Abstract
Background and Purpose
To provide the first correlative study of the hyperdense MCA sign (HMCAS) and gradient-echo (GRE) MRI blooming artifact (BA) with pathology of retrieved thrombi in acute ischemic stroke.
Methods
Noncontrast CT and GRE MRI studies prior to mechanical thrombectomy in 50 consecutive cases of acute MCA ischemic stroke were reviewed, blinded to clinical and pathology data. Occlusions retrieved by thrombectomy underwent histopathologic analysis, including automated quantitative and qualitative rating of proportion composed of red blood cells (RBC), white blood cells (WBC), and fibrin on microscopy of sectioned thrombi.
Results
Among 50 patients, mean age was 66 years and 48% were female. Mean (SD) proportion was 61% (±21) fibrin, 34% (±21) RBC, and 4% (±2) WBC. Of retrieved clots, 22 (44%) were fibrin-dominant, 13 (26%) RBC-dominant and 15 (30%) mixed. HMCAS was identified in 10/20 MCA stroke cases with CT, with mean Hounsfield Unit (HU) density of 61 (SD±8). BA occurred in 17/32 with GRE MRI. HMCAS was more commonly seen with RBC-dominant and mixed than fibrin-dominant clots (100% vs. 67% vs. 20%, p=0.016). Mean percent RBC composition was higher in clots associated with HMCAS (47% vs. 22%, p=0.016). BA was more common in RBC-dominant and mixed clots compared to fibrin-dominant clots (100% vs. 63% vs. 25%, p=0.002). Mean percent RBC was greater with BA (42% vs. 23%, p=0.011).
Conclusions
CT HMCAS and GRE MRI BA reflect pathology of occlusive thrombus. RBC content determines appearance of HMCAS and BA, whereas absence of HMCAS or BA may indicate fibrin-predominant occlusive thrombi.
Keywords: Stroke, cerebral ischemia, thrombus, CT, MRI
Introduction
Acute ischemic stroke may result from a diverse range of underlying disorders, often culminating in obstruction of an artery. The pathophysiologic mechanisms that lead to obstruction of a proximal intracranial artery and resultant downstream ischemia are rarely discerned in the acute phase; however, the role of thrombosis as a cause of obstruction is frequently noted during evaluation. Most therapeutic strategies for acute ischemic stroke focus on clot disruption or resolution of thrombosis.1 In fact, the only two FDA-approved therapies include pharmacologic thrombolysis with intravenous tissue-plasminogen activator (tPA) and endovascular thrombectomy with various devices.2–4 Intravenous tPA does not depend on overt delineation of thrombus, yet subtle neuroimaging findings suggesting thrombosis in proximal intracranial arteries are often viewed as confirmatory evidence of a potentially extensive or destructive event that warrants aggressive treatment.5–7 Prior to most endovascular revascularization procedures for stroke, noninvasive imaging in the form of CT or MRI may similarly reveal features suggestive of a proximal occlusion, yet characterizing such an occlusion typically relies on other approaches. A unique aspect of thrombectomy or clot retrieval from an intracranial artery in the setting of acute ischemic stroke is the opportunity to directly investigate clot composition or the nature of thrombosis or any material that has blocked flow to critically dependent downstream regions of the brain.8–10
Prior studies have analyzed the presence of early vessel signs on CT and MRI suggestive of thrombosis, including the hyperdense middle cerebral artery sign (HMCAS) on CT and blooming artifact (BA) on gradient-echo or other susceptibility-weighted MRI sequences.5–7, 10–17 Many of these studies have correlated these findings as a poor prognostic factor in clinical outcome and diminished likelihood of revascularization.7, 10–13, 17, 18 Most of the studies, however, have not shown angiographic correlation or actual pathologic correlation with the features of the underlying occlusive lesion.
We previously described the initial series of pathologic changes in thrombi retrieved from the proximal intracranial arterial circulation in acute stroke and now provide the first neuroimaging correlative study that may be used to predict clot composition.8 This report describes the unique opportunity to investigate plaque or thrombus constituents that underlie the presence and characteristics of early vessel signs, including HMCAS and BA.
Methods
During the period from May 2001 through March 2007, 85 consecutive cases of acute ischemic stroke were evaluated with CT or MRI prior to endovascular thrombectomy at our center. Noninvasive imaging with CT or MRI was acquired per standard algorithm for acute stroke cases with noncontrast CT or a MRI protocol including gradient-recalled echo (GRE) sequences as previously described.19 GRE images were acquired with slice thickness of 5mm and no gap, TR 800 ms, TE 15 ms, 30° flip angle, 240 field of view, and 256 × 144 matrix size. Selection criteria for this study included acute middle cerebral artery (MCA) occlusions with available noncontrast CT or GRE MRI data acquired immediately prior to endovascular thrombectomy, and available thrombus pathology resulting from any retrieved specimen. CT studies acquired at outside institutions prior to transfer to our center were not included due to incomplete availability, poor quality, and inability to measure Hounsfield Unit (HU) density on non-DICOM format images. As a result, cases without CT or MRI acquired at our center and thrombectomies that did not yield a pathological specimen were excluded from our analyses.
