Abstract
Prominin-1 (Prom1) is recognized as a stem cell marker in several tissues, including blood, neuroepithelium and gut, and in human and mouse embryos and many cancers. Although Prom1 is routinely used as a marker for isolating stem cells, its biological function remains unclear. Here we use a knockout model to investigate the role of Prom1 in the mammary gland. We demonstrate that complete loss of Prom1 does not affect the regenerative capacity of the mammary epithelium. Surprisingly, we also show that in the absence of Prom1, mammary glands have reduced ductal branching, and an increased ratio of luminal to basal cells. The effects of Prom1 loss in the mammary gland are associated with decreased expression of prolactin receptor and matrix metalloproteinase-3. These experiments reveal a novel, functional role for Prom1 that is not related to stem cell activity, and demonstrate the importance of tissue-specific characterization of putative stem cell markers.
Keywords: Tissue-specific stem cells, mammary gland, ductal morphogenesis, tissue regeneration
Introduction
Since its discovery, Prom1 has become recognized as a stem cell marker in several tissues, including neuroepithelium, kidney and intestine as well as human and mouse embryos.(Weigmann et al., 1997; Corbeil et al., 2001; Snippert et al., 2009) It is also postulated to be a cancer stem cell marker in glioblastomas and malignancies of the prostate, liver, colon, lung and others.(Mizrak et al., 2008; Pine et al., 2010) Prom1 is a pentaspan transmembrane protein containing an N-terminal extracellular domain and a C-terminal cytoplasmic tail. It interacts with cholesterol in lipid rafts, but otherwise has no known ligand.(Roper et al., 2000) Although Prom1 is routinely used as a marker for isolating stem cells, its biological function is unclear, and is likely to vary among tissues. Recently, a study of Prom1−/− mice found Prom1 to be required for retinal development, and to be specifically located on membrane evaginations that are precursors to photoreceptive disks.(Zacchigna et al., 2009) Prom1 is also localized to plasma membrane protrusions, including microvilli, in other tissues such as mouse neuroepithelium and kidney proximal tubules.(Weigmann et al., 1997) Due to its location and interactions with cholesterol, Prom1 may function as an organizer of the plasma membrane.(Corbeil et al., 2001)
Prom1 expressing cells of the mouse mammary gland have been previously described as a luminal (cytokeratin 18+) population that exhibits elevated expression of hormone-related genes including those for estrogen receptor α (ERα), progesterone receptor (PR), and prolactin receptor (PrlR). Transplantation of Prom1-enriched cells has demonstrated low regenerative capacity in this population, compared to Prom1− cells or basal cells, suggesting that in normal mammary tissue, Prom1 is not a marker for stem cells.(Sleeman et al., 2007) Nonetheless, Prom1 may have other roles during mammary development and differentiation, and in transformed mammary cells. For example, using in vitro colony assays, a Prom1− luminal cell population has been shown to contain a higher frequency of colony-forming cells, indicating increased luminal progenitor activity in the absence of Prom1.(Sleeman et al., 2007) Additionally, microarray analysis has revealed an increase in Prom1 expression between 4 and 5 weeks of age, soon after the start of pubertal mammary development.(McBryan et al., 2007) Finally, Prom1 expression enriches for cancer stem cell activity in Brca1-associated mouse mammary tumor lines.(Wright et al., 2008)
Here we have used a genetic approach to investigate the functional significance of Prom1 in the mammary gland. Using a knockout model, we demonstrate that the loss of Prom1 does not affect the regenerative capacity of mammary epithelium. Surprisingly, we also show that in the complete absence of Prom1, mammary glands have severely reduced ductal branching, associated with an increased ratio of luminal to basal cells. Prom1−/− luminal cells are also hyper-proliferative under culture conditions. These findings reveal a functional role for Prom1, and provide definitive evidence that Prom1 is not required for stem cell activity in the normal mammary gland.
Results
Prom1−/− tissue retains regenerative capacity
The mouse mammary gland is a useful model for studying stem cell function because the endogenous epithelium can be completely removed, and transplanted epithelium can be tested for its ability to develop into a complete tissue. Accordingly, mammary epithelium from Prom1+/+ and Prom1−/− mice was assayed for its ability to regenerate an entire mammary gland. Prom1−/− tissue was confirmed to be negative for Prom1 expression by immunofluorescence labeling (Figure 1a). Small pieces (1–2 mm2) were taken from post-pubertal, virgin donors and transplanted into the epithelium-divested mammary fat pad of 3 week old nude mice, with Prom1+/+ and Prom1−/− tissue placed contralaterally in the same mouse. After 6 weeks the fat pads were collected as wholemounts and stained for epithelial growth. In 9 of 14 (64%) transplants with Prom1+/+ tissues, full outgrowth was observed. Likewise, 10 of 14 (71%) Prom1−/− transplants produced mammary glands, and we did not detect any difference in the appearance of these outgrowths (Figure 1b). This confirms that stem cell activity is not dependent on Prom1 in the mammary gland.
