology, Neurosurgery, and Psychiatry. 1962;25:126–133. doi: 10.1136/jnnp.25.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P, Vaschillo E, Trost Z, France C. Effects of rhythmical muscle tension at 0.1 Hz on cardiovascular resonance and the baroreflex. Biological Psychology. 2009;81:24–30. doi: 10.1016/j.biopsycho.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Lehrer P, Vaschillo E, Vaschillo B, Lu SE, Scardella A, Siddique M, Habib R. Biofeedback treatment for asthma. Chest. 2004;126:352–361. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback. 2000;25:177–191. doi: 10.1023/a:1009554825745. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo EG, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, Shih WJ, Lin Y, Kuusela TA, Tahvanainen KUO, Hamer RM. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65:796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- Madwed JB, Albrecht P, Mark RG, Cohen RJ. Low-frequency oscillations in arterial pressure and heart rate: a simple computer model. The American Journal of Physiology. 1989;256:H1573–H1579. doi: 10.1152/ajpheart.1989.256.6.H1573. [DOI] [PubMed] [Google Scholar]
- Magosso E, Biavati V, Ursino N. Analysis of cardiovascular instability by mathematical model of baroreflex control. Proceedings of the 23rd Annual EMBS International Conference; 2001. pp. 596–599. [Google Scholar]
- Malpas SC, Burgess DE. Renal SNA as the primary mediator of slow oscillations in blood pressure during hemorrhage. American Journal of Physiology. 2000;279:H1299–H1306. doi: 10.1152/ajpheart.2000.279.3.H1299. [DOI] [PubMed] [Google Scholar]
- McCraty R, Atkinson M, Tomasino D. Impact of a workplace stress reduction program on blood pressure and emotional health in hypertensive employees. Journal of Alternative and Complementary Medicine. 2003;9:355–369. doi: 10.1089/107555303765551589. [DOI] [PubMed] [Google Scholar]
- Naka KK, Tweddel AC, Doshi SN, Goodfellow J, Henderson AH. Flowmediated changes in pulse wave velocity: a new clinical measure of endothelial function. European Heart Journal. 2006;27:302–309. doi: 10.1093/eurheartj/ehi619. [DOI] [PubMed] [Google Scholar]
- Naschitz JE, Bezobchuk S, Mussafia-Priselac R, Sundick S, Dreyfuss D, Khorshidi I, Karidis A, Manor H, Nagar M, Peck ER, Peck S, Storch S, Rosner I, Gaitini L. Pulse Transit Time by R-wave-gated Infrared Photoplethysmography: Review of the Literature and Personal Experience. Journal of Clinical Monitoring and Computing. 2004;18:333–342. doi: 10.1007/s10877-005-4300-z. [DOI] [PubMed] [Google Scholar]
- Nolan RP, Kamath MV, Floras JS, Stanley J, Pang C, Picton P, Young QR. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. American Heart Journal. 2005;149:1137. doi: 10.1016/j.ahj.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Nyklicek I, Wijnen V, Rau H. Effects of baroreceptor stimulation and opioids on the auditory startle reflex. Psychophysiology. 2005;42:213–222. doi: 10.1111/j.1469-8986.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Pagani M, Somers V, Furlan R, Dell’Orto S, Conway J, Baselli G, Cerutti S, Sleight P, Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. American Journal of Physiology: Heart and Circulatory Physiology. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Rau H, Pauli P, Brody S, Elbert T, Birbaumer N. Baroreceptor stimulation alters cortical activity. Psychophysiology. 1993;30:322–325. doi: 10.1111/j.1469-8986.1993.tb03359.x. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. The American Journal of Physiology. 1991;261:H1231–H1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Carr DI, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Tiedt N, Wohlgemuth B, Wohlgemuth P. Dynamic characteristics of heart-rate responses to sine-function work-load patterns in man. Pflugers Archiv: European Journal of Physiology. 1975;355:175–187. doi: 10.1007/BF00581832. [DOI] [PubMed] [Google Scholar]
- van de Vooren H, Gademan MG, Swenne CA, TenVoorde BJ, Schalij MJ, Van der Wall EE. Baroreflex sensitivity, blood pressure buffering, and resonance: what are the links? Computer simulation of healthy subjects and heart failure patients. Journal of Applied Physiology. 2007;102:1348–1356. doi: 10.1152/japplphysiol.00158.2006. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, Ray S. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1-Hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B. Transfer function of the heart rate control system with respiratory input. The classical engineering approach. BIOSIGNALS 2009; International Conference on Bio-inspired Systems and Signal Processing; 2009. pp. 233–238. [Google Scholar]
- Vaschillo EG, Vaschillo B, Jennifer F, Buckman JF, Bates ME, Pandina RJ. Resonances the cardiovascular system: investigation and clinical applications. BIOSIGNALS 2010; International Conference on Bio-inspired Systems and Signal Processing; 2010. pp. 19–27. [Google Scholar]
- Vaschillo E, Lehrer P, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback. 2002;27:1–27. doi: 10.1023/a:1014587304314. [DOI] [PubMed] [Google Scholar]
- Wigertz O. Dynamics of respiratory and circulatory adaptation to muscular exercise in man. A systems analysis approach. Acta Physiologica Scandinavica. 1971;363 Supplementum:1–32. [PubMed] [Google Scholar]
- Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- Yucha CB, Tsai PS, Calderon KS, Tian L. Biofeedback-assisted relaxation training for essential hypertension: who is most likely to benefit? The Journal of Cardiovascular Nursing. 2005;20:198–205. doi: 10.1097/00005082-200505000-00012. [DOI] [PubMed] [Google Scholar]
- Zahedi E, Jaafar R, Ali MA, Mohamed AL, Maskon O. Finger photoplethysmogram pulse amplitude changes induced by flow-mediated dilation. Physiological Measurement. 2008;29:625–637. doi: 10.1088/0967-3334/29/5/008. [DOI] [PubMed] [Google Scholar]


