Abstract
Rationale
Primary cilia are cellular protrusions which serve as mechanosensors for fluid flow. In endothelial cells (EC) they function in transducing local blood flow information into functional responses, like nitric oxide production and initiation of gene expression. Cilia are present on EC in areas of low or disturbed flow and absent in areas of high flow. In the embryonic heart high flow regime applies to the endocardial cushion area, and the absence of cilia here coincides with the process of endothelial-to-mesenchymal transition (EndoMT).
Objective
In this study we investigate the role of the primary cilium in defining the responses of EC to fluid shear stress and in EndoMT.
Methods and Results
Non-ciliated mouse embryonic EC with a mutation in Tg737/Ift88 were used to compare the response to fluid shear stress to that of ciliated EC. In vitro, non-ciliated EC undergo shear-induced EndoMT which is accompanied by downregulation of Klf4. This Tgfβ/Alk5 dependent transformation is prevented by blocking Tgfβ signaling, overexpression of Klf4, or rescue of the primary cilium. In the hearts of Tg737orpk/orpk embryos Tgfβ/Alk5 signaling was activated in areas in which EC would normally be ciliated, but now lack cilia due to the mutation. In these areas EC show increased Smad2 phosphorylation and expression of αSMA.
Conclusions
This study demonstrates the central role of primary cilia in rendering EC prone to shear-induced activation of Tgfβ/Alk5 signaling and EndoMT, and thereby provides a functional link between primary cilia and flow related endothelial performance.
Keywords: cilia, shear stress, endothelial cells, Tg737orpk/orpk, EndoMT, Tgfβ/Alk5, Klf4
INTRODUCTION
Cilia are specialized membrane covered rod-like organelles protruding from virtually all mammalian cells1. Primary cilia are typically present as solitary cellular extensions and are generally immotile, with the exception of cilia on the primitive node of the vertebrate embryo2. Interest in the (dys-) function of primary cilia arose due to their role in olfaction, photoreception, chemosensation, and mechanosensation3, their association with a number of human ciliopathies1,4, and their function as cellular sensory antennae. Biological effects include coordination of cell proliferation and differentiation. Ciliary structure, assembly and maintenance is dependent on microtubule based motor transport of axoneme subunits from the body of the cell into ciliary tips using a bidirectional process termed intraflagellar transport (IFT). Mutations in many of the IFT components lead to defective ciliogenesis5 and are associated with a range of human pathologies such as Orofaciodigital, Bardet-Biedl, Usher, Senior-Loken, and Jeune syndromes. These syndromes can include laterality defects, vestibular impairment, and polycystic kidney disease (PKD)1,4-6.
Endothelial cells lining the heart and blood vessels are constantly exposed to hemodynamic forces of which shear stress represents the drag force on the endothelium exerted by blood flow. The differential response of endothelial cells (EC) to constantly varying flow patterns and velocities requires accurate mechanosensing and mechanotransduction. Primary cilia can sense shear stresses as low as 0.0007 Pa7 and they function as flow sensors in kidney epithelium8, bone matrix lacunae osteocytes and osteoblasts, bile duct epithelium, and vascular endothelium9,10. The function of primary cilium in endothelial shear sensing appears to be biphasic. Fluid flow and the associated ciliary deformation lead to a polycystin-mediated intracellular Ca2+ transient within seconds9,11. Furthermore, the cilium is considered to amplify the cytoskeletal strain, resulting in a prolonged effect on gene expression of shear responsive genes, including Krüppel–like factor 210,12, which further coordinates a major part of the phenotypic response of EC to shear forces. Krüppel –like factor 2 and 4 (KLF2, KLF4) are shear sensitive transcription factors which have been described to coordinate the regulation of endothelial function and the establishment of a quiescent, anti-inflammatory, and anti-thrombotic phenotype13-16. Recent studies point towards a significant degree of mechanistic and functional overlap between KLF2 and KLF4 in EC16. Shear stress induced expression of KLF2 has been shown to be related to the presence of primary cilia9,10, and recent work suggests that cells with abnormal ciliary function or structure are likely to fail to respond to fluid shear stress appropriately9.
Primary cilia are present on EC during embryonic17,18 and adult19 life and their distribution has been described to be spatio-temporaly linked to shear stress patterns in vivo. In adult vasculature, primary cilia are located at atherosclerotic predilection sites where flow is low and oscillatory19. Moreover, EC in areas where shear stress is high, are devoid of cilia19. In the embryonic heart, the presence of ciliated EC is also restricted to areas of low and oscillatory flow, marked by low levels of KLF217,20,21. The endocardial cushions are exposed to high shear stress22 and show high expression of KLF2 in the EC20. Interestingly, these EC are non-ciliated17 and undergo transforming growth factor-beta (Tgfβ) driven endothelial to mesenchymal transition (EndoMT), during which EC transdifferentiate to gain a mesenchymal phenotype and migrate into the cardiac jelly to form the primordia of cardiac valves23. EndoMT is marked by activation of the Tgfβ type I receptor Alk524,25, induction and activation of the transcription factor Snail (Snai1), loss of EC markers like CD31, and gain of expression of mesenchymal markers including αSMA and N-cadherin26,27.
