Abstract
This study examined the gene transfer efficiency and toxicity of 2-kDa polyethylenimine conjugated to gold nanoparticles (PEI2-GNP) in the human cornea in vitro and rabbit cornea in vivo. PEI2-GNP with nitrogen-to-phosphorus (N/P) ratios of up to 180 exhibited significant transgene delivery in the human cornea without altering the viability or phenotype of these cells. Similarly, PEI2-GNP applied to corneal tissues collected after 12 h, 72 h, or 7 days exhibited appreciable gold uptake throughout the rabbit stroma with gradual clearance of GNP over time. Transmission electron microscopy detected GNP in the keratocytes and the extracellular matrix of the rabbit corneas. Additionally, slitlamp biomicroscopy in live animals even 7 days after topical PEI2-GNP application to the cornea detected no inflammation, redness, or edema in rabbit eyes in vivo, with only moderate cell death and immune reactions. These results suggest that PEI2-GNP are safe for the cornea and can be potentially useful for corneal gene therapy in vivo.
Keywords: gold nanoparticles, polyethylenimine, cornea, gene transfer, toxicity
Introduction
Clarity of the cornea, the outermost covering of the eye, is critical for vision1. Both acquired and genetic factors affect corneal transparency resulting in loss of vision1. Topical gene delivery to the cornea offers long-lasting management of corneal disorders and restoration of vision. Recombinant viruses used as a vector for corneal gene therapy have shown high gene transfer efficiency and long-term transgene expression but posed safety and toxicity concerns2-9. The non-viral vectors showed low immunogenicity, incorporation of large DNA, and low cost, but their utility for corneal gene therapy has been hampered by poor transfection efficiency10-13. Thus, more efficacious non-viral vectors for ocular gene therapy are badly needed.
Advances in nanotechnology have produced scores of nanoparticles for such biomedical applications as diagnostic assays and imaging, thermal ablation, and radiotherapy14-17. Because of their small size and large surface area, nanoparticles can readily travel into the target cells and transport big payloads of therapeutics, including DNA and antibodies inside the targeted cell/organ18-23. Although recent studies have shown a high level of gene transfer mediated by nanoparticles in non-ocular tissues24-26, little has been done to explore the potential of nanoparticles for delivering genes in the cornea27.
Gold nanoparticles (GNP) have attracted much attention because of their bio-inertness, non-toxicity, ease of synthesis, and efficient condensation of DNA28. GNP employed as gene delivery vectors have exhibited significant transfection of reporter genes into transformed mammalian cells and subsequent gene expression23,29-32. However, the potential of GNP for in vivo corneal gene therapy has not been examined. Yet the cornea is an ideal target for topical GNP-mediated gene delivery due to the established gene therapy model for testing the efficacy and safety of gene therapy agents because of its immune-privileged status, ease of administering gene therapy reagents, and easy visual monitoring2. In addition, human cornea can be maintained in cultures for weeks to optimize parameters necessary for defining the safety and efficacy of gene delivery vectors2.
In the present study, we tested (i) the potential of PEI2-GNP as a gene therapy vector for the cornea using a human corneal fibroblast in vitro model, (ii) the cellular uptake and clearance of GNP using an in vivo rabbit model, and (iii) the toxicity and safety of PEI2-GNP for the rabbit cornea in vivo.
Methods
Synthesis of polyethylenimine-conjugated gold nanoparticles (PEI2-GNP)
PEI2-GNP were synthesized through conjugation of thiol-modified 2-kDa PEI to GNP as previously described32. Briefly, an aqueous solution containing 0.48 mmol of the thiol-modified PEI was combined with 1.43 mmol of HAuCl4•3H2O and stirred for 10 min before a drop wise addition of 71.4 mmol of NaBH4, followed by stirring for 24 h. The conjugates obtained were dialyzed extensively against water with a 12-kDa cutoff membrane. A portion of the obtained solution was lyophilized and analyzed for elemental content (57.8 % gold, Au/PEI molar ratio of 15). The amount of PEI in the stock solution was calculated based on the mass of the dried solid and the ratio of gold to PEI.
In vivo rabbit model
Twenty-four female New Zealand White rabbits (2.5-3.0 kg) were used in accordance with the tenets of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The study was approved by the Animal Care and Use Committees of the University of Missouri-Columbia and the Harry S. Truman Memorial Veterans’ Hospital (Columbia, MO). All animals received humane care in compliance with the NIH guidelines. Rabbits were anesthetized with an intramuscular injection of the ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (10 mg/kg) cocktail.
In vitro human cornea model
Donor human corneas procured from the eye bank were used in accordance to the Declaration of Helsinki for the use of human tissue. Primary human corneal fibroblasts were generated from human corneas as described previously33. The epithelium and endothelium of the cornea were removed with a scalpel blade, stroma was cut into small pieces and incubated in a humidified CO2 incubator at 37°C in tissue culture flask using Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. The sprouting human corneal fibroblasts were collected and used for experiments. Sixty to seventy confluent cultures were used for experiments.
