Abstract
p53 is frequently wild type (wt) in diffuse large B-cell lymphoma (DLBCL) associated with t(14;18)(q32;q21) that overexpresses BCL2. Nutlin-3a is a small molecule that activates the p53 pathway by disrupting p53–MDM2 interaction. We show that nutlin-3a activates p53 in DLBCL cells associated with t(14;18)(q32;q21), BCL2 overexpression and wt p53, resulting in cell cycle arrest and apoptosis. Nutlin-3a treatment had similar effects on DLBCL cells of activated B-cell phenotype with wt p53. Cell cycle arrest was associated with upregulation of p21. Nutlin-3a-induced apoptosis was accompanied by BAX and PUMA upregulation, BCL-XL downregulation, serine-70 dephosphorylation of BCL2, direct binding of BCL2 by p53, caspase-9 upregulation and caspase-3 cleavage. Cell death was reduced when p53-dependent transactivation activity was inhibited by pifithrin-α (PFT-α), or PFT-μ inhibited direct p53 targeting of mitochondria. Nutlin-3a sensitized activation of the intrinsic apoptotic pathway by BCL2 inhibitors in t(14;18)-positive DLBCL cells with wt p53, and enhanced doxorubicin cytotoxicity against t(14;18)-positive DLBCL cells with wt or mutant p53, the latter in part via p73 upregulation. Nutlin-3a treatment in a xenograft animal lymphoma model inhibited growth of t(14;18)-positive DLBCL tumors, associated with increased apoptosis and decreased proliferation. These data suggest that disruption of the p53–MDM2 interaction by nutlin-3a offers a novel therapeutic approach for DLBCL associated with t(14;18)(q32;q21).
Keywords: nutlin, p53, MDM2, BCL2, t(14;18)(q32;q21), diffuse large B-cell lymphoma
Introduction
The t(14;18)(q32;q21) resulting in overexpression of the antiapoptotic protein BCL2 is a hallmark of follicular lymphoma (FL).1 This translocation is also present in a subset of diffuse large B-cell lymphoma (DLBCL) tumors, thought to be closely related to or transformed from FL.2 The t(14;18), by upregulating BCL2 expression and therefore BCL2-mediated resistance to apoptotic stimuli, is regarded as an initiating oncogenic event in FL, although genetically manipulated animal models have shown that additional genetic events are necessary for lymphoma development.3, 4 Additional genetic events are most certainly involved in transformation of FL to DLBCL.1
The signaling pathway regulated by the tumor suppressor gene p53 is inactivated in most cancers, half of which harbor p53 gene mutations.5 In vitro and in vivo studies have shown, however, that reactivation of p53, either by genetic manipulation or by application of small molecules that specifically target the p53 pathway, can result in the elimination of tumors initiated by transforming events independent of p53.6, 7 Accordingly, recent studies have shown that inhibition of MDM2, a critical negative regulator of p53, by using the recently developed small-molecule nutlin-3a results in significant antitumor activity in various malignancies, including hematopoietic tumors harboring a wild-type (wt) p53 gene.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
p53 mutations are rare in FLs, but, when present, likely have a pathogenetic role in transformation to DLBCL.3, 22, 23 Several studies also have implicated disruption of p53/MDM2 signaling axis in transformation of FL to DLBCL. For example, Sander et al.24 used immunohistochemical and sequencing analysis to show that FLs typically do not overexpress p53 protein, whereas 30% of FLs that transformed to DLBCL overexpressed p53, most of which also had p53 gene mutation. Moller et al.25 showed p53 gene mutations and MDM2 overexpression in 22 and 43% of DLBCLs, respectively. Furthermore, decreased levels of p19ARF, a product of the CDKNA gene and a negative regulator of MDM2, were observed in DLBCLs, either because of homozygous deletion or promoter hypermethylation, in approximately 10–20% of tumors. In aggregate, 62% of DLBCL tumors had aberrations.25 In a comprehensive study of 91 tumor specimens from 29 patients with FL who had transformed to DLBCL, 82% of tumors with mutated p53 were immunopositive for p53, whereas 71% of tumors with wt p53 showed no p53 expression. p53 gene mutations were observed in 28% of transformed tumor samples, but were not observed in FL at diagnosis. High expression of MDM2 was observed in sequential pre- and posttransformation samples and did not correlate with mutational status of p53, indicating a p53-independent mechanism. This study not only confirmed the importance of p53 mutation in the process of transformation but also identified increased expression of MDM2 as a major event in transformation that could be targeted for therapy.26
In this study, we investigated the in vitro and in vivo antitumor potential of nutlin-3a, a functional inhibitor of MDM2 against DLBCL associated with t(14;18)(q32;q21), and whether nutlin-3a-mediated activation of the p53 pathway can overcome the antiapoptotic action of overexpressed BCL2 as a result of t(14;18)(q32;q21). By using an in vitro system with cultured t(14;18)-positive DLBCL cells, or a xenograft lymphoma animal model, our data show that nutlin-3a can activate the p53 pathway inducing cell cycle arrest and apoptosis in t(14;18)-positive DLBCL cells with wt p53, overcoming BCL2 overexpression. Our data also support a role for nutlin-3a in augmenting activation of the intrinsic apoptotic pathway in t(14;18)-positive DLBCL cells induced by BH3 mimetic molecules targeting directly the mitochondria. In addition, nutlin-3a enhanced the activity of classical chemotherapeutic agents (for example, doxorubicin) against DLBCL cells associated with t(14;18) that harbor mutated p53, in part, through activation of p73. In sum, we provide evidence that MDM2 antagonists can be part of a therapeutic strategy for patients with DLBCL associated with t(14;18)(q32;q21).
Materials and methods
Cell lines and reagents
Six DLBCL cell lines of germinal center type harboring t(14;18)(p32;q21) and overexpressing BCL2 were used, including DoHH2, MCA and EJ, harboring a wt p53 gene, and Pfeiffer, MS and BJAB with mutated p53. DoHH2 and Pfeiffer (DLBCL) cells were obtained originally from DSMZ, (Braunschweig, Germany) and ATCC (Manassas, VA, USA), respectively. MS, EJ and MCA (DLBCL) cell lines were established at MD Anderson Cancer Center (by RJF).27 Two DLBCL cell lines with wt p53 and of activated B-cell (ABC) type, OCI-LY3 (with BCL2 gene amplification) and OCI-LY10 were also used. All cells were maintained in RPMI 1640 medium supplemented with 15% fetal bovine serum (Invitrogen, Grand Island, NY, USA), at 37 °C, in a humidified atmosphere containing 5% CO2.
A number of molecules were added to cell cultures in different concentrations as indicated including nutlin-3a, a selective small-molecule antagonist of MDM2 (Calbiochem, San Diego, CA, USA); pifithrin-α (PFT-α), an inhibitor of p53-dependent transactivation of p53-responsive genes; PFT-μ, an inhibitor of p53 translocation to mitochondria; YC-137, a BH3-mimetic; and the chemotherapeutic agent doxorubicin (all from Calbiochem). All experiments were repeated at least twice.