In this study we used transgenic embryonic EC from the IFT88Tg737RPW (or Tg737orpk/orpk) mouse which lack primary cilia9. These were used to investigate the role of the primary cilium in the response of EC to fluid shear stress and Tgfβ, and in EndoMT. Shear stress triggered EndoMT in non-ciliated EC, but not in ciliated cells, a process which depended on Tgfβ signaling and Klf4 regulation. Furthermore, we analysed the hearts of wildtype and Tg737orpk/orpk embryos to address the consequences of the lack of endothelial cilia in vivo. EC lining areas of low shear stress showed enhanced Tgfβ/Alk5 signaling activation and increased expression of the mesenchymal marker αSMA, suggesting that the lack of cilia primes the EC for shear induced activation of Tgfβ signaling and EndoMT.
METHODS
An expanded Methods section is available in the Online Supplement. It includes a detailed description of the culture and use of the EC; of shear stress experiments; Tgfβ stimulation; the use of Tgfβ neutralizing antibody and Alk5 kinase inhibitor compound SB431542; generation and use of constructs in transfection experiments; immunofluorescence studies; Q-PCR for a set of shear and EndoMT markers; Western Blotting for CD31, αSMA, Klf4, Ift88; immunohistochemical analysis of Tg737orpk/orpk embryos; and statistical analysis.
RESULTS
Shear stress induces EndoMT in non-ciliated EC
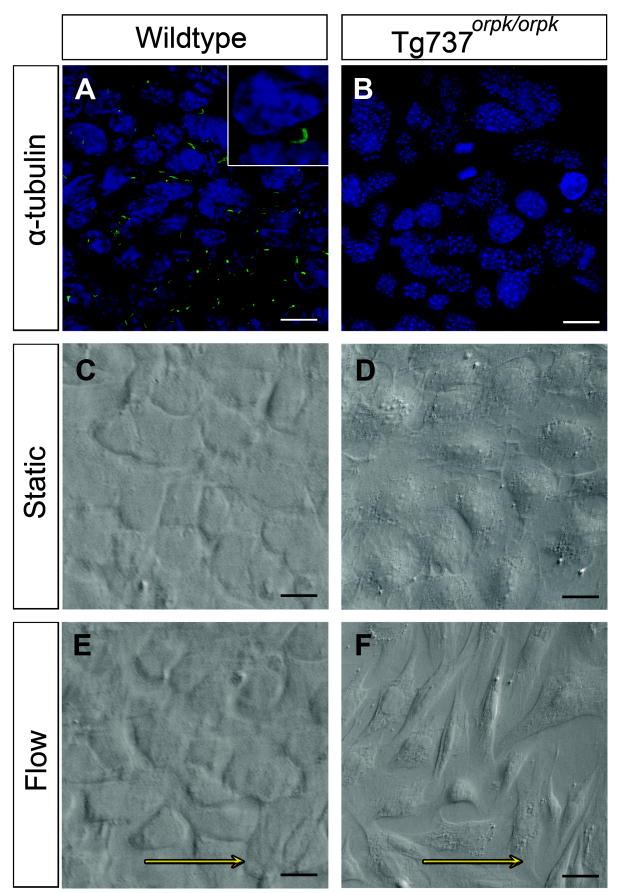
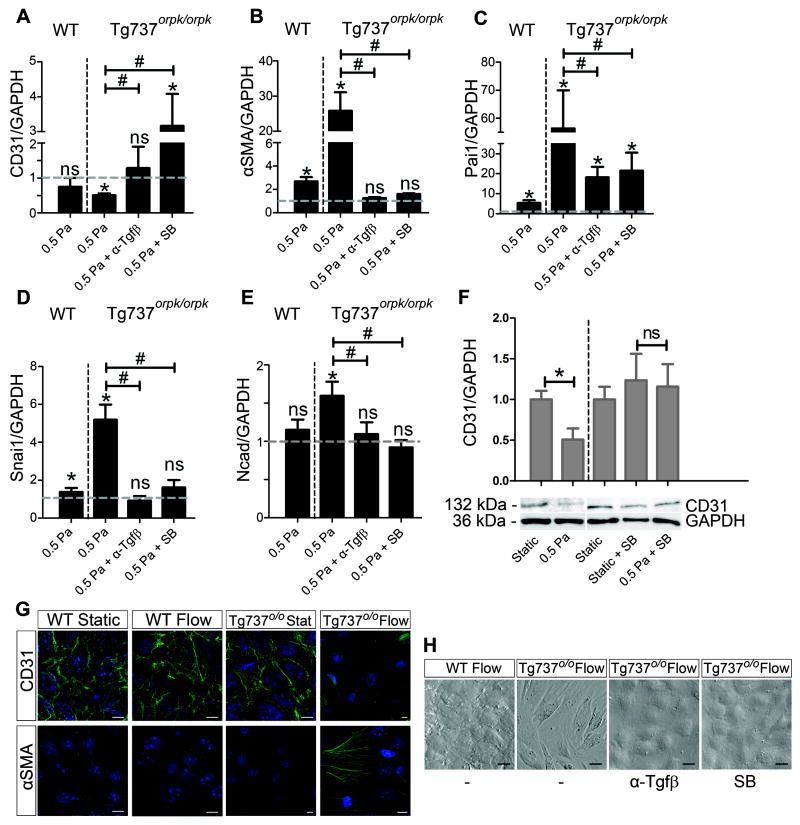
As demonstrated by immunofluorescent staining for acetylated α-tubulin, WT EC present with primary cilia whereas Tg737orpk/orpk EC do not (Figure 1A,B). To study the affect of cilia on the response to fluid flow, WT and Tg737orpk/orpk EC were exposed to 0.5 Pa shear stress for 24 hours. While the WT EC retained their cobblestone morphology (compare Figure 1C and E), non-ciliated Tg737orpk/orpk EC acquired an elongated, fibroblast-like phenotype, randomly orientated with respect to the direction of flow (compare Figure 1D and F). Their morphology resembled that of cells in which EndoMT was induced under static conditions with Tgfβ (Online Figure IA). The ciliation phenotype of the EC did not affect their capacity to undergo Tgfβ ligand-induced EndoMT, as both cell lines showed identical morphological changes. Tgfβ ligand-induced transformation of ciliated WT EC under static conditions was characterized by loss of CD31 expression and induction of mesenchymal markers αSMA, Pai1, Snai1, and Ncad (Online Figure IB). Figure 2 summarizes the effects of shear on Tg737orpk/orpk cells. Shear-induced EndoMT was characterized by the loss of CD31 and gain of αSMA (Figure 2G). Q-PCR analysis confirmed this on the mRNA level and showed that CD31 was downregulated by a factor 2 and αSMA was induced 26-fold under shear stress, compared to the static controls (Figure 2A,B). Pai1, which is a downstream activation marker of the type I Tgfβ receptor Alk5, was induced 56-fold under shear (Figure 2C). Snai1, a downstream target of Tgfβ and a marker for EndoMT, and Ncad, a transmembrane protein characteristic for mesenchymal cells, were both significantly induced by shear stress in Tg737orpk/orpk cells (Figure 2D,E). In comparison, ciliated WT EC did not show