SUMMARY
The sensory branches of the trigeminal nerve encode information about facial expressions, speaking and chewing movements, and stimuli that come into contact with the orofacial tissues. Whatever the cause, damage to the inferior alveolar nerve negatively affects the quality of facial sensibility as well as the patient's ability to translate patterns of altered nerve activity into functionally meaningful motor behaviours. There is no generally accepted, standard method of estimating sensory disturbances in the distribution of the inferior alveolar nerve following injury. Assessment of sensory alterations can be conducted using three types of measures: (i) objective electrophysiological measures of nerve conduction, (ii) sensory testing (stimulus) measures and (iii) patient report. Each type of measure with advantages and disadvantages for use are reviewed.
Keywords: inferior alveolar nerve, orthognathic surgery, altered sensation, nerve injury
Introduction
The sensory branches of the trigeminal nerve encode information about facial expressions, speaking and chewing movements, and stimuli that come into contact with the orofacial tissues. This information is transmitted to those areas of the cerebral cortex that underlie recognition and discernment of somatosensory stimulation and determine `how the face feels'. Only the patient experiences whether his/her sensation has changed and can express whether the alteration causes discomfort or problems in daily life. Much of the research on altered sensory function following orthognathic surgery has focused on reduced or loss of sensation, but, in fact, altered sensation can be experienced as an increase in sensibility as well.
Damage to the inferior alveolar nerve negatively affects the quality of facial sensibility as well as the patient's ability to translate patterns of altered nerve activity into functionally meaningful motor behaviours. A complex of cellular and molecular signalling alterations is immediately initiated following any degree of peripheral nerve injury, and the quality of functional recovery tightly correlates to the molecular responses that attempt to repair and restore the nerve to its pre-injury state. After resolution of inflammation and oedema in and surrounding the nerve, the sensory alteration can be attributed to anatomical or functional changes within the nerve or to changes induced in the central nervous system by the nerve injury (1, 2). In general, three often temporally overlapping phases describe this biological response: the response of the nerve cell body; the active restoration of any loss in the continuity of the proximal and distal segments of the axon and/or reconstitution of axonal diameter and remyelination; and the remodelling of the cortical representation of tissues innervated by the damaged axons (3).
Virtually all of the biological response data is derived from experimental transectional or crush injuries in animal models because the injury can easily be reproduced. The current information on nerve degeneration and regeneration is perhaps most representative of this type of injury but it's reasonable to assume that injuries of the inferior alveolar nerve (IAN) during an orthognathic surgery procedure such as bilateral sagittal split osteotomy (BSSO) activate similar recovery pathways (4). Although transecting nerve injuries during a BSSO procedure are fortunately rare, axonal damage is often severe (axonotmesis), requiring reconnection of axonal sprouts to target tissues, reconstitution of axonal diameter, and remyelination of myelinated afferents (5).
In the normal state, stimulation of the face or lips through facial expression or eating or other contact with the external environment stimulates the sensory receptors of the skin and a profile of neural impulses which describes the pattern of stimulation is elicited. These impulses impact upon the sensory cortex and are associated with the memory of similar previous sensory experiences. Injury to the trigeminal nerve alters this profile resulting in plasticity changes in neural substrates at subcortical and cortical levels within the CNS (6, 7). Thus, after a nerve injury, the same stimulation of the face or lips not only elicits a different, altered profile of neural impulses but the impulses are processed differently which affects the symptoms reported by patients. Symptoms can range from a complete or partial loss of sensation; to non-painful tingling sensation; to increased sensitivity to touch or pressure with or without numbness; to pain. Likely because injury caused during orthognathic surgery is usually a demyelinating injury or a partial axonal lesion (5), most patients do not develop neuropathic pain although approximately 20% of BSSO patients use peripheral neuropathic pain descriptors 6 months after surgery (8) and at least 5% can be classified as experiencing neuropathic pain 1 year after surgery (5).
Inferior alveolar nerve injury during mandibular osteotomy
Because mandibular osteotomies are performed in close proximity to the neurovascular bundle in the mandibular canal, there is a high risk of damage to the inferior alveolar nerve. Electrophysiological signs of injury are observed during all stages of the osteotomy (9). In one study in which electrical recordings were made from the inferior alveolar nerve during surgery, 21 of 38 nerves exhibited decreased conduction velocity, indicative of a demyelinating injury, and 15 exhibited decreased amplitude of the nerve signal, indicative of axonal damage (5, 10). The placement of semi-rigid fixation plates and screws may also cause nerve damage either directly or via compression of the nerve between bony segments after screw fixation (Fig. 1). In a study on monkeys comparing bicortical and monocortical osteosynthesis, signs of Wallerian degeneration, including demyelination, axonal swelling, and axoplasmic darkening after mandibular setback by BSSO were observed with both types of fixation (11). In a clinical study, electrical signals from nerves recorded intraoperatively decreased, indicative of blocked conduction, when the first fixation screw was tightened and the compression between the fragments increased (12).
