Abstract
Upon DNA double-strand break (DSB) induction in mammals, the histone H2A variant, H2AX, becomes rapidly phosphorylated at serine 139. This modified form, termed γ-H2AX, is easily identified with antibodies and serves as a sensitive indicator of DNA DSB formation. This review focuses on the potential clinical applications of γ-H2AX detection in cancer and in response to other cellular stresses. In addition, the role of H2AX in homeostasis and disease will be discussed. Recent work indicates that γ-H2AX detection may become a powerful tool for monitoring genotoxic events associated with cancer development and tumor progression.
Introduction
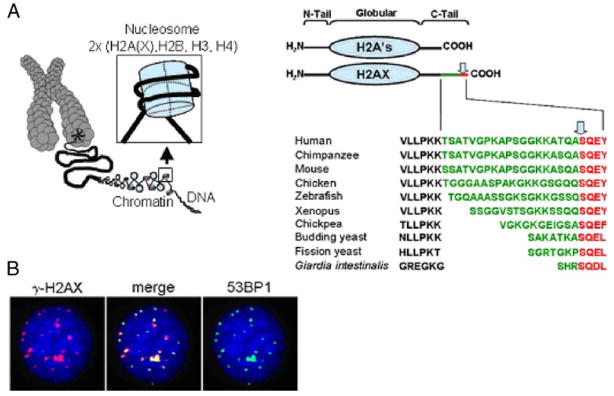
H2AX is a histone H2A variant that constitutes 2–25% of mammalian histone H2A depending on the organism and cell type (Redon et al. 2002; Rogakou et al. 1998). Like most other histone proteins, H2AX is composed of a central globular domain, flanked by N-terminal and C-terminal tails which possess sites for a variety of post-translational modifications such as acetylation, biotinylation, phosphorylation, methylation, and ubiquitination (Cheung et al. 2000; Chew et al. 2006; Goll and Bestor 2002; Rogakou et al. 1998). H2AX is structurally similar to other H2A species except for the presence of a unique COOH terminal tail, containing a serine four residues from the C terminus (omega-4). The omega-4 position of the serine residue as well as the surrounding motif is highly conserved, being present in the protozoa, Giardia intestinalis (Redon et al. 2002; Fig. 1a). Upon induction of a DNA double-strand break (DSB), the H2AX omega-4 serine residue becomes rapidly phosphorylated to form gamma-H2AX (γ-H2AX; Fernandez-Capetillo et al. 2004; Rogakou et al. 1998).
Fig. 1.
a (left panel) H2AX is a component of chromatin and its fundamental packaging unit, the nucleosome. a (right panel) H2AX is composed of a central globular domain, an N-terminal tail and a unique C-terminal tail consisting of an evolutionarily conserved motif (shown in red) and connected by a linker of variable sequence and length (green). The conserved motif contains the omega-4 serine that is phosphorylated upon DNA DSB formation (arrow). b In response to genotoxic stress and upon DNA DSB formation, the H2AX omega-4 serine is phosphorylated (γ-H2AX), which can be visualized using an anti-γ-H2AX antibody as discrete foci that colocalize with other DNA repair proteins. The images depict a HeLa cell 1 h after exposure to 1 Gy of γ-radiation. Red γ-H2AX, green 53BP1, blue DAPI
The proteins responsible for the phosphorylation of the H2AX omega-4 serine are members of the PI3 kinase family, including ataxia telangiectasia mutated (ATM), ATR (AT and Rad3-related protein), and DNA-dependent protein kinase (DNA-PK; Fernandez-Capetillo et al. 2004; Stiff et al. 2004, 2006). Upon DSB induction, one of these kinases phosphorylates many molecules of H2AX in chromatin regions varying from a few Mbp to many tens of Mbp flanking the lesion (Pilch et al. 2003; Rogakou et al. 1999). This phosphorylation event is dynamic, complex, and depends on interactions between MDC1, H2AX, and ATM and other kinases to persist (Savic et al. 2009). This amplified response is easily detected using antibodies to γ-H2AX, manifesting discrete nuclear foci that may be utilized to enumerate the number of DSBs in a cell and/or to examine the co-localization of other DNA repair proteins to the sites of double-strand damage (Sedelnikova et al. 2003; Fig. 1b). This sensitive technique for detecting DNA double-strand damage in cells reveals the presence of γ-H2AX foci in the nuclei of intact primary and cancer cultured cells, as well as in tissues (Bonner et al. 2008; Fernandez-Capetillo et al. 2003; Rogakou et al. 1999). These foci are believed to mark lesions resulting from various kinds of endogenous and exogenous stress (Sedelnikova and Bonner 2006; Sedelnikova et al. 2004a, b). A recent study by Koike et al. presented evidence that phosphorylation and elimination of H2AX in vivo is tissue-specific and depends on different kinases (Koike et al. 2008).
Because of the sensitivity and utility of γ-H2AX detection of DNA DSBs, γ-H2AX has recently been identified as a potentially useful biomarker with clinical implications. This review will focus on the role of γ-H2AX in homeostasis as well as in disease and on the uses of γ-H2AX to aid in the understanding of DNA DSB formation and repair in cancer treatment, and in evaluating various forms of environmental stress. Detailed protocols for γ-H2AX detection in tissue and cellular samples have been addressed elsewhere (Bhogal et al. 2009; Huang et al. 2004; Nakamura et al. 2006; Qvarnstrom et al. 2004) and are reviewed in Bonner et al. (Bonner et al. 2008).