Clinical, radiographic and detailed angiographic data were prospectively acquired as part of ongoing work at our center. These data are routinely acquired and archived in a centralized database. Two board-certified vascular neurologists with accreditation in neuroimaging retrospectively reviewed the noncontrast CT or GRE sequences acquired immediately prior to endovascular thrombectomy, blinded to clinical and angiographic variables as well as the results of pathologic study. The presence or absence of HMCAS was scored on consensus reading by the two neuroimaging experts based on visual inspection. Conspicuity or increased density of the MCA in asymmetric fashion was used to categorize the HMCAS, although specific measures of HU density were not used in this determination.20 After HMCAS rating, HU density measures were obtained of bilateral segments of the MCA. Axial GRE MRI scans were also reviewed in consensus fashion to determine the presence or absence of BA based on visual inspection. BA was defined as an area of hypointensity or signal loss in the proximal MCA, often distorting the margins of the vessel. If CT or MRI artifacts obscured delineation of HMCAS or BA, then the associated imaging dataset of that case was excluded from our analyses.
Digital subtraction angiography was used to confirm the diagnosis of MCA occlusion prior to thrombectomy. MCA occlusions with extension of clot into the ipsilateral internal carotid or anterior cerebral arteries were included in our analyses. Angiographic techniques and the thrombectomy procedure have been described elsewhere.3 Thrombectomy cases included in our analyses were conducted as part of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI), and Multi MERCI trials and as part of routine clinical care following the United States Food and Drug Administration clearance of the Merci Retriever® System.3, 21–23 The MERCI and Multi MERCI trials evaluated the safety and efficacy of endovascular thrombectomy with the Merci Retrieval® System (Concentric Medical, Inc., Mountain View, CA) in the treatment of proximal intracranial arterial occlusions performed within eight hours of stroke symptom onset.3, 21, 22 Mechanical thrombectomy was performed with the Merci Retriever® System and subsequent generation devices in all cases of this report. Serial angiography from the initial diagnostic runs throughout the procedure until completion of thrombectomy was reviewed to assess features of arterial occlusion and corresponding collateral flow.24–26 The presence of occlusion and extent of antegrade perfusion in the downstream territory was measured with the Thrombolysis in Cerebral Infarction (TICI) scale, and collateral perfusion was graded with the ASITN/SIR collateral flow grading system.24
Clot retrieval occurred sequentially throughout the thrombectomy procedure, with variable amounts of thrombus extracted at each stage. After each pass of the device that appeared to reduce clot burden, the catheter was withdrawn and the distal aspect of the helical coil inspected for the presence of thrombus or any particulate material. If no discrete thrombus was identified, the aspirated material was then gently flushed with saline to uncover any smaller fragments that may be obscured. Photographs documented the relationship of thrombotic material with respect to the distal thrombectomy catheter and architecture of the retained clot. Thrombi were then placed on gauze or surgical dressing and photographed from multiple perspectives. Gross measurements of linear thrombus dimensions were taken using a guide. Thrombus material was immediately fixed in 10% phosphate buffered formalin. Formalin-fixed specimens were embedded in paraffin, cut at 8-µm thickness, and stained with hematoxylin and eosin. Histological sections were photographed with an Olympus BX41 microscope with an attached MicroFire digital camera (model S99809). Histological examination was performed without knowledge of the clinical findings and was based on feature-detection analysis of functionally distinct processes, including platelet:fibrin accumulations (thrombosis in flowing blood), linear neutrophil and monocyte deposits (surface adherence interactions), and erythrocyte-rich accumulations (whole-blood coagulation). Clot composition was also categorized as RBC-dominant, fibrin-dominant, or mixed by light microscopy. Further histopathologic analysis included semi-automated quantitative and qualitative measurements for the proportion of red blood cells (RBC), white blood cells (WBC), and fibrin composition from digitized whole slide digital images. Hematoxylin and eosin stained slides were scanned in at 400× magnification using an Aperio Scanscope XT digital scanner (Aperio, Vista, CA). The resulting individual digital image files were large, ranging from 200 MB to 5 GB, and required processing to smaller file sizes so that image analysis software could be used to quantify proportions of components. This processing was done using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA) to assign pseudo-colors to fibrin, red blood cells, and nucleated white blood cells. Pseudo-colorization was conducted with a look up table and automated thresholds to assign specific colors to imaging features of each clot component for calculation of specific content. Image J software (National Institutes of Health, Bethesda, Maryland) was then utilized to quantify the percentage of RBCs, WBCs, and fibrin by area. These pathology studies were repeated for each fragment of clot retrieved from the entire procedure. When multiple clot fragments were retrieved for analysis, the mean values across fragments were used for clot constituents (i.e. RBC, WBC, fibrin).
Descriptive statistical analyses were performed on all clinical, radiographic, angiographic and pathologic data. The presence or absence of early vessel signs including the HMCAS and BA, and the qualitative descriptions of clot pathology were treated as categorical variables in the statistical analyses. Percentages of each specific clot component were treated as continuous variables. The relationship between early vessel signs of thrombosis on CT and MRI and clot composition was probed using both chi-square and ANOVA statistics, with significance noted below the p=0.05 level. Statistical analyses were performed by with the use of SPSS software (version 16.0; SPSS, Inc., Chicago, I.L.).
Results
Among 50 patients who fulfilled entry criteria the mean age was 66 years, 48% were female, and 82% were white. Clinical characteristics are summarized in Table 1. Angiography demonstrated occlusions of the internal carotid artery (ICA) in 52% and MCA in 48%. The Merci Retriever® System was used either alone (78%) or in combination with intravenous (14%) or other treatments (intra-arterial tPA (2%), angioplasty, stenting). The final median TICI score for patients included in this analysis was 2 (2% TICI 0, 22% 1, 40% 2, 36% 3).