Reverse-transcription PCR and direct sequencing of p53 cDNA
Total RNA extraction, synthesis of cDNA, amplification of the entire open reading frame of p53 gene by PCR and sequencing were performed as previously described.12
Colony formation and MTS assays
Colony formation in methylcellulose (Sigma, St Louis, MO, USA) was performed according to the manufacturer's instructions. Briefly, 500 cells in 300 μl of methylcellulose solution were treated with 2, 5 and 10 μg/ml of nutlin-3a or an equivalent amount of dimethyl sulfoxide, and then plated and incubated for 2 weeks. The wells were stained with p-iodonitrotetrazolium violet (Sigma), and colonies were counted using a stereomicroscope.
Cells were treated with nutlin-3a in 96-well plates. A tetrazolium compound (MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) was then added to each well, and the number of viable cells was quantified using the CellTiter 96 AQueous cell proliferation assay (Promega, Madison, WI, USA) and μQuant spectrophotometer (BIO-TEK Instruments, Winooski, VT, USA) according to the manufacturer's instructions.
Cell cycle analysis
Cells were fixed overnight in ice-cold ethanol (70% volume/volume), and stained for 30 min with propidium iodide solution (50 μg/ml propidium iodide, 200 U/ml DNase-free RNase in phosphate buffer solution, pH 7.4; Roche Applied Science, Indianapolis, IN, USA) at 37 °C. DNA content was determined using a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) and the cell cycle was analyzed using ModFit LT software (Verity Software House, Topsham, ME, USA).
Cell viability and apoptosis studies
Cell viability was evaluated using trypan blue exclusion cell counts in triplicate. Annexin V staining (BD Biosciences Pharmingen, San Diego, CA, USA) detected by flow cytometry was used to assess apoptosis according to the manufacturer's instructions. Briefly, the cells were washed in ice-cold phosphate-buffered saline and resuspended in binding buffer at a concentration of 1 × 106 cells/ml. Aliquots of 100 μl of 1 × 105 cells/ml were incubated with 2 μl annexin V–fluorescein isothiocyanate for 15 min, followed by 5 μl propidium iodide for 1 min in dark at room temperature. In all, 1 × 104 ungated cells were then counted using a FACS Calibur flow cytometer (Becton Dickinson). Also, cytospin preparations of nutlin-3a-treated cells were stained with 4',6-diamidino-2-phenylindole and examined by fluorescence microscopy for morphological evidence of apoptosis. Control cells were included in each set of experiments.
Western blot and coimmunoprecipitation studies
Western blot analysis was performed as previously described.28 Antibodies used included: p53, p21, BCL2 and BAX (Dako, Carpinteria, CA, USA); Ser15p-p53, PUMA, Ser70p-BCL2, caspase-3 and cleaved caspase-9 (Cell Signaling Technology, Beverly, MA, USA); BCL-XL (Zymed, South San Francisco, CA, USA); activated caspase-3 (BD Biosciences Pharmingen); MDM2, and p73α (Santa Cruz Biotechnology, Santa Cruz, CA, USA); MDMX (Bethyl, San Antonio, TX, USA); and β-actin (control for protein load; Sigma). Also, coimmunoprecipitation of cell lysates prepared in 1% CHAPS extraction buffer was performed as previously described with slight modifications, using anti-BCL2 (rabbit monoclonal, Cell Signaling Technology).28 Immunocomplexes were analyzed by western blot analysis using anti-p53 and anti-BCL2 (mouse monoclonal, DAKO).
Immunofluorescence
Immunofluorescence was performed as previously described.12 The primary anti-p53 antibody (mouse monoclonal from Dako) was applied overnight at 4 °C and the immunoreaction was detected with Alexa Fluor 488 goat anti-mouse secondary antibody (Molecular Probes, Invitrogen). The 4',6-diamidino-2-phenylindole was used as counterstain. Staining of cells omitting the primary antibody step served as a negative control.
Human tumor xenografts
A total of 14 female severe combined immunodeficiency/beige mice (4- to 6-week old) were obtained from Taconic (New York, NY, USA) and maintained under specific pathogen-free conditions. Mice were injected subcutaneously with 10 × 106 DoHH2 cells and divided randomly in two equal treatment groups. Treatment was started intraperitoneally after the tumors were established (that is, palpable). Nutlin-3a (25 mg/kg, Alexis Biochemicals, San Diego, CA, USA) or the vehicle control was administered daily for a period of 14 days (14 doses). Weight of the mice was measured twice per week. Tumor volumes were measured daily with a caliper and calculated using the formula V=width × height × depth/2. For western blotting, histological and immunohistochemical analyses, tumors (three from each group, after three injections) were snap frozen or fixed in 10% formalin solution and processed routinely. Immunohistochemical analysis and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) were performed as previously described. All animal studies were conducted in accordance with the guidelines of MD Anderson Cancer Center.
Statistical analysis
For evaluating the additive, synergistic or antagonistic effects of different agents, a combination index (CI) was calculated using the Calcusyn Software (Biosoft, Ferguson, MO, USA), according to the Chou–Taladay method. For comparing tumor sizes, p21, Ki-67 and activated caspase-3 immunohistochemical findings and apoptotic indexes (TUNEL), the non-parametric Mann–Whitney U-test and Statview program (Abacus Concepts, Berkeley, CA, USA) were used.
Results
Expression and mutation status of p53 in DLBCL cells
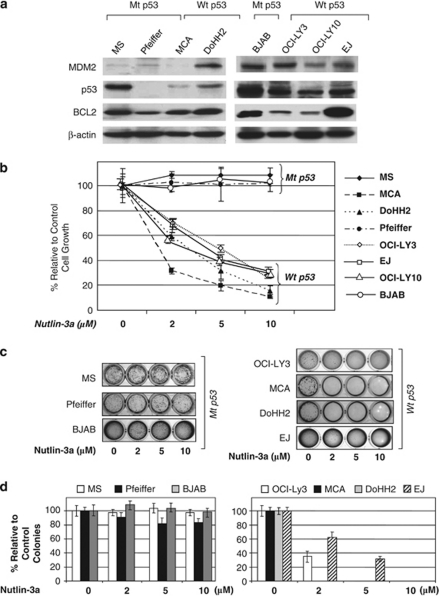
To investigate the mutation status of the p53 gene and expression levels of p53 protein in the DLBCL cell lines, western blot analysis was performed. As shown in Figure 1a, low levels of p53 were seen in DoHH2 and MCA in comparison with MS cells. No p53 protein was detected in Pfeiffer cells. In a separate western blot analysis, EJ, OCI-LY3 and OCI-LY10 cells showed lower levels of p53 than BJAB cells. Direct sequencing of the entire open reading frame of the p53 gene showed that DoHH2, MCA, OCI-LY3 and OCI-LY10 cells harbor wt p53. In MS cells, a non-functional missense mutation (TAC (tyr) to CAC (his)) at codon 234 of exon 7 in the DNA-binding domain of p53 was identified. In BJAB cells, a non-functional missense mutation (CAT (his) to CGT (arg) at codon 578 of exon 6 was identified. We could not amplify a p53 product in Pfeiffer cells, possibly because of a gross deletion, as suggested also by western blot analysis.
Figure 1.