H2AX as a key regulator of the DNA damage response
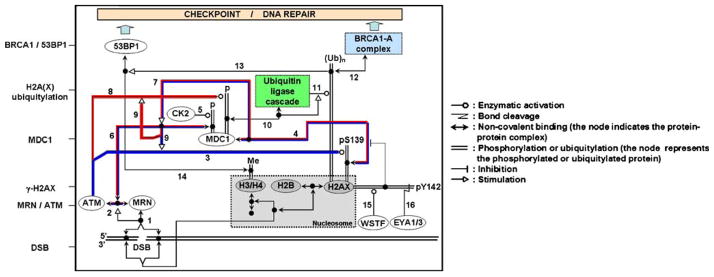
H2AX plays an essential role in the recruitment and accumulation of DNA repair proteins to sites of DSB damage (Fernandez-Capetillo et al. 2003; Fillingham et al. 2006; Paull et al. 2000) including sites of replication fork collapse (Furuta et al. 2003). These proteins include 53BP1, MDC1, RAD51, BRCA1, and the MRE11/RAD50/NBS1 complex which colocalize with γ-H2AX foci. γ-H2AX focus formation also results in the recruitment of proteins of the ubiquitin ligase cascade (RNF8-RNF168-UBC13) which in turn allows the accumulation of the BRCA1-A complex and 53BP1 to the DNA lesion site (Fig. 2; reviewed in (van Attikum and Gasser 2009)). Cohesins, which help maintain chromatid cohesion and are involved in DNA repair, also are localized to DSB sites in a γ-H2AX-dependent manner (Unal et al. 2004). H2AX has also been shown to be a novel component of the Fanconi anemia (FA)/BRCA pathway. Though not an FA gene, H2AX is functionally connected to the pathway to resolve stalled replication forks and prevent chromosome instability (Bogliolo et al. 2007; Lyakhovich and Surralles 2007). The chromatin remodeling complex TIP60-UBC13, which also participates in DNA repair, is recruited to the DSB site by γ-H2AX, allowing γ-H2AX acetylation and ubiquitylation prior to its dephosphorylation/removal from the break site (Ikura et al. 2007).
Fig. 2.
H2AX is a key component of the DNA damage response. This schematic representation illustrates the γ-H2AX-MDC1-BRCA1/53BP1cascade in response to DNA DSB formation after irradiation. Upon DSB formation, the MRN complex (MRE11-RAD50-NBS1) binds to the ends of the DSB (1) and recruits ATM (2). ATM then phosphorylates H2AX on serine 139 to form γ-H2AX (3). This phosphorylation allows the binding of the mediator protein MDC1 (4). The constitutive phosphorylation of MDC1 by CK2 (5) permits the binding of the MRN–ATM complex (via NBS1) to MDC1 (6). The MRN–ATM complex is preferentially recruited at the DSB site because of the presence of the γ-H2AX–MDC1 complex (7). This recruitment of ATM, in turn, enhances the phosphorylation of other proteins at the DSB site, including H2AX (3) and MDC1 (8) itself (feedback loop (9)). MDC1 phosphorylation at the DSB site allows the recruitment of the ubiquitin ligase machinery (10) that will then permit the ubiquitylation H2A and/or H2AX (11). H2A(X) ubiquitylation is necessary for the accumulation of the BRCA1-A complex at break sites via its subunit RAP80 (12). It is generally thought that histone ubiquitylation is necessary for 53BP1 accumulation at the DSB site (13) by providing the chromatin remodeling necessary to expose constitutive H3 and H4 methylated tails (Me) that in turn are recognized by 53BP1 (14). In the absence of DNA damage, H2AX is constitutively phosphorylated by WSTF on tyrosine 142 (15). Following DNA damage, if DNA repair occurs, phosphotyrosine 142 is dephosphorylated by the EYA1/3 phosphatase (16) allowing the binding of MDC1 to γ-H2AX (4). To simplify, the components of the MRN complex, the ubiquitin ligase complex and the BRCA1-A complex are represented by one box each and H2A is not shown. The histones are represented by gray boxes. One isolated node represents another copy of the molecular species that is at the end of the corresponding line. The feedback loops for H2AX and MDC1 phosphorylation are underlined in blue and red, respectively. Symbol conventions (shown at right) are derived from Dr. Kurt Kohn’s molecular interaction maps (Kohn 1999; for further details see http://discover.nci.nih.gov)
The H2AX C-terminal tyrosine residue (Y142) can be also phosphorylated (Cook et al. 2009; Xiao et al. 2009; Fig. 2) by WSTF. Phosphorylation of Y142 regulates γ-H2AX formation. The phosphorylation is constitutive in unstressed cells and dephosphorylated after DNA damage by EYA1 or EYA3 is necessary to allow γ-H2AX formation and the resultant MDC1 binding that leads to the DNA repair response. However, if during the cellular response to genotoxic stress, the cells undergo Y142 phosphorylation prior to DNA repair, the cellular response will switch to apoptosis (Cook et al. 2009).
Additionally, though H2AX is not required for cell cycle checkpoint activation after high doses of ionizing radiation (IR), it is necessary at low doses (Fernandez-Capetillo et al. 2002). Formation of γ-H2AX maintains checkpoint responses while DNA damage is being repaired (Downey and Durocher 2006; Fillingham et al. 2006). Finally, if DNA damage cannot be fixed, cells undergo programmed cell death in which H2AX also plays a role (Lu et al. 2006; Mukherjee et al. 2006). In summary, H2AX with other repair proteins play synergistic roles in DNA damage responses and tumor suppression by facilitating efficient, high-fidelity repair of DNA DSBs (Celeste et al. 2003a,b; Kang et al. 2005).
H2AX roles in disease
Analysis of H2AX null mice indicates that H2AX is required for efficient immunoglobulin class switching, as evidenced by reduced switching to IgG, but not for V(D)J recombination (Bassing et al. 2002; Celeste et al. 2002). Additionally, mice lacking H2AX are more sensitive to radiation and cells cultured from these mice are less efficient at DSB repair, leading to an increased incidence of chromosomal abnormalities (Bassing et al. 2002; Celeste et al. 2002). This implies a role for H2AX in preventing genomic instability associated with cancer. Finally, while female null mice are capable of breeding, males are infertile, indicating a role for H2AX in spermatogenesis (Bassing et al. 2002; Celeste et al. 2002).
