Abstract
Integrin α3A cytoplasmic tail phosphorylation was mapped to amino acid S1042, as determined by mass spectrometry, and confirmed by mutagenesis. This residue occurs within a “QPSXXE” motif conserved in multiple α chains (α3A, α6A, α7A), from multiple species. Phosphorylation of α3A and α6A did not appear to be directly mediated by protein kinase C (PKC) α, β, γ, δ, ε, ζ, or μ, or by any of several other known serine kinases, although PKC has an indirect role in promoting phosphorylation. A S1042A mutation did not affect α3-Chinese hamster ovary (CHO) cell adhesion to laminin-5, but did alter 1) α3-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin (in the presence or absence of phorbol 12-myristate 13 acetate stimulation), and p130CAS (in the absence of phorbol 12-myristate 13 acetate stimulation), 2) the shape of cells spread on laminin-5, and 3) α3-dependent random CHO cell migration on laminin-5. In addition, S1042A mutation altered the PKC-dependent, ligand-dependent subcellular distribution of α3 and F-actin in CHO cells. Together, the results demonstrate clearly that α3A phosphorylation is functionally relevant. In addition, the results strongly suggest that α3 phosphorylation may regulate α3 integrin interaction with the cytoskeleton.
INTRODUCTION
Adhesion receptors in the integrin family regulate many central aspects of cell biology, including cell shape, migration, signaling, cell cycle progression, and apoptosis (Ruoslahti and Reed, 1994; Schwartz et al., 1995; Giancotti, 1997; Sheetz et al., 1998; Boudreau and Jones, 1999; Sanchez-Madrid and del Pozo, 1999). Tyrosine, serine, and threonine residues within integrin β chain cytoplasmic domains may become phosphorylated (Sastry and Horwitz, 1993; Hemler et al., 1994), and this may play a critical role during integrin adhesion, distribution, and signaling functions (Chen et al., 1994; Johansson et al., 1994; Van Nhieu et al., 1996; Blystone et al., 1997; Jenkins et al., 1998).
Integrin α chains, including αL, αM, αX, α3A, and α6A also become phosphorylated, mostly on serine (Chatila et al., 1989; Buyon et al., 1990; Valmu et al., 1991; Pardi et al., 1992; Dumont and Bitonti, 1994), but the significance has not yet been demonstrated. Activation of protein kinase C (PKC), often as a consequence of phorbol ester stimulation, influences the adhesion and spreading activity of many integrins (Wright and Meyer, 1986; Shattil and Brass, 1987; Shimizu et al., 1990; Vuori and Ruoslahti, 1993; Lewis et al., 1996). Adhesion mediated by α6A and α3A integrins is stimulated upon cell treatment with phorbol ester, and correlates with increased phosphorylation of those subunits (Shaw et al., 1990; Hogervorst et al., 1993a; de Melker et al., 1997). However, upon mutation of a critical serine in the α6A tail (occurring within the “QPSXXE” region), there was no loss of cell adhesion (Hogervorst et al., 1993b; Shaw and Mercurio, 1993). Likewise, replacement of α6A or α3A tails with nonphosphorylated α6B or α3B tails had no effect on cell adhesion (Shaw et al., 1993; Delwel et al., 1993; de Melker et al., 1997), indicating again that phosphorylation may not be required. Thus, phosphorylation of the α6A and α3A tails, resulting from PKC activation, has no obvious effect on inside-out integrin signaling.
The α3β1, α6β1, and α6β4 integrins are receptors for various forms of laminin (Sonnenberg et al., 1988; Lee et al., 1992; Eble et al., 1998), and may also recognize other ligands (Hynes, 1992; Chen et al., 1999). In addition, these receptors participate in transdominant inhibition of other integrins (Hodivala-Dilke et al., 1998), phagocytosis (Gresham et al., 1996; Coopman et al., 1996), cell fusion (Ohta et al., 1994), hemidesmosome formation (Jones et al., 1991), and signaling (Mainiero et al., 1997; Wei et al., 1998). For both α6 and α3, alternative splicing within cytoplasmic domains gives rise to A and B isoforms, with the B isoforms (α6B, α3B) having much more limited tissue distributions (Hogervorst et al., 1993a; de Melker et al., 1997). This alternative splicing does not affect α3 or α6 ligand binding specificity.
Here we have used mass spectrometry, and α3 mutagenesis to determine that the α3A cytoplasmic tail is phosphorylated on serine 1042. Because this serine occurs within a QPSXXE motif conserved in multiple integrins and in all animal species tested, we hypothesized that the α3A phosphorylation event should be functionally relevant. Supporting this hypothesis, loss of phosphorylation in the α3 S1042A mutant corresponded to alterations in α3 integrin-dependent signaling, morphology, motility, and in α3 subcellular localization. The results obtained show for the first time that phosphorylation of an integrin α chain “QPSXXE” site indeed can be functionally relevant, and suggest that this phosphorylation may regulate cytoskeletal organization.
MATERIALS AND METHODS
Antibodies
Anti-integrin monoclonal antibodies (mAbs) used were anti-α2 integrin, A2-IIE10 (Bergelson et al., 1994); anti-α3, A3-IVA5, A3-X8, and A3-IIF5 (Weitzman et al., 1993); anti-α4, A4-PUJ1 (Pujades et al., 1996); anti-α5, A5-PUJ2 (Pujades et al., 1996); anti-hamster α5β1, PB1 (Brown and Juliano, 1985); anti-α6, A6-ELE (Lee et al., 1995); and anti-hamster β1, 7E2 (Brown and Juliano, 1988). mAbs to paxillin and p130CAS were obtained from Transduction Laboratories (Lexington, KY). Anti-phosphotyrosine mAb 4G10 was from Upstate Biotechnology (Lake Placid, NY), and anti-focal adhesion kinase (FAK) mAb was from Santa Cruz Biotechnology (Santa Cruz, CA). Other mAbs were anti-CD3, OKT3 (American Type Cell Culture, Rockville, MD); anti-CD98, 6B12 (Kolesnikova, Mannion, Berditchevski, and Hemler, unpublished data); and anti-MHC class I, W6/32. Rabbit polyclonal antibody to the integrin α3 tail was prepared against a peptide (CRQKAEMKSQPSETERLTDDY) coupled through cysteine to carrier protein (keyhole limpet hemocyanin) as previously described (Chan et al., 1991). Polyclonal anti-PKCα was from Santa Cruz Biotechnology. Polyclonal rhodamine-conjugated goat anti-mouse secondary antibodies (for immunofluorescence staining) were from Biosource International (Camarillo, CA). Goat anti-mouse IgG (for cell surface antibody cross-linking) was from Boehringer-Mannheim (Indianapolis, IN). Horseradish peroxidase-conjugated anti-mouse or -rabbit IgG was from Sigma (St. Louis, MO).
Synthetic Peptides, Enzymes, and Kinase Inhibitors
Peptides corresponding to the carboxyl terminus of α3 (RTRALYEAKRQKAEMKSQPSETERLTDDY) and α6 (KKDHYDATYHKAEIHAQPSDKERLTSDA) were synthesized, high pressure liquid chromatography-purified, and masses were verified at the Dana-Farber Cancer Institute molecular biology core facility. Other synthetic peptides include PKC substrate, MARCKS psd peptide (BIOMOL Research Laboratories, Plymouth Meeting, PA); PKCμ substrate, Syntide 2 peptide (Calbiochem-Novabiochem, La Jolla, CA); Ca2+/calmodulin-dependent kinase II (CamKII) substrate, Autocamitide 3 (Life Technologies, Bethesda, MD); glycogen synthase kinase 3 substrate, cAMP response element-binding protein phosphopeptide (New England BioLabs, Beverly, MA); mitogen-activated protein (MAP) kinase substrate, EGFR T669 peptide (Calbiochem-Novabiochem); and casein kinase 2 substrate peptide (Upstate Biotechnology).
Also used were rat brain PKC, containing PKCα, β1, and γ isoforms (Boehringer-Mannheim); recombinant human PKCα, β, ε, μ, and ζ (Calbiochem-Novabiochem); p42 MAP kinase (Erk2), CamKII, and glycogen synthase kinase 3 (New England Biolabs); casein kinase II (Boehringer-Mannheim); and integrin-linked kinase (Dr. Cary Wu, University of Pittsburgh, Pittsburgh, PA). Kinase inhibitors used in this study were PMA (Sigma), chelerythrine chloride (Alexis, San Diego, CA), Go6976 (BIOMOL Research Laboratories), staurosporine (Sigma), KN-62 (Seikagaku America, Rockville, MD), and H8 (Calbiochem-Novabiochem).
Integrin Transfectants and Mutants
K562 cells expressing comparably high levels of wild-type integrin α2, α3, α4, and α6 subunits were described previously (Bazzoni et al., 1998). Integrin cDNAs were ligated into the pFneo expression vector, containing a neomycin selection marker. Transfected cells were cultured in RPMI 1640 media, with 10% fetal calf serum (FCS) and 1 mg/ml G418.