altered CD31 expression under fluid flow, showed only a slight induction of αSMA, Pai1, and Snai1, and did not induce Ncad (Figure 2A-E). Indeed, the majority of WT cells were still ciliated under these conditions (Online Figure IIA). To study the effect of magnitude of shear on ciliation phenotype of EC and their concomitant response to fluid flow, WT and Tg737orpk/orpk EC were exposed to 2.5 Pa shear stress for 24 hours. WT EC became non-ciliated and underwent EndoMT similar to that observed in Tg737orpk/orpk cells (Online Figure IIA). These morphological changes were reflected by expression changes of CD31, αSMA, Pai1, Snai1, and Ncad (Online Figure IIB).
Figure 1. Different responses to shear stress of ciliated and non-ciliated EC.
(A,B) Immunostaining for acetylated α-tubulin showing the presence of cilia in wildtype (WT) EC, and absence of cilia in Tg737orpk/orpk EC. (C,D) WT and Tg737orpk/orpk EC show a cobble-stone morphology under static conditions. (E,F) WT EC retain their phenotype under flow, while Tg737orpk/orpk EC undergo EndoMT. Arrows indicate the direction of flow. Scale bars: A,B 35 μm; C-F 25 μm.
Figure 2. Shear stress induced EndoMT in non-ciliated EC is Tgfβ/Alk5 dependent.
(A-E) Relative mRNA expression of CD31, αSMA, Pai1, Snai1, and Ncad in WT and Tg737orpk/orpk cells under 0.5 Pa shear with and without pan-Tgfβ neutralizing antibody (α-Tgfβ) and SB431542 (SB). Expression is normalized to GAPDH and relative to static shams, as represented by the dashed line. (F) Western Blot analysis and quantification of CD31 protein levels in Tg737orpk/orpk cells under static conditions, and under 0.5 Pa shear stress with and without SB. (G) CD31 and αSMA immunostainings of WT and Tg737orpk/orpk EC under static conditions and after exposure to 0.5 Pa shear stress. Scale bars: 25 μm. (H) Morphology of WT and Tg737orpk/orpk cells under 0.5 Pa shear with and without α-Tgfβ and SB.
EndoMT is Tgfβ/Alk5 kinase dependent
The role of Tgfβ/Alk5 signaling in shear stress-induced EndoMT was investigated using flow in the presence of either a pan-Tgfβ neutralizing antibody (α-Tgfβ) or the Alk5 kinase inhibitor compound SB431542. Depletion of Tgfβ ligand from the culture medium by binding to α-Tgfβ, as well as inhibiting Alk5 kinase activity prevented EndoMT under shear stress in Tg737orpk/orpk EC (Figure 2A-E,F,H). This was marked by the retention of cobblestone morphology (Figure 2H) and CD31 expression, and by the lack of αSMA induction (not shown). After shear stress in the presence of α-Tgfβ, Pai1 induction was reduced from 56-fold to 18-fold, and expression of Snai1, αSMA, Ncad and CD31 remained unchanged compared to the static controls (Figure 2A-E). The response to shear stress in the presence of the SB compound largely mimicked the response in the presence of α-Tgfβ. Pai1 induction under flow was reduced to 26-fold, Snai1, αSMA, and Ncad expression was no longer induced (Figure 2B-E). CD31 mRNA expression was induced under these conditions, but this was not reflected on protein level (Figure 2A,F). Tgfβ-induced Alk5 kinase activation and induction of the downstream signaling pathways therefore plays a critical role in the shear stress response and in EndoMT.