Figure 1. Prom1−/− tissue retains regenerative capacity.
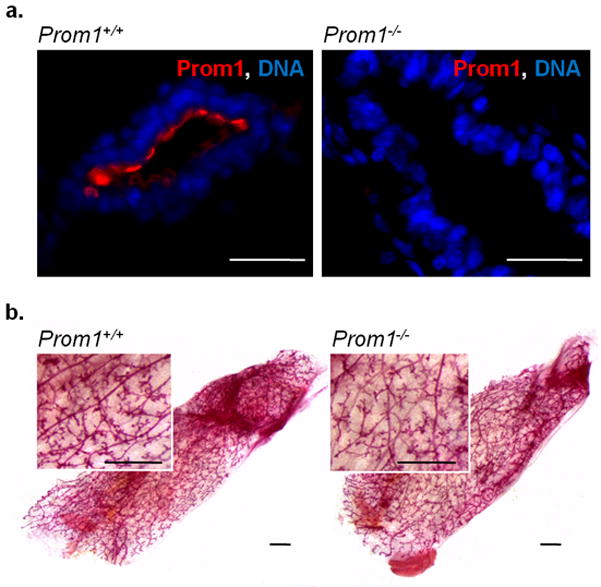
(a) Immunofluorescence shows Prom1 (red) expression on the luminal surface of ducts in Prom1+/+, but not Prom1−/− mammary epithelium; scale bars: 25 μm. (b) Carmine-stained wholemounts of regenerated mammary glands, derived from Prom1+/+ and Prom1−/− tissue; scale bars: 1 mm.
Prom1−/− mammary glands have reduced branching in virgin mice
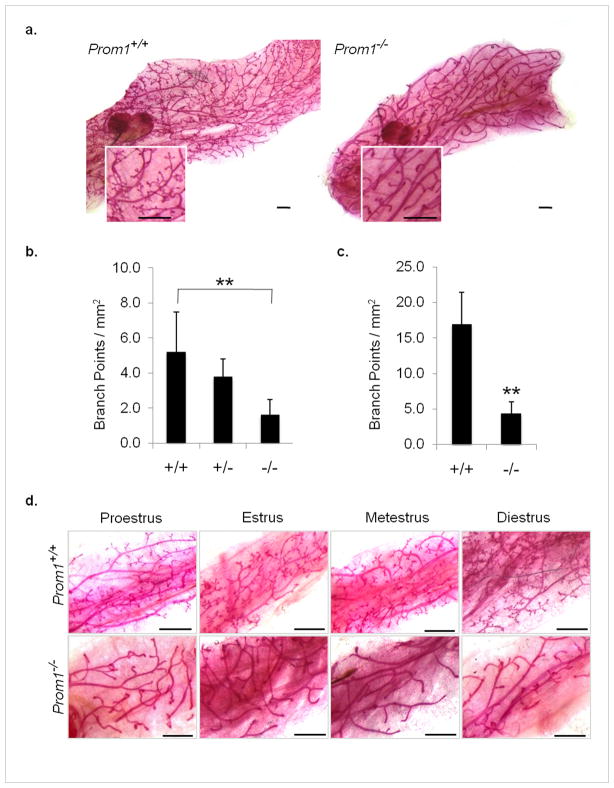
In the absence of a critical role in regeneration, we sought to determine a function for Prom1 in normal mammary gland development. To observe the effects of Prom1 loss on overall gland morphology, we prepared wholemounts from 27 Prom1−/− and 32 age-matched Prom1+/+ control mice, ranging from 4.5–13 weeks of age. Strikingly, the glands of Prom1−/− mice showed reduced branching at all virgin timepoints. At early stages of pubertal development (4.5–5.5 weeks), the elongating ductal trees contained fewer branch points (1.6 ± 0.9 vs. 5.2 ± 2.3 per mm2, n=5). Prom1+/− wholemounts were also observed at this age, and revealed an intermediate phenotype (3.8 ± 1.0 per mm2, n=3) (Figure 2b). By the end of puberty, the primary and secondary ducts of both Prom1−/− and Prom1+/+ glands reached the distal edge of the fat pad, but the Prom1−/− glands were devoid of tertiary branching (Figure 2a,c).
Figure 2. Loss of Prom1 reduces mammary ductal branching.