Integrin α3 cytoplasmic domain point mutants were generated by using polymerase chain reaction methodology, and confirmed by nucleic acid sequencing. After electroporation, G418-resistant Chinese hamster ovary (CHO)-α3 and K562-α3 transfectants were sorted by flow cytometry for high expression, and stable transfectants were maintained in minimal essential (MEM)α+ medium with 10% FCS and G418 (1 mg/ml). NIH3T3 cells stably transfected to express the Trio TGD1 and TGD2 domains as previously described (Seipel et al., 1999). For flow cytometry, cells were incubated with negative control or specific antibody, washed three times, and then stained with fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody. Stained cells were analyzed using a Coulter EPICS XL flow cytometer (Beckman, Coulter Inc., Fullerton, CA).
Cell Radiolabeling and Immunoprecipitation
For 32P-labeling (at ∼1 mCi/107 cells), cells were grown for 3 h or overnight in phosphate-deficient media containing 10% dialyzed fetal calf serum, supplemented with [32P]orthophosphate (NEN Bioscience, Boston, MA). For 35S-labeling (at 1 mCi/5 × 107 cells) cells were grown overnight in methionine- and cysteine-deficient media, containing 10% dialyzed fetal calf serum, supplemented with a mixture of [35S]methionine and [35S]cysteine (NEN Bioscience). Cells were then lysed (in 1% Triton X-100, 25 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, and 10 μg/ml leupeptin, 2 mM sodium vanadate, 2 mM sodium fluoride) for 1 h at 4°C, and insoluble material was pelleted at 12,000 × g for 10 min (Mannion et al., 1996). Proteins were isolated by immunoprecipitation (Berditchevski et al., 1995) by using specific mAb and protein A-Sepharose beads and then were analyzed by SDS-PAGE under nonreducing conditions.
Mass Spectrometry
K562-α3 cells (1 × 109) were treated with either 100 nM PMA in dimethyl sulfoxide or dimethyl sulfoxide alone at 37°C for 30 min, and then lysed at 4°C for 60 min in 1% Triton X-100 lysis buffer (containing 20 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 40 μg/ml leupeptin, 2 mM sodium vanadate, and 2 mM sodium fluoride). After preincubation with protein A-Sepharose and irrelevant Ig-Sepharose beads (to remove nonspecific binding material), the lysate was incubated with mAb A3-IVA5-conjugated Sepharose beads at 4°C for 3 h. After washing, the integrin α3 subunit was eluted by using 50 mM glycine pH 3.0, and neutralized with 0.2 volume of 1 M Tris-HCl, pH 9.0. Following SDS-PAGE under reducing conditions, the 30-kDa α3 light chain protein was visualized by silver staining, excised, and subjected to in-gel trypsin digestion (Williams et al., 1997). Mass analysis, by the matrix-assisted laser desorption ionization-time of flight technique, was carried out using a Voyager DE-STR (Applied Biosystems, Foster City, CA) in reflectron mode. For further analysis of a specific phosphopeptide, the specific gated ion was subjected to post source decay analysis, and the fragment ion masses obtained were compared with predicted y ion and b ion patterns.
In Vitro Protein Kinase Assays
In vitro phosphorylation by PKCμ was assayed as described (Jamora et al., 1999). Other reactions were carried out according to the manufacturer's protocols, in 20-μl volumes, at 30°C for 15 min. For example, reaction mixtures for classical PKCs contained 20 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 0.5 mM CaCl2, 0.25% bovine serum albumin, 100 μg/ml phosphatidylserine (Sigma), 20 μg/ml PMA, 400 μg/ml peptide, 0.25 mU/ml rat brain or recombinant human PKCs, and 5 μCi [γ-32P]ATP. All reactions were stopped by adding 2× Laemmli sample buffer, and reaction products were fractionated on 10–20% Tris-Tricine acrylamide gradient gels (Bio-Rad, Hercules, CA) under reducing conditions. Additional assays of AKT, aurora, and PDK1 kinases were carried out with the assistance of Dr. Patricia McCaffrey, Vertex Pharmaceuticals, Cambridge, MA.
Immunofluorescence and Confocal Microscopy
Circular glass coverslips (12 mm; Fisher Scientific, Pittsburgh, PA) were coated with extracellular matrix proteins (fibronectin in 10 mM NaHCO3, or laminin-5 in phosphate-buffered saline (PBS) containing 0.005% Tween-20) at 4°C overnight. Cells were harvested in PBS with 2 mM EDTA, washed once in serum-free media, and then allowed to spread on coverslips for various times at 37°C in 10% CO2. For some experiments, PMA (at 100 nM) was present during the last 20 min of incubation. Cells were then rinsed in PBS, fixed in PBS containing 3% paraformaldehyde for 10 min, and permeabilized by using 0.1% Brij 99 in PBS for 2 min at 25°C. Nonspecific sites were blocked with 20% goat serum in PBS for 1 h at 25°C or overnight at 4°C. Primary mAbs (1 μg/ml final) were diluted in PBS containing 20% goat serum and incubated with cells for 1 h at 25°C. Coverslips were washed four times with PBS, and then incubated for 30 min with rhodamine-conjugated goat anti-mouse IgG (Biosource International). Finally, coverslips were washed four times with PBS, mounted on glass slides in FluoroSave reagent (Calbiochem), and photographed within 3 d by using a Axioskop fluorescent microscope (Zeiss, Oberkochen, Germany) at 100× magnification.
Confocal microscopy was carried out by using a Zeiss model LSM4 cofocal laser scanning microscope equipped with an external argon-krypton laser (488 and 568 nm). To evalute the fluorescence distribution of F-actin and α3 integrin, horizontal and vertical optical sections were taken at the center of representative cells. Images of 512 × 512 pixels were digitally recorded within 2s and 2x line averaging and printed with a Fujix Pictrography color printer (Fuji, Japan), by using Adobe Photoshop software (Adobe Systems, Mountain View, CA).
Time-Lapse Videomicroscopy
For each sample, an acid-washed glass coverslip was affixed to a 60-mm Petri dish, covering a 12-mm hole. Coverslips were coated overnight at 4°C with either 2 μg/ml rat laminin-5 diluted in PBS containing 0.005% Tween-20 or 2 μg/ml human plasma fibronectin (Collaborative Biomedical Products, Bedford, MA) diluted in 10 mM sodium bicarbonate. The coverslips were then washed three times with MEMα+ medium. Immediately before image acquisition, CHO transfectants were detached with 2 mM EDTA in PBS, washed once with PBS, and plated onto coverslips in serum-free MEMα+ medium containing 100 nM PMA.
Images were acquired by using a Zeiss Axiovert 135 microscope and a video microscope as described (Stipp and Hemler, 2000). Images were captured every 2 min for 2 h, as cells were maintained in a humidified, 37°C, 10% CO2 environment in a custom-built stage incubator. For migration rate determinations, outlines of cells (migrating on the substrate rather than along neighboring cells) were traced using the Scion Image freehand tool, x and y centers were calculated, and the distance moved was determined. For preparation of a video of migrating cells, 50 stacked images (taken at 2-min intervals), were merged using the Scion Image 1.62 program.
Quantitation of Cell Shape by Using Digital Image Analysis
For cell morphology quantitation, cell images were acquired as described for video microscopy, and analyzed by using the Scion Image software (Image 1.62). The periphery of individual cells was traced by using the software's freehand drawing tool, and cell perimeter and actual cell areas were calculated. Then as described previously (Szabo et al., 1995), the deviation of each cell from perfect roundness was calculated by dividing the theoretical maximum area for a given perimeter (perimeter2/4π) by the observed pixel area. The value for a perfectly round cell equals 1.0, and larger values represent increasing levels of deviation from roundness.
RESULTS
Integrin α3A Tail Phosphorylation Requires Serine 1042
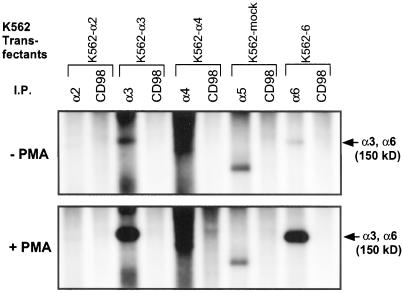
Metabolic 32P-labeling of unstimulated K562-α3 and K562-α6 cells, followed by immunoprecipitation, revealed low-level constitutive phosphorylation of the integrin α3A and α6A subunits (Figure 1, top). Upon cell stimulation with 100 nM PMA (phorbol 12-myristate 13-acetate), α3A and α6A became strongly phosphorylated (Figure 1, bottom). No phosphorylation corresponding to integrin chains (140–150-kDa range) was obtained from α2 or α5 integrins, or from CD98 control immunoprecipitations.
Figure 1.
Phosphorylation of integrin α3A and α6A chains. K562 transfectants, with or without 100 nM PMA stimulation, were labeled with 32P, lysed in 1% Triton X-100, and then immunoprecipitated with relevant anti-integrin α chain mAb, or with anti-CD98 control mAb 6B12. The identity of the labeled protein of ∼100 kDa coimmunoprecipitated with α5 is unknown. The smear of labeling in the α4 lane did not resolve into discrete bands at shorter film exposure times.