Loss of one or both H2AX alleles in mice compromises genomic integrity and increases cancer susceptibility in a p53 null background (Bassing et al. 2003; Celeste et al. 2003a, b). These studies suggest that H2AX functions as a genome caretaker and the expression of both gene alleles is required for optimal protection against tumorigenesis. Cancers to which H2AX-deficient mice are predisposed include T- and B-cell lymphomas as well as solid tumors (Bassing et al. 2003; Celeste et al. 2003a, b). In addition, H2AX/p53 double null mice have shorter life-spans than either single knock-out strain, becoming moribund with lymphomas as early as 6 weeks of age (Bassing et al. 2003; Celeste et al. 2003a, b). Further studies showed that lymphomas from H2AX/p53 double null mice have significant chromosomal abnormalities including complex rearrangements that juxtapose the c-myc oncogene to antigen receptor loci (Bassing et al. 2003; Celeste et al. 2003a, b). These findings support the idea that H2AX has a role as a tumor suppressor.
Combined ATM and H2AX deficiency results in embryonic lethality. The embryonic stem (ES) cells exhibit chromosome aberrations, impaired reactive oxygen species (ROS) regulation, high sensitivity to oxidative stress, and more severe genomic instability than either ATM or H2AX single deficient ES cells (Zha et al. 2008). Since H2AX-deficient ES cells exhibited normal ROS levels, H2AX itself is not essential for the regulation of ROS levels in cells. However, H2AX might be required for the repair of ROS-induced DNA damage and preventing oxidative stress-related genomic instability (Zha et al. 2008). Because a functional H2AX is necessary to ensure genome integrity, its use in therapeutic intervention may be limited.
The human H2AX gene maps to chromosome 11q23, a region that exhibits mutations or deletions in a large number of human cancers and is among the most common cytogenetic abnormalities observed in hematopoietic malignancies such as acute myeloid leukemias and acute lymphoblastic leukemia (Kokandakar et al. 2007; Pui et al. 2003; Rubnitz et al. 1996; Thirman et al. 1993). This chromosome abnormality has also been linked to colorectal cancers (Takagi et al. 2000). Head and neck squamous cell carcinoma is characterized by amplification of chromosomal region 11q13 coupled with the frequent loss of distal 11q, which encodes H2AX as well as other DNA repair factors such as ATM (Parikh et al. 2007). The increased chromosomal instability seen in these cells indicates that loss of 11q and H2AX may contribute to tumor development, progression, and resistance to therapy in this cancer subtype. Additionally, it suggests that other tumors characterized by loss of the distal region of chromosome 11q should be examined for loss of DNA repair efficiency.
These findings have led to the intriguing proposal that human H2AX may be a good candidate gene to indicate susceptibility to lymphomas, leukemia, and other cancers. A study by Novik et al. reported a population-based association of H2AX genetic variants in non-Hodgkins lymphoma (NHL), one of the most commonly diagnosed cancers worldwide (Novik et al. 2007). A G/A single nucleotide polymorphism 417 bp upstream of the H2AX start codon is associated with NHL; the AA genotype is associated with protection from lymphoma, perhaps because the A allele is less easily silenced, while the GG genotype increases lymphoma risk. This is the first study establishing a correlation between an H2AX gene polymorphism and the risk of cancer development in humans. Another recent study has described alterations of H2AX gene copy number in 37% of breast cancer tumor tissues tested (Srivastava et al. 2008).
Further evidence of a tumor-suppressing role for H2AX comes from a study involving human gastrointestinal stromal tumor (GIST) cell lines (Liu et al. 2007). In gastrointestinal stromal tumors, the most common mesenchymal tumors of the gastrointestinal tract, H2AX is downregulated (Liu et al. 2007). Imatinib mesylate, a clinically approved protein kinase inhibitor, has been shown to trigger GIST apoptosis via upregulation of H2AX (Liu et al. 2007). These results imply that increased H2AX expression may help increase tumor sensitivity to chemo- and radiotherapy in a variety of cancers.
In addition to increases in γ-H2AX levels seen in cancer, cells from aging organisms as well as senescing cells in culture display an increased γ-H2AX signal in the absence of any intentional damage. γ-H2AX foci accumulate in senescing human and primate cell cultures as well as in aging mouse tissues including liver, testes, kidney, and lung (Bakkenist et al. 2004; d’Adda di Fagagna et al. 2003; Jeyapalan et al. 2007; Nakamura et al. 2008; Sedelnikova et al. 2004a, b). Moreover, human lymphocytes and fibroblasts from healthy donors exhibit increasing numbers of γ-H2AX foci with increasing age (Sedelnikova et al. 2008). These aging-associated γ-H2AX foci are caused by both dysfunctional telomeres and non-telomeric DNA double-strand damage that may play a causal role in mammalian aging (Nakamura et al. 2008).
γ-H2AX as a biomarker
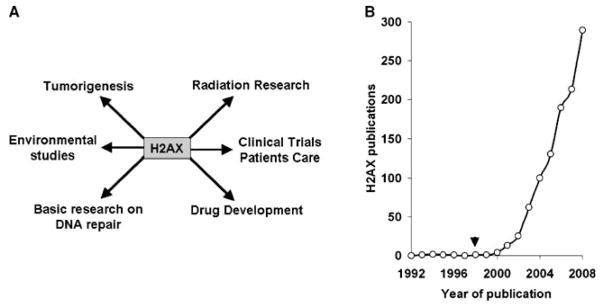
The efficiency of γ-H2AX detection as a biomarker for DNA DSBs makes this protein a good candidate as a therapeutic marker for improving the efficiency of radiation, drug, and other therapies (Halicka et al. 2009; Kao et al. 2006; Kuefner et al. 2009). The use of H2AX in studies examining genome integrity is becoming increasingly common. In addition to basic research studies, H2AX is now also being used in drug development and translational studies (Fig. 3). A highly specific antibody recognizing γ-H2AX in cells was first described by Bonner and colleagues (Rogakou et al. 1999), and antibodies directed against both H2AX and γ-H2AX are commercially available from multiple suppliers (Table 1).