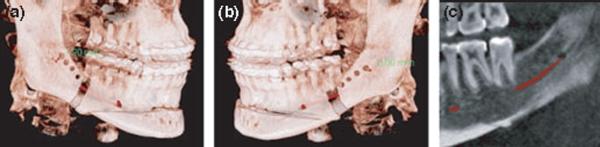
Fig. 1.
3D renderings for a subject who reported substantial sensory loss 1 yr after surgery on the left side of the chin but not the right. (a) On the right side the screws were a minimum of 1.9 mm above the canal, while (b) and (c) show that on the left side, one of the fixation screws was placed through the canal.
Certain anatomical features of the mandible and the intrabony course of the mandibular canal may also increase the risk of nerve damage. The canal passes through the body of the mandible in an anteroposterior direction with considerable inter-subject variation in its vertical and buccolingual position (13). For example, even at 1 year after surgery the proportion of patients who reported persistent sensory loss was higher when the IAN was between the lateral and medial segments of the osteotomy split or had to be freed from the lateral fragment when compared to patients where the nerve was not encountered or was embedded in the medial fragment (14). Other anatomical characteristics of the mandible and mandibular canal that may put the IAN at increased risk of damage are a low corpus height, location of the mandibular canal near the inferior border of the mandible or the lateral cortex of the ramus, and/or a narrow width of the marrow spaces between the mandibular canal and the external cortical surface (10, 15–20). Understanding the importance of the anatomy of the mandible with respect to the risk of inferior alveolar nerve damage has been limited by the reliance on 2D radiographs or 2D measures from CT slices. The introduction of cone-beam CTs into clinical practice offers significant advantages for the visualisation and quantification of how the trajectory of the mandibular canal within the mandible and placement of fixation screws may impact sensory alteration.
Methods of assessment
There is no generally accepted, standard method of estimating sensory disturbances in the distribution of the inferior alveolar nerve following orthognathic surgery on the mandible. Efforts are underway by the Neuropathic Pain Research Consortium (21) and the German Research Network on Neuropathic Pain (22) to establish a protocol that would encompass nearly all aspects of somatosensation. These protocols when validated with respect to use in the clinical or research setting, time necessary to perform, reproducibility, and ease of use may be helpful in assessments following orthognathic surgery as well. Standardisation of assessment methods would facilitate the identification of diagnostic criteria for different types of neurosensory impairment, paralleling those that have been established and are widely used for temporomandibular disorders. Assessment of sensory alterations can be conducted using three types of measures: (i) objective electrophysiological measures of nerve conduction, (ii) sensory testing measures and (iii) patient report.
Electrophysiological testing
The nerve conduction tests for the inferior alveolar nerve are similar to those employed by neurologists to evaluate the integrity of other peripheral nerves. However, the location and course of the inferior alveolar nerve make the procedures logistically difficult to perform, and unacceptable to many patients while awake. Nerve conduction assessments during surgery or post-surgically at even a single time point are relatively rare in the literature (23, 24).
Sensory testing (stimulus measures)
Sensory testing methods derived from the field of psychophysics are used to quantitatively estimate the patient's sensory capacity to detect stimuli applied to the skin or mucosa. Although the stimuli are objective, the response is dependent on the subjective report from the subject. Stimulus measures testing can be categorised as stimulus-detection tasks such as contact detection, two-point discrimination (2PD), light touch perception, and brush stroke discrimination; or as stimulus perception tasks such as two point perception and thermal perception. The stimulus-detection tests are characterised by an unequivocal correct or incorrect response to each stimulus presentation trial while in the stimulus-perception tests there is no correct answer, the response is dependent on the patient's cognitive discernment of the stimulus.