The role of cilia in defining the Klf2/Klf4 response to shear stress
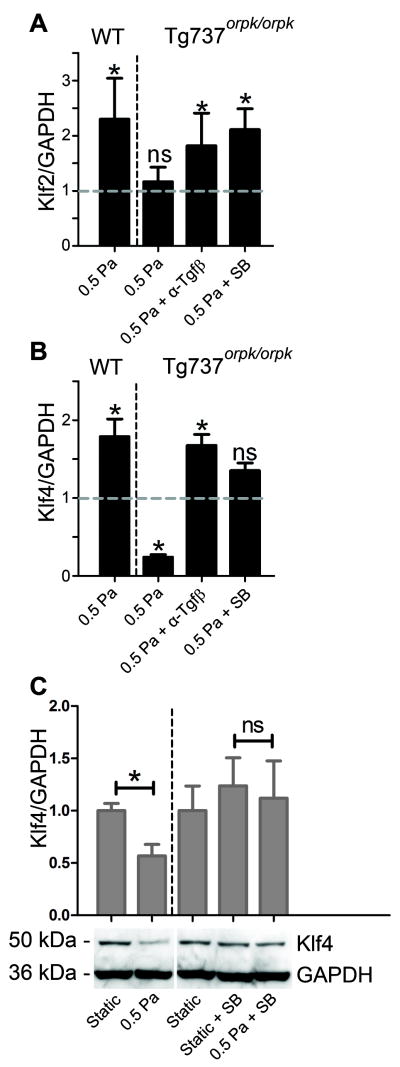
The specific response of WT and Tg737orpk/orpk EC to shear stress was investigated by analyzing the expression levels of Klf2 and Klf4 after exposure to 0.5 Pa shear for 24 hours. WT EC showed a 2.3 and 1.8-fold induction of Klf2 and Klf4, respectively, whereas Tg737orpk/orpk cells failed to induce Klf2 (Figure 3A,B). Remarkably, the latter cells showed a marked downregulation of Klf4 by over 75% under shear stress (Figure 3B). In Tg737orpk/orpk cells shear stress-related upregulation of Klf2 was restored and downregulation of Klf4 was prevented when Tgfβ signaling was inhibited with the SB compound or in the presence of α-Tgfβ (Figure 3A,B). The responses of Klf4 mRNA were accurately reflected at the protein level (Figure 3C).
Figure 3. Klf2 and Klf4 response of WT and Tg737orpk/orpk EC to shear stress.
(A,B) Relative mRNA expression of Klf2 and Klf4 in WT and Tg737orpk/orpk cells under 0.5 Pa shear stress with and without pan-Tgfβ neutralizing antibody (α-Tgfβ) and SB431542 (SB). Expression is normalized to GAPDH and relative to static shams, as represented by the dashed line. (C) Western Blot analysis and quantification of Klf4 protein levels in Tg737orpk/orpk cells under static conditions, and under 0.5 Pa shear stress with and without SB.
Klf4 overexpression prevents shear stress induced EndoMT
To investigate whether shear-induced EndoMT in Tg737orpk/orpk cells was a result of the marked downregulation of Klf4 or is rather the cause of it, Klf4 was transiently overexpressed (Tg737orpk/orpk-Klf4) and transfected cells were exposed to 0.5 Pa shear stress for 24 hours. Tg737orpk/orpk with LacZ overexpression (Tg737orpk/orpk-LacZ) were used as a control. Tg737orpk/orpk-Klf4 showed a 10-fold overexpression of Klf4 mRNA, an effect that was confirmed at the protein level (Figure 4A,B). Under shear stress, Tg737orpk/orpk-Klf4 EC retained their cobblestone morphology, CD31 expression, and did not undergo EndoMT (Figure 4C-E). Pai1 induction was reduced from 34-fold to 9-fold, Snai1 was induced only 1.9-fold, compared to 3.2-fold in the control group, and Ncad was not induced under flow (Figure 4D). Overexpression of Klf4 did not result in the reappearance of the cilium (not shown), demonstrating that prevention of flow induced EndoMT in Tg737orpk/orpk-Klf4 was not a secondary effect of ciliary rescue.
Figure 4. Klf4 overexpression prevents shear stress induced EndoMT.
