Abstract
The authors present the case of a 76-year-old male who presented with right-sided recurrent malignant otitis externa (MOE) and skull-base osteomyelitis. His management involved aggressive antimicrobial therapy and multiple hyperbaric oxygen treatments. After resolution of his right-sided infection, the patient returned a short time later with symptoms and findings consistent with new, left-sided MOE with involvement of the left skull-base. With repeat treatment, the patient is now cured of his infection but poses a challenge to the treating team about future management.
Background
This is a rare case of malignant otitis externa (MOE) that highlights the damage it can cause through the spread to adjacent structures and the recurrence on the contralateral side of the skull. The case also demonstrates that despite extensive antimicrobial therapy, the infection is extremely hard to eradicate and often leaves the patient with residual deficits.
Case presentation
A 76-year-old male was reviewed by our otolaryngology service after being admitted with nausea, vomiting and symptoms consistent with trigeminal neuralgia.
He had a background history of diabetes, aortic stenosis and ischaemic heart disease with an implanted cardiac defibrillator. He had recently been seen in another tertiary hospital for management of right otitis externa and insertion of tympanostomy tubes. At that time, a gallium study showed no scintigraphic evidence of skull osteomyelitis and swabs taken from the right ear were culture negative. He was discharged from their hospital with a course of oral Ciprofloxacin.
Examination revealed an inflamed right external auditory canal (EAC) with minor debris and an inflamed tympanic membrane with a tympanostomy tube in situ. Hearing was reduced in the right ear and a valsalva manoeuvre demonstrated clear fluid escape from the middle ear. Cranial nerve and left ear examination were unremarkable. A provisional diagnosis of right-sided, MOE was made and intravenous antibiotics were commenced after cultures were taken.
Investigations
A gallium scan revealed skull-base osteomyelitis with involvement of the right petrous temporal bone. CT imaging showed evidence of chronic right mastoiditis, otitis externa and otitis media. No organism grew on repeat cultures of the EAC. The patient was not suitable for MRI due to his implanted cardiac defibrillator.
Differential diagnosis
Infection in EAC
-
▶
Localised otitis externa (furuncle), organism usually Staphylococcus aureus
-
▶
Acute diffuse otitis externa (swimmer’s ear)
-
▶
Chronic otitis externa
-
▶
MOE (a large proportion of sufferers are older patients with diabetes, Pseudomonas aeruginosa is the most common organism).
Granulation tissue in the EAC
-
▶
MOE
-
▶
Carcinoma of the ear canal
-
▶
Aspergillus skull-base osteomyelitis.
Treatment
The patient underwent 112 days of therapy (inpatient and ‘hospital in the home’ treatment) which included 3 months of intravenous meropenem and teicoplanin combined with oral fluconazole. Hyperbaric oxygen therapy was also given, and the patient completed a total of 54 treatments.
During this time, the patient developed an ipsilateral grade 5 facial nerve palsy (House–Brackmann) and towards the end of treatment suffered acute renal failure which was thought to be a result of antibiotic-induced interstitial nephritis. The decision was made to cease treatment and the patient remained pain-free on the right side, with a repeat gallium scan showing a satisfactory response to therapy in the right temporal bone.
Outcome and follow-up
At 8-week follow-up, the patient had complete resolution of his infection but his facial nerve palsy had not improved. He remained symptom-free on the right side but complained of new onset left hemicranial pain with tenderness over the left mastoid. Contralateral spread of base of skull infection was suspected but not confirmed with CT imaging. No cause for the new symptoms was identified and it was decided to monitor the patient on a fortnightly outpatient basis.
Six weeks later, the patient complained of worsening left-sided headache and now demonstrated new onset left-sided hearing loss and trismus. He had an elevated erythrocyte sedimentation rate (ESR). Debris was seen in the left EAC and minor serous discharge around the left tympansotomy tube was evident. An audiogram demonstrated new, severe to profound, sensorineural hearing loss.
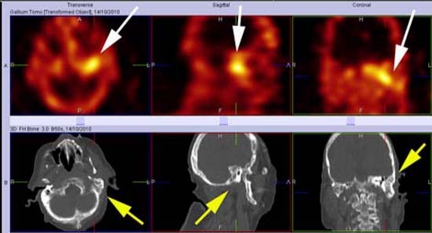
The patient was admitted and re-commenced on intravenous antibiotics. Swabs taken grew P aeruginosa in the left ear. Nuclear imaging revealed a prominent, abnormal focus of activity in the left mastoid and petrous temporal bone that had not been seen on previous studies (figure 1). There was no gallium activity in the right mastoid. Hyperbaric oxygen therapy was repeated and the patient completed an extended course of antibiotic therapy.