PEI2-GNP application on the rabbit cornea
The PEI2-GNP were topically applied onto the cornea using a technique developed in our laboratory. Rabbits were anesthetized and after instilling 2-3 drops of 0.5% proparacaine hydrochloride (Alcon, Fort Worth, TX) a wire lid speculum was placed in the eyelids, corneal epithelial was removed with a #64 surgical blade and dried completely with a sterile surgical sponge. Then, 100 μl of 150 mM PEI2-GNP in a phosphate buffered solution containing 10% glucose was placed topically on the cornea for 5 min using a custom-cloning cylinder (7 mm in diameter). The unabsorbed PEI2-GNP solution was removed and the eyes were copiously washed with BSS.
Cellular viability assay
The effect of PEI2-GNP on the cellular viability was assessed with a trypan blue exclusion assay. Briefly, the cultures at 60-70% confluence were incubated with different amounts of 150 mM PEI2-GNP solution (1.9, 2.8, 3.8, 5.6, and 6.5 μl) for 1h in a humidified CO2 incubator. Thereafter, cultures were washed (2X), incubated for an additional 24h. The cultures were trypsinized and suspended in an equal volume of a 0.4% trypan blue solution (Invitrogen, San Diego, CA). The percent of viable and non-viable cells was determined by counting trypan blue stained cells and unstained white cells with an automated cell counter (Invitrogen).
Clinical eye exam and biomicroscopic studies
The biomicroscopy and clinical eye examination was performed with a slitlamp microscope on rabbits under general anesthesia. The eye was aligned in front of a microscope by placing a rabbit on the platform or holding in one’s lap. Qualitative eye examination of the anterior segment (eyelid, sclera, conjunctiva, iris, lens, and cornea) was performed, and data on the redness, edema, and inflammation were recorded. The level of opacity (haze) in the cornea was measured with a slitlamp in a masked manner using a method reported previously33. Grade 0 was a completely clear cornea; grade 0.5 had a trace haze seen with a careful oblique illumination with slitlamp biomicroscopy; grade 1 had a more prominent haze not interfering with visibility of fine iris details; grade 2 had a mild obscuration of iris details; grade 3 had a moderate obscuration of the iris and lens; and grade 4 had a complete opacification of the stroma in the area of the ablation.
Tissue collection
Rabbits were euthanized with pentobarbitone (100 mg/kg) under general anesthesia. Corneas were removed with forceps and sharp Westcott scissors, embedded in liquid optimal cutting temperature compound (Sakura FineTek, Torrance, CA), snap-frozen, and maintained at −80°C. Tissue sections (7 μm) were cut and maintained at −80°C for staining.
In vivo toxicity and keratocyte apoptosis
The in vivo toxicological response of PEI2-GNP on the cornea was determined with a TUNEL assay (ApopTag; Millipore, Temecula, CA) that detects apoptosis and, to a lesser extent, necrosis. The corneal sections were fixed in acetone at −20°C for 10 min and TUNEL assay was performed following manufacturer’s instructions. A rhodamine-conjugated apoptotic cells (red) and DAPI-stained nuclei were viewed and photographed with a fluorescence microscope (Leica, Wetzlar, Germany) equipped with a digital SpotCam RT KE camera system (Diagnostic Instruments, Sterling Heights, MI). The tissue sections prepared from de-epithelialized rabbit cornea without application of PEI2-GNP served as a control.
Immunocytochemistry
The immunological reaction in the cornea to PEI2-GNP application was examined by performing CD11b immunostaining. The rabbit corneal sections (7 μm) were stained with CD11b primary antibody (BD Pharmingen, San Jose, CA; 1:50 dilution) for 90 min, followed by the AlexaFlour 594 anti-rat IgG secondary antibody (1:500 dilution) for 60 min. The DAPI-stained nuclei and CD11b-stained cells in tissue sections were viewed and photographed under a fluorescent microscope (Leica) equipped with a digital SpotCam RT KE camera system (Diagnostic Instruments). The rabbit corneas receiving PBS instead of PEI2-GNP were used as controls.
Silver staining
The distribution and localization of PEI2-GNP in the rabbit cornea in vivo was determined with a silver staining kit (Molecular Probes, Invitrogen) following manufacturer’s protocol. The 7μm corneal sections were counterstained with silver staining solution. The resultant deposits of metallic silver around GNP were visualized using light microscopy and photographed with an oil immersion lens (Leica).
Neutron activation analysis (NAA)
Instrumental neutron activation analysis (NAA) was used to quantify the amount of gold in rabbit corneas to determine the uptake of GNP in corneal tissue. The NAA measurements were performed at the University of Missouri Research Reactor using the 411.8 keV gamma-ray from 198-Au (half-life of 2.7 days). The 198-Au was produced by the neutron capture reaction on 197-Au. For the NAA measurements, samples (rabbit corneas) were loaded in polyethylene transfer tubes in sets of nine and irradiated for 90 sec in a thermal flux density of approximately 5 × 1013 n cm-2 s-1. The samples were then allowed to decay for 24 to 48 h and live time counted for 20 min in a high-resolution γ-ray spectrometry system. The Au comparator standards irradiated concurrently with the samples yielded a relative specific activity of (237 ± 6) × 103 counts/μg of Au (n = 9) with a relative standard deviation of 2.5%.