Table 1.
Clinical Characteristics of Study Population
| Clinical Characteristic | Population Variable (n=50) |
|---|---|
| Age (yrs) | Mean 66 ± SD 21 |
| Sex | |
| Female | 48% |
| Race | |
| White | 82% |
| Black | 10% |
| Asian | 6% |
| Hispanic | 2% |
| Diabetes | 12% |
| History of hypertension | 66% |
| Coronary artery disease | 26% |
| Atrial fibrillation | 14% |
| History of smoking | 12% |
| Baseline NIHSS score | Median 19 (IQR15–22) |
| Intravenous tPA | 14% |
| Intra-arterial tPA | 2% |
| Day 90 mRS | Median 3 (IQR 1–5) |
NIHSS = National Institutes of Health Stroke Scale
tPA = tissue Plasminogen Activator
mRS = modified Rankin Scale
SD = Standard Deviation
IQR = Interquartile Range
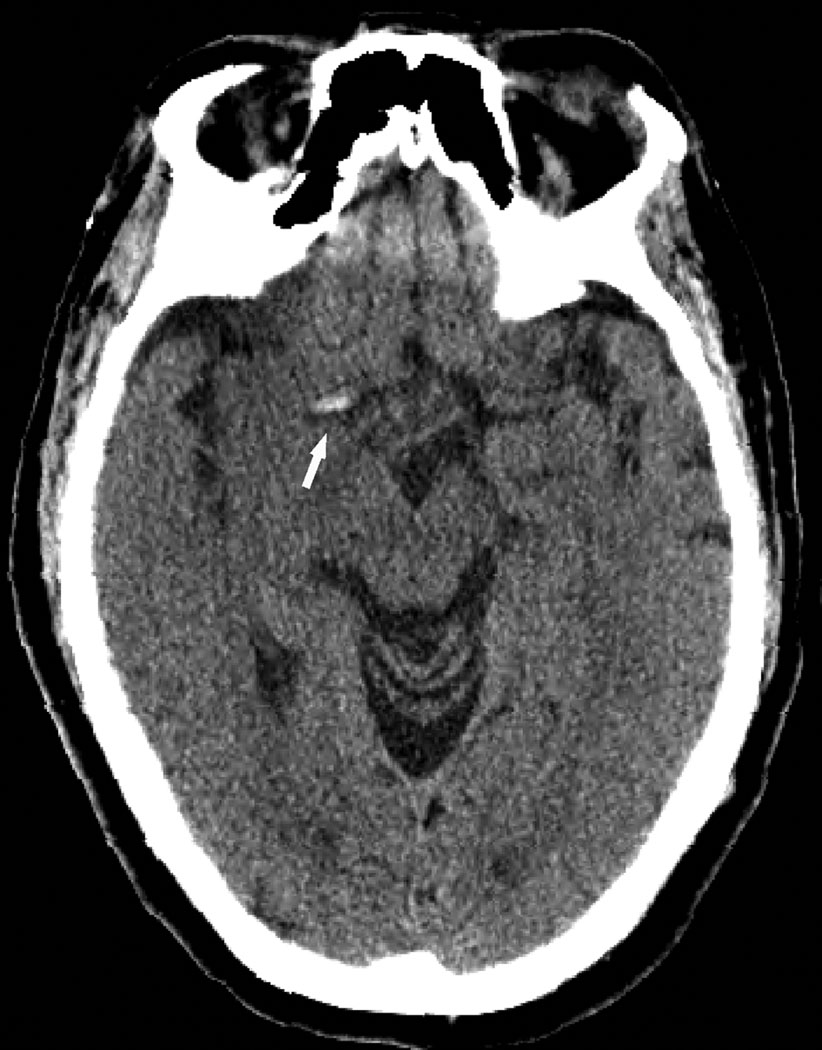
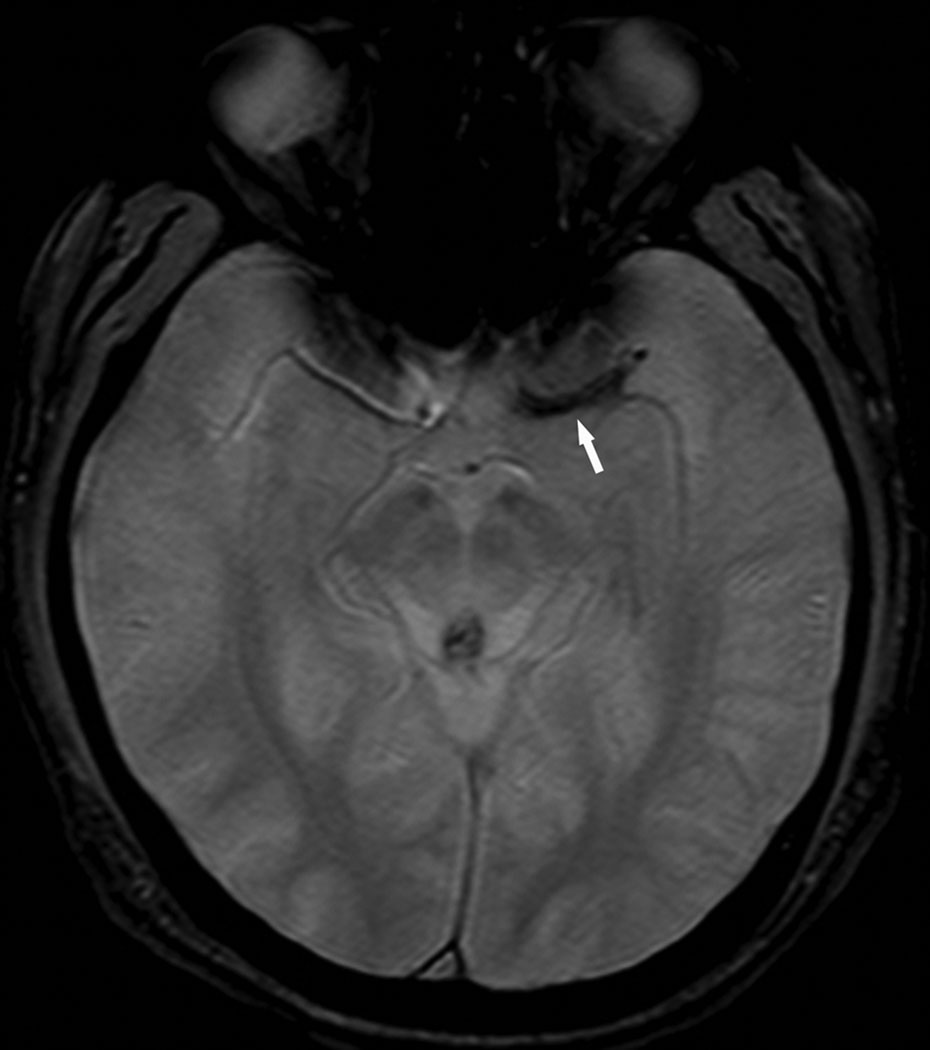
A total of 20 CT scans were included for analysis of which 10 demonstrated HMCAS (Fig. 1). The HMCAS revealed a mean HU of 61 (SD±8) across all cases. There were 32 MRI scans reviewed with 17 (53%) demonstrating BA (Fig. 2). The two patients who had both CT and MRI at our institution prior to angiography were found to have both HMCAS and BA, respectively. Acquisition of CT prior to MRI was utilized for screening purposes in cases where MRI contraindications could not be immediately assessed. In these cases, the vessel signs were situated in the exact same vascular anatomical location.
Figure 1.
Noncontrast CT scan of the head reveals a right hyperdense middle cerebral artery sign (HMCAS, arrow) associated with acute left hemiparesis.
Figure 2.
GRE MRI demonstrates blooming artifact (BA, arrow) in the left middle cerebral artery.