Fig. 3.
a H2AX is being studied in other areas besides basic research on DNA repair, including drug development, translational studies, radiation research, and environmental studies. As cancer cells and tumors often exhibit high levels of γ-H2AX, it is now considered to be a cancer biomarker. b Since its discovery as a DNA double-strand damage marker in 1998 (arrow), the number of papers published each year since 1992 containing H2AX in the title and/or abstract has continually increased (source: PubMed)
Table 1.
Commercial availability of human H2AX and γ-H2AX antibodies and peptides
| Company | Location | H2AX antibody | γ-H2AX antibody | Peptide |
|---|---|---|---|---|
| Abcam Inc. | Cambridge, MA | (R) | (M) (R) | H2AX and γ |
| AbD Serotec | Raleigh, NC | (R) | ||
| Abgent | San Diego, CA | (M) | ||
| Abnova Corp. | Taipei, Taiwan | (M) | (R) | rH2AX |
| ABR Affinity Bioreagents Inc. | Golden, CO | (R) | (M) (R) | |
| Acris Antibodies, GmbH | Hiddenhausen, Germany | (M) (R) | (M) (R) | rH2AX |
| Active motif | Carlsbad, CA | (R) | ||
| Assay Designs/ Stressgen Bioreagents Inc. | Ann Arbor, MI | (M) (R) | rH2AX | |
| Bethyl Laboratories Inc. | Montgomery, TX | (R) | (R) | H2AX and γ |
| Biolegend | San Diego, CA | (R) | (M) | |
| Biovision Inc | Mountain View, CA | (R) | ||
| Calbiochem | San Diego, CA | (R) | (R) | |
| Cell Sciences | Canton, MA | (R) | ||
| Cell Signaling Tech. Inc. | Danvers, MA | (R) | (R) | γ |
| Epitomics, Inc. | Burlingame, CA | (R) | ||
| GeneTex Inc. | San Antonio, TX | (M) (R) | (M) (R) | |
| GenWay biotech, Inc. | San Diego, CA | (R) | (R) | |
| Hycult Biotechnology | Uden, The Netherlands | (R) | ||
| LifeSpan Biosciences Inc. | Seattle, WA | (M) (R) | (M) (R) | |
| MBL international | Woburn, MA | (R) | (R) | |
| Millipore | Billerica, MA | (R) | (M) | rH2AX |
| Novus Biologicals Inc. | Littleton, CO | (M) (R) | (M) (R) | rH2AX |
| OriGene, Inc. | Rockville, MD | (R) | ||
| Proteintech Group Inc. | Chicago, IL | (R) | ||
| Raybiotech, Inc. | Norcross, GA | (R) | ||
| R&D Systems | Minneapolis, MN | (M) | (R) | |
| Santa Cruz Biotechnologies Inc. | Santa Cruz, CA | (G) | (R) | |
| Sigma-Aldrich Co | St. Louis, MO | (M) | (R) | |
| Signalway Antibody | Pearland, TX | (R) | (R) | γ |
| Trevigen Inc. | Gaithersburg, MD | (R) |
(R) rabbit, (M) mouse, (G) goat polyclonal, H2AX H2AX peptide, γ γ-H2AX peptide, rH2AX recombinant H2A
γ-H2AX detection provides a considerably more sensitive, efficient, and reproducible measurement of the amount of DNA damage compared to other techniques such as pulsed field gel electrophoresis and comet assays (Sedelnikova and Bonner 2006). Induction of γ-H2AX after exposure to pleiotropic DNA-damaging agents can be measured by immunofluorescence, flow cytometry, or western blotting (Huang et al. 2005; Kao et al. 2006). Exposure to sources of IR, including X-rays, γ-radiation, α-particles, and heavy ions leads to the direct induction of DSBs in cellular DNA (Desai et al. 2005; Hanasoge and Ljungman 2007; Hu et al. 2005; Rogakou et al. 1999; Usami et al. 2006). In addition, treatment of cells with cytotoxic agents, including but not limited to DNA synthesis inhibitors, DNA alkylating agents, topoiso-merases I and II inhibitors, bleomycin, and hydrogen peroxide, also lead to the formation of DSBs which induce γ-H2AX formation (Furuta et al. 2003; Horikawa et al. 2000; Huang et al. 2003; Liu et al. 2003; Olive et al. 2004; Sedelnikova et al. 2004a, b; Ward and Chen 2001). This DNA damage presumably occurs during the repair or attempted repair of other non-DSB DNA lesions, many of which occur because of interference with replication and transcription complex progression. Thus, the central position of γ-H2AX in DNA DSB detection/repair may give it a significant role in new cancer drug development and treatment optimization through clinical trials ((Hochhauser et al. 2009; Karagiannis and El-Osta 2006; Karp et al. 2008) reviewed in (Bonner et al. 2008)).
Persistence of γ-H2AX foci after the initial induction of DNA damage indicates that some of the damage remains unrepaired, making γ-H2AX an attractive candidate for the rapid assessment of radiation sensitivity in individuals and cell lines (Hamasaki et al. 2007) leading to the identification of cell lines and human subjects with defective DNA repair (Porcedda et al. 2006, 2009; Taneja et al. 2004). Therefore, γ-H2AX may be useful as a biodosimeter (Marchetti et al. 2006) for exposure to IR and as a predictor of radiosensitivity (Olive and Banath 2004; Porcedda et al. 2006) making γ-H2AX a potentially useful tool to enhance the clinical efficacy of radiation treatment, a procedure indicated for approximately 60% of cancer patients (Perez et al. 2004).