Transmission electron microscopy (TEM)
Rabbit corneas were fixed in TEM fixative and processed at the University of Missouri Electron Microscopy Core. The 85nm ultrathin corneal sections were visualized and imaged using JEOL 1400 TEM (Tokyo, Japan).
Agarose gel retardation assay
The GNP-plasmid complexes were prepared by mixing 1 μg of plasmid DNA with various concentration of PEI2-GNP at predetermined N-to-P ratios (these ratios depict the molar ratio of nitrogen of PEI and the phosphate of DNA). The DNA:PEI2-GNP mixture was incubated at 37°C for 30 min, loaded on 1% agarose gel containing ethidium bromide, and subjected to electrophoresis with a Tris-acetate running buffer.
Transfection of human corneas in vitro
Seventy percent confluent primary cultures of human corneal fibroblasts were used for these studies. The DNA-GNP polyplexes were prepared at a N-to-P ratio of 180. Briefly, appropriate amounts of the stock solution of 150 mM PEI2-GNP in 300 μl of water were added dropwise with constant stirring to 7.5 μg of plasmid DNA (pTRUF11 expressing GFP gene) in 300 μl of 20 mM aqueous PBS containing 10% glucose. The resulting solutions were incubated at 37°C for 30 min and diluted to 7.5 ml with DMEM containing 10% FBS. Then 2.5 ml of this mixture was added to each well in a 6-well plate. The transfection mixture was removed after 6 h, and the cells were allowed to grow till they reached a 90% confluence.
Statistical analysis
The results were expressed as mean ± standard error of the mean. Statistical analysis was performed using a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test. A value of p < 0.05 was considered statistically significant.
Results
Effect of PEI2-GNP on cell viability and morphology
To test the potential of PEI2-GNP as ocular gene delivery vectors, we first evaluated their toxicity. Figure 1 depicts cellular viability of human corneal fibroblast cultures exposed to PEI2-GNP at the N/P ratios of 60, 90, 120, 180, and 210 (the N/P ratio is that of PEI’s monomer to DNA’s monomer). Only at the N/P ratio of 210 was an appreciable (13%, P < 0.01) decrease in the live cell count observed (Figure 1); lower concentrations of PEI-GNP did not significantly alter the cellular viability (P > 0.05). Since the N/P ratio of 180 was the highest well tolerated dose of PEI2-GNP, it was used in all subsequent studies.
Figure 1.
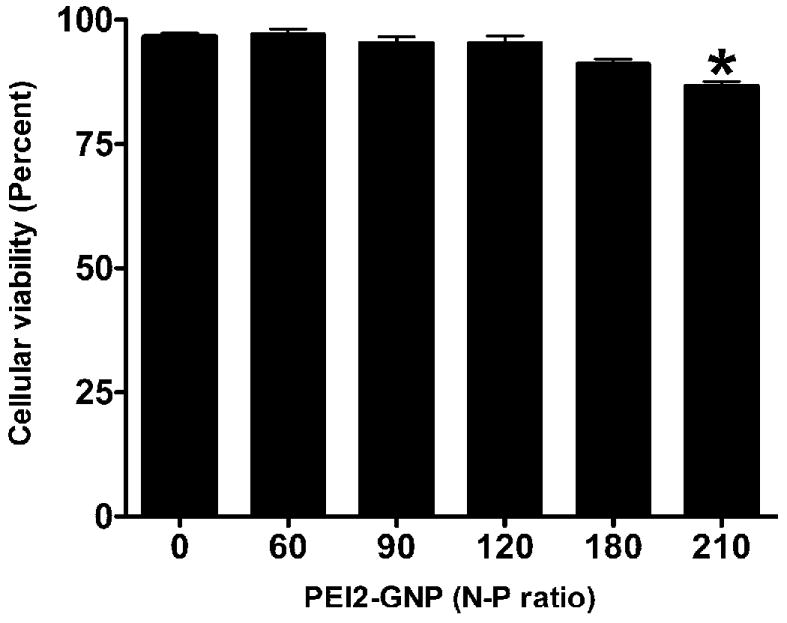
Dose-dependent effect of PEI2-GNP on human corneal fibroblast viability. Cultures were exposed to five different concentrations of PEI2-GNP (N/P ratios of 60, 90, 120, 180, and 210) for 1 h, and cellular viability was quantified with a trypan blue assay.