For evaluation of nerve injury following an osteotomy, evaluation of the results from either type of test requires a comparison with pre-surgical estimates obtained from the same facial area, or an unaffected skin site, or control subjects without nerve injury. The extent to which bias may be introduced in the interpretation of the longitudinal changes in stimulus measures by the timing of the baseline pre-orthognathic surgical estimate (for example, pre-orthodontic treatment versus pre-surgery) or by the type of dento-facial disharmony is currently unknown, but of concern. For example, in a cross-sectional study, patients in pre-surgical orthodontic treatment showed some degree of hypersensitivity to pressure and pinch pain compared to healthy controls suggesting that sensitisation had already begun prior to the surgery (25). Because of the length of time required for pre and post surgical orthodontics, there are currently no sufficiently sized longitudinal studies that have incorporated pre and post-treatment quantitative sensory testing to separate the contribution of orthodontic from surgical trauma. Even if pre-surgical comparison estimates are used, multiple other issues may confound or bias the interpretation of the sensory differences over time: (i) Is the exact same part of the nerve distribution being tested at all time points? (ii) How much variability in the responses can be attributed to uncontrolled variation in stimulus delivery that may be introduced by changes in force or pressure of the delivery or the duration of the stimulus delivery? (iii) How much variability may be introduced by differences in the environment or patient's behaviour (coffee or food intact, sleepiness) at different time points? and (iv) If the stimuli feel qualitatively abnormal on the skin, does this impact the patient's response? For example, light touch (nylon filaments) and brush stroke stimuli may evoke abnormal unpleasant tactile percepts suggestive of punctate and dynamic allodynia, respectively (26). If a control site at a different location on the face is used, is the presumption that sensibility would be the same in the absence of therapy at the control and test site valid?
Sensory testing measures are intended to provide a quantification and a basis for judgments regarding changes in the severity, location and spatial extent of sensory alteration over time. Theoretically, the various measures quantify the extent of persistent injury in different axonal fibre types. For example, contact (touch) detection (CTD) threshold is thought to assess the functional integrity of the large diameter Aβ mechanoreceptors while thermal perception tests assess the functional integrity of small diameter Aδ myelinated thermoreceptors and unmyelinated C-fibre thermoreceptors (27, 28). However, subtle differences in the testing procedure with the same stimuli can result in substantially different threshold estimates and in differences in the interpretation of the results. For example, 2PD threshold assesses the subject's ability to discriminate two from one point of contact based in part on spatial information, while the two-point perception (2PP) threshold assesses the subject's subjective interpretation or discernment of the overall pattern of tactile stimulation, i.e. `were two distinct points of contact felt?' This distinction is often overlooked.
The choice of sensory testing measures for post-surgical follow-up (Fig. 2) then is highly dependent on the purpose of assessment (nerve fibre injury versus tactile / spatial acuity; longitudinal observation versus treatment effect). The contact detection and warmth perception thresholds appear to be the most sensitive and useful stimulus detection and perception thresholds, respectively, for assessing trigeminal nerve injury (1, 29, 30). Estimates of the CD threshold obtained postoperatively have been shown to correlate significantly with objectively assessed nerve injury intraoperatively (15) and with objectively assessed persistent nerve injury and patient report of altered sensation for up to 1 year after surgery (24, 31).
Fig. 2.
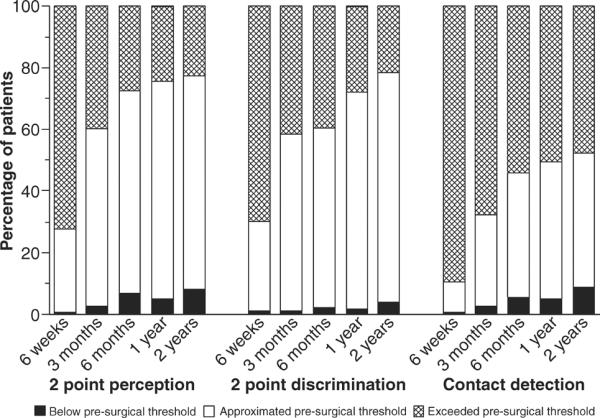
Percentage of 186 patients whose two-point perception, two-point discrimination, and contact detection thresholds obtained on the chin after a mandibular osteotomy approximated the presurgical value (between ½ and 2 times the presurgical threshold indicated in yellow); exceeded the presurgical threshold value (more than 2 times higher indicated in green); or were below the presurgical threshold (at least ½ the presurgical threshold indicated in blue). Note that the time course of recovery differed for the three measures of somatosensory function, with less recovery observed for contact detection than either two-point perception or discrimination.
However, contact detection alone would not necessarily be sufficient for the differentiation of a treatment effect that was primarily focused on cognitive changes rather than nerve repair. For example, at 2 years after surgery, patients who used sensory retraining exercises following orthognathic surgery responded that they were able to `feel' two distinct points of skin contact at shorter separations, on average, than patients who used only opening exercises, even though the exercises were discontinued at 6 months postsurgery (32, 33). However, the two groups did not differ in average contact detection or 2PD thresholds. These results suggest that nerve recovery was similar for the two groups but the patients who had participated in the sensory retraining exercises had cognitively accommodated to the sensory alteration, resulting in an apparent improved sensory function as indicated by testing of 2PP. Although two point perception is similar to the 2PD threshold, it is a biased estimator dependent on the subject's ability to discriminate two from one point of contact, which is heavily influenced by cognitive factors as well as subjects' discriminative capacities (34). This cognitive treatment effect over a 2-year period after surgery was corroborated by the sensory retraining patients' reports of the presence of less altered sensation and less burden associated with the alteration than was reported by those patients who had not received the sensory retraining (35–37).