Nutlin-3a inhibits the growth of diffuse large B-cell lymphoma (DLBCL) cell lines associated with t(14;18)(q32;q21), depending on the presence or absence of p53 mutation. (a) p53 expression levels were assessed in six DLBCL associated with t(14;18) cell lines and two DLBCL cell lines of activated B-cell type using western blot analysis. Immunoblots showed high levels of p53 in MS and BJAB, whereas DoHH2, MCA, EJ, OCI-LY3 and OCI-LY10 expressed comparatively lower levels. No p53 protein was observed in Pfeiffer cells. MDM2 protein levels are also shown. These data suggested that p53 might be mutated in BJAB and MCA, deleted in Pfeiffer and unmutated (wild type, wt) in the remaining cell lines. This was verified by amplification of p53 cDNA and direct sequencing of the entire open reading frame of p53 in these cells. (b) MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay after 48 h showed dose-dependent decrease of cell viability on treatment with nutlin-3a in cell lines with wt p53, whereas no loss of cell viability was observed in cell lines with mutant (mt) p53. (c, d) Colony formation assay after treatment with nutlin-3a showed inhibition of growth of cells with wt p53, but not that of MS, BJAB and Pfeiffer cells, which harbored mt p53. At 2 weeks after treatment, a dose of 2–10 μ nutlin-3a completely inhibited the formation of colonies in cells with wt p53, whereas a dose up to 10 μ had no significant effect on cells with mt p53.
Nutlin-3a inhibits the growth of DLBCL cells depending on p53 mutation status
To investigate the functional status of the p53 pathway, the cell lines were treated with nutlin-3a. Treatment with nutlin-3a showed remarkable antiproliferative activity against DoHH2, EJ and MCA cells (with wt p53), but not against MS, BJAB and Pfeiffer cells (with mutant (mt) p53). MTS assay at 48 h after incubation with 10 μ of nutlin-3a showed that growth of DoHH2 and MCA cells was inhibited by 85 and 89%, respectively (Figure 1b). Similarly, treatment with nutlin-3a also decreased cell proliferation of OCI-LY3 and OCI-LY10 cells, both of ABC type with wt p53 (Figure 1b). More importantly, nutlin-3a treatment resulted in a dose-dependent decrease of the colony-forming ability of DoHH2, MCA, OCI-LY3 and EJ cells, whereas no appreciable effect was observed on Pfeiffer, BJAB and MS cells up to a concentration of 10 μ (Figure 1b). The colony-forming assay is not contributory for OCI-LY10 cells, as they lack the colony-forming ability in methylcellulose.
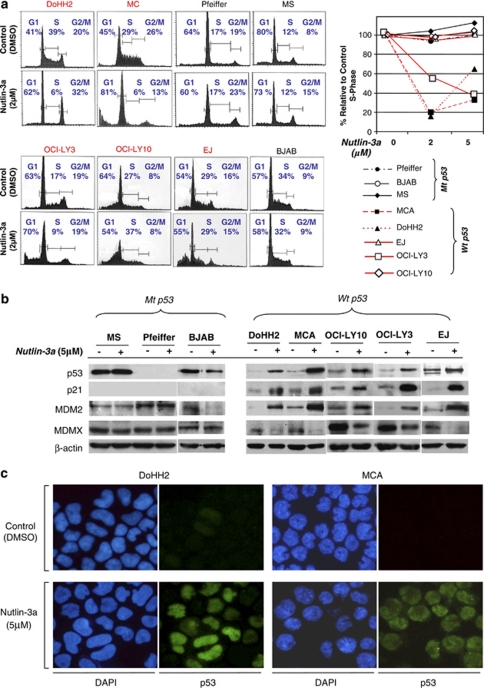
Nutlin-3a induces cell cycle arrest in t(14;18)-positive DLBCL cells through activation of the p53 pathway and upregulation of p21
To investigate the effects of p53 activation on cell cycle progression, we analyzed the cell cycle. Nutlin-3a treatment of DLBCL cells associated with t(14;18) and harboring wt p53 showed a substantially reduced S-phase fraction (Figure 2a). At 24 h after treatment with 2 μ of nutlin-3a, the S-phase fraction of DoHH2 and MCA cells decreased by ∼80 and 85%, respectively. In DoHH2 cells, an increase in G1- and G2/M-phase fractions was observed, indicating cell cycle arrest at the G1 and G2/M checkpoints. Under similar treatment conditions, an increase of the G1 phase along with an increase of the sub-G1-phase fraction (indicator of cell death) was observed in MCA, indicating both cell cycle arrest in G1 phase and cell death. However, treatment of EJ cells with 2 μm nutlin-3a resulted only in an increased sub-G1 fraction without any change in any other phases of the cell cycle. This result indicates cell death, without any arrest in either G1– or G2–M phase. Treatment of OCI-LY3 cells with 2 μm nutlin-3a resulted in increases of the G1- and sub-G1 phases along with a decreased S-phase fraction, indicating both cell cycle arrest in G1 phase and cell death (Figure 2a). However, similar treatment of OCI-LY10 cells resulted predominantly in induction of cell death, as evident by the considerable increase of the sub-G1 phase. No cell cycle arrest in G1– or G2–M phase or decrease of S phase was observed (Figure 2a). In contrast, in t(14;18)-positive DLBCL cell lines with mt p53 (Pfeiffer, MS or BJAB), neither cell cycle arrest nor apoptotic cell death was observed with nutlin-3a treatment at any concentration (Figure 2a).
Figure 2.
Nutlin-3a induces cell cycle arrest and/or cell death in diffuse large B-cell lymphoma (DLBCL) associated with t(14;18)(q32;q21) through activation of the p53 pathway. (a) At 24 h after treatment with 2 μ nutlin-3a in DLBCL cell lines with wild-type (wt) p53 resulted in G1 and G2/M phase cell cycle arrest with decreased S phase (DoHH2), cell cycle arrest in G1 phase, decreased S phase accompanied by cell death (MCA and OCI-Ly3) or cell death without cell cycle arrest and decreased S phase (OCI-LY10 and EJ). No cell death or cell cycle arrest was seen in cells that lacked p53 or with mutant p53 (Pfeiffer, MS and BJAB). (b) Western blot analysis at 24 h after treatment with 5 μ nutlin-3a showed increased expression of p21, in parallel with increased p53 levels in cells with wt p53 along with upregulation of MDM2 and downregulation of MDMX. No such changes were detected in Pfeiffer, BJAB and MS cells treated with nutlin-3a. (c) At 12 h following treatment with nutlin-3a, DoHH2 and MCA cells had increased levels of p53 visualized by immunofluorescence. p53 localization was predominantly nuclear. The 4',6-diamidino-2-phenylindole was used as counterstain of the nuclei.
Western blot analysis showed stabilization of wt p53 protein levels in DoHH2, MCA, OCI-LY3, OCI-LY10 and EJ cells on treatment with nutlin-3a, whereas no such effects were seen in BJAB and MS cells, both with mt p53 (Figure 2b). The cyclin-dependent kinase inhibitor p21, a known transcriptional target of p53, showed increased levels, indicating that stabilized wt p53 is functional and capable of inducing p21 expression (Figure 2b). Increased p53 protein levels were also shown by immunofluorescence in nutlin-3a-treated cells with wt p53 (Figure 2c).