Light microscopy images were evaluated to observe any phenotypic or morphologic changes in human corneal fibroblast cultures after the application of PEI2-GNP. Figure 2 shows a representative image of DMEM-treated control and the PEI2-GNP treated cultures. Control cultures demonstrated a classic spindle-shaped morphology. The PEI2-GNP treatment caused no observable changes in morphology (Supplementary data Figure S1). Throughout the study period, both the control and the treated cell culture populations continued to demonstrate a healthy spindle-shaped morphology and progressed normally to confluence at about 36 h.
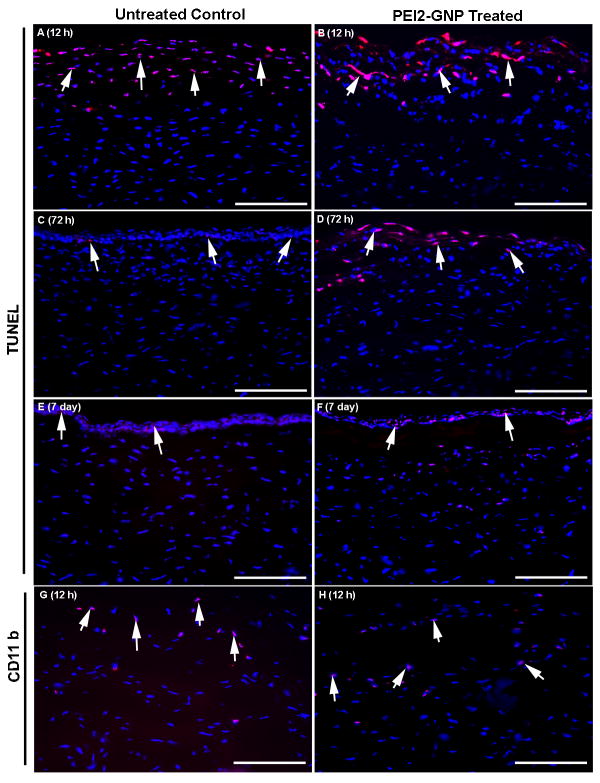
Figure 2.
Representative images showing cytotoxicity of PEI2-GNP to the rabbit cornea in vivo evaluated with TUNEL assay (A-F) and CD11b immunocytochemistry. The GNP-untreated (A, E) and GNP-treated (B, F) corneas did not show any statistically significant difference in TUNEL positive cells (red) at 12 h or 7days. However, a significant difference in the TUNEL-positive cells in GNP-untreated (C) and GNP-treated (D) corneas was detected at 72 h. The detection of apoptosis at 12 hours in untreated control and treated corneas was due to the vector-delivery technique, which involves removal of epithelium and is known to cause transient keratocyte apoptosis. The corneal epithelium in rabbit typically regenerates completely in 48-72h. The removal of epithelium is frequently used in eye clinic to treat epithelial defects, and was found useful for defining tissue-selective gene therapy modalities for the cornea. Images G-H show immunological response of cornea to PEI2-GNP application in vivo. The GNP-untreated (G) and PEI2-GNP–treated (H) rabbit corneas subjected to CD11b immunostaining showed moderate CD11b-positive cells (red) in the anterior stroma at 12 h. Less than 3 CD11b-positive cells were detected at 72 h or 7 days (data not shown). Nuclei are stained blue with DAPI. Scale bar denotes 100 μm.
Effect of PEI2-GNP on cell death in the rabbit corneas in vivo
The foregoing encouraging in vitro studies prompted us to examine the toxicity of PEI2-GNP to the cornea using an in vivo rabbit model by measuring keratocyte apoptosis. Figure 2 (A-F) shows the keratocyte death detected by the TUNEL assay in control corneas and rabbit corneas exposed to a single 100-μl PEI2-GNP topical application for 5 min. A significant number of TUNEL positive cells (30 ± 7) were detected in the anterior stroma at the tested time point of 12 h. Similar level of TUNEL staining was also detected in untreated corneas subjected to epithelial scraping suggesting that most of the apoptotic cells death noted at 12 h was due to scrape injury rather than the PEI2-GNP application. At 72 h, PEI2-GNP treated corneas still showed TUNEL positive cells, whereas few, if any, TUNEL positive cells were detected in the untreated corneas. By day 7, fewer than four TUNEL positive cells were seen in the stroma of PEI2-GNP treated corneas, and this number was significantly (P < 0.01) below that observed at either 12 h or 72 h. By day 7, natural corneal healing was completed, and the TUNEL positive cells represent only normal corneal epithelial cell replenishment by apoptosis. However, the treated corneas showed fewer DAPI stained keratocyte nuclei below the regenerated epithelium compared to the control corneas suggesting that the PEI2-GNP application delays repopulation of keratocytes in the anterior stroma.
Effect of PEI2-GNP on immune response in the rabbit corneas in vivo
To test the possibility of inflammatory response due to the PEI2-GNP application, the treated rabbit corneal sections were stained for the CD11b antigen specific for activated granulocytes. As seen in Figure 2 (G,H), a comparable number of CD11b-positive cells (10-15) were detected in PEI2-GNP treated and untreated rabbit corneas at 12 h post application. Only 1-3 Cd11b-positive cells were detected in the treated corneas at 72 h and 7 days after the PEI2-GNP application (data not shown).