Patient self-report
The debate over which type of assessment (sensory testing or patient report) should be considered the `gold standard' of nerve injury and recovery is unwarranted. To the clinician considering microsurgery for nerve repair the objective, electrophysiological measure of nerve integrity or stimulus detection measures may be most valuable; but if the consideration is the patient's accommodation to the altered sensitivity (32, 33, 35–37) or the effect of medication (38), then stimulus perception measures or patient self-report may be the preferred measure. And to the patient, the changes in sensitivity and sensation and the extent to which the change causes discomfort or problems with daily life is paramount regardless of sensory testing estimates of residual nerve injury. The reports in the literature of the prevalence and qualitative nature of altered sensation following orthognathic surgery as assessed by patient report have been highly variable. This variability reflects differences in study design,; the length of time after surgery when the patient is queried; and most importantly, the disparity in the intent and response options of the patient queries (39–47). For example, studies that focus on loss of sensitivity (39, 40) or even exclude patients who report a sensation other than reduced sensitivity (41) ignore the paresthesias / dysesthesias that patients commonly report either alone or in combination with loss of sensitivity (Table 1, Fig. 3) (8, 25, 42).
Table 1.
Examples of variation in design and intent of patient self-report assessment with respect to post-surgical altered sensation
| Study | Design | Assessment |
|---|---|---|
| Al-Bishri (42) | Retrospective | Any altered sensation (verbal descriptors) |
| Al-Bishri (43) | Retrospective | Any altered sensation (yes/no) |
| Baad-Hanson (25) | Cross-sectional | Any altered sensation (yes/no) |
| Cunningham (41) | Prospective | Loss of sensation (normal to completely numb) |
| Essick (44) | Prospective | Any altered sensation (yes/no) |
| Lemke (47) | Prospective | Loss of sensation (normal vs. any reduction) |
| Nesari (40) | Retrospective | Loss of sensation (normal vs. any reduction) |
| Phillips (35) | Prospective | Any altered sensation (yes/no) |
| Phillips (8) | Prospective | Any altered sensation (verbal descriptors) |
| Teerijoki-Oksa (24) | Prospective | Any altered sensation (yes/no) |
| Thuer (45) | Prospective | Any altered sensation (verbal descriptors) |
| Westermark (39) | Prospective | Loss of sensation (normal to completely numb) |
| Ylikontiola (14) | Prospective | Loss of sensation (normal to completely numb) |
| Ylikontiola (46) | Prospective | Loss of sensation (normal to completely numb) |
Fig. 3.
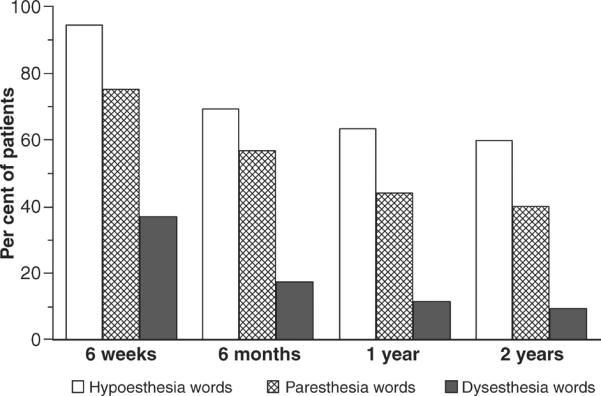
Percent of 186 patients who choose words associated with hypoesthesia, paresthesia, and/or dysesthesia to describe their altered sensation at 4 visits during the first two years after orthognathic surgery. Note that at all time points after surgery the percent of patients exceeds 100% indicating that patients frequently chose words associated with more than one category of altered sensation.
There are at least six dimensions that may be addressed in a patient self-report:
Does the patient perceive any change in sensation?
Where?
When? Spontaneously, evoked or both?
How does the patient describe the change? Decreased or increased sensitivity, abnormal sensation, or pain?
What does the patient perceive as the functional or behavioural sequelae in daily life?
How much of a burden or problem do these sequelae cause?