Extracted thrombi were occasionally retrieved as a single mass, though most were retrieved in multiple fragments. These multiple retrieval specimens were obtained at various stages of each procedure and the time to clot retrieval varied extensively. There was no correlation between the amount of thrombus retrieved and recanalization or reperfusion status. The orientation of the occlusive thrombus within the vessel could not be unequivocally established due to the nature of the clot retrieval procedure and catheter manipulation. In some cases, however, intact clots on gross examination and histopathology could be readily oriented in space.
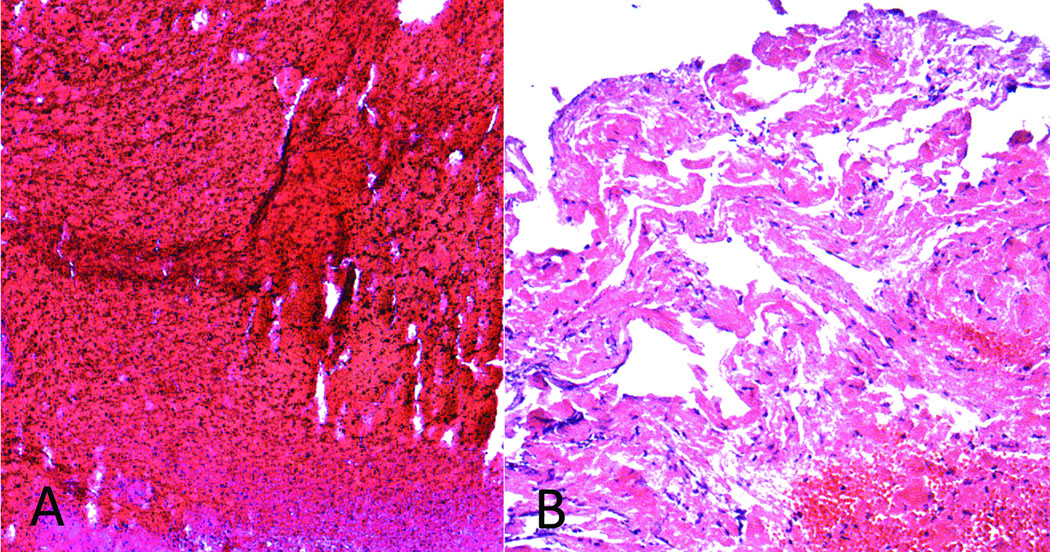
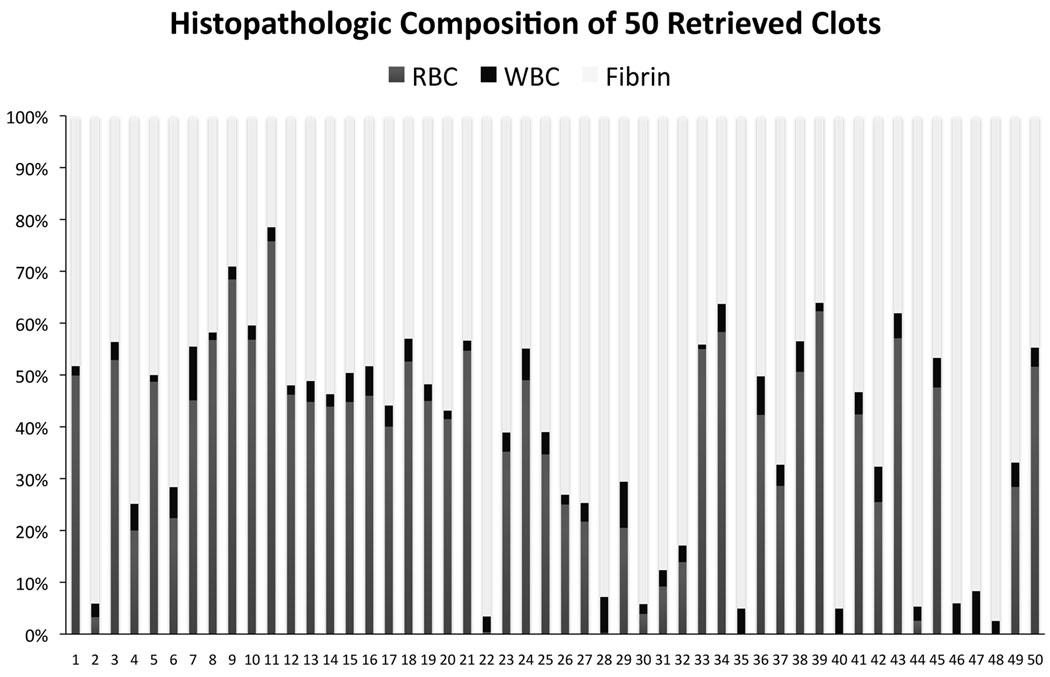
Across all retrieved thrombi, mean (SD) proportion of components was 61% (±21) fibrin, 34% (±21) RBC, and 4% (±2) WBC. Of the retrieved clots, 22 (44%) were classified as fibrin-dominant, 13 (26%) RBC-dominant and 15 (30%) mixed (Fig. 3). A broad distribution of pathology was noted across all cases as depicted in Figure 4. WBC composition was consistently marginal across all cases. In cases with multiple fragments obtained, there was no change in composition with successive clots retrieved. Over the 6-year period of this study, from the first retrieval case ever performed with the Merci Retriever® System to a period more than 2 years after introduction to clinical practice, there was no change in pathologic findings that may have implicated potential variation in technical aspects of the endovascular procedure. We have recently published an autopsy study describing patients with poor outcomes following this procedure.27
Figure 3.