(a) Severely reduced ductal side branching is apparent in post-pubertal Prom1−/− mammary glands; scale bars: 1 mm. Quantification of branch points per mm2 at (b) 4.5–5.5 weeks and (c) 7.5–9 weeks; n=3–5 mice. (d) Estrus-matched samples from 7.5–9 week old virgin mice confirm that reduced branching in Prom1−/− glands is independent of estrus stage; scale bars: 1 mm. **p<0.01, bars=SD.
We investigated whether this branching defect was caused by an inability of Prom1−/− tissue to respond to basic fibroblast growth factor (bFGF). As has been recently demonstrated, bFGF is sufficient to induce de novo branching from fragments of mammary epithelium suspended in Matrigel. Virgin mammary tissues, or organoids, from 7–8 week old Prom1−/− and Prom1+/+ mice were used for this analysis. Throughout the period of observation (3 weeks), there was no significant difference in the extent of growth or the extent of branching between organoids from the two genotypes (Supplementary Figure 1). These results indicate that Prom1−/− epithelium responds normally to bFGF, and thus it is unlikely that the FGF signaling pathway is at the core of the branching phenotype.
Since the abundance of side branches/alveolar budding on mammary ducts can fluctuate throughout the estrus cycle (Joshi et al., 2010), we sought to determine if these phases affected the Prom1 phenotype. We correlated mammary wholemounts with the estrus stage of each animal at the time of collection, and found that reduced branching was observed in Prom1−/− mice at all stages of the estrus cycle (Figure 2d).
Interestingly, in mammary glands formed from transplanted epithelium, the extent of ductal branching was variable, but not significantly less in those derived from Prom1−/− epithelium (Figure 1b). This is strong evidence that the branching phenotype observed in virgin Prom1−/− mice is due to stromal and/or systemic effects. A similar phenotype is observed in prolactin receptor (PrlR) knockout mice, which fail to produce side branches because PrlR activity in the ovary is required to stimulate the release of progesterone, a mediator of branching morphogenesis.(Oakes et al., 2008) Like Prom1−/−, the PrlR−/− branching phenotype can be rescued by transplanting PrlR−/− epithelium into the fat pad of a wildtype mouse.(Ormandy et al., 2003)
Prom1−/− mammary glands have an increased ratio of luminal to basal cells
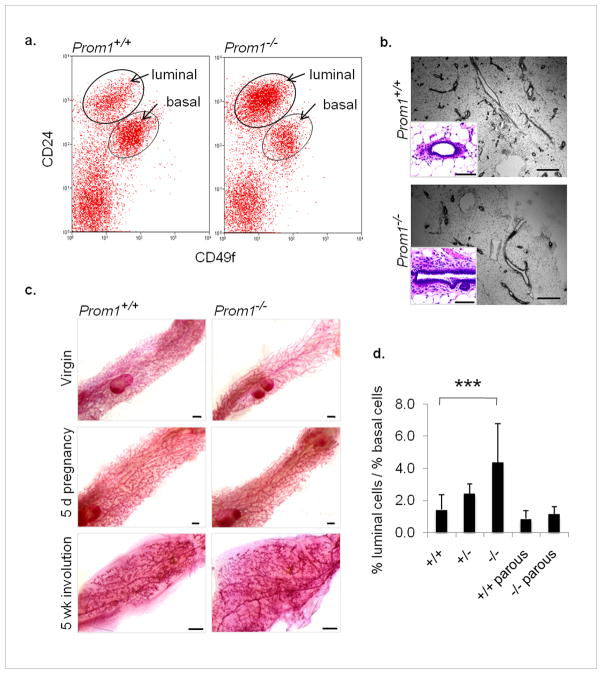
Next we asked if the absence of Prom1, and the consequent branching abnormalities, affected the cellular composition of the ducts. Flow cytometry is an efficient method for characterizing mammary cells according to their expression of cell surface markers. The proteins CD24 (heat stable antigen) and CD49f (α6-integrin) can be used to distinguish epithelial luminal cells (CD24hiCD49flo), epithelial basal cells (CD24medCD49fhi), and non-epithelial cells (CD24−).(Sleeman et al., 2006) We collected mammary cells from Prom1−/− mice and Prom1+/+ controls, and compared their distribution among the CD24/CD49f populations. We found that Prom1−/− mammary glands consistently had a luminal to basal ratio that was over 3-fold higher than in Prom1+/+ mice (Figure 3a). Immunofluorescence and hematoxylin-eosin (H&E) staining did not reveal an aberrant accumulation of luminal cells in Prom1−/− glands (Figure 3b and Supplementary Figure 2a); however, Prom1−/− glands contained a higher proportion of large ducts, which tend to have thicker luminal layers than do small terminal ducts.(Sekhri et al., 1967) Thus the altered ratio may be due to the lack of side branches which contribute to the surface area of the epithelium, and thus the frequency of basal cells. Cell polarization was analyzed by immunofluorescence labeling for the tight junction protein ZO1, and was found to be normal in Prom1−/− glands (Supplementary Figure 2b).