Clinical examination of PEI2-GNP treated rabbit eyes using slitlamp biomicroscopy
To test the effect of PEI2-GNP application on cornea transparency, redness, and cellular infiltration, slitlamp biomicroscopy was performed in rabbit eyes. Figure 3 shows slit-lamp images of the eyes of untreated (control) and the treated corneas at 12 h and 7 days after the PEI2-GNP application. A mild purple coloration in the cornea was noted at 12 h, suggesting the uptake of colored PEI2-GNP solution. A qualitative comparison between the control and the treated corneas revealed no significant difference in the degree of redness, edema, or cellular infiltration at either 12 h or 7 days post-application.
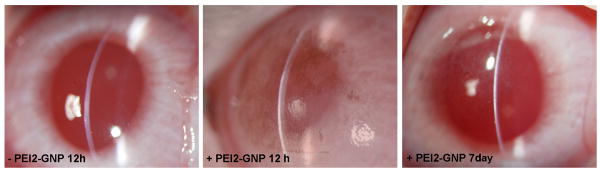
Figure 3.
Representative clinical eye examination images performed with slitlamp microscope in PEI2-GNP-treated and untreated (control) rabbit corneas at 12 h and 7 days post application. No opacity, redness or inflammation are seen. PEI2-GNP-treated corneas show mild purple coloration at 12 h confirming GNP uptake and clearing of coloration detected on day 7 suggest no GNP accumulation in the cornea.
Total uptake of GNP in rabbit corneas in vivo was analyzed using neutron activation (NAA). The remarkably high levels of gold detected in the PEI2-GNP treated rabbit corneas (Table 1) suggest substantial uptake of GNP.
Table 1. Gold uptake in PEI2-GNP-treated rabbit corneas in vivo.
The total gold content was quantified with neutron activation analysis in corneas at 12 h, 72 h, and 7 days post PEI2-GNP application. Values are mean ± standard error.
| Time after PEI2-GNP application | Gold (ppm) |
|---|---|
| 12 h | 332 ± 38 |
| 72 h | 326 ± 37 |
| 7 days | 319 ± 40 |
| 1 month | 259 ± 10 |
The GNP may be cleared from the cornea via multiple mechanisms, such as washing off with tears, diffusion into aqueous fluid, or clearance by leukocytes. To test the in vivo clearance, rabbit corneas were subjected to NAA at 12 h, 72 h, 7 days and 1 month after the PEI2-GNP treatment. Table 1 depicts an average gold content in rabbit corneas at the four different time points following a single PEI2-GNP application: 332, 326, 319 and 259 ppm, respectively. These results suggest that PEI2-GNP clear from the cornea in vivo slowly.
PEI2-GNP distribution, tracking, and localization in the rabbit corneas in vivo
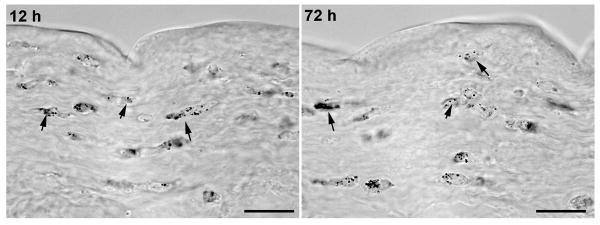
Figure 4 shows the localization of GNP in the corneal stroma detected by silver staining. Following a 5-min topical application, GNPs were distributed uniformly throughout the anterior and mid-stroma in rabbit corneas.
Figure 4.
Representative images of rabbit corneal sections showing localization and distribution of GNP detected with silver staining. Stained gold nanoparticles (black) were detected throughout the stroma of PEI2-GNP-treated rabbit corneas. Scale bar denotes 20 μm.
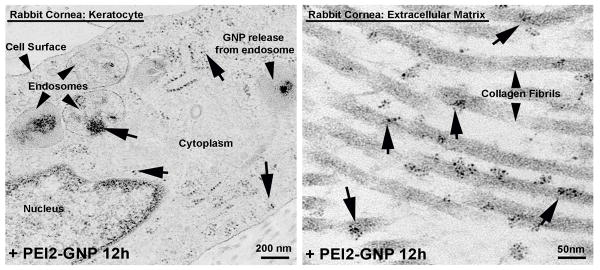
Prompted by the NAA and silver staining results that confirmed the uptake and presence of GNPs in rabbit cornea in vivo, we investigated the sub-cellular localization of PEI2-GNP in the corneal cells in vivo using transmission electron microscopy (TEM). As is evident from Figure 8, PEI2-GNP was detected inside keratocytes (Figure 5A), as well as in the extracellular matrix of corneal stroma (Figure 5B). Moreover, the TEM images (Figure 5A) reveal GNP inside endosomes, suggesting that topical application of PEI-GNP occurs via an endocytic mechanism. This inference is in agreement with the observation that intravenous PEI-GNP polyplexes are taken up via endocytosis.