(A,B) QPCR and Western Blot analysis, respectively, showing Klf4 overexpression in Tg737orpk/orpk EC which were transfected with either LacZ (Tg737o/o-LacZ, sham) or Klf4 (Tg737o/o-Klf4) expression constructs. Transfection with Klf4 results in a 10 fold overexpression on mRNA level, and a 2.2 fold overexpression on protein level. (C) Images of Tg737orpk/orpk-LacZ and Tg737orpk/orpk-Klf4 cells under static conditions and under 0.5 Pa shear stress. Arrows indicate the direction of flow. Scale bars: 25 μm. (D) Q-PCR showing relative mRNA expression of CD31, αSMA, Pai1, Snai1, and Ncad in Tg737orpk/orpk-LacZ and Tg737orpk/orpk-Klf4 cells under 0.5 Pa shear stress. Expression is normalized to GAPDH and relative to static shams, as represented by the dashed line. (E) Western Blot analysis and quantification of CD31 protein in Tg737orpk/orpk-LacZ and Tg737orpk/orpk-Klf4 cells under static conditions and 0.5 Pa shear stress.
Rescue of cilia prevents shear stress induced transformation in EC
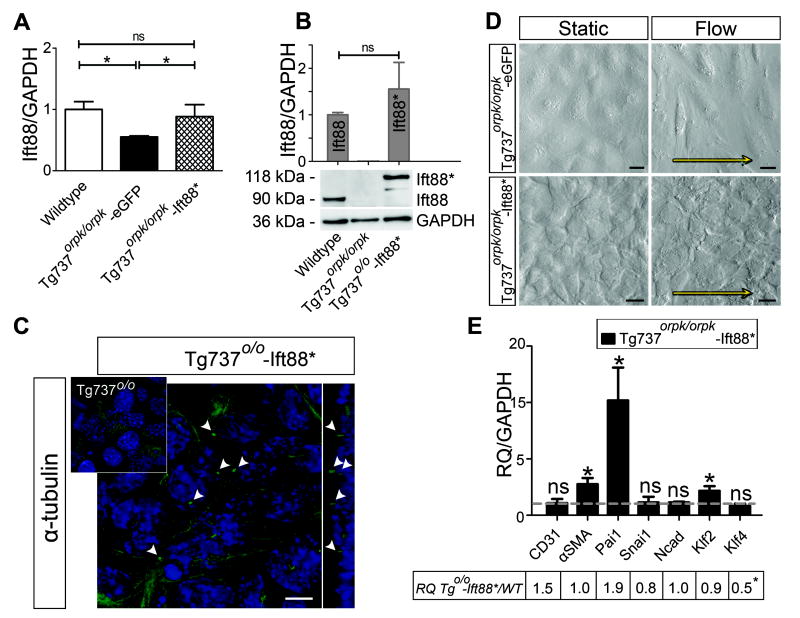
Tg737orpk/orpk EC were stably transfected with Ift88-mCherry cDNA to generate Tg737orpk/orpk–Ift88*. Figure 5(A,B) shows that Ift88 mRNA and Ift88* protein levels in rescued cells were comparable to the Ift88 levels in WT EC. Rescue of Ift88 in Tg737orpk/orpk EC led to a structural rescue of the cilia, with a normal distribution of one primary cilium per single cell (Figure 5C). To confirm functional rescue, Tg737orpk/orpk–Ift88* cells were subjected to 0.5 Pa shear stress for 24 hours, a condition which induced EndoMT in non-rescued cells. Cells now retained their cobblestone morphology and did not undergo EndoMT (Figure 5D). Q-PCR analysis showed induction of Pai1 and αSMA similar in magnitude to that of the WT EC under shear, without any changes in the expression levels of CD31, Snai1, and Ncad (Figure 5E). The table in Figure 5E shows the ratios of relative (to static controls) expression levels of genes of interest in Tg737orpk/orpk–Ift88*, compared to WT EC under shear. The expression profiles of these two cell types under shear stress are comparable and rescue of the cilia led to induction of Klf2 and lack of Klf4 downregulation under flow.
Figure 5. Rescue of Ift88 leads to functional rescue of the cilia and prevents shear stress induced EndoMT in Tg737orpk/orpk EC.
(A,B) Q-PCR and Western Blot analysis, respectively, showing Ift88 levels in WT, Tg737orpk/orpk stably transfected with pEGFP-N1 (Tg737o/o-eGFP, sham) and Ift88-mCherry/Ift88* (Tg737o/o-Ift88*) expression constructs. Transfection with Ift88* results in a 2 fold increase in mRNA level of Ift88* in Tg737orpk/orpk EC and normalizes protein levels to those of Ift88 in WT EC. (C) Confocal images with optical cross section of Tg737orpk/orpk cells, immunostained for acetylated tubulin, showing the presence of cilia in rescued Tg737orpk/orpk-Ift88* EC but not in non-rescued cells (inset). Arrowheads point towards primary cilia and scale bar represents 10μm. (D) Images of Tg737orpk/orpk-Ift88* cells under static conditions and under 0.5 Pa shear stress. Arrows indicate the direction of flow and scale bars represent 25 μm. (E) Q-PCR showing relative mRNA expression of CD31, αSMA, Pai1, Snai1, Ncad, Klf2, and Klf4 in Tg737orpk/orpk-Ift88* cells under 0.5 Pa shear stress. Expression is normalized to GAPDH and relative to static shams, as represented by the dashed line. The table shows the ratios of relative mRNA expression levels of Tg737orpk/orpk–Ift88* and WT EC under shear stress.