Figure 3. Deficient branching is rescued by pregnancy.
(a) Flow cytometric analysis of mammary epithelial luminal (CD24hiCD49flo) and basal (CD24medCD49fhi) populations; representative dot plots of Prom1+/+ and Prom1−/− samples (n=16). (b) H&E-stained sections reveal normal morphology, but fewer small ducts in Prom1−/− glands; scale bars: 500 μm, inserts 50 μm. (c) Wholemounts from virgin, 5 day pregnant, and involuted (5 week post-lactation) mammary glands; scale bars: 1 mm. (d) Quantification of the ratio of luminal to basal epithelial cells in Prom+/+ and Prom1−/− mammary glands from virgin (n=16) and parous (n=4–6) mice; average ratio from virgin Prom1+/− mice (n=3) is also shown. ***p<0.001, bars=SD.
Deficient branching in Prom1−/− mammary glands is rescued by pregnancy
Pregnancy is marked by a dramatic increase in mammary branching morphogenesis, in response to sudden changes in hormone and growth factor signaling. We found that branching was restored to normal levels by early pregnancy (5 days), such that Prom1−/− glands became indistinguishable from Prom1+/+ glands; this recovery was permanent, as involuted (post-lactational) glands were also identical (Figure 3c). In the normal gland, Prom1 expression is highest in the virgin, and decreases steadily throughout pregnancy (Supplementary Figure 3). This suggests that alternative mechanisms for branching are involved in pregnancy-induced growth compared to pubertal development. Not surprisingly, we found that the recovery of side branching during pregnancy also restored the luminal to basal ratio of epithelial cells in Prom1−/− glands to that of Prom1+/+ controls, as measured in involuted parous samples (Figure 3d).
Prom1−/− cells have increased proliferation in colony assays
In view of the aberrant branching patterns observed during pubertal development, we investigated the role of progenitor cells in Prom1−/− mammary glands. Epithelial cells (CD24+) were collected from Prom1+/+ and Prom1−/− tissue by FACS, and then grown with irradiated fibroblasts for 7 days. This assay is designed to show the frequency of progenitor cells, or colony-forming cells, which corresponds to the number of individual colonies formed per 1000 plated cells.(Asselin-Labat et al., 2007) Interestingly, we did not observe a significant difference in the number of colonies formed by Prom1+/+ versus Prom1−/− cells (57.7 ± 28.2 vs. 61.1 ± 26.7). However, we did find that colonies from Prom1−/− cells were significantly larger than those from Prom1+/+ cells (Figure 4a,b). The large colony size was due to an increase in cell number, rather than an increase in the size of individual cells. Immunofluorescence labeling of colonies with antibodies for cytokeratin 18 and smooth muscle actin (markers of luminal and basal cells, respectively) revealed that the colonies grown in this assay were composed entirely of luminal cells (Supplemental Figure 4a).
Figure 4. Prom1−/− cells have increased proliferation in vitro.
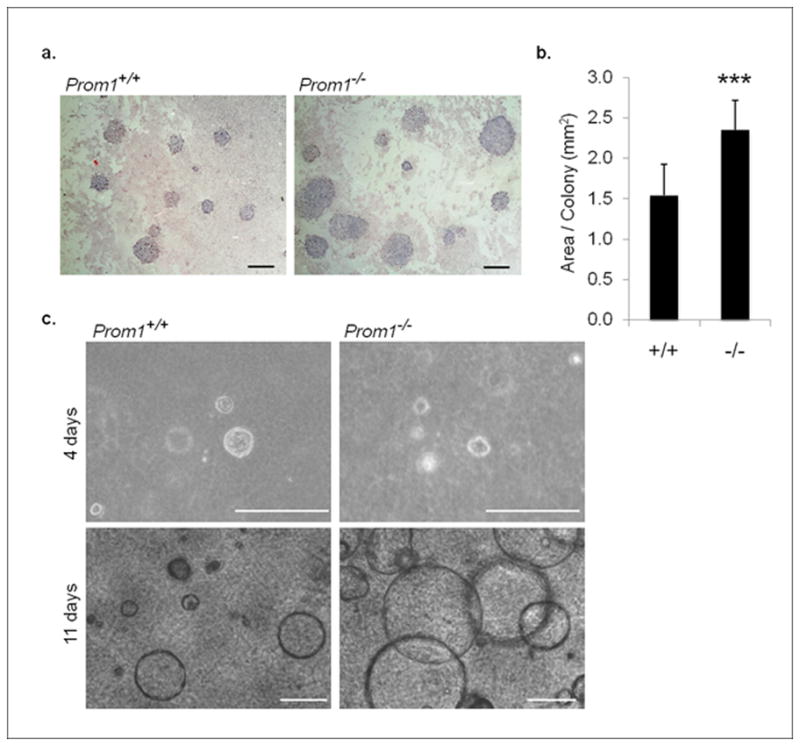
(a) Giemsa-stained 2D culture assays, revealing colonies derived from FACS-sorted epithelial cells; scale bars: 2 mm. (b) Quantification of the area of individual colonies from Prom1+/+ and Prom1−/− cells (n=12). (c) Spherical colonies from Prom1+/+ and Prom1−/− mammary epithelial cells in 3D culture, shown after 4 days and 11 days of growth; scale bars: 200 μm. ***p<0.001, bars=SD.