Figure 1.
Single photon emission CT (SPECT) imaging of the skull in three planes. The top row of images demonstrates prominent abnormal focal accumulation of gallium activity in the left mastoid and petrous temporal bone (white arrows) which is indicative of recrudescent osteomyelitis. The CT images in the bottom row correlate to the nuclear images above and clearly show the area of anatomy in question (yellow arrows).
The patient’s left-sided osteomyelitis has resolved and there has been no recurrence on the right side. His ESR has returned to normal levels. The patient still has a right-sided, grade 5, facial nerve palsy and significant bilateral hearing loss (figure 2). He presents a therapeutic challenge for future auditory rehabilitation. Hearing aids may contribute to recurrent otitis externa with cochlear implantation being relatively contraindicated.
Figure 2.
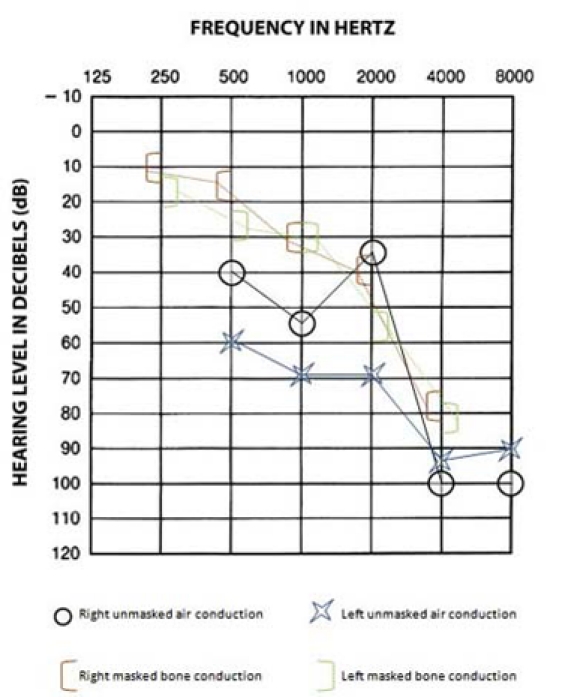
An audiogram showing air and bone conduction. Taken after commencement of treatment for the contralateral, left-sided malignant otitis externa. The audiogram demonstrates dramatic high frequency sensorineural hearing loss in both ears.
Discussion
MOE is an infection that originates in the EAC and progresses from an initial cellulitis, to chondritis, osteitis and finally osteomyelitis.1 Once the bony skull is involved, there is progressive replacement of compact bone with granulation tissue. When this process involves the stylomastoid foramen, facial nerve paralysis occurs. Parotitis and trismus secondary to masseter myositis and involvement of the temporomandibular joint can also occur.2
The diagnosis of MOE should be considered in all diabetic or immunocompromised patients with otitis externa.3 Diabetes remains the most important associated condition with various mechanisms explaining its role, including microangiopathy and enhanced adherence of microorganisms in diabetic patients.4
The causative agent for MOE in most cases is P aeruginosa, which is a gram-negative, obligate aerobic bacillus not normally found in the EAC.1–3 It also commonly causes benign acute otitis externa (swimmer’s ear) and colonises after significant water exposure or minor trauma.3 There have also been reported cases of fungal MOE which is less commonly associated with diabetic patients and most commonly involves the organism Aspergillus fumigatus.2
Patients with MOE typically present with severe otalgia and may complain of aural fullness, otorrhea and hearing loss.1–3 5 The otalgia may be worse at night and the hearing loss is initially conductive in nature. Patients may have a history of recent ear trauma or aural irrigation.2 Examination revealed a swollen and tender EAC with granulation tissue commonly seen on the floor of the canal.2 3 The tympanic membrane may appear normal. Facial nerve paralysis and jugular foramen syndrome are poor prognostic signs.1
A culture of the purulent discharge typically reveals P aeruginosa and sensitivity testing to all antipseudomonas antibiotics should be sought.1 Biopsies of abnormal tissue should be examined to rule out malignancy.2 The ESR is a non-specific test that can be utilised to monitor the response to antibiotic therapy.1 Non-diabetic patients should be evaluated for diabetes.