Each of these dimensions provides a unique perspective about the alteration. `Does' allows for the possibility that the clinician's examination might not assess those aspects of impaired nerve function sensed by the patient or that the patient has accommodated sufficiently that the impairment is not noticeable. `Where' provides insight into the nerve distribution affected. `When' provides information relevant to the severity of the sensory alteration. For example, a patient who experiences burning pain on the chin only when exposed to cold temperatures exhibits cold allodynia but if the burning pain occurs spontaneously, the patient is diagnosed with neuropathic pain (26, 48). Verbal descriptors target the range of altered sensations experienced by patients following injury in the trigeminal region (Fig. 3) (8, 49). Patient self-report has been shown to be quite reliable: given the same instructions by multiple examiners, patients provided identical responses when requested to describe alterations that occur spontaneously or were elicited by touch (50). Indeed, similarly constructed verbal descriptor scales have been shown to be useful in identifying and classifying neuropathic pain disorder (51–53). The perceived effect on daily life provides an indication of the patient's level of accommodation to the altered sensation. At 6 months after BSSO, almost three-fourths of patients report at least mild difficulty in their everyday life related to numbness; slightly less report difficulty caused by the lips being less sensitive, or difficulty related to unusual sensations; and about 20% report problems related to facial pain (54). The altered sensation is reflected in patient reports of the functional or behavioural sequelae such as difficulty in eating, and kissing, food particles remaining on their face, drooling, and the inability to feel their face during smiling and speaking (47, 54–56). The total area over the face affected by the alteration also affects patients' perceptions. Patients who report altered sensation on both the upper lip and lower face report more problems related to their social interactions and the recovery of self-confidence than patients who report alteration on only one jaw or no alteration at all (57).
The experience of persistent altered sensation following a nerve injury is complex, and like acute (1 h to 1 week) post-operative pain, is likely influenced by demographic, environmental, and psychosocial characteristics as well as genetic factors. Age and psychological well-being have also been shown to impact symptoms of altered sensation following orthognathic surgery (14, 33, 36, 37, 58). Older patients and those with elevated psychological distress prior to surgery are more likely to report or exhibit persistent altered sensation 2 years after BSSO (35); more interference with daily life activities (36); greater impairment in 2PD (33), and more post-surgical oral health problems (59). To what extent the effect of age is a result of cognitive versus physiological changes is not known. The indication that psychological distress affects a patient's sensitivity to altered sensation and difficulty accommodating to that alteration is supported by the conclusion from a recent evidence-based literature review (60) on clinical recovery: `preoperative consideration of attitudinal (expectations, optimism) and mood (anxiety, depression) factors will assist the surgeon in estimating both the speed and extent of recovery.' Results from diverse types of surgery indicate that patients who are psychologically distressed prior to surgery tend to report more discomfort or difficulty with symptoms, general health, and overall recovery in the first few months after surgery than those who are not distressed (61–67).
Conclusion
The personal and societal cost of altered sensation can be considerable: lost productivity, increased utilisation of health care services, decreased quality of life, and potential negative impacts on the emotional and psychological well-being of the individual. Persistent, postoperative altered sensation, whether pain or numbness, is an important health issue and yet prevention, treatment, and management are poorly understood. Strategies for individualising patient management and new therapies for enhancing afferent nerve repair and reducing persistent altered sensation require an improved understanding of the pattern of neurosensory recovery and the factors that influence that pattern.
Acknowledgment
The authors wish to acknowledge the assistance provided by Aaron Abate, research patient care coordinator, and Debora Price, applications programmer. This project was supported in part by NIH grants DE013967 and DE005215
References
- 1.Essick G. Psychophysical assessment of patients with post-traumatic neuropathic trigeminal pain. J Orofac Pain. 2004;18:345–354. [PubMed] [Google Scholar]