Non-ciliated EC activate Tgfβ signaling in vivo
In order to assess the in vivo consequences of (non-)ciliation of EC for Tgfβ/Alk5 signaling activation, the hearts of E11.5 wild type and Tg737orpk/orpk mouse embryos were analyzed for endothelial expression of phosphorylated Smad2 (P*Smad2) and αSMA (Figure 6). EC of Tg737orpk/orpk embryos lining areas of low shear, i.e. in the atria, on the ventricular septum and on trabeculations show increased expression of phospho-Smad2 compared to their wild type littermates, pointing towards enhanced Tgfβ/Alk5 activation. Tg737orpk/orpk embryos also have αSMA positive cells in these areas, whereas all EC in the WT embryos were negative for αSMA. Moreover, the subendocardial space of the ventricular septum and trabeculations is increased in the mutant embryos suggesting increased extracellular matrix production and deposition. Western Blot analysis of P*Smad2 levels in WT and Tg737orpk/orpk EC after exposure to 2 hours of shear stress showed an increase of P*Smad2 levels by 30% in the WT EC, compared to a 2.2 fold increase in the Tg737orpk/orpk cells, confirming enhanced P*Smad2 signaling in non-ciliated cells (Online Figure III).
Figure 6. Lack of cilia primes EC for shear induced activation of Tgfβ signaling, in vivo.
Transverse sections through the hearts of wild type (A,C,E,G,I) and Tg737orpk/orpk (B,D,F,H,J) mouse embryos of stage E11.5 comparing the expression of αSMA and phosphorylated Smad2 (P*Smad2). Boxed areas in A and B are magnified in C-J in these or adjacent sections. αSMA positive cells are observed in the endothelium of the ventricular septum and trabeculations of Tg737orpk/orpk embryos (compare C and D). The EC of Tg737orpk/orpk embryos have higher expression of P*Smad2 compared to wild type littermates (compare E and F). The subendothelial space of the ventricular septum and trabeculations, representing cardiac jelly, is increased in the mutants (compare C,E and D,F). The atria of wildtype animals are lined by EC negative for αSMA and P*Smad2, whereas these cells stain positive for both markers in the Tg737orpk/orpk embryos. A=Atrium, AVC=Atrioventricular Canal, V=Ventricle, VS=Ventricular Septum. Scale bars: A,B 100μm; C-J 50μm.
DISCUSSION
In this study we used EC deficient in functional IFT88 protein28 and thus lacking the ability to form cilia. They provide a robust model to analyse the functional role of primary cilia in response to fluid flow. Although the effects of ciliary dysfunction on kidney epithelial cells and their response to flow has been the subject of numerous studies, the effects of dysfunctional primary cilia on EC remain largely unknown. In vivo EC primary cilia are present in areas of low and disturbed shear stress17,19, where they have been suggested to play an important role in mechanosensing10. In our model, ciliated EC retained an endothelial phenotype in response to 0.5 Pa shear stress in vitro, whereas non-ciliated Tg737orpk/orpk EC underwent EndoMT and adopted a fibroblast-like phenotype. When exposed to higher shear levels, which resulted in the de-ciliation of WT EC, these cells also underwent EndoMT and gained a phenotype that closely resembled that of Tg737orpk/orpk EC under flow. It is therefore plausible that WT EC disassemble their cilia under high laminar shear stress, as has previously been reported for chicken and human EC17,29, priming the cells for shear induced EndoMT. In this respect shear induced EndoMT of non-ciliated cells should be clearly distinguished from flow induced endothelial alignment which has been described extensively. Here, the direction of cellular elongation was random with respect to the direction of flow, and the changes in phenotype were accompanied by the loss of endothelial markers and the gain of mesenchymal and transition markers. EC of various origins have been demonstrated to undergo EndoMT in vitro when exposed to Tgfβ26. We confirmed these findings in the WT and Tg737orpk/orpk EC and identified Tgfβ/Alk5 kinase activity to be essential in shear stress mediated EndoMT. Since treatment with either Tgfβ blocking antibodies or Alk5 kinase inhibitor prevented shear-induced transformation, an autocrine mechanism by which flow activates Tgfβ production or promotes its bioavailability which in turn activates signaling by binding to its receptors on EC, appears feasible. However this requires further confirmation.
A typical response of endothelial cells to shear stress is upregulation of the zinc finger transcription factor KLF2. In vivo, expression of KLF2 in the embryonic and adult cardiovascular system is confined to areas of high shear stress20,30. In vitro, KLF2 is induced by flow21,31 and acts as a regulator of endothelial function through regulation of multiple genes14,30. Here we confirm the shear dependent upregulation of Klf2 in ciliated EC but also show that the absence of cilia in Tg737orpk/orpk cells prevents this induction. This is in line with previous results showing attenuation of KLF2 induction in de-ciliated chicken EC10. Like KLF2, KLF4 has been reported to be expressed in EC in a shear dependent manner32. Ciliated EC show a similar response in our experimental setting. Non-ciliated cells, however, show a dramatic downregulation of this transcription factor. We show that this downregulation of Klf4 not only coincides with EndoMT but is in fact required for this transition. Preventing downregulation by artificial overexpression of Klf4 or by blocking Tgfβ signaling also prevents shear stress induced EndoMT, as demonstrated by the retention of CD31 expression and a cobblestone phenotype, and by the lack of induction of transition markers like αSMA, Pai1, Snail, and Ncad. This is in line with studies that show that Klf4 potently represses the expression of smooth muscle differentiation markers, including αSMA33, in vascular smooth muscle cells and is rapidly induced following vascular injury34. Smooth muscle differentiation also largely depends on Tgfβ signaling33 and probably involves a similar transition mechanism.