Classification of retrieved thrombi as red blood cell-dominant (A) and fibrin-dominant (B).
Figure 4.
Clot composition based on histopathology, including red blood cell (RBC), white blood cell (WBC), and fibrin percentage. Retrieved clots are numbered from 1 to 50 in order of historical entry into our study.
No correlation was noted between the type of baseline imaging modality (i.e. CT or MRI) and gross or histopathological findings. There were also no differences between the timeline between baseline diagnostic imaging acquisition to clot retrieval (mean 86 ± SD 32 mins), and the resultant thrombus constituents or composition.
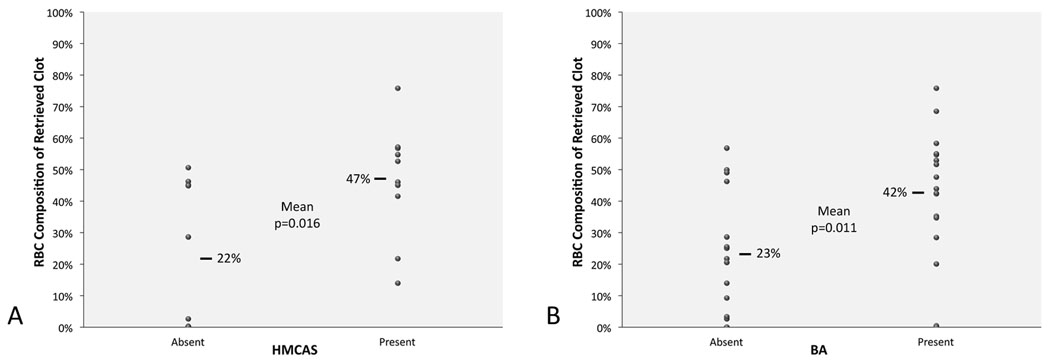
HMCAS on CT was more commonly seen with RBC-dominant and mixed than fibrin-dominant clot pathology (100% vs. 67% vs. 20%, p=0.016). Mean percent RBC composition was higher in clots with HMCAS (47% vs. 22%, p=0.016), although HU density was not correlated with clot composition. BA was also more common in RBC-dominant and mixed clots compared to fibrin-dominant clots (100% vs. 63% vs. 25%, p=0.002). The consistently low percentage of WBC content across all cases was not a determinant of HMCAS or BA. Mean percent RBC was greater with BA (42% vs. 23%, p=0.011). The presence of either early vessel sign (i.e. HMCAS or BA) did not correlate with clinical or radiographic factors. Multivariate regression analyses did not identify predictors of HMCAS or BA other than RBC content (Fig. 5). In the 2 cases with both CT and MRI, the complete concordance of HMCAS and BA was associated with RBC-dominant clots with elevated RBC composition on quantitative analyses. Absence of HMCAS or BA was more common with small, fibrin-rich specimens.