It has been found that elevated levels of γ-H2AX are present in a number of human cancer model systems, including cervical cancer cells (Banath et al. 2004; Yu et al. 2006), melanoma cells (Warters et al. 2005), colon carcinomas, fibrosarcoma, osteosarcoma, glioma, and neuroblastoma cells (Sedelnikova and Bonner 2006). These results suggest that an increased level of DNA damage is a general characteristic of cancer development (Banath et al. 2004; Bartkova et al. 2005; Gorgoulis et al. 2005; Sedelnikova and Bonner 2006; Warters et al. 2005; Yu et al. 2006). Moreover, colonocytes from ulcerative colitis patients, a chronic inflammatory disease that predisposes patients to colorectal cancer show an increase in γ-H2AX content (Risques et al. 2008). For these reasons, detection of γ-H2AX through human biopsies and/or aspirates could be used for early cancer screening and to monitor cancer therapy (Sedelnikova and Bonner 2006).
γ-H2AX as a therapeutic target
While H2AX and the PI3 kinases that phosphorylate H2AX have both been proposed as potential therapeutic targets, no drugs directed against these targets are known to be currently in clinical use or development. However, PI3 kinase inhibitors have been developed for research purposes and are available through AstraZeneca/KuDos (Hickson et al. 2004; Veuger et al. 2003). Because H2AX is ubiquitous to all cells, serves a structural role in the integrity of chromatin, and has a relatively long half-life in the cell, the H2AX protein itself may be a problematic drug target. Many commercial H2AX and γ-H2AX peptides and antibodies are available from a variety of companies (listed in Table 1); however, no therapeutic antibodies or peptides are known to be currently in clinical use or development. The lack of therapeutic antibodies may also be attributed at least in part to the strong similarity of H2AX both in structure and sequence to other, essential, H2A histone species.
Inhibition of the phosphorylation of H2AX is probably a more practical therapeutic strategy than alteration of H2AX levels. Peptide inhibitors of H2AX phosphorylation may be useful as chemotherapeutic agents (Kao et al. 2006; Taneja et al. 2004). The effect of H2AX peptides on IR sensitivity was examined using human squamous cell carcinoma cell lines that were either radiosensitive (SCC-61) or radioresistant (SQ-20B). The peptide mimics were found to inhibit γ-H2AX focal formation in both cell lines in response to 3 Gy IR and to decrease cell survival following irradiation (Taneja et al. 2004). These results indicate that H2AX could potentially be targeted to enhance the efficiency of radiation therapy. Additionally, inhibition of H2AX phosphorylation through interference with upstream kinase activities may be an attractive target for drug development. Caffeine and wortmannin which inhibit H2AX phosphorylation are also radiosensitizers (Wang et al. 2005). However, though many tumor cell lines exhibit higher spontaneous levels of γ-H2AX, inhibition of H2AX phosphorylation may be expected to deleteriously affect all cells, not just cancer cells (Yu et al. 2006).
Several patents have been filed pertaining to H2AX. A patent describing a method to detect DNA DSBs using an antibody to γ-H2AX (WO20010104158) was filed by Dr. William M. Bonner of the National Institutes of Health. In addition to this, several more patents have been filed dealing with specific applications of DNA DSB detection to ascertain the genotoxicity of a drug or compound. These include a patent filed by AstraZeneca for the use of γ-H2AX detection in determining the effectiveness of Chk1 inhibitors (WO2006087557), a patent filed by Axys Pharmaceuticals for the use of γ-H2AX detection in determining the effectiveness of HDAC inhibitors (WO2006042035), and a patent filed by Vector Tobacco and New York Medical University for the use of γ-H2AX in approaches to identify less harmful tobacco and tobacco products (WO2005113821). There has also been a patent filed by Dr. Thanos Halazonetis of the University of Geneva, Switzerland for the use of γ-H2AX in detecting pre-cancerous lesions (WO2006105142). Finally, Dr. David Brenner of Columbia University of New York has filed a patent for using γ-H2AX in a system and method for high-throughput radiation biodosimetry (WO2008073168).
γ-H2AX as an indicator of environmental health risks
Induction of γ-H2AX is found following exposure of cells to suspected DNA-damaging compounds such as cigarette smoke, polycyclic aromatic compounds, dinitrobenzo[e] pyrene, norethindrone, chromium, crude oil, electromagnetic fields, microwaves from mobile phones, and extreme heat, all demonstrating a potential role for γ-H2AX detection in determining potential genotoxics (Albino et al. 2004; Gallmeier et al. 2005; Hunt et al. 2007; Ibuki et al. 2007; Kawanishi et al. 2009; Luo et al. 2006; Markova et al. 2005; Mattsson et al. 2009; Peterson-Roth et al. 2005; Shao et al. 2004; Toyooka and Ibuki 2005, 2006, 2009). The radiation induced bystander effect, which can be monitored through DSB induction, can also be tracked using γ-H2AX formation (Sokolov et al. 2007). Additionally, outside of Earth’s atmosphere, the biological effects of high charge and energy ions during space exploration are a major concern for astronaut health. Toward this end, γ-H2AX may be useful in elucidating the effects of space travel-induced DNA damage (Desai et al. 2005; Redon et al. 2009). Finally, γ-H2AX could be used to monitor people exposed to other sources of radiation, answering growing concerns about terrorism threats from dirty bombs.
Conclusions
As discussed above, γ-H2AX is a sensitive indicator of DNA DSBs and is therefore a potentially useful tool in the detection of genotoxic stress. Such an indicator could be valuable in monitoring cancer development and progression as well as other instances of cell stress. Future work in this field will be directed at moving the γ-H2AX detection assay to the clinic where it will be used as a practical means to detect cancer and monitor therapeutic progress. Additionally, the γ-H2AX focus formation assay is a powerful tool to further dissect the cellular response to DNA damage. This technique could be used to identify new potential target proteins for cancer therapeutics as well as to elucidate additional roles for proteins known to participate in the maintenance of genome stability.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute, NIH. We would like to thank Dr. Kurt Kohn for assistance with the design of the Molecular Interaction Map.