To ensure that the differences in response to shear stress and the process of EndoMT were specific for the lack of cilia on EC, Ift88 was stably overexpressed in Tg737orpk/orpk cells. This resulted in the re-appearance of primary cilia in a normal distribution pattern of one per cell. Tg737orpk/orpk–Ift88* EC responded to shear stress in a manner similar to the WT EC, reflected by the induction of Klf2, lack of downregulation of Klf4 and the retention of an endothelial phenotype under flow. This identifies the primary cilium as a necessary element to retain endothelial quiescence under low flow conditions. Together, this confirms the central role of primary cilia in defining a functional response to fluid flow in EC, as is illustrated in Figure 7. In short, ciliated EC retain their cobblestone morphology under shear stress, whereas non-ciliated EC undergo flow mediated EndoMT. This transition is preceded by the activation of Tgfβ/Alk5 signaling and downregulation of transcription factor Klf4. Shear stress induced EndoMT in non-ciliated cells can be prevented in vitro by interfering with Tgfβ signaling, by rescuing the cilium, or by induction of Klf4 expression.
Figure 7. Model for the role of primary cilia in shear response of EC.
Depending on the flow pattern EC can be ciliated or not. The center picture shows a scanning electron micrograph of an endothelial cilium. When ciliated EC (+) are exposed to shear stress they show induction of Klf2 and Klf4 and retain a cobblestone endothelial phenotype (green arrow). However, when non-ciliated cells (-) are exposed to flow they fail to induce Klf2 and downregulate Klf4. They lose their endothelial characteristics and acquire a mesenchymal or transition phenotype (red arrow/cells) in a Tgfβ/Alk5 dependent manner. This potentially pathological transition can be prevented by e.g. blocking Tgfß signaling or by inducing Klf4 expression.
Our in vivo data confirm the relation between the absence of primary cilia and activation of Tgfβ signaling. In the hearts of Tg737orpk/orpk embryos EC which would normally be ciliated17,18 now showed increased Smad2 phosphorylation and αSMA expression, indicating activation of Tgfβ signaling. This is supported by increased production of extracellular matrix resulting in more cardiac jelly, as observed in the Tg737orpk/orpk embryos. However, these embryos did not show concomitant excessive EndoMT in the low-flow areas. Apparently, other factors, most probably locally secreted by the myocardium, inhibit the final transition into mesenchymal cells in these embryos. This is in line with other studies which suggest an EndoMT-stimulatory environment specifically in the atrioventricular canal and outflow tract of the embryonic heart due to paracrine signaling. As a consequence of the high levels of shear stress20,22,35-38 the EC in these areas are already non-ciliated during cushion EndoMT stages in WT embryos, and altered EndoMT due to lack of cilia in Tg737orpk/orpk mice was not expected. Indeed, in the Tg737orpk/orpk embryos no abnormalities with respect to Tgfβ activation patterns in areas of high shear or cushion development were identified. Whether cilia and altered flow induced EndoMT play a role under pathological conditions in adult mutant mice, such as atherosclerosis or heart failure related cardiac fibrosis39, will need to be determined in future studies.
Appreciation of ciliary function in development and normal human physiology has led to reanalysis of a number of human syndromes which have previously been associated merely on the basis of similar clinical features. Several ciliopathies are characterized by gross cardiac anomalies, probably related to the failed orientation of heart looping along the left-right axis, corresponding to the high incidence of situs inversus in these patients2,40,41. Here we provide data to suggest that EC might contribute to the phenotypes observed in these syndromes, in which case their defects in the cardiovascular system could be masked by the gross anomalies of disturbed left-right asymmetry. Studies have shown mice with mutations leading to complete loss of cilia or defective mechanosensation to have 100% penetrance of intracardiac defects, next to the prominent left-right patterning abnormalities2,42. In contrast, only about 40% of mice with structurally normal, but immotile, cilia have intracardiac defects which are mostly associated with abnormal left-right development43. These observations lead to the belief that cilia have a broader role in heart development than through their function in node cilia alone18. Furthermore, endothelial dysfunction and increased carotid intima-media thickness has been reported with increased frequency in adult PKD patients44, pointing towards a role for cilia in vascular function.
Supplementary Material
Acknowledgments
The authors thank Venus C. Roper (Cell Biology, University of Alabama at Birmingham) for harvesting the embryos, and Emile de Heer and Wim Corver (Pathology, LUMC) for generously providing the antibodies. Sebastiaan Blankevoort (Anatomy and Embryology, LUMC) is acknowledged for preparation of the artwork in Figure 7. C.L. Mummery (Anatomy and Embryology, LUMC) is gratefully acknowledged for help with the manuscript.