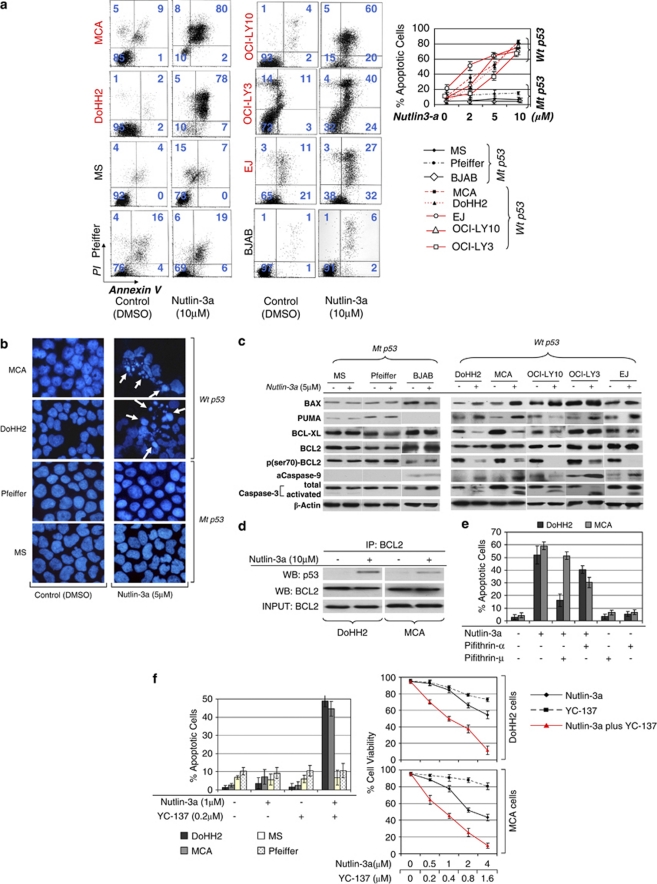
Nutlin-3a induces apoptotic cell death in t(14;18)-positive DLBCL cells as well as DLBCL cells of ABC type through activation of the p53 apoptotic pathway
To investigate the nature of nutlin-3a-induced suppression of cell viability, annexin V staining and flow cytometry were performed. As illustrated in Figure 3a, there was a concentration-dependent increase in annexin V binding in DoHH2, MCA, EJ, OCI-LY3 and OCI-LY10 cells, but not in Pfeiffer, MS and BJAB cells. At 24 h following treatment with 10 μ of nutlin-3a, annexin V binding was increased by ∼80, 74 and 58% in DoHH2, MCA and EJ cells, respectively. In OCI-LY3 and OCI-LY10 cells, annexin V binding was increased by 63 and 74%, respectively. No significant increase in annexin V binding was observed in nutlin-3a-treated Pfeiffer, BJAB and MS cells that harbor mt p53 (Figure 3a). Also, 4',6-diamidino-2-phenylindole staining and fluorescence microscopy demonstrated nuclear condensation and fragmentation, morphologic evidence of apoptotic cell death, in nutlin-3a-treated wt p53 cells (Figure 3b).
Figure 3.
Nutlin-3a induces apoptotic cell death of t(14;18)-positive diffuse large B-cell lymphoma (DLBCL) and ABC-type DLBCL cells through activation of the p53 pathway. (a) At 48 h after incubation with 10 μ nutlin-3a, a considerable increase in annexin V binding was observed in DLBCL cells with wild-type (wt) p53, indicating apoptotic cell death. No significant change was observed in Pfeiffer, BJAB and MS cells, which have mutant p53 (left panel). Annexin V binding corresponding to various concentrations of nutlin-3a treatment is depicted in the diagrams of the right panel (b). Microscopic examination of 4',6-diamidino-2-phenylindole-stained preparations of DoHH2 and MCA cell at 48 h after treatment with 5 μ nutlin-3a showed morphologic evidence of apoptosis, including nuclear condensation and fragmentation, whereas no such changes were observed in nutlin-3a-treated Pfeiffer and MS cells. (c) Western blot analysis of DLBCL cells with wt p53 after treatment with nutlin-3a showed increased levels of the proapoptotic proteins BAX and PUMA in all cell lines with wt p53, known transcriptional targets of p53. Also, substantially decreased levels of BCL-XL levels were observed after nutlin-3a treatment in DoHH2, OCI-LY10 and MCA cells, without much change in OCI-LY3 and EJ cells. Whereas the levels of total BCL2 remained constant, the p-Ser70BCL2 levels decreased dramatically after nutlin-3a treatment in all the cells with wt p53. In addition, western blot analysis demonstrated cleavage of caspase-3, accompanied by activation of caspase-9. By contrast, no significant changes in the levels of proapoptotic or antiapoptotic proteins, or in activation of caspase-3 or -9, were observed in nutlin-3a-treated Pfeiffer and MS cells. Lysates were prepared at 24 h following nutlin-3a treatment. (d) Coimmunoprecepitation of DoHH2 and MCA cell lysates treated with 10 μ of nutlin-3a or control (dimethyl sulfoxide) showed that nutlin-3a induced binding of BCL2 by p53 protein, suggesting that non-transcriptional mechanisms are also involved in nutlin-3a-induced cell death of t(14;18)-positive DLBCL cells. (e) Preincubation of DoHH2 and MCA cells with 25 μ pifithrin-α (PFT-α) or 4.8 μ PFT-μ (the maximum non-toxic dose) rescued a substantial number of nutlin-3a-treated cells from apoptotic cell death. PFT-μ, an agent that inhibits the interaction of p53 with antiapoptotic proteins without affecting p53 transactivation function, rescued a greater proportion of nutlin-3a-treated DoHH2 cells and a smaller proportion of MCA cells, whereas PFT-α rescued a larger proportion of nutlin-3a-treated-MCA cells and a smaller proportion of DoHH2 cells. (f) Treatment with a comparatively small dose of nutlin-3a (1 μ) enhanced dramatically the cytotoxicity of YC-137, a BH3 mimetic in DoHH2 and MCA cells, with no effect observed in Pfeiffer and MS cells. Combined treatment with nutlin-3a and YC-137 synergistically induced decreased viability of DoHH2 and MCA cells (average combination index (CI)=0.53 and CI=0.35, respectively).
To examine the possible mechanisms underlying nutlin-3a-mediated cell death, western blot analysis was performed. As shown in Figure 3c, alterations in the levels of pro- and antiapoptotic proteins involved in the intrinsic apoptotic pathway were detected in nutlin-3a-treated DLBCL cells. Specifically, an increase of the proapoptotic proteins BAX and PUMA, both transcriptional targets of p53, was observed in DoHH2, MCA and EJ cells (wt p53) at 24 h after nutlin-3a treatment. In ABC-DLBCL cell lines, BAX was increased in both OCI-LY3 and OCI-LY10 cells, whereas PUMA level was increased only in OCI-LY3 cells (Figure 3c). Also, levels of the antiapoptotic protein BCL-XL were decreased in DOHH2, MCA and OCI-LY10 cells, but not in OCI-LY3 and EJ, whereas the levels of BCL2 remained almost constant in these cell lines (Figure 3c). Because the antiapoptotic activity of BCL2 is dependent not only on protein levels but also on the its phosphorylation status, we also examined p-Ser70BCL2 levels.29, 30 All DLBCL cell lines with wt p53 (DoHH2, MCA, OCI-LY10, OCI-LY3 and EJ) showed a significant decrease of p-Ser70BCL2 levels after nutlin-3a treatment. In contrast, these changes were not observed in nutlin-3a-treated Pfeiffer, BJAB and MS cells with mt p53 (Figure 3c).