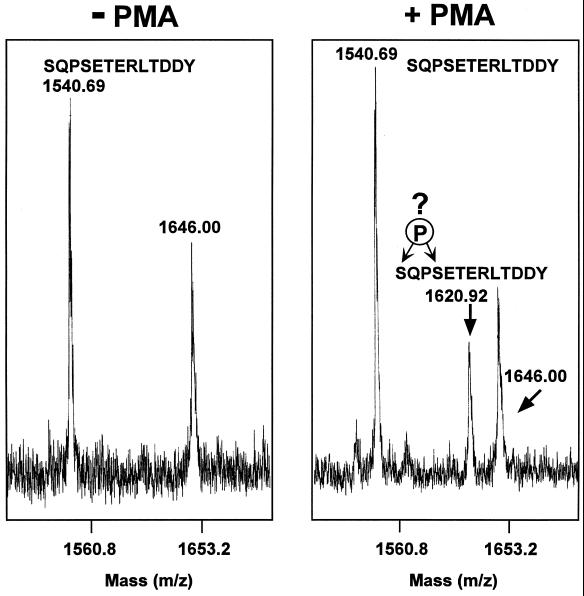
To determine the site of α3A cytoplasmic tail phosphorylation, K562-α3 cells were treated with or without PMA, the ∼30-kDa reduced α3A light chain was isolated, purified by SDS-PAGE under reducing conditions, and then trypsinized fragments were subjected to mass spectrometry analysis. Following PMA treatment, a major peak of 1620.92 m/z was obtained, exactly corresponding to monophosphorylated α3-derived “SQPESETERLTDDY” peptide (Figure 2, right). A peak corresponding to unphosphorylated peptide (1540.69 m/z) was also present. Considering that phosphorylated peptides are typically recovered with markedly lower efficiency, the relative intensity of the unphosphorylated and phosphorylated peaks indicates a substantial level of phosphorylation. Without PMA stimulation, only the unphosphorylated peptide was observed (Figure 2, left). None of the other α3-derived peptides appeared to be phosphorylated, and no peaks corresponding to di- or triphosphorylated “SQPSETERLTDDY” peptide were obtained (our unpublished results). Previous studies, in two different cell lines, showed that PMA-induced phosphorylation of α3β1 occurs almost exclusively on serine (Hogervorst et al., 1993a; Dumont and Bitonti, 1994). Thus, we assume that phosphorylation should occur on one of the two serines present in the 1620.92 m/z peptide (Figure 2, right).
Figure 2.
Identification of a monophosphorylated α3 peptide. The light chain of α3 was isolated from K562-α3 cells, digested with trypsin, and the resulting peptides were analyzed by mass spectrometry. In the absence of cell treatment with 100 nM PMA, a peptide of 1540.69 m/z was obtained. In the presence of PMA treatment, α3 yielded an additional peptide of 1620.92 that exactly corresponds to the predicted size of monophosphorylated SQPSETERLTDDY peptide. The peak of 1646.00 corresponds to a background peptide, not derived from the α3 subunit.
To identify the specific phosphorylated residue, the gated ion of 1620.92 m/z was subjected to post source decay fragmentation analysis (Table 1). Fragments with m/z corresponding to the indicated y10, y11, y13, b2, b3, and b4 ions are entirely consistent with phosphorylation occurring on serine 1042, at the y10/b4 position. The results are not consistent with phosphorylation of serine or threonine at any other position in the SQPSETERLTDDY peptide.
Table 1.
Fragmentation results for α3-derived phosphopeptide of 1620.9 mass
| Residue | y ions present
|
b ions present | |
|---|---|---|---|
| y ion | y - H3PO4 | ||
| y13 S b1 | 1620.6 | 1522.7 | — |
| y12 Q b2 | — | — | 216.1 |
| y11 P b3 | 1405.6 | (1307.6) | (313.2) |
| y10 pS b4 | 1308.5* | (1210.5) | (480.2) |
| y9 E b5 | 1141.5 | N/A | — |
| y8 T b6 | 1012.5 | N/A | — |
Gated ion of 1620.9, corresponding to phosphopeptide, was subjected to post source decay analysis. Fragment ions obtained are indicated either without parentheses (definitive) or with brackets (less definitive).
—, fragment peaks that could not be distinguished.
N/A, not applicable.
The 1308.5 peak is particularly intense.
None of the fragment masses underlined should appear if the serine at the y13-b1 position was phosphorylated instead of the serine at y10-b4.
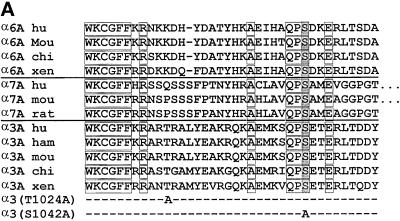
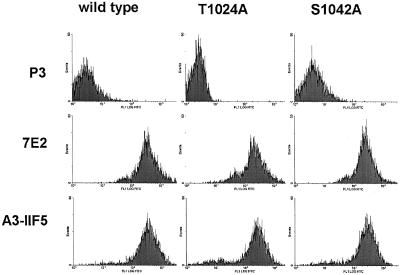
Alpha3 S1042 occurs within a highly conserved QPSXXE motif, and is the only serine or threonine residue conserved among the α6A, α7A, and α3A tails from multiple species (Figure 3A). Serine phosphorylation of the α6A tail also may occur within the conserved QPSXXE motif (Hogervorst et al., 1993b; Shaw and Mercurio, 1993). To confirm that α3 serine 1042 is critical for phosphorylation, an S1042A mutation was prepared, in addition to a control T1024A mutation (Figure 3A, bottom). The S1042A-α3, T1024A-α3, and wild-type α3A subunits were stably expressed at comparable levels in CHO cells as seen by flow cytometry (Figure 4A) and by Western blotting (Figure 4B), and in K562 cells as determined by flow cytometry (our unpublished results). Metabolic 32P labeling established that integrin α3 phosphorylation was indeed lost in the S1042A mutant, but not in wild-type α3 or T1024A-α3 cells (Figure 3B). No phosphorylation of hamster α5β1 was seen in CHO cell control lanes, and no phosphorylation of CD98 was seen K562 cell control lanes.
Figure 3.
Confirmation of the α3A phosphorylation site. (A) For α6A, α7A, and α3A tail sequences from multiple species, conserved residues are boxed, and the putative serine phosphorylation site is shaded. This site, within a shared “QPSXXE” motif, was previously suggested to be the α6A phosphorylation site (Hogervorst et al., 1993b). Point mutations in the α3A tail (T1024A, S1042A) are also indicated. (B, top). CHO cells and K562 cells expressing either human wild-type α3, or mutated α3 (T1024A or S1042A) were treated with 100 nM PMA, labeled with 32P, and then lysed. Immunoprecipitations of human α3 were carried out by using mAb A3-X8; hamster α5β1 was precipitated with mAb PB1, and human CD98 was precipitated by using mAb 6B12. (B, bottom) CHO and K562 transfectants were also labeled with [35S]methionine, and immunoprecipitates were carried out by using the same antibodies.
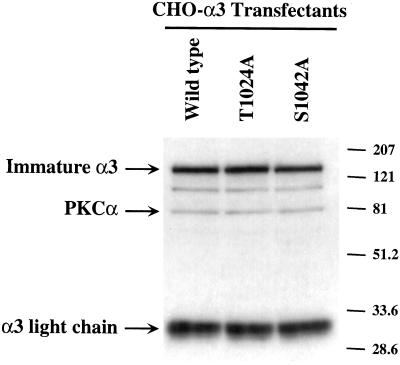
Figure 4.
Expression of wild-type and mutant α3 integrins in CHO cells. (A) α3-transfected CHO cells were detached, and then incubated with either negative control mAb P3, mAb to human integrin α3 (A3-IIF5), or mAb to hamster β1 (7E2), and then analyzed by flow cytometry. Mean fluorescence intensity (MFI values) for wild-type, T1024A, and S1042A-α3 were 28, 22, and 25, respectively. (B) From equivalent amounts of CHO cell lysate (lysis in 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS), integrin α3 subunit was immunoprecipitated (by using mAb A3-IIF5), resolved by SDS-PAGE under reducing conditions, and then Western blotting was carried out by using a mixture of polyclonal antibodies to the α3 cytoplasmic tail, and to PKCα.
What Kinase Phosphorylates the QPSXXE Site?