Radiologic examinations are required to assess the extent and severity of the disease. A CT scan is used initially to evaluate soft tissue involvement and skull-base osteomyelitis.2 It is of limited use for monitoring the response to treatment as the initial demineralisation changes detected at the onset of disease persist despite resolution.1–3 MRI is of limited use in detecting bony changes but is better than CT at identifying soft tissue changes.2 Again, changes initially identified do not resolve with disease resolution so MRI is not utilised to monitor the response to antibiotic therapy. Nuclear imaging has been the mainstay for the diagnosis and follow-up of patients with MOE.3 The bony involvement of early MOE can be detected with a technetium scan (bone scan) and ongoing response to therapy is best monitored with a gallium scan as it is a sensitive indicator of active infection.1 2
Treatment for MOE requires initial hospitalisation for aggressive targeted antibiotic therapy with adjuvant antifungal treatment, if a fungal pathogen is suspected.1 2 Common regimes include combination antipseudomonal and aminoglycoside therapy or monotherapy with Ciprofloxacin or a third generation cephalosporin such as Ceftazidime3 Early debridement of the EAC with cultures should be repeated during the early phase of management.1 Good glycaemic control, monitoring of renal function and drug levels, and periodic hearing tests are also essential.1 Duration of treatment depends on the response as determined by patient symptoms, inflammatory blood markers and nuclear imaging.1 2
Hyperbaric oxygen has been utilised in advanced cases of MOE with significant skull-base involvement with some success.6 It is postulated that elevating the partial pressure of oxygen from hypoxic levels to normal or above normal levels, results in greater oxidative killing of bacteria.3 However, a Cochrane review in 2005, looking at hyperbaric oxygen as an adjuvant treatment for MOE found no trials to demonstrate that the addition of hyperbaric oxygen therapy offers a better outcome than current treatments alone and concluded that further research is still required.7
Most cases of MOE with skull-base osteomyelitis are cured.2 Prognostic indicators of poor outcome include facial paralysis, intracranial extension and involvement of multiple cranial nerves.8 Recurrences can occur up to 1 year after cessation of therapy, so patients should not be considered cured until after that time has elapsed.4
Learning points.
-
▶
Osteomyelitis of the skull base is a complication of MOE.
-
▶
Obtaining a positive culture is difficult if prior treatment has been undertaken.
-
▶
MOE with involvement of the skull base can lead to serious complications such as cranial nerve palsies and hearing loss. Early diagnosis and aggressive antimicrobial treatment are essential.
-
▶
Management of diabetes is essential for a good outcome following treatment for MOE.
-
▶
Recurrences of MOE can occur up to 1 year after cessation of therapy, so a patient should not be considered cured until after that time has elapsed.
-
▶
Monitoring for the side effects of antibiotic treatment is essential.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Snow JB, Ballenger JJ. Ballenger’s Otorhinolaryngology Head and Neck Surgery. Ontario: BC Decker; 2003 [Google Scholar]
- 2.Sreepada GS, Kwartler JA. Skull base osteomyelitis secondary to malignant otitis externa. Curr Opin Otolaryngol Head Neck Surg 2003;11:316–23 [DOI] [PubMed] [Google Scholar]
- 3.Carfrae MJ, Kesser BW. Malignant otitis externa. Otolaryngol Clin North Am 2008;41:537–49, viii–ix [DOI] [PubMed] [Google Scholar]
- 4.Rubin J, Yu VL. Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy. Am J Med 1988;85:391–8 [DOI] [PubMed] [Google Scholar]
- 5.Alva B, Prasad KC, Prasad SC, et al. Temporal bone osteomyelitis and temporoparietal abscess secondary to malignant otitis externa. J Laryngol Otol 2009;123:1288–91 [DOI] [PubMed] [Google Scholar]
- 6.Davis JC, Gates GA, Lerner C, et al. Adjuvant hyperbaric oxygen in malignant external otitis. Arch Otolaryngol Head Neck Surg 1992;118:89–93 [DOI] [PubMed] [Google Scholar]
- 7.Phillips JS, Jones SEM. Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst Rev 2005;2:CD004617. [DOI] [PubMed] [Google Scholar]
- 8.Chen CN, Chen YS, Yeh TH, et al. Outcomes of malignant external otitis: survival vs mortality. Acta Otolaryngol 2010;130:89–94 [DOI] [PubMed] [Google Scholar]