In addition to enhancing the antiapoptotic activity of BCL2, serine-70 phosphorylation of BCL2 has been shown to inhibit the interaction of p53 with BCL2, one of the transcription-independent mechanisms of p53-mediated proapoptotic functions. For this reason, we investigated whether nutlin-3a-induced cell death is associated with physical interaction of the p53 and BCL2 proteins.31 Coimmunoprecipitation studies showed that nutlin-3a treatment induced binding of BCL2 by p53 in DoHH2 cells and to a lesser degree in MCA cells (Figure 3d).
To further elaborate on the apoptotic mechanisms, and clarify the functional importance of the changes associated with nutlin-3a-induced cell death revealed in our analysis, we studied the apoptotic effects in nutlin-3a-treated DoHH2 and MCA cells pretreated with PFT-α, an inhibitor of p53 transcriptional activity, or PFT-μ, an inhibitor of p53 protein translocation to mitochondria.32, 33 As shown in Figure 3e, both PFT-α and PFT-μ rescued a significant proportion of nutlin-3a-treated DoHH2 and MCA cells, suggesting that p53 transcriptional and posttranscriptional mechanisms involve direct targeting of antiapoptotic proteins by p53. Interestingly PFT-μ rescued a larger proportion of nutlin-3a-treated DoHH2 cells compared with PFT-α, whereas the opposite was found for MCA cells, a finding reflected in our western blot analysis and coimmunoprecipitation data.
It must be added that although PFT-μ was protective at the higher non-toxic dose of 4.8 μ, above 5 μ induced significant cell death in both DoHH2 and MCA cells, an unexpected finding (data not shown). Similar data have been reported recently in chronic lymphocytic leukemia cells.34 Also, in higher doses, above 8 μ, PFT-μ induced significant cell death in Pfeiffer and MS cells (mt p53). For comparison, we similarly assessed ALK+ anaplastic large cell lymphoma cells that did not show significant cytotoxicity up to a concentration of 10 μ, suggesting that this phenomenon is cell-type specific.
Nutlin-3a sensitizes t(14;18)-positive DLBCL cells with wt p53 to activation of the intrinsic apoptotic pathway
Following up on our data that showed that nutlin-3a-mediated activation of p53 affects the intrinsic apoptotic pathway, we investigated whether nutlin-3a treatment can facilitate activation of the intrinsic apoptotic pathway mediated by YC-137, a BH3 mimetic, that directly targets the antiapoptotic proteins BCL2 and BCL-XL.35 The results showed that a small concentration of nutlin-3a dramatically increased the cytotoxicity of YC-137, specifically in cells with wt p53. (Figure 3f). Furthermore, combining YC-137 and nutlin-3a synergistically increased cytotoxicity in both DoHH2 and MCA cells (average CI=0.53 and CI=0.35, respectively; Figure 3f).
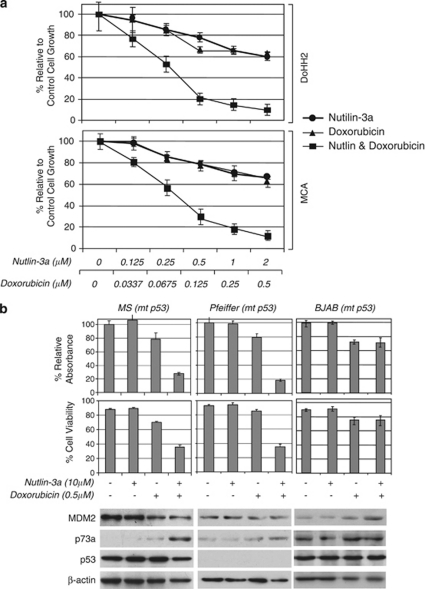
Nutlin-3a enhances the antiproliferative activity of chemotherapeutic agents against t(14;18)-positive DLBCL cells with wt p53
Previous studies have shown that nutlin-3a can enhance the antitumor activity of standard chemotherapeutic agents against cells harboring wt p53.36 To investigate whether this applies to DLBCL cells associated with t(14;18)(q32;q21), we combined nutlin-3a and doxorubicin treatment in our in vitro system. As shown in Figure 4a, treatment of DoHH2 and MCA cells with nutlin-3a and doxorubicin for 48 h showed synergistic antiproliferative activity (average CI=0.34 and CI=0.29, respectively).
Figure 4.
Nutlin-3a enhances the antitumor activity of chemotherapeutic agents against diffuse large B-cell lymphoma cells harboring wild-type or mutant (mt) p53. (a) Combined treatment for 48 h with nutlin-3a and doxorubicin synergistically inhibited the growth of DoHH2 and MCA cells (average combination index (CI)=0.34 and CI=0.29, respectively). (b) Treatment with 10 μ nutlin-3a for 48 h enhanced the inhibitory effect of 0.5 μ doxorubicin treatment by 50 and 60% on the growth of MS and Pfeiffer cells, respectively, and substantially decreased the cell viability by 34 and 50%, respectively. Nutlin-3a treatment, by itself, had no effect on the growth and survival of these cells (upper panel). Western blot analysis showed that combined treatment of nutlin-3a and doxorubicin induced increased levels of the p53-related protein p73a in both MS and Pfeiffer cells that harbor mt p53 (lower panel).
Nutlin-3a also enhances the antiproliferative activity of chemotherapeutic agents against DLBCL cells with mt p53 via p73
As it is known that p53 gene mutation is associated with progression of FL to DLBCL in a subset of cases, and recent studies have shown that high doses of nutlin-3a can induce cytotoxicity of cancer cells harboring mt p53 gene, through effects on partner proteins of MDM2 other than p53, we investigated the effect of nutlin-3a on the antitumor activity of doxorubicin against DLBCL cells harboring mt p53.37, 38, 39 As shown in Figure 4b, application of 10 μ nutlin-3a for 48 h had no effect on the growth and cell viability of MS and Pfeiffer cells. However, nutlin-3a enhanced the cytotoxicity and inhibitory effect of 0.5 μg/ml of doxorubicin, reducing cell viability in these cell lines from 70 to 36% and 85 to 35%, respectively, and reducing cell growth from 78 to 28% and 79 to 19%, respectively (Figure 4b). Western blot analysis showed that nutlin-3a combined with a relatively low dose of doxorubicin induced p73α expression, another MDM2 partner protein. However, in BJAB cells, combined treatment of doxorubicin and nutlin-3a did not have a synergistic effect on doxorubicin cytotoxicity. This may be attributable to the lack of significant induction of p73α expression in BJAB cells after combined treatment (Figure 4b). The explanation for the lack of effect in BJAB cells is not clear. In aggregate, the findings suggest that combined genotoxic and non-genotoxic treatment may have effect in DLBCL cells with t(14;18)(q32;q21) and mt p53, depending on the ability of the combined treatment to upregulate p73α expression and signaling.