Footnotes
Communicated by E. A. Nigg.
Contributor Information
Jennifer S. Dickey, Email: dickeyj@mail.nih.gov, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. National Cancer Institute, Building 37, Room 5050A, 9000 Rockville Pike, Bethesda, MD 20892, USA
Christophe E. Redon, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Asako J. Nakamura, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Brandon J. Baird, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Olga A. Sedelnikova, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
William M. Bonner, Email: bonnerw@mail.nih.gov, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. National Cancer Institute, Building 37, Room 5050A, 9000 Rockville Pike, Bethesda, MD 20892, USA
References
- Albino AP, Huang X, Jorgensen E, Yang J, Gietl D, Traganos F, Darzynkiewicz Z. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: a new assay for carcinogens. Cell Cycle. 2004;3:1062–1068. [PubMed] [Google Scholar]
- Bakkenist CJ, Drissi R, Wu J, Kastan MB, Dome JS. Disappearance of the telomere dysfunction-induced stress response in fully senescent cells. Cancer Res. 2004;64:3748–3752. doi: 10.1158/0008-5472.CAN-04-0453. [DOI] [PubMed] [Google Scholar]
- Banath JP, Macphail SH, Olive PL. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004;64:7144–7149. doi: 10.1158/0008-5472.CAN-04-1433. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bassing CH, Chua KF, Sekiguchi J, et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci USA. 2002;99:8173–8178. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassing CH, Suh H, Ferguson DO, et al. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- Bhogal N, Jalali F, Bristow RG. Microscopic imaging of DNA repair foci in irradiated normal tissues. Int J Radiat Biol. 2009 doi: 10.1080/09553000902785791. (in press) [DOI] [PubMed] [Google Scholar]
- Bogliolo M, Lyakhovich A, Callen E, et al. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO J. 2007;26:1340–1351. doi: 10.1038/sj.emboj.7601574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. gammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Difilippantonio S, Difilippantonio MJ, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003a;114:371–383. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003b;5:675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Chew YC, Camporeale G, Kothapalli N, Sarath G, Zempleni J. Lysine residues in N-terminal and C-terminal regions of human histone H2A are targets for biotinylation by biotinidase. J Nutr Biochem. 2006;17:225–233. doi: 10.1016/j.jnutbio.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Desai N, Davis E, O’Neill P, Durante M, Cucinotta FA, Wu H. Immunofluorescence detection of clustered gamma-H2AX foci induced by HZE-particle radiation. Radiat Res. 2005;164:518–522. doi: 10.1667/rr3431.1. [DOI] [PubMed] [Google Scholar]
- Downey M, Durocher D. gammaH2AX as a checkpoint maintenance signal. Cell Cycle. 2006;5:1376–1381. doi: 10.4161/cc.5.13.2899. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Chen HT, Celeste A, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Celeste A, Nussenzweig A. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle. 2003;2:426–427. [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Fillingham J, Keogh MC, Krogan NJ. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84:568–577. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- Furuta T, Takemura H, Liao ZY, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- Gallmeier E, Winter JM, Cunningham SC, Kahn SR, Kern SE. Novel genotoxicity assays identify norethindrone to activate p53 and phosphorylate H2AX. Carcinogenesis. 2005;26:1811–1820. doi: 10.1093/carcin/bgi132. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Histone modification and replacement in chromatin activation. Genes Dev. 2002;16:1739–1742. doi: 10.1101/gad.1013902. [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Halicka HD, Ozkaynak MF, Levendoglu-Tugal O, et al. DNA damage response as a biomarker in treatment of leukemias. Cell Cycle. 2009;8:1720–1724. doi: 10.4161/cc.8.11.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki K, Imai K, Nakachi K, Takahashi N, Kodama Y, Kusunoki Y. Short-term culture and gammaH2AX flow cytometry determine differences in individual radiosensitivity in human peripheral T lymphocytes. Environ Mol Mutagen. 2007;48:38–47. doi: 10.1002/em.20273. [DOI] [PubMed] [Google Scholar]
- Hanasoge S, Ljungman M. H2AX phosphorylation after UV-irradiation is triggered by DNA repair intermediates and is mediated by the ATR kinase. Carcinogenesis. 2007;28(11):2298–2304. doi: 10.1093/carcin/bgm157. [DOI] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- Hochhauser D, Meyer T, Spanswick VJ, et al. Phase I study of sequence-selective minor groove DNA binding agent SJG-136 in patients with advanced solid tumors. Clin Cancer Res. 2009;15:2140–2147. doi: 10.1158/1078-0432.CCR-08-1315. [DOI] [PubMed] [Google Scholar]
- Horikawa I, Yawata T, Barrett JC. Cellular senescence mechanisms independent of telomere shortening and telomerase: other barriers to cell immortalization and carcinogenesis. J Anti-Aging Med. 2000;3:373–382. [Google Scholar]
- Hu B, Han W, Wu L, et al. In situ visualization of DSBs to assess the extranuclear/extracellular effects induced by low-dose alpha-particle irradiation. Radiat Res. 2005;164:286–291. doi: 10.1667/rr3415.1. [DOI] [PubMed] [Google Scholar]
- Huang X, Traganos F, Darzynkiewicz Z. DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle. 2003;2:614–619. [PubMed] [Google Scholar]
- Huang X, Halicka HD, Darzynkiewicz Z. Detection of histone H2AX phosphorylation on Ser-139 as an indicator of DNA damage (DNA double-strand breaks) Curr Protoc Cytom. 2004;Chapter 7(Unit 7):27. doi: 10.1002/0471142956.cy0727s30. [DOI] [PubMed] [Google Scholar]
- Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif. 2005;38:223–243. doi: 10.1111/j.1365-2184.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CR, Pandita RK, Laszlo A, et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007;67:3010–3017. doi: 10.1158/0008-5472.CAN-06-4328. [DOI] [PubMed] [Google Scholar]
- Ibuki Y, Toyooka T, Shirahata J, Ohura T, Goto R. Water soluble fraction of solar-simulated light-exposed crude oil generates phosphorylation of histone H2AX in human skin cells under UVA exposure. Environ Mol Mutagen. 2007;48:430–439. doi: 10.1002/em.20292. [DOI] [PubMed] [Google Scholar]
- Ikura T, Tashiro S, Kakino A, et al. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27:7028–7040. doi: 10.1128/MCB.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Ferguson D, Song H, Bassing C, Eckersdorff M, Alt FW, Xu Y. Functional interaction of H2AX, NBS1, and p53 in ATM-dependent DNA damage responses and tumor suppression. Mol Cell Biol. 2005;25:661–670. doi: 10.1128/MCB.25.2.661-670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J, Milano MT, Javaheri A, Garofalo MC, Chmura SJ, Weichselbaum RR, Kron SJ. gamma-H2AX as a therapeutic target for improving the efficacy of radiation therapy. Curr Cancer Drug Targets. 2006;6:197–205. doi: 10.2174/156800906776842957. [DOI] [PubMed] [Google Scholar]
- Karagiannis TC, El-Osta A. Modulation of cellular radiation responses by histone deacetylase inhibitors. Oncogene. 2006;25:3885–3893. doi: 10.1038/sj.onc.1209417. [DOI] [PubMed] [Google Scholar]
- Karp JE, Flatten K, Feldman EJ, et al. Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia: a preclinical and phase I trial of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra) combined with etoposide. Blood. 2008 doi: 10.1182/blood-2008-08-172726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi M, Watanabe T, Hagio S, et al. Genotoxicity of 3, 6-dinitrobenzo[e]pyrene, a novel mutagen in ambient air and surface soil, in mammalian cells in vitro and in vivo. Mutagenesis. 2009;24(3):279–284. doi: 10.1093/mutage/gep007. [DOI] [PubMed] [Google Scholar]
- Kohn KW. Molecular interaction map of the mammalian cell cycle control and DNA repair systems. Mol Biol Cell. 1999;10:2703–2734. doi: 10.1091/mbc.10.8.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Sugasawa J, Yasuda M, Koike A. Tissue-specific DNA-PK-dependent H2AX phosphorylation and gamma-H2AX elimination after X-irradiation in vivo. Biochem Biophys Res Commun. 2008;376:52–55. doi: 10.1016/j.bbrc.2008.08.095. [DOI] [PubMed] [Google Scholar]
- Kokandakar HR, Tembhare PR, Mamoon A, Mulay VM, Bhople KS. Acute basophilic leukaemia: a case report. Indian J Pathol Microbiol. 2007;50:443–446. [PubMed] [Google Scholar]
- Kuefner MA, Grudzenski S, Schwab SA, et al. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Invest Radiol. 2009;44 (8):440–446. doi: 10.1097/RLI.0b013e3181a654a5. [DOI] [PubMed] [Google Scholar]
- Liu JS, Kuo SR, Beerman TA, Melendy T. Induction of DNA damage responses by adozelesin is S phase-specific and dependent on active replication forks. Molecular Cancer Therapeutics. 2003;2:41–47. [PubMed] [Google Scholar]
- Liu Y, Tseng M, Perdreau SA, et al. Histone H2AX is a mediator of gastrointestinal stromal tumor cell apoptosis following treatment with imatinib mesylate. Cancer Res. 2007;67:2685–2692. doi: 10.1158/0008-5472.CAN-06-3497. [DOI] [PubMed] [Google Scholar]
- Lu C, Zhu F, Cho YY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Yang J, Zeng QL, Zhu XM, Qian YL, Huang HF. 50-Hertz electromagnetic fields induce gammaH2AX foci formation in mouse preimplantation embryos in vitro. Biol Reprod. 2006;75:673–680. doi: 10.1095/biolreprod.106.052241. [DOI] [PubMed] [Google Scholar]
- Lyakhovich A, Surralles J. New roads to FA/BRCA pathway: H2AX. Cell Cycle. 2007;6:1019–1023. doi: 10.4161/cc.6.9.4223. [DOI] [PubMed] [Google Scholar]
- Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82:605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- Markova E, Hillert L, Malmgren L, Persson BR, Belyaev IY. Microwaves from GSM mobile telephones affect 53BP1 and gamma-H2AX foci in human lymphocytes from hypersensitive and healthy persons. Environ Health Perspect. 2005;113:1172–1177. doi: 10.1289/ehp.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson A, Lundstedt S, Stenius U. Exposure of HepG2 cells to low levels of PAH-containing extracts from contaminated soils results in unpredictable genotoxic stress responses. Environ Mol Mutagen. 2009;50:337–348. doi: 10.1002/em.20486. [DOI] [PubMed] [Google Scholar]
- Mukherjee B, Kessinger C, Kobayashi J, Chen BP, Chen DJ, Chatterjee A, Burma S. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006;5:575–590. doi: 10.1016/j.dnarep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sedelnikova OA, Redon C, et al. Techniques for gamma-H2AX detection. Methods Enzymol. 2006;409:236–250. doi: 10.1016/S0076-6879(05)09014-2. [DOI] [PubMed] [Google Scholar]
- Nakamura AJ, Chiang YJ, Hathcock KS, Horikawa I, Sedelnikova OA, Hodes RJ, Bonner WM. Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence. Epigenetics Chromatin. 2008;1:6. doi: 10.1186/1756-8935-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novik KL, Spinelli JJ, Macarthur AC, et al. Genetic variation in H2AFX contributes to risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:1098–1106. doi: 10.1158/1055-9965.EPI-06-0639. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331–335. doi: 10.1016/j.ijrobp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP, Sinnott LT. Phosphorylated histone H2AX in spheroids, tumors, and tissues of mice exposed to etoposide and 3-amino-1, 2, 4-benzotriazine-1, 3-dioxide. Cancer Res. 2004;64:5363–5369. doi: 10.1158/0008-5472.CAN-04-0729. [DOI] [PubMed] [Google Scholar]
