Abstract
Prostatic abscess is rare. Its potentially serious course requires a high level of clinical suspicion and prompt and effective treatment. The causative germs are usually either enterobacteria or Enterococcus. The authors highlight the importance of considering epidemiological and clinical aspects in the early diagnosis and treatment. Prostatic abscess due to community-acquired methicillin resistant Staphylococcus has three typical characteristics: skin entry point, periprostatic compromise, and anaemia and low prothrombin.
Background
Prostatic abscess comprises less than 1% of all prostatic diseases. Its diagnosis is usually difficult and late, since related symptoms are common to other pathologies. Mortality was up to 30% in the pre-antibiotic era, with the most common causative germ being Neisseria gonorrhoeae. Current mortality is 3–16%, with enterobacteria being the most common pathogens, especially Escherichia coli (approximately 70%). The incidence of prostatic abscesses decreased dramatically after the advent of broad-spectrum antibiotics, such as fluoroquinolones and trimethoprim-sulfamethoxazole (TMP-SMX). This case report discusses the importance of considering epidemiological and clinical aspects in the early diagnosis and treatment of prostatic abscess.
In recent years, there has been a substantial increase in the incidence and virulence of infections due to methicillin resistant Staphylococcus aureus (MRSA). Those cases which are community acquired, affecting healthy children and young adults without predisposing risk factors for hospital-acquired infections, are called CA-MRSA.
Most cases of CA-MRSA are mild, predominantly affecting the skin and soft tissues. CA-MRSA infection is an emerging disease worldwide. In Uruguay, CA-MRSA infection was first reported in 2001, followed by a large outbreak in 2004.
Case presentation
A 59-year-old white male developed progressive lower urinary tract symptoms and fever over a period of 30 days. Initially, there was dysuria, frequency and a weak urinary stream. Subsequently, fever of up to 40°C developed, along with chills and malaise, and rectal and perineal pain. The patient also had suppurated lesions in both nasal cavities. Concomitantly, the patient’s wife had suppurated lesions on the left hand and forearm. The patient had associated comorbidities: alcoholism, smoking, chronic bronchitis, dyslipidaemia, insulin-dependent diabetes, ischaemic cardiopathy and intermittent claudication. A family doctor treated the patient with TMP-SMX for a period of 20 days, with partial improvement, although fever and malaise persisted. Therefore, ciprofloxacin 500 mg twice a day was also prescribed. A urine culture at that point was negative.
As the overall condition of the patient worsened over the next 5 days, he was taken to the emergency room, where he presented with hypotension, signs of septic shock and respiratory failure. He was admitted to the intensive care unit, where he received haemodynamic and respiratory support and was empirically treated with imipenem. Blood tests at admission revealed serum creatinine 2.92 mg/dl, glucose 2.53 g/l, alkaline phosphatase 2260 UI/l, prothrombin time 50%, haemoglobin 8.5 g/dl and 33 000 leucocytes/ml. An abdominal ultrasound did not reveal any abnormalities. The patient improved. History confirmed symptoms in the lower urinary tract, with pain in the perineum and pyuria, and digital rectal examination showed a large and painful prostate, with a soft and warm area in the left prostatic lobe. During the third day after admission, bacteriological tests revealed two blood cultures positive for CA-MRSA, which prompted the initiation of culture-specific antibiotics with TMP-SMX 800 mg/160 mg per os twice a day and vancomycin 1 g intravenously twice a day for 10 days.
On the fifth day after admission, a thoraco-abdominal CT was performed. The prostate was of heterogeneous density, with hypodense areas bilaterally and with lack definition of its lateral limits; both seminal vesicles were distended (figure 1 and 2).
Figure 1.