- 2.Becerra L, Morris S, Bazes S, Gostic R, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 5.Jääskeläinen SK, Teerijoki-Oksa T, Virtanen A, Tenovuo O, Forssell K, Forssell H. Sensory regeneration following intraoperatively verified trigeminal nerve injury. Neurology. 2004;62:1951–1957. doi: 10.1212/01.wnl.0000129490.67954.c2. [DOI] [PubMed] [Google Scholar]
- 6.Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- 7.Kaas JH, Collins CE. Anatomic and functional reorganization of somatosensory cortex in mature primates after peripheral nerve and spinal cord injury. Adv Neurol. 2003;93:87–95. [PubMed] [Google Scholar]
- 8.Phillips C, Essick G, Zuniga J, Tucker M, Blakey GH., III Qualitative descriptors used by patients following orthognathic surgery to portray altered sensation. J Oral Maxillofac Surg. 2006;64:1751–1760. doi: 10.1016/j.joms.2005.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panula K, Finne K, Oikarinen K. Neurosensory deficits after bilateral sagittal split ramus osteotomy of the mandible – influence of soft tissue handling medial to the ascending ramus. Int J Oral Maxillofac Surg. 2004;33:543–548. doi: 10.1016/j.ijom.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Teerijoki-Oksa T, Jääskeläinen SK, Forssell K, Forssell H, Vahatalo K, Tammisalo T, et al. Risk factors of nerve injury during mandibular sagittal split osteotomy. Int J Oral Maxillofac Surg. 2002;31:33–39. doi: 10.1054/ijom.2001.0157. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Zhao Q, Tang J, Zheng Z, Qi MC. Changes in the inferior alveolar nerve following sagittal split osteotomy in monkeys: a comparison of monocortical and bicortical fixation. Br J Oral Maxillofac Surg. 2007;45:265–267. doi: 10.1016/j.bjoms.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Ueki K, Takatsuka S, et al. Somatosensory evoked potential to evaluate the trigeminal nerve after sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:146–152. doi: 10.1067/moe.2001.112331. [DOI] [PubMed] [Google Scholar]
- 13.Rajchel J, Ellis E, III, Fonseca RJ. The anatomical location of the mandibular canal: its relationship to the sagittal ramus osteotomy. Int J Adult Orthod Orthognath Surg. 1986;1:37–47. [PubMed] [Google Scholar]
- 14.Ylikontiola L, Kinnunen J, Oikarinen K. Factors affecting neurosensory disturbance after mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2000;58:1234–1239. doi: 10.1053/joms.2000.16621. [DOI] [PubMed] [Google Scholar]
- 15.Teerijoki-Oksa T, Jaaskelainen S, Forssell K, Virtanen A, Forssell H. An evaluation of clinical and electrophysiologic tests in nerve injury diagnosis after mandibular sagittal split ostetomy. Int J Oral Maxillofac Surg. 2003;32:15–23. doi: 10.1054/ijom.2002.0325. [DOI] [PubMed] [Google Scholar]
- 16.Susarla SM, Dodson TB. Preoperative computed tomography imaging in the management of impacted mandibular third molars. J Oral Maxillofac Surg. 2007;65:83–88. doi: 10.1016/j.joms.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto R, Nakamura A, Obno K, Michi K. Relationship of the mandibular canal to the lateral cortex of the mandibular ramus as a factor in the development of neurosensory disturbance after bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2002;60:490–495. doi: 10.1053/joms.2002.31843. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa K, Ueki K, Takatsuka S, Yamamoto E. Trigeminal nerve hypesthesia after sagittal split osteotomy in setback cases: correlation of postoperative computed tomography and long-term trigeminal somatosensory evoked potentials. J Oral Maxillofac Surg. 2003;61:898–903. doi: 10.1016/s0278-2391(03)00295-7. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji Y, Muto T, Kawakami J, Takeda S. Computed tomo-graphic analysis of the position and course of the mandibular canal: relevance to the sagittal split ramus osteotomy. Int J Oral Maxillofac Surg. 2005;54:243–246. doi: 10.1016/j.ijom.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Muto T, Shigeo K, Yamamoto K, Kawakami J. Computed tomography morphology of the mandibular ramus in prognathism: effect on the medial osteotomy of the sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2003;61:89–93. doi: 10.1053/joms.2003.50014. [DOI] [PubMed] [Google Scholar]
- 21.Walk D, Sehgal N, Moeller-Bertram T, et al. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin J Pain. 2009;25:632–640. doi: 10.1097/AJP.0b013e3181a68c64. [DOI] [PubMed] [Google Scholar]
- 22.Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Takazakura UK, Nakagawa K, Marukawa K, Shimada M, Shimiul A, Yamamoto A. A comparison of postoperative hypoesthesia between two types of sagittal split ramus osteotomy and intraoral vertical ramus osteotomy, using the trigeminal somatosensory-evoked potential method. Int J Oral Maxilofac Surg. 2007;36:11–14. doi: 10.1016/j.ijom.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Teerijoki-Oksa T, Jaaskelainen SK, Forssell K, Forssell H. Recovery of nerve injury after mandibular sagittal split osteotomy. Diagnostic value of clinical and electrophysiologic tests in the follow-up. Int J Oral Maxillofac Surg. 2004;33:134–140. doi: 10.1054/ijom.2003.0463. [DOI] [PubMed] [Google Scholar]