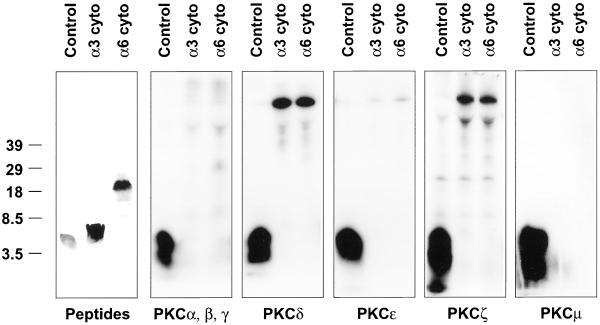
Stimulation of α3 and α6 phosphorylation by the phorbol ester PMA suggests a role for PKC. Indeed, it was previously suggested that several PKC isoforms (α, β, γ, δ, ε) may directly phosphorylate the HAQPSDKER site in α6A (Gimond et al., 1995). However, study of preferred PKC phosphorylation motifs (Woodgett et al., 1986; Nishikawa et al., 1997) revealed that the α6A site lacks potentially important basic residues in the −2, −3, −4, and +3 positions, and contains an unfavorable acidic residue in the +1 and +3 positions. Also, the α3A “KSQPSETER” sequence is an especially unlikely PKC site, because it lacks basic residues at the −2, −3, +2, and +3 positions, while containing unfavorable acidic residues at the +1 and +3 positions. To investigate experimentally whether PKC might directly mediate phosphorylation, α3 peptide (RTRALYEAKRQKAEMKSQPSETERLTDDY) and α6 peptide (KKDHYDATYHKAEIHAQPSDKERLTSDA) were tested for in vitro phosphorylation (Figure 5). Little phosphorylation of the α3A or α6A peptides was observed for any of the PKC isozymes tested, whereas positive control peptides were well phosphorylated. Also, we attempted to duplicate exactly the conditions in which PKC-mediated α6A phosphorylation was previously observed (Gimond et al., 1995), but again we saw no α6A or α3A peptide phosphorylation (our unpublished results). In other in vitro kinase assays, the α3A and α6A peptides were not phosphorylated by casein kinase II, calmodulin-dependent kinase II, integrin-linked kinase, glycogen synthase kinase 3, MAP kinase (ERK2), AKT, aurora, or PDK1. Thus, the serine kinase directly responsible for mediating α3 and α6 phosphorylation remains to be identified.
Figure 5.
PKC fails to phosphorylate α3A or α6A peptides. Peptides corresponding to the α3 and α6 cytoplasmic tails were subjected to in vitro kinase assays, and 32P-labeled peptides were resolved by acrylamide gels. A relevant positive control peptide was included for each enzyme. The left panel shows the positions of α3 and α6 peptides, as determined by Coomassie blue staining. Although phosphorylated background material appeared in a few lanes, the PKC assays produced no phosphorylated material comigrating with either the α3 or α6 peptides.
It is assumed that PKC must be involved in α3A and α6A phosphorylation because PMA stimulation of this phosphorylation has been seen in many studies, and inhibitors of PKC abolished PMA-induced α6A phosphorylation (Tentori et al., 1995). Confirming the involvement of PKC, PMA-induced phosphorylation of both α3 and α6 was substantially inhibited by PKC inhibitors chelerythrine (10 μM), Go6976 (0.5 μM), calphostin C (2.5 μM), and the PKA/PKC inhibitor staurosporine (2.5 μM), but not by inhibitors of protein tyrosine kinase (genestein, 25 μg/ml), PKA (H8, 2.5 μM), CamKII (KN62, 10 μM), or PI3-kinase (wortmannin, 100 nM) (our unpublished results).
Functional Relevance of α3 S1042 Phosphorylation: Cell Adhesion, Integrin Signaling, and Cell Morphology
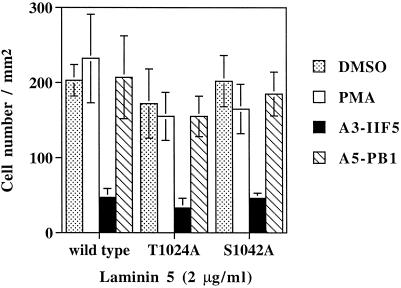
Before proceeding to studies of “outside-in” signaling through α3 integrin, we first needed to know whether inside-out signaling would be altered, leading to changes in cell adhesion. CHO cells expressing wild-type α3, T1024A-α3, and S1042-α3 all showed similar levels of adhesion to surfaces coated with laminin-5, either in the presence or absence of PMA (Figure 6). Inhibition by anti-α3 mAb A3-IIF5 confirmed that adhesion was mostly due to transfected human α3 integrin. As expected, anti-hamster α5β1 mAb (PB1) had no effect on cell adhesion to laminin-5. These results are consistent with previous results, in which loss of α3A or α6A phosphorylation sites (due to serine mutations, or cytoplasmic tail exchanges) had no effect on cell adhesion mediated by α3A or α6A integrins (Hogervorst et al., 1993b; Shaw and Mercurio, 1993; Shaw et al., 1993; Delwel et al., 1993; de Melker et al., 1997).
Figure 6.
Cell adhesion of CHO-α3 transfectants. Cells were tested for adhesion to laminin-5 (coated at 2 μg/ml), and attached cells were analyzed by using the Cytofluor 2300 measurement system (Millipore, Bedford, MA) as previously described (Chan et al., 1992; Bazzoni et al., 1995). Cells were untreated, incubated with 100 nM PMA at the start of the assay, or were preincubated with either antihuman α3 (A3-IIF5) or anti-hamster α5 (PB1) mAbs at 4°C for 30 min before the adhesion assay. Background binding (assessed by using bovine serum albumin-coated wells) was typically less than 5% of the total and was subtracted from experimental values. Results are reported as mean ± SD of triplicate determinations. This experiment was repeated multiple times, with each experiment yielding no significant differences between mutant and wild-type α3 transfectants.
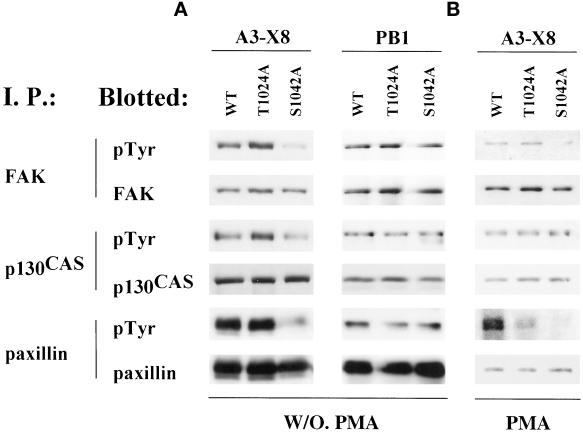
Integrin α chain cytoplasmic tails often may regulate integrin-dependent “outside-in” signaling (Shaw et al., 1995; Wei et al., 1998). To analyze the potential role of α3 S1042 phosphorylation during α3 integrin signaling, unstimulated CHO-α3 transfectants were plated on immobilized anti-α3 A3-X8 antibody. In CHO cells, the S1042A mutant, compared with wild type α3 or the T1024A mutant, showed diminished tyrosine phosphorylation of FAK, p130CAS, and paxillin (Figure 7A). No differences in tyrosine phosphorylation of FAK, p130CAS, and paxillin were seen when cells were plated on surfaces coated with mAb PB1, to engage the hamster α5β1 integrin (Figure 7A, right). Also, PMA-stimulated CHO cells were plated on immobilized anti-α3 antibody (Figure 7B), and again, the S1042A mutant cells showed diminished tyrosine phosphorylation of FAK and paxillin. However, tyrosine phosphorylation of p130CAS was not reduced in Figure 7B. In the absence of α3 integrin engagement, stimulation of CHO cells in suspension by PMA alone was sufficient to induce markedly elevated tyrosine phosphorylation of p130CAS (our unpublished results). Thus, α3-dependent stimulation of p130CAS phosphorylation is likely to be obscured by the overriding effects of PMA. In other experiments, engagement of α3 integrin (by using mAb A3-X8) caused no fluctuations in c-Src tyrosine phosphorylation or in MAP kinase activity (our unpublished results). Thus, alterations in protein tyrosine phosphorylation were selective rather than global.
Figure 7.
Signaling through wild-type and mutant integrin α3. (A) After detaching, washing, and resuspending in serum-free MEMα+ media, CHO-α3 transfectant cells were allowed to spread on surfaces coated with either anti-human α3 mAb X8 (10 μg/ml) or anti-hamster α5 mAb PB1 (10 μg/ml) at 37°C in10% CO2 for 60 min. Cells were then lysed in RIPA buffer, and immunoprecipitations (I. P.) were carried out using antibodies to the indicated proteins. After SDS-PAGE under reducing conditions, proteins were transferred to nitrocellulose membranes, and blotted with anti-phosphotyrosine mAb, and then stripped and reblotted with antibodies to the indicated proteins. (B) CHO transfectants were treated with 100 nM PMA for 30 min before lysis, and then analyzed as in A.
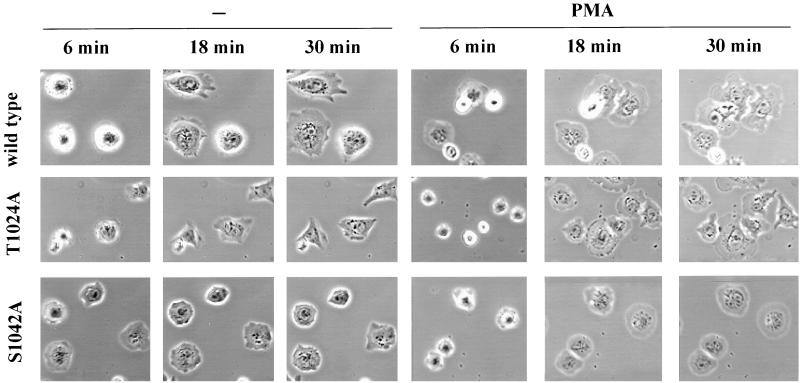
For each mutant, the percentage of cell spreading was also calculated (Table 2). After 18–30 min of attachment, in either the presence or absence of PMA; wild-type α3, S1042A-α3, and T1024A-α3 CHO cells all showed similar spreading on laminin-5 (65–83% after 18 min, 94–99% after 30 min). The S1042A mutant showed slightly enhanced spreading after 6 min of attachment, but this was not a highly significant difference.