- Parikh RA, White JS, Huang X, et al. Loss of distal 11q is associated with DNA repair deficiency and reduced sensitivity to ionizing radiation in head and neck squamous cell carcinoma. Genes, Chromosomes Cancer. 2007;46:761–775. doi: 10.1002/gcc.20462. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RK. Principles and practice of radiation oncology. Lippincott, Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- Peterson-Roth E, Reynolds M, Quievryn G, Zhitkovich A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol Cell Biol. 2005;25:3596–3607. doi: 10.1128/MCB.25.9.3596-3607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- Porcedda P, Turinetto V, Lantelme E, et al. Impaired elimination of DNA double-strand break-containing lymphocytes in ataxia telangiectasia and Nijmegen breakage syndrome. DNA Repair (Amst) 2006;5:904–913. doi: 10.1016/j.dnarep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Porcedda P, Turinetto V, Orlando L, et al. Two-tier analysis of histone H2AX phosphorylation allows the identification of Ataxia Telangiectasia heterozygotes. Radiother Oncol. 2009;92:133–137. doi: 10.1016/j.radonc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Pui CH, Chessells JM, Camitta B, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17:700–706. doi: 10.1038/sj.leu.2402883. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom OF, Simonsson M, Johansson KA, Nyman J, Turesson I. DNA double strand break quantification in skin biopsies. Radiother Oncol. 2004;72:311–317. doi: 10.1016/j.radonc.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Redon C, Dickey JS, Bonner WM, Sedelnikova O. gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res. 2009;43:1171–1178. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risques RA, Lai LA, Brentnall TA, et al. Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology. 2008;135:410–418. doi: 10.1053/j.gastro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Behm FG, Downing JR. 11q23 rearrangements in acute leukemia. Leukemia. 1996;10:74–82. [PubMed] [Google Scholar]
- Savic V, Yin B, Maas NL, et al. Formation of dynamic gamma-H2AX domains along broken DNA strands is distinctly regulated by ATM and MDC1 and dependent upon H2AX densities in chromatin. Molecular Cell. 2009;34:298–310. doi: 10.1016/j.molcel.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Bonner WM. GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5:2909–2913. doi: 10.4161/cc.5.24.3569. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233–235. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004a;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Panyutin IV, Neumann RD, Bonner WM, Panyutin IG. Assessment of DNA damage produced by 125I-triplex-forming oligonucleotides in cells. Int J Radiat Biol. 2004b;80:927–931. doi: 10.1080/09553000400017648. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci USA. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle. 2007;6:2210–2212. doi: 10.4161/cc.6.18.4682. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Gochhait S, Gupta P, Bamezai RN. Copy number alterations of the H2AFX gene in sporadic breast cancer patients. Cancer Genet Cytogenet. 2008;180:121–128. doi: 10.1016/j.cancergencyto.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Stiff T, Walker SA, Cerosaletti K, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25:5775–5782. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Futamura M, Yamaguchi K, Aoki S, Takahashi T, Saji S. Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut. 2000;47:268–271. doi: 10.1136/gut.47.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja N, Davis M, Choy JS, Beckett MA, Singh R, Kron SJ, Weichselbaum RR. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem. 2004;279:2273–2280. doi: 10.1074/jbc.M310030200. [DOI] [PubMed] [Google Scholar]
- Thirman MJ, Gill HJ, Burnett RC, et al. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993;329:909–914. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Ibuki Y. Co-exposure to benzo[a]pyrene and UVA induces phosphorylation of histone H2AX. FEBS Lett. 2005;579:6338–6342. doi: 10.1016/j.febslet.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Ibuki Y. New method for testing phototoxicity of polycyclic aromatic hydrocarbons. Environ Sci Technol. 2006;40:3603–3608. doi: 10.1021/es060182i. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Ibuki Y. Cigarette sidestream smoke induces phosphorylated histone H2AX. Mutat Res. 2009;676:34–40. doi: 10.1016/j.mrgentox.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE, Koshland D. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Molecular cell. 2004;16:991–1002. doi: 10.1016/j.molcel.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Usami N, Maeda M, Eguchi-Kasai K, Maezawa H, Kobayashi K. Radiation-induced gamma-H2AX in mammalian cells irradiated with a synchrotron X-ray microbeam. Radiat Prot Dosimetry. 2006;122:307–309. doi: 10.1093/rpd/ncl434. [DOI] [PubMed] [Google Scholar]
- van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19(5):207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- Wang H, Wang M, Wang H, Bocker W, Iliakis G. Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J Cell Physiol. 2005;202:492–502. doi: 10.1002/jcp.20141. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Warters RL, Adamson PJ, Pond CD, Leachman SA. Melanoma cells express elevated levels of phosphorylated histone H2AX foci. J Invest Dermatol. 2005;124:807–817. doi: 10.1111/j.0022-202X.2005.23674.x. [DOI] [PubMed] [Google Scholar]
- Xiao A, Li H, Shechter D, et al. WSTF regulates the H2A. X DNA damage response via a novel tyrosine kinase activity. Nature. 2009;457:57–62. doi: 10.1038/nature07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, MacPhail SH, Banath JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst) 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Zha S, Sekiguchi J, Brush JW, Bassing CH, Alt FW. Complementary functions of ATM and H2AX in development and suppression of genomic instability. Proc Natl Acad Sci USA. 2008;105:9302–9306. doi: 10.1073/pnas.0803520105. [DOI] [PMC free article] [PubMed] [Google Scholar]