Figure 5.
Representative transmission electron microscopy images of PEI2-GNP-treated rabbit corneas demonstrating the presence and intracellular trafficking of GNP in keratoctes (A) and extracellular matrix (B). GNP can be seen in the endosomes near the cell surface depicting their uptake by endocytosis. Ruptured endosome represents the release of GNP into the cytoplasm.
Characterization of PEI2-GNP polyplexes by gel retardation assay
The agarose gel electrophoresis of mixtures of PEI2-GNP and plasmid DNA at various N/P ratios were run (Supplementary data Figure S2). The PEI2-GNP completely inhibited electrophoretic migration of the plasmid at N to P ratios exceeding 4, suggesting complex formation between the positively charged PEI2-GNP and the negatively charged DNA. This complexation results in the exclusion of the ethidium bromide dye34, as is evident in the gel by the lack of ethidium bromide staining of DNA retained in the wells (Supplementary data Figure S2).
PEI2-GNP mediated gene transfer
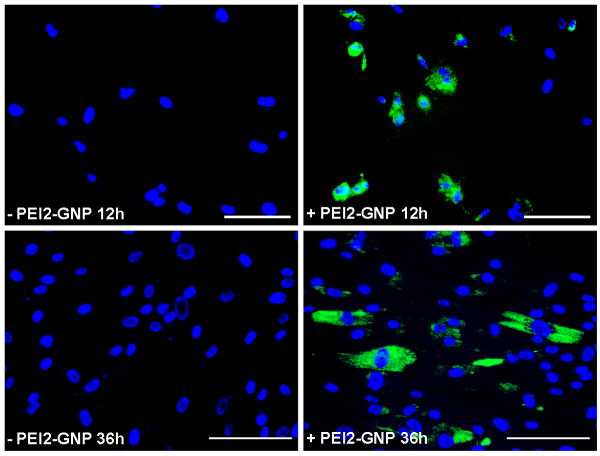
Next we examined the ability of PEI2-GNP to transfect human corneal fibroblasts by exposing cultures to PEI2-GNP polyplexes expressing green fluorescent marker protein gene. Figure 6 demonstrates GFP expression observed in corneal fibroblasts at 12 and 36 h post-transfection. At 12 h, nearly 50% of human corneal fibroblasts showed GFP expression indicating that PEI2-GNP is an efficient carrier for delivering plasmid DNA into corneal cells. The level of GFP gene expression at 36 h was considerably higher, suggesting a time-dependent increase of delivered gene expression in the cells. At 36 h, the number of GFP-positive cells remained unaltered, while the number of DAPI-stained nuclei has increased, likely due to cell division.
Figure 6.
Florescence microscopy images of PEI2-GNP: plasmid treated human corneal fibroblast cultures showing delivered GFP expression. The cultures were exposed to mixtures made of PEI2-GNP and plasmid expressing GFP (N/P ratio of 180). Nuclei are stained blue with DAPI; scale bar denotes 100 μm.
Discussion
The gene delivery vector plays a pivotal role for the success of gene therapy. Of the many nanoparticles available, GNP were selected for corneal gene therapy studies because of their bio-inertness and ease of conjugation to polymeric scaffolds23,29-32. While several researchers have demonstrated successful gene delivery with cationic polymer-conjugated GNP23,29-32,35, we conjugated GNP to PEI because of the many unique properties of this polycation, including its ability to efficiently condense nucleic acids and protect them from nuclease degredation32,36. In evaluating the DNA condensing ability of PEI2-GNP using gel retardation assay, we observed inhibition of plasmid migration that confirmed effective polyplex formation between it and PEI2-GNP. Additionally, in exhibiting a “proton-sponge” effect that facilitates DNA release from endocytic vesicles, PEI accepts protons pumped into endocytic vesicles during acidification leading to a further influx of protons and chloride ions and ultimately resulting in osmotic swelling and rupture of endocytic vesicles37. TEM data obtained in this study indeed detected ruptured endosomes inside corneal keratocytes.
The results obtained in this study also suggest that PEI2-GNP are safe and efficient gene delivery vectors for corneal cells because up to an N/P ratio of 180, they did not alter cellular phenotype or viability. At the N/P ratio of 210 showed moderate loss of cellular viability which may be due to either gold or PEI. Since PEI alone has been shown to exhibit moderate cytotoxicity36,38,39, we hypothesized that its interaction with polyanionic cellular components32,36 were responsible for the cytotoxic effects observed at a high N/P ratio of PEI2-GNP.