Similarly, in a three-dimensional culture assay, only luminal cells successfully generated spherical colonies under the culture conditions. While there was no difference in the number of colonies that began to form, or in their structural organization, the colonies derived from Prom1−/− cells were dramatically larger by the second week of culture (Figure 4c). These results demonstrate that Prom1−/− cells, when isolated from a mammary gland and placed as single cells into culture conditions, divide significantly more than their corresponding Prom1+/+ controls. One possible explanation is that Prom1 functions as a repressor of cell division and that in its absence, luminal cells divide more frequently. However, if this were the case, we may expect to observe increased proliferation of luminal cells in Prom1−/− mammary glands in vivo. We stained mammary gland sections from Prom1+/+ and Prom1−/− pubertal mice for proliferation markers, including proliferating cell nuclear antigen (PCNA), phospho-histone H3, and the thymidine analog EdU, and did not observe a statistically significance difference between the genotypes (data not shown). In fact, reverse transcription-PCR (RT-PCR) for cyclin D1(Ccnd1) cDNA, and Western blot analysis for the activation of ERK, suggested lower levels of proliferation in Prom1−/− pubertal mammary tissue (Supplementary Figure 4b,c).
In light of these observations, an alternative explanation for the accelerated growth of Prom1−/− colonies is that incomplete branching morphogenesis during puberty results in the persistence of a reservoir of luminal cells with high replicative potential. To further test this possibility, colony assays were repeated using cells from mice that had undergone a complete cycle of pregnancy, lactation (3 weeks), and involution (at least 5 weeks). As stated above, involuted mammary glands from Prom1+/+ and Prom1−/− look identical, with both showing complete branching morphogenesis (Figure 3c). Surprisingly, accelerated growth was still observed in colonies derived from parous Prom1−/− cells, compared to parous Prom1+/+ cells (Supplemental Figure 4d). Thus, it appears that mammary cells from Prom1−/− mice retain an increased proliferative capacity in culture conditions, independent of the developmental status of the gland from which they were isolated. This raises the intriguing possibility that Prom1−/− luminal cells are perpetually maintained in a more progenitor-like state.
Hormone receptors and gene expression in Prom1−/− mice
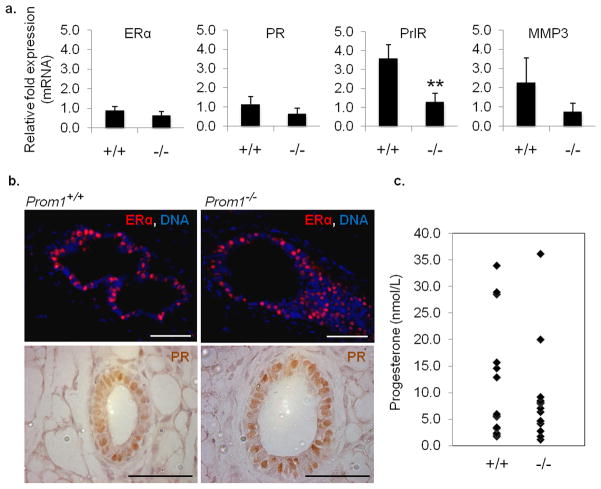
Previous research has indicated that Prom1 expression distinguishes between two luminal populations in the mammary gland: a hormone receptor positive, or ‘hormone sensing’ population (CD24hiProm1+), and a hormone receptor negative population (CD24hiProm1−).(Sleeman et al., 2007) We investigated the expression and distribution of hormone receptors in the Prom1−/− mammary gland. Surprisingly, quantitative RT-PCR (qRT-PCR) did not reveal a significant difference in the mRNA levels of ERα or PR (Figure 5a). Using immunofluorescence and immunohistochemistry, we found abundant expression of ERα and PR proteins in the luminal layer of Prom1−/− epithelium, similar to Prom1+/+ controls (Figure 5b), demonstrating that a population of hormone sensing luminal cells exists even in the absence of Prom1. However, in contrast to ERα and PR, PrlR transcripts were found to be significantly decreased in Prom1−/− mammary tissue during early (4–5 week old) pubertal development. Inasmuch as PrlR−/− mice show a similar lack of side branching in virgin mice, this may be evidence of a functional link between Prom1 expression and the PrlR signaling pathway. Since both proteins can be located in lipid rafts, Prom1 may interact with and stabilize PrlR, leading to downstream signaling.(Piazza et al., 2009) In addition to PrlR, gene transcripts for matrix metalloproteinase-3 (MMP3), which is known to affect side branching in the pubertal mammary gland (Wiseman et al., 2003), were also down-regulated in Prom1−/− glands. MMP3 functions in the stroma surrounding mammary epithelium, and mediates side branching by degrading the basement membrane. Whether the observed decrease in MMP3 expression is a cause of the branching phenotype, or an effect of aberrant signaling through other pathways, remains to be determined.