Table 2.
Analysis of CHO-α3 transfectants spread on laminin-5
| Cell
spreading (%)a
|
Cell
symmetryb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| W/O PMA | W/PMA | W/O PMA | W/PMA | |||||
| 6 | 18 | 30 | 6 | 18 | 30 | |||
| Wild Type | 37 ± 2 | 73 ± 24 | 95 ± 4 | 43 ± 9 | 83 ± 2 | 99 ± 2 | 1.66 ± 0.41 (22) | 1.90 ± 0.49 (31) |
| T1024A | 39 ± 8 | 65 ± 12 | 95 ± 4 | 44 ± 5 | 79 ± 2 | 99 ± 2 | 1.80 ± 0.76 (16) | 2.33 ± 0.80 (29) |
| S1042A | 58 ± 15 | 79 ± 19 | 94 ± 5 | 53 ± 11 | 77 ± 7 | 98 ± 3 | 1.21 ± 0.18 (17)* | 1.21 ± 0.13 (23)** |
Cell spreading (%) represents the number of spread cells divided by total cells counted × 100. Spread cells were readily defined as cells that were no longer phase-bright in the light microscope, as they began to show a flattened morphology. Results were obtained at three different time points (6, 18, 30 min), and each number represents mean ± SD from three experiments, each using at least 20 cells.
Cell symmetry was measured (see MATERIALS AND METHODS) after CHO transfectants were allowed to spread on laminin-5 (coated at 2 μg/ml) for 30 min, in either the presence or absence of 100 nM phorbol ester. Cell symmetry values represent mean ± SD for (N) cells measured.
In the absence of PMA, S1042 cells are significantly more symmetrical than either wild-type or T1024 cells (p < 0.005).
In the presence of PMA, S1042 cells are significantly more symmetrical than either wild-type or T1024 cells (p < 0.0001).
In contrast to this quantitative similarity in numbers of spread cells, there was a pronounced qualitative difference in their morphologies. Spread S1042A-CHO cells generally showed a much more symmetric, rounded shape compared with the others. This was observed both with and without PMA stimulation (Figure 8, bottom row). For cells spread for 30 min on laminin-5, deviation from perfect roundness was quantitated. For each measured cell perimeter, the maximum possible area (assuming perfect roundness) was divided by the actual area, such that larger ratios correspond to increased deviation from a perfect rounded symmetry. As indicated (Table 2, right columns) S1042A-CHO cells were significantly more rounded than the wild-type or T1024A-CHO cells, regardless of the presence or absence of phorbol ester. The T1024 control mutant may have slightly increased asymmetry compared with wild type α3 (Table 2), but this difference is not nearly as significant as the loss of asymmetry in the S1042 mutant.
Figure 8.
Spreading of CHO-α3 cells on laminin-5. Mutant and wild-type CHO transfectants were allowed to spread on laminin-5 (coated at 2 μg/ml) in the presence or absence of treatment with 100 nM PMA. Images of typical cells were recorded at 6, 18, and 30 min after cell plating. Magnification, 40×.
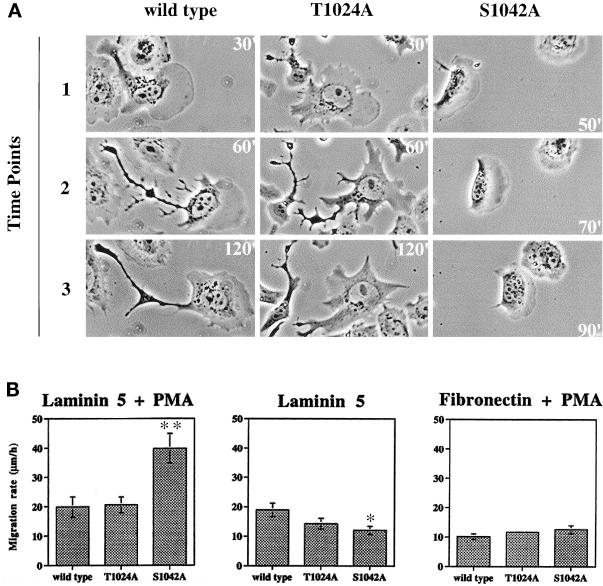
Confirming and extending results in Figure 8, representative time-lapse images (Figure 9A, and attached video) showed that PMA-stimulated S1042A cells have a much more rounded “half-moon” shape, with many fewer cellular projections, and generally more regular lamellipodia. In addition, the S1042A cells lacked the elongated rear retraction tails frequently observed for wild-type and T1024A cells. Analysis of many cells in this experiment showed the same consistent differences between S1042A cells and T1024A or wild-type cells. On the other hand, differences between wild-type α3 and T1024A cells were minimal.
Figure 9.
Altered migration of α3-S1042A CHO cells. (A) CHO transfectants migrating on laminin-5 (coated at 2 μg/ml) in the presence of 100 nM PMA were photographed at the indicated times after initial plating. Note that S1042A cells have migrated the same distance as the other cells, but in less than half the time. Magnification, 80×. Figure 9A results can also be seen in a short video. (B) Random migration rates for CHO-α3 transfectants were measured on laminin-5 (2 μg/ml), or on fibronectin (2 μg/ml), with or without PMA stimulation. Cell positions were recorded at 2-min intervals for 2 h (see MATERIALS AND METHODS). Each column represents the mean accumulated migrated distance per hour for at least 20 cells (±SD). ∗∗S1042A migration rate is greater than wild-type (p < 0.01); ∗Migration rate is less than wild-type (p < 0.012).
Functional Relevance of α3 S1042 Phosphorylation: Cell Migration and Integrin Distribution
As an apparent consequence of being unencumbered by retraction tails, the S1042A cells moved more rapidly when stimulated by PMA. For example in Figure 9A, wild-type cells took longer (90 min) to move about the same distance that S1042A cells moved in 40 min. Quantitation of many time-lapse video images for many different cells confirmed that migration of PMA-stimulated CHO-S1042A cells was markedly higher than the migration rate of CHO-wild-type α3, or CHO-T1024A cells (Figure 9B, left). If PMA-stimulated cells were plated on fibronectin instead of laminin-5, no significant migration differences were seen (right). Also if PMA stimulation was omitted, CHO-S1042A cells did not show elevated random migration on laminin-5. Instead, they migrated at a rate (12.0 ± 1.4 μm/h; p < 0.012) that was diminished compared with the migration rate of CHO-wild-type α3 (18.9 ± 2.2 μm/h) (Figure 9B, center). In the absence of PMA, it appears that diminished ability to form asymmetrical projections may contribute to diminished migration, rather than enhanced migration.
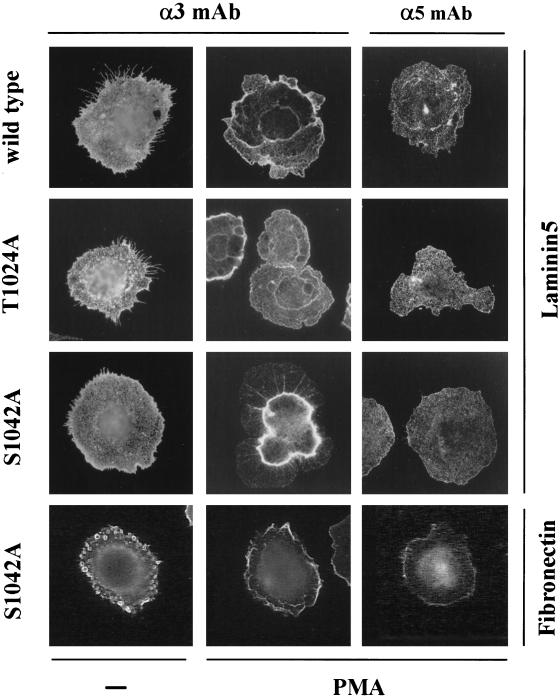
Because PMA can influence so many diverse cellular processes (Ron and Kazanietz, 1999), it is difficult to know precisely why the S1042A mutation yields such different results in the presence of PMA, compared with the absence of PMA. Nonetheless, a possibly important clue was revealed upon analysis of α3 integrin distribution by immunofluorescent staining of permeabilized CHO cells. The S1042A-α3 integrin, in 10–20% of PMA-stimulated S1042A-CHO cells, was aberrantly localized in a central internal ring (Figure 10A). This distinctive staining pattern was seen only on S1042A-α3 cells that were stimulated by PMA, and spread on laminin-5. It was not seen on unstimulated S1042A-α3 cells on laminin-5, stimulated S1042A-α3 cells on fibronectin, or on stimulated wild-type α3, or T1024A-α3 cells on laminin-5. The central ring-like staining was also observed in nonpermeabilized PMA-stimulated S1042A-α3 cells (our unpublished results), consistent with the “ring” of α3 integrin being on the cell surface. A confocal microscopy Z section (side view), showed the α3 integrin “ring” structure located on the dorsal surface of the cell, and clearly absent from the basal surface (Figure 10B, lower left).