The topical route of application was chosen for the in vivo studies since it is the most convenient way of delivering drugs to the eye and it avoids pharmacokinetic challenges associated with systemic administration. Lack of edema, redness, or cellular infiltration after PEI2-GNP treatment in rabbit cornea revealed by slitlamp biomicroscopy suggests a lack of toxicity in corneal gene therapy. However, a mild to moderate keraotocyte death demonstrated by the TUNEL assay and a decrease in cellular density of keratocytes in the anterior stroma suggest that more studies are needed to identify optimal dose of PEI2-GNP for the rabbit eye. Our TUNEL staining and decreased keratocyte density observed at 72 h and 7 days after PEI2-GNP application are consistent with a report that that PEI caused activation of the mitochondrial pathway of apoptosis resulting in a delayed phase of cell death.40
Identification of an efficient gene delivery vector for the stroma can lead to the development of gene therapy modalities for treating corneal diseases and disorders. One of the most intriguing findings of this study is the detection of GNP inside keratocytes and the extracellular matrix. The presence in keratocytes substantiates PEI2-GNP’s potential use as a gene delivery vector, whereas its detection in the extracellular matrix demonstrates that GNP can serve as a vector for drug delivery.
The presence of GNPs in the eye may affect refraction and could pose unknown challenges. To address this concern, the residence time of GNP in the cornea and their clearance rate from the corneal tissue were monitored. To determine the latter, total gold content in PEI2-GNP treated rabbit corneas was analyzed and found to be progressively clearing from the cornea. Based on the rabbit’s day-to-day behavioral activities and biomicroscopy studies, we deduce that GNP presence in the cornea does not impair vision, however, complete clearance of GNP from the cornea may take months. While the clearance of gold nanoparticles via renal excretion has been reported as the main route of GNP clearance 41,42, this route of clearance is not available to the cornea since it is an avascular tissue; furthermore, GNP were topically applied using a cloning cylinder for a short time, thus restricting GNP contact with neighboring vascular tissues. Perhaps GNP clearance in the cornea occurs through slow diffusion into tears or the aqueous humor.
In conclusion, the low toxicity, rapid uptake, and slow clearance of PEI2-GNP observed in the rabbit cornea in vivo suggest that it offers an attractive platform for delivering therapeutic genes to the cornea. Additionally, after releasing its nucleic acid cargo, the vector does not remain in the cornea but diffuses out of the tissue. Thus PEI2-GNP may offer safe and efficacious gene therapy modalities for treating corneal diseases.
Supplementary Material
Representative light microscopy images showing the effects of PEI2-GNP (N/P ratio of 180) on the human corneal fibroblast phenotype. Both untreated (control) and PEI2-GNP-treated human corneal fibroblast cultures exhibited a typical spindle-shape morphology at 24 and 36 h after application revealing that the tested N/P 180 dose does not induce phenotypic changes. Scale bar denotes 10 μm.
Agarose gel electrophoresis assay showing effective complexation of plasmid DNA by the PEI2-GNP. PEI2-GNP completely inhibited plasmid DNA migration at the N/P ratios above 4 suggesting effective complex formation. DNA denotes plasmid DNA (1 μg) without PEI2-GNP showing its normal migration. No DNA depicts PEI2-GNP without plasmid DNA. L denotes 1 kb plus the DNA ladder.
Acknowledgments
We thank Heartland Eye Bank, St. Louis, MO, for donor human corneas and Jason M. Newman and Eric T. Hansen (medical) and Tyler C. Cebulko and Yasaman J. Hemmat (undergraduate) students for their help.
Supported by: 1I01BX000357-01 Veteran Health Affairs Merit (RRM), RO1EY17294 National Eye Institute, NIH (RRM), RO1EB000244 National Institutes of Health (AMK) and Research to Prevent Blindness Unrestricted grants.
Footnotes
Conflict of Interest Statement: None of the authors have any conflict of interest.