Figure 5. Gene expression and hormone levels are altered in Prom1−/− mice.
(a) Relative abundance of mRNA from the mammary glands of young (4–5 week old) Prom1+/+ and Prom1−/− mice, showing no significant difference for ERα and PR expression, but 3-fold decreased expression of both PrlR and MMP3 in Prom1−/− tissue (n=3–4). (b) Immuno-staining for ERα and PR proteins in Prom1+/+ and Prom1−/− mammary ducts; scale bars: 50 μm. (c) Quantification of serum progesterone levels (n=14). **p<0.01, bars=SD.
In addition to hormone receptor expression, we compared the levels of serum progesterone between the genotypes, since this hormone has a direct role in mammary branching, and it is secreted from the ovary in a PrlR-dependent manner. Blood serum samples were collected from 14 Prom1+/+ and 14 Prom1−/− mice immediately prior to euthanization at 7 weeks of age. There was variability across both sets of samples, which is likely due to estrus cycling. While a statistically significant difference was not observed, there was a trend toward lower progesterone levels in Prom1−/− mice (Figure 5c). This is suggestive of, but not definitive proof for a hormone-mediated mechanism of branching deficiency in Prom1−/− mice, which would agree with our findings that either pregnancy or transplantation into Prom1+/+ hosts abolishes the phenotype. Such a mechanism may involve the ovary via PrlR expression, as previously described, or other factors such as cilia. Loss of cilia function in the ovary results in decreased hormone secretion, and a consequent retardation of mammary ductal development.(Johnson et al., 2008) Furthermore, it was recently shown that primary cilia contribute to branching morphogenesis in the mammary gland, and that cilia-deficient glands resemble those of Prom1−/− mice.(McDermott et al., 2010) We analyzed the distribution of cilia in both ovarian and mammary tissue from Prom1−/− and Prom1+/+ mice, but no significant difference was observed (Supplemental Figure 5 a,b). However, as Prom1 is known to localize to membrane protrusions, it remains possible that the functionality of cilia is impaired in the absence of Prom1, leading to defects in downstream signaling. .
Discussion
Prom1 expression is widespread throughout the differentiated tissues of many organs, including liver, lung and colon, as well as most (if not all) glandular epithelia (Karbanova et al., 2008; Shmelkov et al., 2008), and the function of Prom1 must be studied in a tissue-specific manner. The data presented here demonstrate a role for Prom1 in mouse mammary gland development, specifically in branching morphogenesis, and demonstrate conclusively that Prom1 is not required for stem cell activity in the mouse mammary gland. Using Prom1−/− mice, we have shown that side branching is severely reduced in pubertal and adult virgin mice, and that this phenotype is unaffected by normal estrus cycling. Side branching is, however, recovered by pregnancy, suggesting that pregnancy-associated branching mechanisms, such as those involving Jak2-Stat5 signaling, occur normally. Additionally, we have demonstrated that Prom1 loss leads to hyper-proliferation of mammary epithelial cells in primary culture conditions. Examination of the expression profile of Prom1 in the NKI 295 breast cancer dataset suggests that it is unlikely that Prom1 is a tumor suppressor as Prom1 expression was not significantly decreased (or increased) in patients with lymph nodes metastases (p=0.15), distant metastases (p=0.22), 5-year recurrence (p=0.96), 5-year survival (p=0.23), or overall death (p=0.28). Prom1 expression was, however, significantly lower (p=8.09E-11) in samples that expressed the estrogen receptor gene (Esr1).(van de Vijver et al., 2002) This is interesting because previous work has shown that Prom1 and ERα expression usually coincide (Sleeman et al., 2007), and is indicative of the altered nature of ERα+ cells in the context of cancer.