- 25.Baad-Hansen L, Arima T, Arendt-Nielsen L, Neumann-Jensen B, Svensson P. Quantitative sensory tests before and 1 ½ years after orthognathic surgery: a cross-sectional study. J Oral Rehab. 2010;37:313–321. doi: 10.1111/j.1365-2842.2010.02059.x. [DOI] [PubMed] [Google Scholar]
- 26.Arning K, Baron R. Evaluation of symptom heterogeneity in neuropathic pain using assessments of sensory functions. Neurother J Am Soc Exp NeuroTher. 2009;6:738–748. doi: 10.1016/j.nurt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Essick G. Comprehensive clinical evaluation of perioral sensory function. Oral Maxillofac Surg Clin North Am. 1992;4:503–526. [Google Scholar]
- 28.Greenspan J. Quantitative assessment of neuropathic pain. Curr Pain Headache Rep. 2001;5:107–113. doi: 10.1007/s11916-001-0078-y. [DOI] [PubMed] [Google Scholar]
- 29.Campbell R, Shamaskin R, Harkins S. Assessment of recovery from injury to inferior alveolar and mental nerves. Oral Surg Oral Med Oral Pathol. 1987;64:519–526. doi: 10.1016/0030-4220(87)90024-7. [DOI] [PubMed] [Google Scholar]
- 30.Essick G, Patel J, Trulsson M. Mechanosensory and thermosensory changes across the border of impaired sensitivity to pinprick after mandibular nerve injury. J Oral Maxillofac Surg. 2002;60:1250–1266. doi: 10.1053/joms.2002.35721. [DOI] [PubMed] [Google Scholar]
- 31.Essick GK, Phillips C, Turvey TA, Tucker M. Facial altered sensation and sensory impairment after orthognathic surgery. Int J Oral Maxillofacial Surg. 2007;36:577–582. doi: 10.1016/j.ijom.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essick G, Phillips C, Zuniga J. Effect of facial sensory retraining on sensory thresholds. J Dent Res. 2007;86:571–575. doi: 10.1177/154405910708600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essick GK, Phillips C, Kim SK, Zuniga J. Sensory retraining following orthognathic surgery: effect on threshold measures of sensory function. J Oral Rehabil. 2009;36:415–426. doi: 10.1111/j.1365-2842.2009.01954.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Boven R, Johnson K. A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain. 1994;11:149–167. doi: 10.1093/brain/117.1.149. [DOI] [PubMed] [Google Scholar]
- 35.Phillips C, Kim S, Essick G, Tucker M, Turvey TA. Sensory retraining following orthognathic surgery: effect on Patient Report of the Presence of Altered Sensation. Am J Ortho Dentofac Orthop. 2009;136:788–794. doi: 10.1016/j.ajodo.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Phillips C, Kim S, Tucker M, Turvey TA. Sensory retraining: burden in daily life related to altered sensation after orthognathic surgery, a randomized clinical trial. Orthod Craniofac Res. 2010;13:1–12. doi: 10.1111/j.1601-6343.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips C, Essick G, Preisser JS, Turvey TA, Tucker M, Lin D. Sensory Retraining following orthognathic surgery: effect on patient perception of altered sensation. J Oral Maxillofac Surg. 2007;65:1162–1173. doi: 10.1016/j.joms.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo K, Tanaka Y, Terumitsu M, Someya G. Efficacy of steroid treatment for sensory impairment after orthognathic surgery. J Oral Maxillofac Surg. 2004;62:1193–1197. doi: 10.1016/j.joms.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Westermark A, Englesson L, Bongenhielm U. Neurosensory function after sagittal split osteotomy of the mandible: a comparison between subjective evaluation and objective assessment. Int J Adult Orthodon Orthognath Surg. 1999;14:268. [PubMed] [Google Scholar]
- 40.Nesari S, Kahnberg KE, Rasmusson L. Neurosensory function of the inferior alveolar nerve after bilateral sagittal ramus osteotomy: a retrospective study of 68 patients. Int J Oral Maxillofac Surg. 2005;34:495–498. doi: 10.1016/j.ijom.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham LL, et al. A comparison of questionnaire versus monofilament assessment of neurosensory deficit. J Oral Maxillofac Surg. 1996;54:454–459. doi: 10.1016/s0278-2391(96)90120-2. [DOI] [PubMed] [Google Scholar]
- 42.Al-Bishri A, Rosenqist J, Sunzel B. On neurosensory disturbance after sagittal split osteotomy. J Oral Maxillofac Surg. 2004;62:1472–1476. doi: 10.1016/j.joms.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Al-Bishri A, Barghash Z, Rosenquist J, Sunzel B. Neurosensory disturbance after sagittal split and intraoral vertical ramus osteotomy: as reported in questionnaires and patients' records. Int J Oral Maxillofac Surg. 2005;34:247–251. doi: 10.1016/j.ijom.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Essick GK, Austin S, Phillips C, Kiyak HA. Short-term sensory impairment after orthognathic surgery. Oral Maxillofac Surg Clin North Am. 2001;13:295–313. [Google Scholar]