Disclosures: None of the authors have any Commercial Relationship or other disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qazi Y, Wong G, Monson B, Stringham J, Ambati BK. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull. 2010;81:198–210. doi: 10.1016/j.brainresbull.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–59. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Ghosh A, Siddapa C, Mohan RR. Ocular Surface: Gene Therapy. In: Besharse J, Dana R, Dartt DA, editors. Encyclopedia of the Eye. Elsevier; 2010. pp. 185–194. [Google Scholar]
- 4.Sharma A, Ghosh A, Hansen ET, Newman JM, Mohan RR. Transduction efficiency of AAV 2/6, 2/8 and 2/9 vectors for delivering genes in human corneal fibroblasts. Brain Res Bull. 2010;81:273–8. doi: 10.1016/j.brainresbull.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Tovey JC, Ghosh A, Mohan RR. AAV serotype influences gene transfer in corneal stroma in vivo. Exp Eye Res. 2010;91:440–8. doi: 10.1016/j.exer.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan RR, Schultz GS, Hong JW, Mohan RR, Wilson SE. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003;76:373–83. doi: 10.1016/s0014-4835(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, Hauswirth WW, Castro MG, Schultz GS, Ljubimov AV. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. Mol Vis. 2008;14:2087–96. [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter T, Yang J, Dannowski H, Vogt K, Volk HD, Pleyer U. Effects of interleukin-12p40 gene transfer on rat corneal allograft survival. Transpl Immunol. 2007;18:101–7. doi: 10.1016/j.trim.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci. 2010;51:1970–80. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrieu-Soler C, Bejjani RA, de Bizemont T, Normand N, BenEzra D, Behar-Cohen F. Ocular gene therapy: a review of nonviral strategies. Mol Vis. 2006;12:1334–47. [PubMed] [Google Scholar]
- 11.Dannowski H, Bednarz J, Reszka R, Engelmann K, Pleyer U. Lipid-mediated gene transfer of acidic fibroblast growth factor into human corneal endothelial cells. Exp Eye Res. 2005;80:93–101. doi: 10.1016/j.exer.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Pleyer U, Groth D, Hinz B, Keil O, Bertelmann E, Rieck P, Reszka R. Efficiency and toxicity of liposome-mediated gene transfer to corneal endothelial cells. Exp Eye Res. 2001;73:1–7. doi: 10.1006/exer.2001.1005. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CN, Yang LC, Wu PC, Kuo HK, Kuo CJ, Tai MH. Dehydrated form of plasmid expressing basic fibroblast growth factor-polyethylenimine complex is a novel and accurate method for gene transfer to the cornea. Curr Eye Res. 2005;30:1015–24. doi: 10.1080/02713680500330512. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine. 2006;1:413–26. doi: 10.2217/17435889.1.4.413. [DOI] [PubMed] [Google Scholar]
- 17.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6:865–78. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 18.Ravi Kumar M, Hellermann G, Lockey RF, Mohapatra SS. Nanoparticle-mediated gene delivery: state of the art. Expert Opin Biol Ther. 2004;4:1213–24. doi: 10.1517/14712598.4.8.1213. [DOI] [PubMed] [Google Scholar]
- 19.Ragusa A, García I, Penadés S. Nanoparticles as nonviral gene delivery vectors. IEEE Trans Nanobioscience. 2007;6:319–30. doi: 10.1109/tnb.2007.908996. [DOI] [PubMed] [Google Scholar]
- 20.Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed Engl. 2008;47:1382–95. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- 21.Jin S, Leach JC, Ye K. Nanoparticle-mediated gene delivery. Methods Mol Biol. 2009;544:547–57. doi: 10.1007/978-1-59745-483-4_34. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury EH, Akaike T. Bio-functional inorganic materials: an attractive branch of gene-based nano-medicine delivery for 21st century. Curr Gene Ther. 2005;5:669–76. doi: 10.2174/156652305774964613. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano. 2008;2:2213–8. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda-Mamiya R, Noiri E, Isobe H, Nakanishi W, Okamoto K, Doi K, Sugaya T, Izumi T, Homma T, Nakamura E. In vivo gene delivery by cationic tetraamino fullerene. Proc Natl Acad Sci USA. 2010;107:5339–44. doi: 10.1073/pnas.0909223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102:11539–44. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–30. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res. 2008;48:319–24. doi: 10.1016/j.visres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. doi: 10.1016/j.jconrel.2009.12.006. in press. [DOI] [PubMed] [Google Scholar]
- 29.Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. Gold nanoparticle-mediated transfection of mammalian cells. Bioconjug Chem. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Li P, Li G, Wang J, Wang E. The effect of nocodazole on the transfection efficiency of lipid-bilayer coated gold nanoparticles. Biomaterials. 2009;30:1382–8. doi: 10.1016/j.biomaterials.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Zhang X, Yu X, Zha X, Fu Q, Liu B, Wang X, Chen Y, Chen Y, Shan Y, Jin Y, Wu Y, Liu J, Kong W, Shen J. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29:111–7. doi: 10.1016/j.biomaterials.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA. 2003;100:9138–43. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR. Trichostatin A inhibits corneal haze in vitro and in vivo. Invest Ophthalmol Vis Sci. 2009;50:2695–701. doi: 10.1167/iovs.08-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–63. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 35.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49:3280–94. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005;22:373–80. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 38.Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23:1868–76. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 39.Hoon Jeong J, Christensen LV, Yockman JW, Zhong Z, Engbersen JF, Jong Kim W, Feijen J, Wan Kim S. Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials. 2007;28:1912–7. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–5. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006;79:248–53. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 42.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–9. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative light microscopy images showing the effects of PEI2-GNP (N/P ratio of 180) on the human corneal fibroblast phenotype. Both untreated (control) and PEI2-GNP-treated human corneal fibroblast cultures exhibited a typical spindle-shape morphology at 24 and 36 h after application revealing that the tested N/P 180 dose does not induce phenotypic changes. Scale bar denotes 10 μm.
Agarose gel electrophoresis assay showing effective complexation of plasmid DNA by the PEI2-GNP. PEI2-GNP completely inhibited plasmid DNA migration at the N/P ratios above 4 suggesting effective complex formation. DNA denotes plasmid DNA (1 μg) without PEI2-GNP showing its normal migration. No DNA depicts PEI2-GNP without plasmid DNA. L denotes 1 kb plus the DNA ladder.