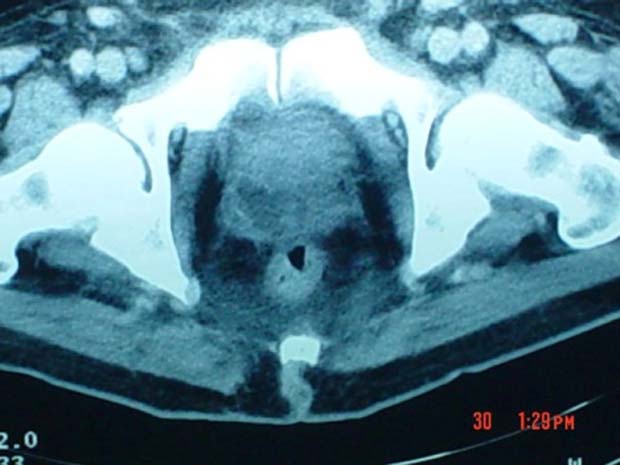
CT image of the prostatic abscess showing a multiloculated intraprostatic collection, with lack of definition of the periprostatic limits and oedema of the anterior rectal wall.
Figure 2.
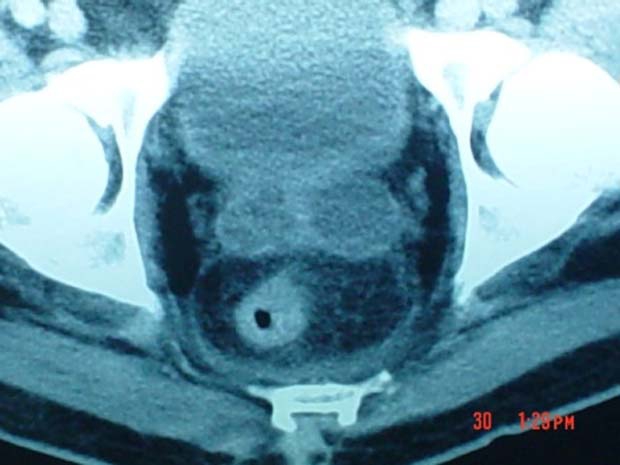
Pelvic CT showing distended seminal vesicles.
A diagnosis of prostatic abscess was made, and there was a strong suspicion that MRSA was the aetiological agent. A transrectal ultrasound was then performed and revealed significant intraparenchymatous collection. An ultrasound-guided needle was used to puncture the main intraparenchymal collections and obtained approximately 20 ml of purulent material, which was sent for further bacteriological studies (figure 3).
Figure 3.
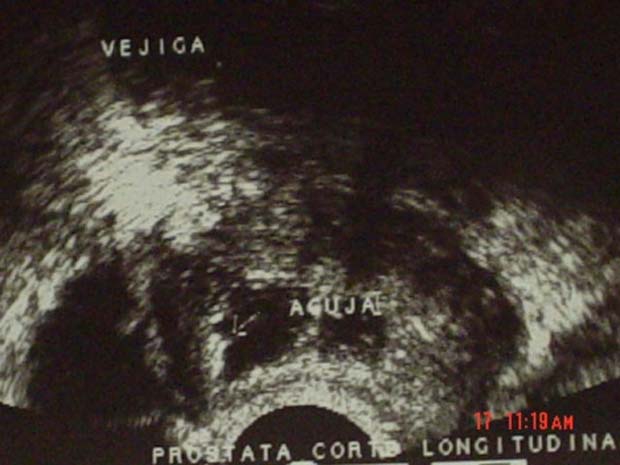
Transrectal ultrasound following drainage of the main intraparenchymal collection.
Cultures of the material revealed a CA-MRSA, which confirmed the highly unusual diagnosis of a urological origin of sepsis caused by this germ. Antibiotic treatment was maintained, and a transoesophageal ultrasound to identify associated bacterial endocarditis was negative. A CT control of the prostatic abscess performed 10 days after drainage showed a significant reduction in the areas of intraprostatic collections, with the persistence of distension of both seminal vesicles. At 25 days after drainage, a transrectal ultrasound revealed recovery of the normal prostatic anatomy, without residual abscesses. The patient was discharged after completion of 6 weeks of antibiotic therapy with TMP-SMX and 30 days of ciprofloxacin.