A defined, molecular mechanism for Prom1 activity has yet to be described in any tissue. In the context of the mammary gland, we have attempted to correlate Prom1 expression with known signaling pathways that are involved in mammary development. Prolactin, acting in the ovary, mediates the release of progesterone, which promotes side branching during puberty via PrlR. Additionally, MMP3 directly mediates branching by degrading the basement membrane around the ducts.(Wiseman et al., 2003) Both PrlR and MMP3 deficiencies result in mammary phenotypes similar to that of the Prom1−/− mouse. Not surprisingly, a corresponding down-regulation of these molecules was observed in the mammary tissue of Prom1−/− mice. A tendency toward lower progesterone levels was also found. Taken together, these results indicate a complex network of factors that involves both systemic hormones and local mediators of branching morphogenesis in the virgin gland. A direct interaction between these factors and Prom1 itself has not yet been achieved. Indeed, the molecular behavior of Prom1 still requires investigation, both in the context of mammary gland development, and in other tissues and cells in which Prom1 is expressed.
In conclusion, these experiments reveal a novel role for Prom1 in mammary gland development that is independent of stem cell activity and regeneration, and further demonstrate the importance of understanding the functional roles, if any, of putative stem/cancer stem cell markers.
Methods
Mice
Prom1−/− mice were generated as previously described.(Zacchigna et al., 2009) Both Prom1−/− and Prom1+/+ control mice had a 50/50 129/Swiss background. Vaginal smears for estrus staging were taken concurrent with tissue collection, fixed in acetone, and stained with hematoxylin. Blood samples were collected under terminal anesthesia. Hormone levels were measured with the DELFIA progesterone immunoassay (A066-101, PerkinElmer) at the Addenbrooke’s Mouse Biochemistry Laboratory (Cambridge, UK). All experiments involving Prom1−/− and Prom1+/+ control mice were performed according to the guidelines for the care and use of laboratory animals approved by the institutional ethical animal care committee of Katholieke Universiteit Leuven.
Mammary fat pad transplantation
Endogenous mammary epithelium was cleared from 3 week old female nude (CD-1-Foxn1nu) mice by surgically removing the proximal portion of the inguinal fat pads (nipple to lymph nodes). Small (1–2 mm2) fragments of mammary tissue were placed into the remaining portion of the fat pads, and allowed to grow for 6 weeks. Transplant surgeries involving nude mice were done at the National Cancer Institute and University of Cambridge, and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the UK Home Office guidelines; these procedures were approved by the National Cancer Institute Animal Care and Use Committee and the local ethical committee at the University of Cambridge.
Wholemounts and histology
Wholemounts were soaked overnight in Carnoy’s fixative, stained with Carmine Alum, and cleared in ethanol and xylene. Frozen sections were cut from fresh mammary tissue that was fixed in 4% buffered paraformaldehyde for 1 hour and embedded in OCT matrix (LAMB/OCT, Thermo Fisher). Sections were cut at 6–10 μm, blocked in 5% normal goat serum (G9023, Sigma), and labeled with antibodies for 1 hour at room temperature. Antibodies used were anti-Prom1 clone 13A4 (14-1331, eBioscience) and Cy3 anti-rat IgG (A10522, Invitrogen). Nuclei (DNA) were counterstained with Hoechst 33342. Paraffin-embedded sections (5 μm thick) were prepared from mammary tissue fixed in 4% buffered paraformaldehyde overnight. For immunofluorescence, antibodies used were anti-ERα (sc-542, Santa Cruz), anti-cytokeratin 18 (65028, Progen), anti-cytokeratin 14 (ab53115, Abcam), anti-ZO1 (MAB1520, Chemicon), anti-PCNA (sc-56, Santa Cruz), anti-phospho-histone H3 (9701S, Cell Signaling), anti-acetylated α-tubulin (T7451, Sigma), Cy3 anti-rabbit IgG (C2306, Sigma), Cy3 anti-mouse IgG (C2181, Sigma), Cy3 anti-rat IgG, Alexa Fluor 488 anti-rabbit IgG (A11008, Invitrogen), and Alexa Fluor 488 anti-mouse IgG (A11001, Invitrogen). EdU was administered by intraperitoneal injection of 250 μg in PBS, 24 hours before tissue collection, and detected per manufacturer’s instructions (C10339, Invitrogen). H&E staining was according to standard protocols. Immunohistochemistry was performed using an antibody against progesterone receptor (A0098, Dako), HRP-conjugated anti-rabbit antibody, and standard DAB protocols.
bFGF branching assay
Mammary branching was done as previously described.(Ewald et al., 2008) Briefly, mammary tissue was digested with trypsin, collagenase, and DNase. Organoids were resuspended in Matrigel (354230, BD) and incubated in minimal media [DMEM/F-12, 1% ITS (I3146, Sigma), 1% pen/strep], or minimal media plus 2.5 nM bFGF (F0291, Sigma).