Figure 5.
Red blood cell (RBC) composition of retrieved clots correlates with early vessel signs, including (A) the hyperdense middle cerebral artery sign (HMCAS) and (B) blooming artifact (BA).
Our analyses revealed no correlation between imaging findings (HMCAS or BA) or thrombus histopathology with baseline variables, including stroke severity, or subsequent outcomes. Thrombus histopathology was unrelated to final determination of stroke etiology or mechanism (e.g. cardioembolism or atherosclerosis) and was not predictive for successful extraction. Similarly, there were no differences in imaging or histopathological features with respect to the timing of clot extraction.
Discussion
Noninvasive imaging modalities such as CT and MRI have delineated vessel abnormalities attributed to occlusive thrombus in acute ischemic stroke for more than 20 years, without pathologic corroboration of the nature of the underlying thrombus.5, 15 Our findings provide the initial radiologic-pathologic correlation that early vessel signs (including the HMCAS on CT and BA on GRE MRI) reflect underlying clot pathology. The HMCAS and BA are commonly encountered in the triage of acute stroke patients, resulting in much speculation to date about the type or composition of intravascular thrombus and related expected outcome with various revascularization strategies. Definitive statements about clot composition, such as our observations, must rely on comprehensive evaluation of clinical variables, noninvasive imaging, angiography, and gross examination with histopathology.8 Furthermore, detailed pathologic examination of the thrombus is possible only with mechanical thrombectomy, unlike the situation with intravenous or intra-arterial thrombolysis, aspiration, or angioplasty and stenting. Our findings reveal several novel observations about imaging of occlusive thrombus in acute ischemic stroke.
Acute MCA occlusion due to thrombus may reveal early vessel findings in only a fraction of cases and perhaps more importantly, the absence of such subtle imaging abnormalities does not rule out thrombotic occlusion. The HMCAS or BA was noted in about half of all our cases with successful thrombectomy. Initial descriptions of the HMCAS cited a much higher incidence, yet most successive studies reported detection rates around 50%, consistent with our findings.5–7, 11 HMCAS detection is undoubtedly influenced by variable methodology including blinding, quantitative measures of HU and other baseline factors.20 Our results are also consistent with previously reported detection rates for BA, although stroke mechanism differentiated by cardioembolism or large artery atherosclerosis may affect conspicuity of BA.12–14 Relatively greater thrombus burden associated with cardioembolism may increase BA conspicuity.13 Absence of BA in 47% of our cases was generally associated with fibrin-rich thrombi, a potential target for pharmacologic fibrinolysis. Only limited data were available to correlate HMCAS with BA as primary use of MRI and rapid triage to thrombectomy often obviate the need for CT.14 HMCAS has been reported in as low as 15% of cases evaluated with routine use of CT alone before thrombolysis depending on case series and therapeutic benefit may be achieved irrespective of this finding.28 Early vessel findings in other territories such as the posterior cerebral artery still await pathologic correlation.10, 13, 14, 29, 30
The HMCAS and BA reflect RBC content, a thrombus constituent, yet not the principal target of fibrinolysis. Classification of thrombi as RBC-dominant was noted in every case in which either HMCAS or BA were identified. These early vessel findings were increasingly infrequent with fibrin-rich thrombi. The percentage of RBC was also closely linked with these imaging findings. Measurement of HU within the HMCAS yielded values consistent with recently lodged emboli, although it remains difficult to ascribe these density changes to a particular clot consituent.10, 20, 31 As we did not discern any correlation between HU density and RBC quantitative measures, one may conclude that the mere presence or absence of HMCAS using simple visual inspection is likely sufficient in distinguishing the presence of a RBC-rich clot or “red thrombus”. The susceptibility effect of BA on GRE MRI has been ascribed to local ferromagnetic field distortion associated with RBC components, as well. The HMCAS and BA are therefore indirect markers of occlusive thrombi, reflecting trapped RBC more closely than the fibrin mesh targeted by most arterial revascularization procedures developed to date for stroke. It remains possible, however, that mechanical thrombectomy specimens ensnare additional constituents and adjacent red thrombi during the endovascular procedure, itself.
The potential to distinguish “red thrombi” from “white thrombi” has been a longstanding and elusive expectation of diagnostic imaging modalities.32 Our previous findings on the initial analyses of clots causing ischemic stroke in humans questioned whether such traditional distinctions of “red versus white clots” are truly applicable, as much heterogeneity was observed among pathologic specimens.8 A subsequent report also described marked heterogeneity in thrombi.9 Prediction of clot composition from CT or MRI may therefore be difficult, especially if one assumes that the HMCAS or BA reflects the original embolus rather than secondary components promoted by stasis proximal and distal to the occlusion site. Our findings on the HMCAS and BA that accentuate RBC content may also suggest that stasis and fresh thrombus are more common in such cases. Although it remains challenging to reconstruct the spatial orientation of the retrieved fragment with respect to the HMCAS or BA, limited reperfusion (TICI 0 or 1) in 24% of cases raises the possibility that RBC content was augmented by stasis. This hypothesis underscores the role of flow derangements in cerebral ischemia, up against the clot face and in distal segments filled via collateral perfusion.25 Stasis has previously been invoked in determining thrombus composition at the embolic source yet not at the recipient site.10, 33 Angiography may be indispensible in distinguishing such factors. Interestingly, we found no correlation between amount of clot retrieved and subsequent reperfusion, suggesting that other aspects of ischemic pathophysiology beyond thrombosis will be essential in future therapeutic strategies for stroke.