Nutlin-3a treatment inhibits growth of t(14;18)-positive DLBCL tumors harboring wt p53 in vivo
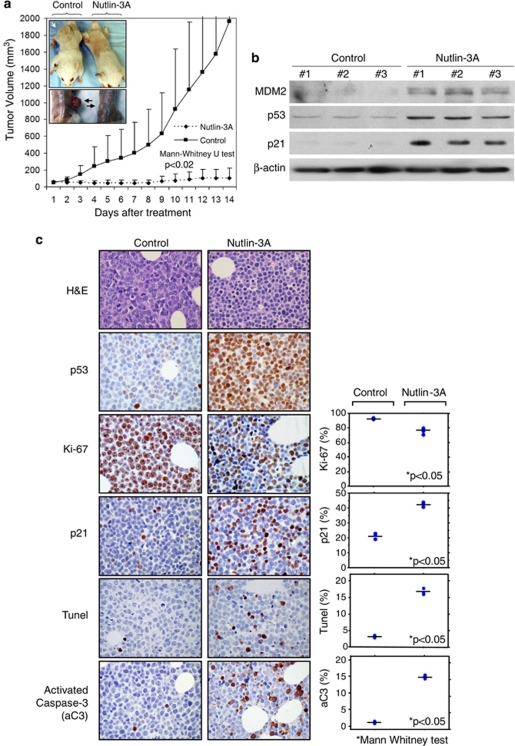
To further investigate the therapeutic potential of nutlin-3a-mediated activation of p53, we employed a human xenograft lymphoma animal model using the DoHH2 cell line. Nutlin-3a was well tolerated, without significant weight loss or other obvious toxicity signs in severe combined immunodeficiency–beige mice. After two weeks of nutlin-3a treatment of severe combined immunodeficiency–Beige mice with already established (palpable) subcutaneous DoHH2 tumors, the mean tumor volume in the control group was 1965.5 mm3, whereas in the nutlin-3a-treated group, the mean tumor volume was 107.5 mm3 (P<0.02, Mann–Whitney U-test; Figure 5a). In addition, two of the nutlin-3a-treated mice had no palpable tumors at the end of treatment. Western blot analysis of six tumors (three from each group) after three injections showed upregulation of p53, and p53 transcriptional targets p21 and MDM2, specifically in the nutlin-3a-treated tumors, supporting nutlin-3a-mediated activation of the p53 pathway (Figure 5b). Immunohistochemical analysis of these tumors showed that the mean proliferation index (Ki-67) was 92.8 and 75.4%, and the percentage of p21-positive lymphoma cells was 13.8 and 42.3% in the control and treated groups, respectively (P<0.05, Mann–Whitney U-test). Also, the average apoptotic index, assessed by TUNEL, was 3.1 and 16.6%, and the percentage of activated caspase-3-positive lymphoma cells was 2.1 and 15.7% in the control and treated groups, respectively (P<0.05, Mann–Whitney U-test; Figure 5c). In aggregate, these results support the interpretation that nutlin-3a treatment induces p53-mediated cell cycle arrest and apoptosis in t(14;18)-positive DLBCL cells in vivo.
Figure 5.
Nutlin-3a inhibits the growth of t(14;18)-positive diffuse large B-cell lymphoma tumors with wild-type p53 in vivo. (a) Severe combined immunodeficiency–Beige mice with already established (palpable) subcutaneous DoHH2 lymphomas were treated daily with nutlin-3a or vehicle for 2 weeks, and tumor volume was recorded. Error bars show s.d. from the mean. At the end of treatment, the average tumor volume in the control group was 1965.5 mm3, whereas in the nutlin-3a-treated group, the average tumor volume was 107.5 mm3 (*P<0.02, Mann–Whitney U-test). Inset shows the subcutaneous tumors (arrows). (b) Western blot analysis of six tumors after three injections showed upregulation of p53 and the p53 transcriptional targets p21 and MDM2 specifically in the nutlin-3a-treated tumors, verifying nutlin-3a-mediated activation of the p53 pathway. (c) Immunohistochemical analysis of tumors showed that the proliferation index (Ki-67) was 92.8 and 75.4%, and the percentage of p21-positive tumor cells was 13.8 and 42.3% in the control and treated groups, respectively (P<0.05, Mann–Whitney U-test), supporting p53-mediated cell cycle arrest of the lymphoma cells. Also, the apoptotic index assessed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) was 3.1 and 16.6%, and the percentage of activated caspase-3-positive lymphoma cells was 2.1 and 15.7% in the control and treated groups, respectively (P<0.05, Mann–Whitney U-test), verifying the ability of nutlin-3a to induce apoptosis in t(14;18)-positive diffuse large B-cell lymphoma cells in vivo. Hematoxylin and eosin and p53 immunostaining of the tumors are also shown in the upper two rows (DAB as chromogen and hematoxylin as counterstain, original magnification × 400).
Discussion
Molecules such as nutlin-3a that disrupt the p53–MDM2 interaction and activate the p53 pathway affect normal and neoplastic cells differently. In normal cells, nutlin-3a predominantly induces reversible biological effects including cell cycle arrest. In cancer cells with wt p53, on the other hand, nutlin-3a additionally induces irreversible apoptosis, but in a highly variable manner.9, 36 One hypothesis put forward to explain this highly heterogeneous apoptotic response is that a definite downstream block of the apoptotic machinery may be involved in unresponsive cells.9 We show here, using as a paradigm wt p53 DLBCL cells that carry t(14;18)(q32;q21) and overexpress BCL2, that isolated overexpression of BCL2 in this context cannot impede the execution of the p53-mediated apoptotic program. Because t(14;18)(q32;q21) and BCL2 overexpression are regarded as an initiating oncogenic event in FL, and also seem to have a definite role in the pathogenesis of a subset of DLBCL tumors, our data provide further evidence that nutlin-3a-induced activation of the p53 pathway may overcome initiating oncogenic events, unrelated to the p53 pathway, in agreement with data from recent studies.1, 6, 40
These results also shed some light on the findings of a recent study examining the in vivo biologic effects of low-dose irradiation in FL cells.41 Activation of the p53 pathway, induced in this context by irradiation-induced DNA damage, was found to result in cell cycle arrest and massive apoptosis.41 The authors, surprised by the simultaneous detection of p53 pathway activation and high expression of BCL2 in FL cells, as well as immunological tissue activation, speculated that tissue microenvironment changes, including immunological activation may have a predominant role in the induction of apoptosis of BCL2-overexpessing lymphoma cells.41 Although the tissue microenvironment may have an important role, our results provide evidence that specific activation of the p53 pathway can result in apoptosis overcoming the antiapoptotic effect of overexpressed BCL2 in a cell-autonomous manner in B-cell lymphoma cells characterized by t(14;18).