Figure 10.
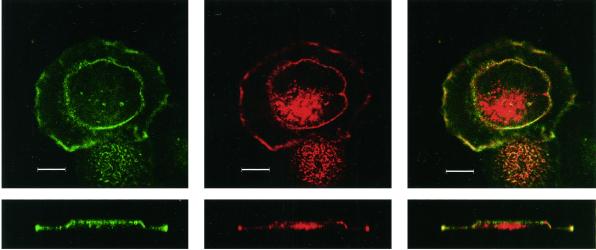
Altered subcellular distribution of α3 integrin S1042A mutant. (A) Transfected CHO cells were spread on laminin-5 (coated at 4 μg/ml), or fibronectin (coated at 4 μg/ml), in the presence or absence of PMA (100 nM). After 30 min, cells were fixed, permeabilized, and stained with anti-human α3 integrin mAb A3-X8 or anti-hamster α5 mAb PB1 followed by rhodamine-conjugated secondary antibody, and photography was carried out as described in MATERIALS AND METHODS. Magnification, 100×. (B) Distribution of F-actin and α3 integrin in CHO-α3-S1042A mutant cells was examined by confocal microscopy. The S1042A cells were treated with PMA on a laminin-5-coated coverslip and labeled with Texas Red conjugated-phalloidin (center panels) and Alexa 488-conjugated α3 integrin mAb X8 (left panels). An overlay of both colors is shown (right panels). Horizontal (xy, upper panels) and vertical (z, lower panels) sections were taken through the center of the cell. Bar, 10 μm.
Double staining of PMA-treated S1042A-CHO cells revealed that α3 partially overlapped with F-actin in both the central ring, and at the periphery of the cell, as seen in confocal XY sections (Figure 10B, top) and Z sections (Figure 10B, bottom). Although a substantial proportion of the S1042A α3 colocalized with F-actin, there was a large amount of additional F-actin, in the center of the cell, that failed to colocalize with α3. In cells that did not show a central α3 ring (PMA-treated T1024A and wild-type cells), there was a partial overlap between α3 integrin and F-actin, but were no apparent differences between cells in terms of either the extent or location of the α3 integrin-actin overlap (our unpublished results). In the absence of PMA stimulation, there again was no α3 ring staining, and there were no discernible differences in α3 integrin–F-actin colocalization patterns (our unpublished results).
DISCUSSION
α Tail Phosphorylation Site
Although the α3A cytoplasmic tail was known to be phosphorylated (Dumont and Bitonti, 1994; de Melker et al., 1997), the site had not been identified. Here we have used mass spectrometry to identify the key monophosphorylated α3 peptide, to show that a substantial level of α3 phosphorylation occurs upon PMA stimulation, and to identify S1042 as the site of phosphorylation in human α3A. This result was confirmed by mutagenesis, as an S1042A mutant was no longer phosphorylated. These results are consistent with previous mapping of α3A phosphorylation to serine (Hogervorst et al., 1993a; Dumont and Bitonti, 1994). In α3A, S1042 is part of a “QPSXXE” motif highly conserved among the α3A, α6A, and α7A tails in all animal species examined (including human, rodent, chicken, and frog). No other serine or threonine is fully conserved among α3A, α6A, and α7A of different species (Figure 3A). Our results, together with previous α6A serine mutagenesis results (Hogervorst et al., 1993b), now firmly establish the “QPSXXE” motif as a site for serine phosphorylation.
Enzymes Involved in Phosphorylation
Based on stimulation by PMA, and inhibitor studies, protein kinase C is clearly implicated as playing a key role in α3A and α6A phosphorylation. However, our results strongly suggest that PKC is not directly responsible for the phosphorylation. Neither α3 nor α6 peptide was phosphorylated in vitro by any isoform of PKC tested, and neither the α3 nor α6 “QPSXXE” sites resemble a preferred PKC phosphorylation site. Previously, a peptide containing the C-terminal 28 residues of α6A was phosphorylated in vitro by PKC (Gimond et al., 1995). However, it was not established that serine phosphorylation occurred within the QPSXXE site, and thus those results might not be pertinent to the results reported here.
We hypothesize that α3A and α6A may be phosphorylated by the same kinase. These cytoplasmic tails are 1) 63% similar in sequence, 2) both phosphorylated within a similar QPSXXE motif, 3) both phosphorylated at similarly low constitutive levels in CHO and K562 cells, 4) both phosphorylated at similarly high levels upon PMA stimulation, and 5) both similarly sensitive to PKC inhibitors. For these reasons, we assume that there are essential similarities in the roles of α3A and α6A phosphorylation during outside-in signaling events, and that meaningful extrapolations can be made between the two integrins (see below). Confirmation of our hypothesis regarding the use of the same kinase awaits identification of the serine kinase directly responsible. Despite assay of several kinases in vitro, and scanning of abundant available information on known kinase site specificities, we were unable to identify any serine kinases as likely candidates. Possibly the relevant kinase may reside among the hundreds of vertebrate serine kinases not yet characterized.
Signaling through “QPSXXE” Sites and Morphological Consequences
Although integrin α chain phosphorylation has been known for at least 13 years (Kantor et al., 1987), the functional relevance of this had not been elucidated. Our results now provide strong evidence that α3 S1042 phosphorylation plays a key role during α3 integrin outside-in signaling. Loss of α3 phosphorylation in the S1042A mutant correlates with loss of α3 integrin signaling through p130CAS, FAK, and paxillin, and loss of α3-dependent formation of asmmetrical cellular morphology. Tyrosine phosphorylation of p130CAS, FAK, and paxillin was diminished, even though cell attachment to (and low-level spreading on) immobilized anti-α3 mAb was not different between wild-type and mutant α3 CHO transfectants. Thus, altered signaling is not secondary to changes in adhesion or amount of cell spreading. Although our α3 transfectants were not tested for signaling differences on laminin-5 (because laminin-5 was not available in sufficient quantity) we anticipate that the S1042A mutant should again show diminished signaling.
The α3 S1042 mutation was associated with the loss of both signaling, and cellular projections, either in the presence or absence of PMA stimulation. Thus, constitutive α3 phosphorylation as observed here (Figure 1) and elsewhere (Kantor et al., 1987; Hogervorst et al., 1993a) appears to be as functionally important as PMA-induced phosphorylation. Neither constitutive nor PMA-stimulated CHO cell adhesion was altered by the α3 S1042 mutation. Thus, inside-out signaling of the α3Aβ1 integrin is not regulated by α3 tail phosphorylation. In agreement with the results seen here, phosphorylation within the α6 QPSXXE site also is not involved in the regulation of cell adhesion (Hogervorst et al., 1993).
We hypothesize that phosphorylation of α3 S1042 leads to an α3 integrin-dependent regulation of the actin cytoskeleton that is required for both signaling and formation of cellular projections. Obviously, if α3 integrin phosphorylation facilitates the specific formation of cellular projections, then this must involve regulation of the cytoskeleton. Furthermore, phosphorylations of FAK, paxillin, and p130CAS, and their recruitment into focal adhesions in normal cells, are all dependent on the actin cytoskeleton (Burridge et al., 1992; Lipfert et al., 1993; Nojima et al., 1995; Manie et al., 1997). Additional evidence for α3 phosphorylation regulating the actin cytoskeleton comes from phorbol ester-stimulated cells expressing the α3 S1042A mutation. In that case, upon engagement of the α3 integrin with laminin-5, there was a specific redistribution of both the integrin itself, and F-actin, into a central ring structure. At present it is unclear whether the α3 S1042A mutation (and by inference α3 phosphorylation) causes increased or decreased integrin association with the cytoskeleton. In this regard, selective detergent extraction experiments yielded no discernible difference between wild-type and mutant α3 in terms of integrin extractability from cells spread on laminin-5 (our unpublished results).
The specific mechanism by which α3 phosphorylation might alter outside-in signaling is unclear. It is possible that phosphorylation of wild-type α3 could lead to movement of the α3 tail, and uncovering of the β1 subunit, which then facilitates signaling and cytoskeletal rearrangement. For example, removal of α chain tails was previously shown to enhance β tail-dependent signaling, and cytoskeletal reorganization (Briesewitz et al., 1993; LaFlamme et al., 1992; Ylänne et al., 1993). Inability of mutant α3 to become phosphorylated would prevent uncovering of the β1 subunit, and thus hinder subsequent signaling and cytoskeletal reorganization events. Another possibility is that S1042 phosphorylation regulates specific biochemical interactions of other key proteins with the α3A integrin. In this regard, dephosphorylation of α6A correlated with integrin association with an intermediate filament-related protein (Baker et al., 1997), but it was not established whether the interaction was direct, or dependent on α6A phosphorylation.