Discussion
In this patient there was a significant delay before a CT scan was obtained, which should have been performed on admission to the intensive care unit.
Treatment of prostatic abscesses usually consists of the use of broad-spectrum antibiotics active against Gram-negative germs and with good prostatic penetration. Surgical drainage is an option depending on the size of the abscess and on the patient’s response to antibiotic treatment1–3; it is usually accomplished with a transrectal ultrasound-guided needle, and can be performed under local anaesthesia, with low morbidity.2 4 5 Other options, such as a transperineal approach and transurethral resection, are also possible. Open surgical drainage is reserved for failures of the minimally invasive therapies or for severely compromised prostates with predominant extraprostatic components.1 2 6
Immunosuppression (diabetes, chronic renal insufficiency, viral infections) are well-known factors predisposing to prostatic abscesses, as well as a previous history of urethral manipulation or of symptoms of lower urinary tract obstruction.2 In this case, although the patient was relatively young and without pre-existing urological complaints, diabetes was an important risk factor.7 8 So far, there have only been four additional reports in the medical literature describing prostatic compromise by CA-MRSA, in three of which there was also immunosuppression.9–12
Initially, we did not consider CA-MRSA as a possible aetiological agent, due to the rarity of prostatic compromise by this germ. The usual route of transmission of CA-MRSA is person-to-person, from infected individuals with suppurated cutaneous lesions or from asymptomatic carriers of the germ, with the nasal cavities identified as the usual foci of colonisation in roughly 30% of cases.13 Lesions are contagious in infected patients until suppuration, and also in asymptomatic carriers for a variable period of time.7 13 14
We have recently reported a series of 13 patients with retroperitoneal infections due to CA-MRSA in whom suppurated skin lesions were present in 100% of the cases. In this case, once CA-MRSA was identified in blood cultures, the patient was questioned about previous suppurated cutaneous infections, and suppurated lesions were identified in both nasal cavities. The patient’s wife reported having three suppurated skin lesions during the same period.13 We therefore presume that the suppurated skin lesions in the wife and in the patient’s nasal cavities were the sources of the CA-MRSA. Prostatic colonisation by CA-MRSA, although currently rare, might not be so in the near future due to the recently increased prevalence of this germ.
Prostatic abscesses present heterogeneously, and it is often difficult to distinguish them from the much more common prostatitis. Digital rectal examination may reveal an abscess or fluctuation.3 6 15 In a series of 31 prostatic abscesses, the most frequent finding at digital rectal examination was pain, in 55% of cases, whereas fluctuation was present in 29%.
If a clinical suspicion of a prostatic abscess arises, imaging studies such as transrectal ultrasound or CT are required promptly, and transrectal ultrasound is usually the first choice, since it allows for the simultaneous needle drainage of prostatic collections.4 16 Pelvic CT is usually better for the evaluation of extraprostatic collections.7
Another characteristic of invasive CA-MRSA infections is abnormal laboratory tests suggesting anaemia or hypoprothrombinaemia. As described in retroperitoneal CA-MRSA infections,7 this patient also presented with these findings.
Regarding antibiotic sensitivity, CA-MRSA is usually 100% sensitive in vitro to TMP-SMX (as seen in this patient), vancomycin, gentamycin and quinolones such as gatifloxacin and moxifloxacin.
The medical treatment initially adopted was administration of TMP-SMX, but the patient’s immunocompromise led to rapid evolution to prostatic abscesses and to progression of the infection. The change to a more specific antibiotic regimen, and the transrectal drainage of the prostatic collections were useful in the successful treatment of this patient.
Learning points.
-
▶
This report highlights specific aspects of prostatic infection by CA-MRSA.
-
▶
The epidemiology should be considered when searching for the aetiological agent of a severe genitourinary infection.