- 45.Thuer U, Ingervall B, Vuillemin T. Functional and sensory impairment after sagittal split osteotomies. Int J Adult Orthod Orthognath Surg. 1997;12:263–272. [Google Scholar]
- 46.Ylikontiola L, Kinnunen J, Oikarinen K. Comparison of different tests assessing neurosensory disturbances after bilateral sagittal split osteotomy. Int J Oral Maxillofac Surg. 1998;27:417–421. doi: 10.1016/s0901-5027(98)80028-3. [DOI] [PubMed] [Google Scholar]
- 47.Lemke RR, Clark GM, Bays RA, Tiner BD, Rugh JD. Effects of hypesthesia on oral behaviors of the orthognathic surgery patient. J Oral Maxillofac Surg. 1998;56:153–157. doi: 10.1016/s0278-2391(98)90856-4. [DOI] [PubMed] [Google Scholar]
- 48.Burchiel K. Trigeminal neuropathic pain. Acta Neurochir Suppl (wien) 1993;58:145–149. doi: 10.1007/978-3-7091-9297-9_33. [DOI] [PubMed] [Google Scholar]
- 49.Zuniga JR, Essick GK. A contemporary approach to the clinical evaluation of trigeminal nerve injures. Oral Maxillofac Surg Clin N Am. 1992;4:353–367. [Google Scholar]
- 50.Feldman S, Essick G, Zuniga JR, Phillips C. Interexaminer reliability of three subjective clinical neurosensory tests. Int J Adult Ortho Orthogn Surg. 1997;12:273–275. [Google Scholar]
- 51.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 52.Marchettini P. The burning case of neuropathic pain wording. Pain. 2005;114:313–314. doi: 10.1016/j.pain.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire. Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Phillips C, Essick G, Blakey GH, III, Tucker M. Relationship between patients' perceptions of post-surgical sequelae and altered sensation following BSSO. J Oral Maxillofac Surg. 2007;65:597–607. doi: 10.1016/j.joms.2005.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bothur S, Blomqvist JE. Patient perception of neurosensory deficit after sagittal split osteotomy in the mandible. Plast Reconstr Surg. 2003;111:373–377. doi: 10.1097/01.PRS.0000036049.37768.37. [DOI] [PubMed] [Google Scholar]
- 56.Essick GK. Invited Discussion of: Effects of hypesthesia on oral behaviors of the orthognathic surgery patient (Lemke RR, Clark GM, Bays RA, Tiner BD, Rugh JD) J Oral Maxillofac Surg. 1998;56:158–160. doi: 10.1016/s0278-2391(98)90856-4. [DOI] [PubMed] [Google Scholar]
- 57.Harvey WS, Phillips CL, Essick GK. Neurosensory impairment and patient perception of recovery following orthognathic surgery. J Dent Res. 2001;80(Special Issue):187. [Google Scholar]
- 58.Nishioka GJ, Zysset MK, Van Sickels JE. Neurosensory disturbance with rigid fixation of the bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 1987;45:20–26. doi: 10.1016/0278-2391(87)90081-4. [DOI] [PubMed] [Google Scholar]
- 59.Scott AA, Hatch JP, Rugh JD, Rivera SM, Hoffman TJ, Dolce C, et al. Psychosocial predictors of high-risk patients undergoing orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1999;14:113–124. [PubMed] [Google Scholar]
- 60.Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14:397–405. doi: 10.5435/00124635-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Eli I, Schwartz-Arad D, Baht R, Ben-Tuvim H. Effect of anxiety on the experience of pain in implant insertion. Clin Oral Implants Res. 2003;14:115–118. doi: 10.1034/j.1600-0501.2003.140115.x. [DOI] [PubMed] [Google Scholar]
- 62.Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain. 2005;21:439–445. doi: 10.1097/01.ajp.0000135236.12705.2d. [DOI] [PubMed] [Google Scholar]
- 63.Klages U, Kianifard S, Ulusoy O, Wehrbein H. Anxiety sensitivity as predictor of pain in patients undergoing restorative dental procedures. Community Dent Oral Epidemiol. 2006;34:139–145. doi: 10.1111/j.1600-0528.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 64.Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, Adamatti LC. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdom-inal surgery. Acta Anaesthesiol Scand. 2002;46:1265–1271. doi: 10.1034/j.1399-6576.2002.461015.x. [DOI] [PubMed] [Google Scholar]
- 65.Ozalp G, Sarioglu G, Tuncel G, Aslan K, Kadiogullari N. Preoperative emotional states in patients with breast cancer and postoperative pain. Acta Anaesthesiol Scand. 2003;47:26–29. doi: 10.1034/j.1399-6576.2003.470105.x. [DOI] [PubMed] [Google Scholar]
- 66.Trief PM, Grant W, Fredrickson B. A prospective study of psychological predictors of lumbar surgery outcome. Spine. 2000;25:2616–2621. doi: 10.1097/00007632-200010150-00012. [DOI] [PubMed] [Google Scholar]
- 67.Kiecolt-Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery. Perspectives from Psychoneuroimmunology. Am Psychol. 1998;53:1209–1218. doi: 10.1037//0003-066x.53.11.1209. [DOI] [PubMed] [Google Scholar]