Compared with α6A, signaling through α6B yielded diminished paxillin phosphorylation (Shaw et al., 1995). This result is perhaps explained by the inability of α6B to undergo serine phosphorylation. However, α6B also showed diminished MAP kinase activation capability (Wei et al., 1998), whereas our α3 S1042A mutation did not affect MAP kinase activity. Most likely, functional deficiencies in α6B arise from more than just the absence of a serine phosphorylation site. Alternatively, α3A and α6A may differ in signaling through MAP kinase, or CHO cells may differ from the macrophage cell line used elsewhere (Wei et al., 1998).
Role for α Chain Phosphorylation during Cell Motility
With α3 phosphorylation playing a key role during α3-dependent signaling and cell morphology, it is perhaps not surprising that it would also influence cell motility. In the absence of PMA stimulation, the S1042A mutation caused a small, but significant decrease in random migration on laminin-5. This deficiency in motility most likely results from diminished signaling and a diminished ability to form cellular projections that are necessary for motility. In this regard, activation and tyrosine phosphorylation of p130CAS, FAK, and paxillin are all associated with increased cell motility (Cary et al., 1998; Petit et al., 2000).
Other studies also suggest a positive correlation between “QPSXXE” phosphorylation and cell migration in the absence of phorbol ester stimulation. For example, metastatic activities of melanoma (Dumont and Bitonti, 1994) and Lewis lung carcinoma cells (Tentori et al., 1995) coincided with α3A and α6A phosphorylation, respectively. Also, integrin α6A was 2–4-fold better than α6B in supporting the haptotactic migration of macrophages (Shaw and Mercurio, 1994; Wei et al., 1998) and lymphocytes (Gimond et al., 1998) toward laminin-1. This latter difference could at least partly be due to the absence of a serine phosphorylation site in the α6B cytoplasmic tail.
Whereas α3A serine phosphorylation seems to promote migration in the absence of PMA stimulation, it may restrict migration in the presence of PMA stimulation. Indeed, the S1042A mutation caused a marked increase in PMA-stimulated, α3-dependent random cell motility of CHO cells. These effects were highly specific, because increased motility was not seen unless α3 was engaged, and PMA was added. We suggest that PMA-stimulated phosphorylation of wild-type α3 facilitates α3-dependent signaling through molecules such as FAK and p130CAS, leading to formation of asymmetrical cellular projections that in this case restrict rather than enhance cell migration.
Why does α3A serine phosphorylation promote migration in the absence of PMA, but restrict migration in the presence of PMA stimulation? In this regard, it is well appreciated that many processes, and molecules such as FAK, can both positively and negatively impact cell motility (Ilic et al., 1995; Palecek et al., 1997; Cary et al., 1998). We suggest that constitutive levels of α3 phosphorylation, in the absence of PMA stimulation, may contribute to a level of signaling and cytoskeletal interaction that is optimal for migration. Conversely, the very high levels of phorbol ester-induced phosphorylation of α3 and other PKC substrates (e.g., cytoskeletal proteins) may lead to signaling and/or cytoskeletal rearrangements that are beyond the optimal level, and thereby impair cell migration. It is only in PMA-stimulated cells that the S1042A-α3 integrin is localized into a dorsal, ring-like structure, separate from the α3 staining at the periphery of the cell. This result provides support for our suggestion that α3 integrin interactions with the cytoskeleton are markedly altered in PMA-stimulated cells.
Summary and Conclusions
Here we identified the α3A phosphorylation site, and showed that α3A phosphorylation may strongly influence cell signaling, morphology, and motility, most likely by affecting integrin-dependent cytoskeletal organization. The relevance of integrin phosphorylation was well supported by the high conservation of the serine phosphorylation “QPSXXE” motif in the α3A, α6A, and α7A integrin subunits of all species analyzed. All of these results point to the α3A and α6A cytoplasmic tails playing a central role in downstream outside-in integrin signaling pathways. Although not yet tested, we predict that phosphorylation of the α6A and α7A tails may also strongly influence cell morphology and motility. It remains to be determined whether α3 S1042 will play a critical role in other α3 integrin functions such as phagocytosis (Gresham et al., 1996; Coopman et al., 1996), cell fusion (Ohta et al., 1994), and transdominant inhibition of other integrins (Hodivala-Dilke et al., 1998).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants CA-86712 and CA-42368 (to M.E.H.)
Abbreviations used:
- CHO
Chinese hamster ovary
- FAK
focal adhesion kinase
- FCS
fetal calf serum
- mAb
monoclonal antibody
- MEM
minimal essential medium
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13 acetate
Footnotes
Online version of this article contains video material for Figure 9. Online version is available at www.molbiolcell.org.
REFERENCES
- Baker SE, Skalli O, Goldman RD, Jones JC. Laminin-5 and modulation of keratin cytoskeleton arrangement in FG pancreatic carcinoma cells: involvement of IFAP300 and evidence that laminin-5/cell interactions correlate with a dephosphorylation of α 6A integrin. Cell Motil Cytoskeleton. 1997;37:271–286. doi: 10.1002/(SICI)1097-0169(1997)37:3<271::AID-CM9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Ma L, Blue M-L, Hemler ME. Divalent cations and ligands induce conformational changes that are highly divergent among β1 integrins. J Biol Chem. 1998;273:6670–6678. doi: 10.1074/jbc.273.12.6670. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Shih D-T, Buck CA, Hemler ME. MAb 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Berditchevski F, Bazzoni G, Hemler ME. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J Biol Chem. 1995;270:17784–17790. doi: 10.1074/jbc.270.30.17784. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, St. John N, Kawaguchi S, Pasqualini R, Berdichevsky F, Hemler ME, Finberg RW. The I domain is essential for echovirus 1 interaction with VLA-2. Cell Adhes Commun. 1994;2:455–464. doi: 10.3109/15419069409004455. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Williams MP, Slater SE, Brown EJ. Requirement of integrin β3 tyrosine 747 for β3 tyrosine phosphorylation and regulation of αVβ3 avidity. J Biol Chem. 1997;272:28757–28761. doi: 10.1074/jbc.272.45.28757. [DOI] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL. Extracellular matrix and integrin signaling: the shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- Briesewitz R, Kern A, Marcantonio EE. Ligand-dependent and -independent integrin focal contact localization: the role of the α chain cytoplasmic domain. Mol Biol Cell. 1993;4:593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985;228:1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Monoclonal antibodies to distinctive epitopes on the α and β subunits of the fibronectin receptor. Exp Cell Res. 1988;177:303–318. doi: 10.1016/0014-4827(88)90464-8. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon JP, Slade S, Reibman J, Abramson SB, Philips MR, Weissmann G, Winchester R. Constitutive and induced phosphorylation of the α- and β-chains of the CD11/CD18 leukocyte integrin family. J Immunol. 1990;144:191–197. [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BMC, Elices MJ, Murphy E, Hemler ME. Adhesion to VCAM-1 and fibronectin: comparison of α4β1 (VLA-4) and α4β7 on the human cell line JY. J Biol Chem. 1992;267:8366–8370. [PubMed] [Google Scholar]
- Chan BMC, Wong J, Rao A, Hemler ME. T cell receptor dependent, antigen specific stimulation of a murine T cell clone induces a transient VLA protein-mediated binding to extracellular matrix. J Immunol. 1991;147:398–404. [PubMed] [Google Scholar]
- Chatila TA, Geha RS, Arnaout MA. Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J Cell Biol. 1989;109:3435–3444. doi: 10.1083/jcb.109.6.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Almeida EA, Huovila AP, Takahashi Y, Shaw LM, Mercurio AM, White JM. Evidence that distinct states of the integrin alpha6beta1 interact with laminin and an ADAM. J Cell Biol. 1999;144:549–561. doi: 10.1083/jcb.144.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, O'Toole TE, Ylänne J, Rosa JP, Ginsberg MH. A point mutation in the integrin β3 cytoplasmic domain (S752>P) impairs bidirectional signaling through αIIbβ3 (platelet glycoprotein IIB-IIIA) Blood. 1994;84:1857–1865. [PubMed] [Google Scholar]
- Coopman PJ, Thomas DM, Gehlsen KR, Mueller SC. Integrin α3β1 participates in the phagocytosis of extracellular matrix molecules by human breast cancer cells. Mol Biol Cell. 1996;7:1789–1804. doi: 10.1091/mbc.7.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melker AA, Sterk LM, Delwel GO, Fles DL, Daams H, Weening JJ, Sonnenberg A. The A and B variants of the α3 integrin subunit: tissue distribution and functional characterization. Lab Invest. 1997;76:547–563. [PubMed] [Google Scholar]
- Delwel GO, Hogervorst F, Kuikman I, Paulsson M, Timpl R, Sonnenberg A. Expression and funciton of cytoplasmic variants of the integrin α6 subunit in transfected K562 cells. J Biol Chem. 1993;268:25865–25875. [PubMed] [Google Scholar]