-
▶
Three characteristics should be given special consideration: (1) previous suppurated skin lesions in the patient or close contacts, (2) a tendency towards periorgan extension and multiple foci, and (3) anaemia and hypoprothrombinaemia, which are typically present in CA-MRSA invasive infections.
-
▶
Attention to these characteristics may result in a higher diagnostic yield of these infections, and to the prompt institution of specific treatments, reducing the associated morbidity and mortality.
Footnotes
Competing interests None.
Patient consent Not obtained.
References
- 1.Bosquet SM, Gimeno AV, Palmero MJL, et al. Absceso prostático: Revisión de la literatura y presentación de un caso. Actas Urol Esp 2005;29:100–4 [DOI] [PubMed] [Google Scholar]
- 2.Collado A, Palou J, García-Penit J, et al. Ultrasound-guided needle aspiration in prostatic abscess. Urology 1999;53:548–52 [DOI] [PubMed] [Google Scholar]
- 3.Ludwig M, Schroeder-Printzen I, Schiefer HG, et al. Diagnosis and therapeutic management of 18 patients with prostatic abscess. Urology 1999;53:340–5 [DOI] [PubMed] [Google Scholar]
- 4.Oliveira P, Andrade JA, Porto HC, et al. Diagnosis and treatment of prostatic abscess. Int Braz J Urol 2003;29:30–4 [DOI] [PubMed] [Google Scholar]
- 5.Gögüs C, Ozden E, Karaboga R, et al. The value of transrectal ultrasound guided needle aspiration in treatment of prostatic abscess. Eur J Radiol 2004;52:94–8 [DOI] [PubMed] [Google Scholar]
- 6.Granados EA, Riley G, Salvador J, et al. Prostatic abscess: diagnosis and treatment. J Urol 1992;148:80–2 [DOI] [PubMed] [Google Scholar]
- 7.Abreu DA, Osorio F, Guido LG, et al. Retroperitoneal infections by community acquired methicillin resistant Staphylococcus aureus. J Urol 2008;179:172–6 [DOI] [PubMed] [Google Scholar]
- 8.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerging Infect Dis 2003;9:978–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker SD, Horger DC, Keane TE. Community-acquired methicillin-resistant Staphylococcus aureus prostatic abscess. Urology 2004;64:808–10 [DOI] [PubMed] [Google Scholar]
- 10.Shindel AW, Darcy MD, Brandes SB. Management of prostatic abscess with community-acquired methicillin-resistant Staphylococcus aureus after straddle injury to the urethra. J Trauma 2006;61:219–21 [DOI] [PubMed] [Google Scholar]
- 11.Pierce JR, Jr, Saeed Q, Davis WR. Prostatic abscess due to community-acquired methicillin-resistant Staphylococcus aureus. Am J Med Sci 2008;335:154–6 [DOI] [PubMed] [Google Scholar]
- 12.Lin MY, Rezai K, Schwartz DN. Septic pulmonary emboli and bacteremia associated with deep tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2008;46:1553–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guía para el manejo global de las infecciones por Staphilococcus aureus meticilino resistente de origen comunitario(SAMR-com). Ministerio de Salud Publica, Uruguay, Agosto de 2004. http://www.msp.gub.uy (accessed 25 October 2004). [Google Scholar]
- 14.Ma XX, Galiana A, Pedreira W, et al. Community-acquired methicillin-resistant Staphylococcus aureus, Uruguay. Emerging Infect Dis 2005;11:973–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberger M, Cytron S, Servadio C, et al. Prostatic abscess in the antibiotic era. Rev Infect Dis 1988;10:239–49 [DOI] [PubMed] [Google Scholar]
- 16.Barozzi L, Pavlica P, Menchi I, et al. Prostatic abscess: diagnosis and treatment. AJR Am J Roentgenol 1998;170:753–7 [DOI] [PubMed] [Google Scholar]