The prognostic significance of the HMCAS and BA in the setting of arterial revascularization may be inherently flawed without consideration of the interaction between flow and thrombi in cerebral arteries.25, 26 Many studies have attempted to define prognostic aspects of early vessel findings or their predictive role in revascularization, yet such outcomes are likely multifactorial, including considerations of how thrombus composition is not just the cause but also the result of impaired flow.7, 10, 11, 13, 16–18 Despite an unequivocal link between the HMCAS and BA with RBC-dominant pathology, undue emphasis should not persuade clinicians to establish stroke etiology or plan revascularization strategies based on this finding alone. Our finding that imaging features of HMCAS or BA cannot alone predict successful clot extraction warrants investigation of other potential influential factors, as recanalization may be affected by many features in a given case. Further correlative studies should evaluate the impact of these imaging signs with various endovascular approaches, incorporating angiographic features to characterize flow.
The unique opportunity that permitted this comprehensive analysis of early vessel findings with thrombus pathologic findings also imposed several limitations. Availability and quality of baseline imaging immediately prior to angiography resulted in further selection of a cohort already limited to candidates deemed suitable for mechanical thrombectomy. Our findings are limited by significant bias associated with excluding many cases, as the results relate only to clots in the proximal MCA that could be retrieved. Resilient occlusions and those with complete disintegration could not be studied and were thereby excluded from our analyses. It remains possible that some thrombi reflected changes of intravenous tPA prior to angiography or even changes associated with standard procedural heparin administration. As noted above, the orientation of clot fragments is speculative and other retained fragments may have differed in composition. Finally, our classification of clot types is also imperfect as most specimens were heterogeneous in nature, with considerable variation across cases.
Conclusions
Our novel observations provide the first correlative study of early vessel signs in acute ischemic stroke with underlying clot composition. The HMCAS and BA are not ubiquitous in thrombotic MCA occlusion and failure to discern these subtle findings should not deter arterial revascularization strategies. Further studies are underway to delineate more detailed aspects of clot composition including molecular features and architecture with respect to flow.
Acknowledgements
Sources of Funding
This work has been funded by NIH-NINDS Awards K23 NS054084 (DSL) and P50 NS044378.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict(s) of Interest/Disclosure(s)
All authors were employed by the University of California (UC), which holds a patent on retriever devices for stroke, at the time of this work. The UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled in the MR RESCUE multicenter clinical trial and the Concentric Merci Registry.
Dr. Liebeskind is a scientific consultant regarding trial design and conduct to Concentric Medical (modest) and CoAxia (modest).
Dr. Kidwell is Principal Investigator of the NIH-funded MR RESCUE trial (P50 NS044378).
Dr. Tateshima is a scientific advisor of Reverse Medical (modest), which makes a device to treat acute stroke.
Dr. Duckwiler is a medical advisor and stockholder of Concentric Medical.
Dr. Vinters is supported in part by the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine.
Dr. Saver is a scientific consultant to AGA Medical (modest), Boehringer Ingelheim (modest), Bristol Myers Squibb (modest), CoAxia (modest), Concentric Medical (modest), Ev3 (modest), FibroGen (modest), ImaRx (modest), Sanofi Aventis (modest), and Talecris (modest). He receives support for editorial work in MedReviews (modest).
References
- 1.Liebeskind DS. Reperfusion for acute ischemic stroke: Arterial revascularization and collateral therapeutics. Curr Opin Neurol. 2010;23:36–45. doi: 10.1097/WCO.0b013e328334da32. [DOI] [PubMed] [Google Scholar]
- 2.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, Pile-Spellman J, Segal A, Vinuela F, Saver JL. Merci 1: A phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke. 2004;35:2848–2854. doi: 10.1161/01.STR.0000147718.12954.60. [DOI] [PubMed] [Google Scholar]
- 4.The penumbra pivotal stroke trial: Safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 5.Tomsick TA, Brott TG, Olinger CP, Barsan W, Spilker J, Eberle R, Adams H. Hyperdense middle cerebral artery: Incidence and quantitative significance. Neuroradiology. 1989;31:312–315. doi: 10.1007/BF00344173. [DOI] [PubMed] [Google Scholar]
- 6.Tomsick TA, Brott TG, Chambers AA, Fox AJ, Gaskill MF, Lukin RR, Pleatman CW, Wiot JG, Bourekas E. Hyperdense middle cerebral artery sign on ct: Efficacy in detecting middle cerebral artery thrombosis. AJNR Am J Neuroradiol. 1990;11:473–477. [PMC free article] [PubMed] [Google Scholar]
- 7.Tomsick T, Brott T, Barsan W, Broderick J, Haley EC, Spilker J, Khoury J. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol. 1996;17:79–85. [PMC free article] [PubMed] [Google Scholar]