- Dumont JA, Bitonti AJ. Modulation of human melanoma cell metastasis and adhesion may involve integrin phosphorylation mediated through protein kinase C. Biochem Biophys Res Commun. 1994;204:264–272. doi: 10.1006/bbrc.1994.2454. [DOI] [PubMed] [Google Scholar]
- Eble JA, Wucherpfennig KW, Gauthier L, Dersch P, Krukonis E, Isberg RR, Hemler ME. Recombinant soluble human α 3β 1 integrin: purification, processing, regulation, and specific binding to laminin-5 and invasin in a mutually exclusive manner. Biochemistry. 1998;37:10945–10955. doi: 10.1021/bi980175+. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Gimond C, Baudoin C, van der Neut R, Kramer D, Calafat J, Sonnenberg A. Cre-loxP-mediated inactivation of the α 6A integrin splice variant in vivo: evidence for a specific functional role of α 6A in lymphocyte migration but not in heart development [published erratum appears in J. Cell Biol. (1998)143, following 1412] J Cell Biol. 1998;143:253–266. doi: 10.1083/jcb.143.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimond C, de Melker A, Aumailley M, Sonnenberg A. The cytoplasmic domain of α 6A integrin subunit is an in vitro substrate for protein kinase C. Exp Cell Res. 1995;216:232–235. doi: 10.1006/excr.1995.1029. [DOI] [PubMed] [Google Scholar]
- Gresham HD, Graham IL, Griffin GL, Hsieh JC, Dong LJ, Chung AE, Senior RM. Domain-specific interactions between entactin and neutrophil integrins. G2 domain ligation of integrin α 3β 1 and E domain ligation of the leukocyte response integrin signal for different responses. J Biol Chem. 1996;271:30587–30594. doi: 10.1074/jbc.271.48.30587. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Weitzman JB, Pasqualini R, Kawaguchi S, Kassner PD, Berdichevsky FB. Structure, biochemical properties, and biological functions of integrin cytoplasmic domains. In: Takada Y, editor. Integrin: The biological Problem. Ann Arbor: CRC Press; 1994. pp. 1–35. (Abstract). [Google Scholar]
- Hodivala-Dilke KM, Dipersio CM, Kreidberg JA, Hynes RO. Novel roles for α 3β 1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol. 1998;142:1357–1369. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F, Admiraal LG, Niessen C, Kuikman I, Janssen H, Daams H, Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol. 1993a;121:179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, Noteboom E, Sonnenberg A. The role of phosphorylation in activation of the α6Aβ1 laminin receptor. J Biol Chem. 1993b;268:18427–18430. [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Jenkins AL, Nannizzi-Alaimo L, Silver D, Sellers JR, Ginsberg MH, Law DA, Phillips DR. Tyrosine phosphorylation of the β 3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- Johansson MW, Larsson E, Lüning B, Pasquale EB, Ruoslahti E. Altered localization and cytoplasmic domain-binding properties of tyrosine-phosphorylated β1 integrin. J Cell Biol. 1994;126:1299–1309. doi: 10.1083/jcb.126.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JCR, Kurpakus MA, Cooper HM, Quaranta V. A function for the integrin α6β4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor RRS, Mattes MJ, Lloyd KO, Old LJ, Albino AP. Biochemical analysis of two cell surface glycoprotein complexes: VCA-1 and VCA-2. Relationship to VLA T cell antigens. J Biol Chem. 1987;262:15158–15165. [PubMed] [Google Scholar]
- LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin α 6β 4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Berditchevski F, Cheng GC, Hemler ME. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res. 1995;76:209–214. doi: 10.1161/01.res.76.2.209. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Cheresh DA, Schwartz MA. Protein kinase C regulates α Vβ 5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1993;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of α 6β 4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manie SN, Beck AR, Astier A, Law SF, Canty T, Hirai H, Druker BJ, Avraham H, Haghayeghi N, Sattler M, Salgia R, Griffin JD, Golemis EA, Freedman AS. Involvement of p130(Cas) and p105(HEF1), a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells. J Biol Chem. 1997;272:4230–4236. doi: 10.1074/jbc.272.7.4230. [DOI] [PubMed] [Google Scholar]
- Mannion BA, Berditchevski F, Kraeft S-K, Chen LB, Hemler ME. TM4SF proteins CD81 (TAPA-1), CD82, CD63 and CD53 specifically associate with α4β1 integrin. J Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- Ohta H, Tsurudome M, Matsumura H, Koga Y, Morikawa S, Kawano M, Kusugawa S, Komada H, Nishio M, Ito Y. Molecular and biological characterization of fusion regulatory proteins (FRPs): anti-FRP mAbs induced HIV-mediated cell fusion via an integrin system. EMBO J. 1994;13:2044–2055. doi: 10.1002/j.1460-2075.1994.tb06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness [published erratum appears in Nature (1997) 388, 210] Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Pardi R, Inverardi L, Rugarli C, Bender JR. Antigen-receptor complex stimulation triggers protein kinase C-dependent CD11a/CD18-cytoskeleton association in T lymphocytes. J Cell Biol. 1992;116:1211–1220. doi: 10.1083/jcb.116.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C, Teixidó J, Bazzoni G, Hemler ME. Integrin cysteines 278 and 717 modulate VLA-4 ligand binding and also contribute to α4/180 formation. Biochem J. 1996;313:899–908. doi: 10.1042/bj3130899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Seipel K, Medley QG, Kedersha NL, Zhang XA, O'Brien S, Serra-Pagés C, Hemler ME, Streuli M. Trio amino-terminal guanine nucleotide exchange factor domain expression promotes actin cytoskeleton reorganization, cell migration and anchorage-independent cell growth. J Cell Sci. 1999;112:1825–1834. doi: 10.1242/jcs.112.12.1825. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Brass LF. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J Biol Chem. 1987;262:992–1000. [PubMed] [Google Scholar]
- Shaw LM, Lotz MM, Mercurio AM. Inside-out integrin signaling in macrophages: analysis of the role of the α6Aβ1 and α6Bβ1 integrin variants in laminin adhesion by cDNA expression in an α6 integrin-deficient macrophage cell line. J Biol Chem. 1993;268:11401–11408. [PubMed] [Google Scholar]
- Shaw LM, Mercurio AM. Regulation of α6β1 integrin laminin receptor function by the cytoplasmic domain of the α6 subunit. J Cell Biol. 1993;123:1017–1025. doi: 10.1083/jcb.123.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Mercurio AM. Regulation of cellular interactions with laminin by integrin cytoplasmic domains: the A and B structural variants of α6β1 integrin differentially modulate the adhesive strength, morphology, and migration of macrophages. Mol Biol Cell. 1994;5:679–690. doi: 10.1091/mbc.5.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Messier JM, Mercurio AM. The activation dependent adhesion of macrophages to laminin involves cytoskeleton anchoring and phosphorylation of the α6β1 integrin. J Cell Biol. 1990;110:2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Turner CE, Mercurio AM. The α6Aβ1 and α6Bβ1 integrin variants signal differences in the tyrosine phosphorylation of paxillin and other proteins. J Biol Chem. 1995;270:23648–23652. doi: 10.1074/jbc.270.40.23648. [DOI] [PubMed] [Google Scholar]
- Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (β 1) integrin receptors on T cells. Nature. 1990;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;360:487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Hemler ME. Transmembrane-4-superfamily proteins CD151 and CD81 associate with α3β1 integrin and selectively contribute to α3β1-dependent neurite outgrowth. J Cell Sci. 2000;113:1871–1882. doi: 10.1242/jcs.113.11.1871. [DOI] [PubMed] [Google Scholar]
- Szabo MC, Teague TK, McIntyre BW. Regulation of lymphocyte pseudopodia formation by triggering the integrin α4β1. J Immunol. 1995;154:2112–2124. [PubMed] [Google Scholar]
- Tentori L, Leonetti C, Aquino A. Temozolomide reduces the metastatic potential of Lewis lung carcinoma (3LL) in mice: role of α6 integrin phosphorylation. Eur J Cancer. 1995;31A:746–754. doi: 10.1016/0959-8049(94)00521-6. [DOI] [PubMed] [Google Scholar]
- Valmu L, Autero M, Siljander P, Patarroyo M, Gahmberg CG. Phosphorylation of the β-subunit of CD11/CD18 integrins by protein kinase C correlates with leukocyte adhesion. Eur J Immunol. 1991;21:2857–2862. doi: 10.1002/eji.1830211130. [DOI] [PubMed] [Google Scholar]
- Van Nhieu GT, Krukonis ES, Reszka AA, Horwitz AF, Isberg RR. Mutations in the cytoplasmic domain of the integrin β1 chain indicate a role for endocytosis factors in bacterial internalization. J Biol Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- Williams KR, LoPresti M, Stone K. Internal protein sequencing or SDS-PAGE-separated proteins: optimization of an in-gel digest protocol. In: Marshak DP, editor. Techniques VIII. San Diego: Academic Press; 1997. pp. 79–90. (Abstract). [Google Scholar]
- Woodgett JR, Gould KL, Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986;161:177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Wright SD, Meyer BC. Phorbol esters cause sequential activation and deactivation of complement receptors on polymorphonuclear leukocytes. J Immunol. 1986;136:1759–1764. [PubMed] [Google Scholar]
- Ylänne J, Chen Y, O'Toole TE, Loftus JC, Takada Y, Ginsberg MH. Distinct functions of integrin α and β subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.