Nutlin-3a-induced apoptotic cell death of DLBCL associated with t(14;18) was associated with various changes in the levels of proapoptotic and antiapoptotic proteins involving the intrinsic apoptotic pathway, including increased PUMA and BAX and decreased BCL-XL, although the levels of BCL2 remained constant. Similar changes also have been reported after nutlin-3a treatment of other types of hematopoietic neoplastic cells, including Hodgkin lymphoma, anaplastic large cell lymphoma, Burkitt lymphoma, chronic lymphocytic leukemia and mantle cell lymphoma cells facilitating apoptotic cell death.10, 12, 18, 20, 42, 43
In addition, we show that nutlin-3a-induced apoptosis of t(14;18)-positive DLBCL cells was associated with decreased levels of p-Ser70BCL2 levels. Phosphorylation of multiple residues in the flexible loop regulatory region of BCL2, including serine-70, is regulated by multiple kinase signaling pathways, including MAPK and JNK, as well as by various phosphatases, including PP2A, and has been shown to enhance the antiapoptotic function of BCL2.30 Also, it has been shown that this phosphorylation decreases direct binding of BCL2 by activated p53, inhibiting one of the central non-transcriptional mechanisms of p53-mediated apoptotic death.31 In our in vitro system, we showed that nutlin-3a-induced apoptosis of DLBCL cells involves transcriptional as well as non-transcriptional mechanisms including direct binding of BCL2 by nutlin-3a-activated p53, and therefore nutlin-3a-induced dephosphorylation of BCL2 may facilitate this phenomenon. The exact mechanisms of nutlin-3a-induced dephosphorylation of BCL2 are uncertain. However, the fact that BCL2 is known to be predominantly unphosphorylated in the G1 phase of the cell cycle in combination with the nutlin-3a-induced G1 cell cycle arrest may, in part, explain this finding.44, 45 Also, other investigators have reported involvement of non-transcriptional mechanisms in nutlin-3a-induced cell death in other hematologic malignancies after activation of the p53 pathway, further supporting our data.10, 11, 46 In our in vitro system, transcriptional and non-transcriptional p53-mediated mechanisms seemed to act in concert, overcoming collectively the antiapoptotic function of BCL2 as shown by previous studies.47 However, our data are in contrast with a recent study on chronic lymphocytic leukemia suggesting an antagonistic relationship between these two mechanisms.46 It is possible that the exact interrelationship of these mechanisms varies in different cell types.
Recently, new pharmacological agents that directly target mitochondria inducing activation of the apoptotic machinery have been introduced in experimental therapeutics.35 Prominent among these agents are molecules that directly inhibit antiapoptotic proteins such as BCL2.48 These agents are already being tested in clinical trials of various cancer types including hematologic malignancies.49 We show here that nutlin-3a can synergistically enhance the antitumor effect of such agents, augmenting their therapeutic potential. Other studies have shown that a combination of nutlin-3a with BCL2 inhibitors can result in synergistically enhanced apoptosis in acute myeloid leukemia.45, 50 These findings further support our data.
Recent studies have shown that high concentrations of nutlin-3a can induce an antitumor effect in cases of non-functional and mt p53, partly, through activation of other partners of MDM2, including p73 or E2F1, depending on the particular cell context.38, 39 Accordingly, we have shown that nutlin-3a can enhance the cytotoxicity of classical chemotherapeutic agents against DLBCL cells associated with t(14;18) and mutated p53 associated with increased p73 expression. Our data are further supported by recent studies showing that nutlin-3a can induce increased expression of p73 in anaplastic large cell lymphoma or mantle cell lymphoma cells harboring mt p53, and synergize with chemotherapy for enhanced antitumor activity.20, 43 As p53 mutation can be associated with progression of FL to DLBCL, our data suggest that nutlin-3a combined with classical chemotherapy could be an experimental therapeutic strategy in this context.1, 22, 23 Also, the results of our in vivo animal study show that nutlin-3a treatment was well tolerated by the animals and exhibited significant antitumor activity against DLBCL. Previous in vivo animal studies also have shown that nutlin-3a treatment has minimal side effects.8, 9, 14 In addition, Mohammad et al.51 have employed a different small molecule MDM2-inhibitor and a different animal model with FL and showed extended survival of the treated animals.51 These data further are in accord with our findings.
In conclusion, our data suggest that inhibition of the p53–MDM2 interaction by nutlin-3a can activate the p53 pathway, resulting in cell cycle arrest and apoptosis in DLBCL cells with t(14;18)(q32;q21) and functional p53, and that nutlin-3a can enhance the efficacy of chemotherapy in cases with wt or mutated p53. Therefore, agents like nutlin-3a targeting the p53–MDM2 interaction may be valuable additions to novel therapeutic strategies for patients with B-cell lymphomas characterized by t(14;18)(q32;q21) and BCL2 overexpression.
The authors declare no conflict of interest.
References
- Harris NL, Nathwani BN, Swerdlow SH, de Jong D, Jaffe ES, Yoshino T, et al. Follicular LymphomaIn: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (eds).World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues,(4th edn)IARC Press; 2008220–226. [Google Scholar]
- Stein H, Chan JKC, Warnke RA, Gatter KC, Chan WC, Campo E, et al. Diffuse large B-cell lymphoma, not otherwise specifiedIn: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds).World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues,(4th edn)IARC Press: Lyon, France; 2008233–237. [Google Scholar]
- de Jong D. Molecular pathogenesis of follicular lymphoma: a cross talk of genetic and immunologic factors. J Clin Oncol. 2005;23:6358–6363. doi: 10.1200/JCO.2005.26.856. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, McQueen T, O'Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakos E, Thomaides A, Medeiros LJ, Li J, Leventaki V, Konopleva M, et al. Inhibition of p53-murine double minute 2 interaction by nutlin-3A stabilizes p53 and induces cell cycle arrest and apoptosis in Hodgkin lymphoma. Clin Cancer Res. 2007;13:3380–3387. doi: 10.1158/1078-0432.CCR-06-2581. [DOI] [PubMed] [Google Scholar]
- Stuhmer T, Chatterjee M, Hildebrandt M, Herrmann P, Gollasch H, Gerecke C, et al. Nongenotoxic activation of the p53 pathway as a therapeutic strategy for multiple myeloma. Blood. 2005;106:3609–3617. doi: 10.1182/blood-2005-04-1489. [DOI] [PubMed] [Google Scholar]
- Sarek G, Kurki S, Enback J, Iotzova G, Haas J, Laakkonen P, et al. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J Clin Invest. 2007;117:1019–1028. doi: 10.1172/JCI30945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Zhu N, Findley HW, Zhou M. MDM2 antagonist nutlin-3 is a potent inducer of apoptosis in pediatric acute lymphoblastic leukemia cells with wild-type p53 and overexpression of MDM2. Leukemia. 2008;22:730–739. doi: 10.1038/leu.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz M, Stuhmer T, Vassilev LT, Bargou RC. Pharmacologic activation of p53-dependent and p53-independent apoptotic pathways in Hodgkin/Reed-Sternberg cells. Leukemia. 2007;21:772–779. doi: 10.1038/sj.leu.2404565. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Barbarotto E, Tiribelli M, Zerbinati C, di Iasio MG, Gonelli A, et al. Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107:4122–4129. doi: 10.1182/blood-2005-11-4465. [DOI] [PubMed] [Google Scholar]
- Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de Frias M, Castano E, et al. MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood. 2006;107:4109–4114. doi: 10.1182/blood-2005-08-3273. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Sebasigari D, Jin L, Rudelius M, Davies-Hill T, Miyake K, et al. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res. 2009;15:933–942. doi: 10.1158/1078-0432.CCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakos E, Atsaves V, Li J, Leventaki V, Andreeff M, Medeiros LJ, et al. Stabilization and activation of p53 downregulates mTOR signaling through AMPK in mantle cell lymphoma. Leukemia. 2009;23:784–790. doi: 10.1038/leu.2008.348. [DOI] [PubMed] [Google Scholar]
- Drakos E, Atsaves V, Schlette E, Li J, Papanastasi I, Rassidakis GZ, et al. The therapeutic potential of p53 reactivation by nutlin-3a in ALK+ anaplastic large cell lymphoma with wild-type or mutated p53. Leukemia. 2009;23:2290–2299. doi: 10.1038/leu.2009.180. [DOI] [PubMed] [Google Scholar]
- Martinez-Climent JA, Alizadeh AA, Segraves R, Blesa D, Rubio-Moscardo F, Albertson DG, et al. Transformation of follicular lymphoma to diffuse large cell lymphoma is associated with a heterogeneous set of DNA copy number and gene expression alterations. Blood. 2003;101:3109–3117. doi: 10.1182/blood-2002-07-2119. [DOI] [PubMed] [Google Scholar]
- Lo Coco F, Gaidano G, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood. 1993;82:2289–2295. [PubMed] [Google Scholar]
- Sander CA, Yano T, Clark HM, Harris C, Longo DL, Jaffe ES, et al. p53 mutation is associated with progression in follicular lymphomas. Blood. 1993;82:1994–2004. [PubMed] [Google Scholar]
- Moller MB, Ino Y, Gerdes AM, Skjodt K, Louis DN, Pedersen NT. Aberrations of the p53 pathway components p53, MDM2 and CDKN2A appear independent in diffuse large B cell lymphoma. Leukemia. 1999;13:453–459. doi: 10.1038/sj.leu.2401315. [DOI] [PubMed] [Google Scholar]
- Davies AJ, Lee AM, Taylor C, Clear AJ, Goff LK, Iqbal S, et al. A limited role for TP53 mutation in the transformation of follicular lymphoma to diffuse large B-cell lymphoma. Leukemia. 2005;19:1459–1465. doi: 10.1038/sj.leu.2403802. [DOI] [PubMed] [Google Scholar]
- Ford RJ, Goodacre A, Ramirez I, Mehta SR, Cabanillas F. Establishment and characterization of human B-cell lymphoma cell lines using B-cell growth factor. Blood. 1990;75:1311–1318. [PubMed] [Google Scholar]
- Leventaki V, Drakos E, Medeiros LJ, Lim MS, Elenitoba-Johnson KS, Claret FX, et al. NPM-ALK oncogenic kinase promotes cell cycle progression through activation of JNK/cJun signaling in anaplastic large cell lymphoma. Blood. 2007;110:1621–1630. doi: 10.1182/blood-2006-11-059451. [DOI] [PubMed] [Google Scholar]
- May WS, Tyler PG, Ito T, Armstrong DK, Qatsha KA, Davidson NE. Interleukin-3 and bryostatin-1 mediate hyperphosphorylation of BCL2 alpha in association with suppression of apoptosis. J Biol Chem. 1994;269:26865–26870. [PubMed] [Google Scholar]
- Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, Anderson J, May WS. Bcl2's flexible loop domain regulates p53 binding and survival. Mol Cell Biol. 2006;26:4421–4434. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- Steele AJ, Prentice AG, Hoffbrand AV, Yogashangary BC, Hart SM, Lowdell MW, et al. 2-Phenylacetylenesulfonamide (PAS) induces p53-independent apoptotic killing of B-chronic lymphocytic leukemia (CLL) cells. Blood. 2009;114:1217–1225. doi: 10.1182/blood-2008-11-190587. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Kepp O, Tajeddine N, Kroemer G. Targeting p53 to mitochondria for cancer therapy. Cell cycle. 2008;7:1949–1955. doi: 10.4161/cc.7.13.6222. [DOI] [PubMed] [Google Scholar]
- Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Aonuma M, Lee SH, Fukutake S, McCormick F. E2F-1 transcriptional activity is a critical determinant of Mdm2 antagonist-induced apoptosis in human tumor cell lines. Oncogene. 2008;27:5303–5314. doi: 10.1038/onc.2008.164. [DOI] [PubMed] [Google Scholar]
- Lau LM, Nugent JK, Zhao X, Irwin MS. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997–1003. doi: 10.1038/sj.onc.1210707. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK. Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene. 2007;26:3473–3481. doi: 10.1038/sj.onc.1210136. [DOI] [PubMed] [Google Scholar]
- Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Knoops L, Haas R, de Kemp S, Majoor D, Broeks A, Eldering E, et al. In vivo p53 response and immune reaction underlie highly effective low-dose radiotherapy in follicular lymphoma. Blood. 2007;110:1116–1122. doi: 10.1182/blood-2007-01-067579. [DOI] [PubMed] [Google Scholar]
- Renouf B, Hollville E, Pujals A, Tetaud C, Garibal J, Wiels J. Activation of p53 by MDM2 antagonists has differential apoptotic effects on Epstein-Barr virus (EBV)-positive and EBV-negative Burkitt's lymphoma cells. Leukemia. 2009;23:1557–1563. doi: 10.1038/leu.2009.92. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Chen Q, Voorhees PM, Young KH, Bruey-Sedano N, Yang D, et al. Inhibition of the p53 E3 ligase HDM-2 induces apoptosis and DNA damage--independent p53 phosphorylation in mantle cell lymphoma. Clin Cancer Res. 2008;14:5416–5425. doi: 10.1158/1078-0432.CCR-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Iwase S, Kikuchi J, Terui Y, Nakamura M, Yamada H, et al. Phosphorylation of Bcl-2 protein by CDC2 kinase during G2/M phases and its role in cell cycle regulation. J Biol Chem. 2000;275:21661–21667. doi: 10.1074/jbc.M906893199. [DOI] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778–2786. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- Steele AJ, Prentice AG, Hoffbrand AV, Yogashangary BC, Hart SM, Nacheva EP, et al. p53-mediated apoptosis of CLL cells: evidence for a transcription-independent mechanism. Blood. 2008;112:3827–3834. doi: 10.1182/blood-2008-05-156380. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- Pellecchia M, Reed JC. Inhibition of anti-apoptotic Bcl-2 family proteins by natural polyphenols: new avenues for cancer chemoprevention and chemotherapy. Curr Pharm Des. 2004;10:1387–1398. doi: 10.2174/1381612043384880. [DOI] [PubMed] [Google Scholar]
- Lock R, Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1181–1189. doi: 10.1002/pbc.21433. [DOI] [PubMed] [Google Scholar]
- Wade M, Rodewald LW, Espinosa JM, Wahl GM. BH3 activation blocks Hdmx suppression of apoptosis and cooperates with Nutlin to induce cell death. Cell Cycle. 2008;7:1973–1982. doi: 10.4161/cc.7.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad RM, Wu J, Azmi AS, Aboukameel A, Sosin A, Wu S, et al. An MDM2 antagonist (MI-319) restores p53 functions and increases the life span of orally treated follicular lymphoma bearing animals. Mol Cancer. 2009;8:115. doi: 10.1186/1476-4598-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]