- 8.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 9.Almekhlafi MA, Hu WY, Hill MD, Auer RN. Calcification and endothelialization of thrombi in acute stroke. Ann Neurol. 2008;64:344–348. doi: 10.1002/ana.21404. [DOI] [PubMed] [Google Scholar]
- 10.Molina CA. Imaging the clot: Does clot appearance predict the efficacy of thrombolysis? Stroke. 2005;36:2333–2334. doi: 10.1161/01.STR.0000185933.44619.1b. [DOI] [PubMed] [Google Scholar]
- 11.von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, Sartor K. Sensitivity and prognostic value of early ct in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994;15:9–15. discussion 16–18. [PMC free article] [PubMed] [Google Scholar]
- 12.Rovira A, Orellana P, Alvarez-Sabin J, Arenillas JF, Aymerich X, Grive E, Molina C, Rovira-Gols A. Hyperacute ischemic stroke: Middle cerebral artery susceptibility sign at echo-planar gradient-echo mr imaging. Radiology. 2004;232:466–473. doi: 10.1148/radiol.2322030273. [DOI] [PubMed] [Google Scholar]
- 13.Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on t2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36:2379–2383. doi: 10.1161/01.STR.0000185932.73486.7a. [DOI] [PubMed] [Google Scholar]
- 14.Assouline E, Benziane K, Reizine D, Guichard JP, Pico F, Merland JJ, Bousser MG, Chabriat H. Intra-arterial thrombus visualized on t2* gradient echo imaging in acute ischemic stroke. Cerebrovasc Dis. 2005;20:6–11. doi: 10.1159/000086120. [DOI] [PubMed] [Google Scholar]
- 15.Gacs G, Fox AJ, Barnett HJ, Vinuela F. Ct visualization of intracranial arterial thromboembolism. Stroke. 1983;14:756–762. doi: 10.1161/01.str.14.5.756. [DOI] [PubMed] [Google Scholar]
- 16.Bastianello S, Pierallini A, Colonnese C, Brughitta G, Angeloni U, Antonelli M, Fantozzi LM, Fieschi C, Bozzao L. Hyperdense middle cerebral artery ct sign. Comparison with page 17 angiography in the acute phase of ischemic supratentorial infarction. Neuroradiology. 1991;33:207–211. doi: 10.1007/BF00588219. [DOI] [PubMed] [Google Scholar]
- 17.Leys D, Pruvo JP, Godefroy O, Rondepierre P, Leclerc X. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke. 1992;23:317–324. doi: 10.1161/01.str.23.3.317. [DOI] [PubMed] [Google Scholar]
- 18.Mattle HP, Arnold M, Georgiadis D, Baumann C, Nedeltchev K, Benninger D, Remonda L, von Budingen C, Diana A, Pangalu A, Schroth G, Baumgartner RW. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke. 2008;39:379–383. doi: 10.1161/STROKEAHA.107.492348. [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind DS, Kidwell CS. Advanced mr imaging of acute stroke: The university of california at los angeles endovascular therapy experience. Neuroimaging Clin N Am. 2005;15:455–466. doi: 10.1016/j.nic.2005.06.002. xiii. [DOI] [PubMed] [Google Scholar]
- 20.Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10:419–423. doi: 10.1159/000016101. [DOI] [PubMed] [Google Scholar]
- 21.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (merci) trial, part i. AJNR Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP, Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE. Mechanical thrombectomy for acute ischemic stroke: Final results of the multi merci trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 23.Becker KJ, Brott TG. Approval of the merci clot retriever: A critical view. Stroke. 2005;36:400–403. doi: 10.1161/01.STR.0000153056.25397.ff. [DOI] [PubMed] [Google Scholar]
- 24.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 25.Liebeskind DS. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. x. [DOI] [PubMed] [Google Scholar]
- 26.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 27.Yin NS, Benavides S, Starkman S, Liebeskind DS, Saver JA, Salamon N, Jahan R, Duckwiler GR, Tateshima S, Vinuela F, Vespa PM, Chute DJ, Vinters HV. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: A clinicopathologic study of 5 patients. Stroke. 2010;41:938–947. doi: 10.1161/STROKEAHA.109.576793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Janjua N, Divani AA. Is iv tissue plasminogen activator beneficial in patients with hyperdense artery sign? Neurology. 2006;66:1171–1174. doi: 10.1212/01.wnl.0000208407.69544.5a. [DOI] [PubMed] [Google Scholar]
- 29.Bettle N, Lyden PD. Thrombosis of the posterior cerebral artery (pca) visualized on computed tomography: The dense pca sign. Arch Neurol. 2004;61:1960–1961. doi: 10.1001/archneur.61.12.1960. [DOI] [PubMed] [Google Scholar]
- 30.Krings T, Noelchen D, Mull M, Willmes K, Meister IG, Reinacher P, Toepper R, Thron AK. The hyperdense posterior cerebral artery sign: A computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke. 2006;37:399–403. doi: 10.1161/01.STR.0000199062.09010.77. [DOI] [PubMed] [Google Scholar]
- 31.Cobelli R, Zompatori M, De Luca G, Chiari G, Bresciani P, Marcato C. Clinical usefulness of computed tomography study without contrast injection in the evaluation of acute pulmonary embolism. J Comput Assist Tomogr. 2005;29:6–12. doi: 10.1097/01.rct.0000148274.45419.95. [DOI] [PubMed] [Google Scholar]
- 32.Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: Value of ct in estimation of the prognosis of thrombolysis phantom study. Radiology. 2003;228:126–130. doi: 10.1148/radiol.2273020530. [DOI] [PubMed] [Google Scholar]
- 33.Liebeskind DS. Venous hemodynamics may enhance collateral perfusion and the fibrinolytic milieu in paradoxical embolism. Stroke. 2009;40:e30–e31. doi: 10.1161/STROKEAHA.108.541441. [DOI] [PMC free article] [PubMed] [Google Scholar]