SOURCES OF FUNDING B.K.Y. is supported by the NIH P30 DK074038 Hepato/Renal Fibrocystic Diseases Core Center and P.t.D. by Leducq Foundation and Centre for Biomedical Genetics.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- Alk5
Transforming Growth Factor-β Type I Receptor/ Activin-Like Kinase 5
- αSMA
α-smooth muscle actin
- CD31
Platelet Endothelial Cell Adhesion Molecule-1, PECAM-1
- EC
Endothelial cell
- EndoMT
Endothelial to Mesenchymal Transition
- Ift
Intraflagellar transport
- Ift88/Tg737
Intraflagellar transport protein 88
- IFT88Tg737RPW
ORPK mouse, Tg737orpk/orpk
- Klf2
Krüppel-like Factor 2
- Klf4
Krüppel-like Factor 4
- Ncad
N-cadherin
- ORPK
Oak Ridge Polycystic Kidney
- Pai1
Plasminogen activator inhibitor-1
- Phospho-Smad2
Phosphorylated Smad2 protein
- PKD
Polycystic Kidney Disease
- Snai1
Snail homolog 1
- WT
Wild type
Footnotes
DISCLOSURES None declared.
REFERENCE LIST
- 1.D’Angelo A, Franco B. The dynamic cilium in human diseases. Pathogenetics. 2009;2:3. doi: 10.1186/1755-8417-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 3.Satir P, Christensen ST. Overview of Structure and Function of Mammalian Cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 4.Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum JL, Witman GB. Intraflagellar transport. Nature Reviews Molecular Cell Biology. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 6.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith HL, Cokelet GR, Gaehtgens P. Robin Fahraeus: evolution of his concepts in cardiovascular physiology. Am J Physiol. 1989;257:H1005–H1015. doi: 10.1152/ajpheart.1989.257.3.H1005. [DOI] [PubMed] [Google Scholar]
- 8.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 9.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid-shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hierck BP, Van der Heiden K, Alkemade FE, van de Pas S, van Thienen JV, Groenendijk BCW, Bax WH, Van der Laarse A, DeRuiter MC, Horrevoets AJG, Poelmann RE. Primary Cilia Sensitize Endothelial Cells for Fluid Shear Stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 11.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Lil XG, Elia AEH, Lu WN, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 12.Poelmann RE, Van der Heiden K, Gittenberger-de Groot AC, Hierck BP. Deciphering the endothelial shear stress sensor. Circulation. 2008;117:1124–1126. doi: 10.1161/CIRCULATIONAHA.107.753889. [DOI] [PubMed] [Google Scholar]
- 13.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJG. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 15.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 16.Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun. 2010;391:984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Heiden K, Groenendijk BCW, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- 18.Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn. 2008;237:2304–2314. doi: 10.1002/dvdy.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJBM, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Groenendijk BCW, Hierck BP, Gittenberger-de Groot AC, Poelmann RE. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn. 2004;230:57–68. doi: 10.1002/dvdy.20029. [DOI] [PubMed] [Google Scholar]
- 21.Groenendijk BCW, Van der Heiden K, Hierck BP, Poelmann RE. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology (Bethesda) 2007;22:380–389. doi: 10.1152/physiol.00023.2007. [DOI] [PubMed] [Google Scholar]
- 22.Vennemann P, Kiger KT, Lindken R, Groenendijk BCW, Stekelenburg-de Vos S, ten Hagen TLM, Ursem NTC, Poelmann RE, Westerweel J, Hierck BP. In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart. J Biomech. 2006;39:1191–1200. doi: 10.1016/j.jbiomech.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13:301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 25.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGFb type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman JM, Michaud EJ, Schoeb TR, ydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekker RJ, van Thienen JV, Elderkamp YW, Seppen J, de Vries CJM, Biessen EAL, van Berkel TJ, Pannekoek H, Horrevoets AJG. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular-tone regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dekker RJ, Van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJG. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 32.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan FF, Liu YF, Liu Y, Zhao YX. KLF4: a novel target for the treatment of atherosclerosis. Med Hypotheses. 2008;70:845–847. doi: 10.1016/j.mehy.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor b isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 36.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamagishi T, Ando K, Nakamura H. Roles of TGFbeta and BMP during valvulo-septal endocardial cushion formation. Anat Sci Int. 2009;84:77–87. doi: 10.1007/s12565-009-0027-0. [DOI] [PubMed] [Google Scholar]
- 38.Hierck BP, Van der Heiden K, Poelmann RE. Fluid shear stress and inner curve remodeling of the embryonic heart. Choosing the right lane! ScientificWorldJournal. 2008;8:212–222. doi: 10.1100/tsw.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 40.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 41.McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–392. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 42.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Icardo JM, Sanchez de Vega MJ. Spectrum of heart malformations in mice with situs solitus, situs inversus, and associated visceral heterotaxy. Circulation. 1991;84:2547–2558. doi: 10.1161/01.cir.84.6.2547. [DOI] [PubMed] [Google Scholar]
- 44.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, Alisir S, Turgut F, Mercanoglu F, Ecder T. Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2004;43:854–860. doi: 10.1053/j.ajkd.2004.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.