Fluorescence-activated cell sorting (FACS)
Mammary tissue was digested overnight at 37°C in DMEM/F-12 containing 1 mg/ml collagenase (10103578001, Roche) and 1,000 U/ml hyaluronidase (H3506, Sigma) then further dissociated with 5 mg/ml dispase (D4693, Sigma), 0.1 mg/ml DNase (D4513, Sigma), and 0.05% trypsin-EDTA. Red blood cells were removed with ammonium chloride. Hematopoietic lineage cells were labeled and excluded using the following antibodies: biotin anti-CD31 (13-0311, eBioscience), biotin anti-CD45 (13-0451, eBioscience), biotin anti-TER-119 (13-5921, eBioscience), and Streptavidin-PE-Texas Red (551487, BD). Dead cells were labeled and excluded with propidium iodide. Epithelial cells were labeled with PE anti-CD24 (12-0242, eBioscience) and Alexa Fluor 647 anti-CD49f (313610, BioLegend) antibodies. Cells were sorted using a MoFlo XDP sorter (Beckman Coulter).
Colony assays
FACS-sorted epithelial cells (250 cells/ml) were collected in EpiCult-B medium (05610, Stem Cell Technologies) with supplements and 10,000 cells/ml irradiated 3T3 fibroblasts and plated on Nunclon polystyrene dishes (150288, VWR). Cells were left undisturbed for 7 days, then fixed with methanol/acetone and stained with Giemsa. Colonies were photographed and measured using ImageJ (http://rsb.info.nih.gov/ij/). For immunofluorescence, colonies were labeled with the following antibodies: anti-α-smooth muscle actin (ab5694, Abcam), anti-cytokeratin 18, Alexa Fluor 488 anti-rabbit IgG, and Cy3 anti-mouse IgG. For three-dimensional culture, FACS sorted cells were resuspended in a mixture of collagen and basement membrane extract (BME) as previously described.(Jechlinger et al., 2009)
Molecular analysis
RNA was extracted from frozen tissues using TRIzol Reagent (15596, Invitrogen) and the RNeasy Mini Kit (74104, Qiagen). cDNA was prepared using the SuperScript First-Strand Synthesis System for RT (11904, Invitrogen). SYBR Green JumpStart Taq ReadyMix (S4438, Sigma) was used for qRT-PCR. Primer sequences were as follows: ERα, 5′-GCTTTGGTGTGAAGGGTCAT -3′ and 5′-CTCTGGGCGACATTCTTCTC -3′; PR, 5′-GGTCCCCCTTGCTTGCA -3′ and 5′-CAGGACCGAGGAAAAAGCAG -3′; PrlR, 5′-GCTCACCTCCACAGAGAAGC -3′ and 5′-CGTTCTGGATTTTACACGGG -3′; MMP3, 5′-ACATGGAGACTTTGTCCCTTTTG -3′ and 5′-TTGGCTGAGTGGTAGAGTCCC -3′; Ccnd1, 5′-GCAGGAGAGGAAGTTGTTGG -3′ and 5′-AGACCTTTGTGGCCCTCTGT -3′; Gapdh, 5′-ACCACAGTCCATGCCATCAC -3′ and 5′-TCCACCACCCTGTTGCTGTA -3′. Western blot analysis was performed according to standard protocols, using the following antibodies: anti-phospho-ERK (9106, Cell Signaling), anti-pan-ERK (610124, BD), and HRP-conjugated anti-mouse (P0447, Dako).
Supplementary Material
Organoids from Prom1+/+ and Prom1−/− mammary tissue grown in Matrigel for 7 days, with or without bFGF supplementation; scale bars: 100 μm.
Tissue sections from Prom1+/+ and Prom1−/− mice labeled with antibodies against (a) cytokeratin 18 (K18, red) and cytokeratin 14 (K14, green), and (b) ZO1 (red) and K14 (green), showing normal distribution in the mammary ducts of both genotypes; scale bars: 50 μm.
Immunofluorescence of Prom1 (red) expression in virgin mammary tissue and throughout pregnancy; scale bars: 50 μm.
(a) Individual colonies established in 2D culture, labeled for K18 (red) and smooth muscle actin (SMA, green); scale bars: 100 μm. (b) Western blot analysis of ERK activation (phosphorylation) in mammary tissue from 4–5 week old Prom1+/+ and Prom1−/− mice, with a β-actin loading control. (c) RT-PCR analysis of cyclin D1 (Ccnd1) cDNA levels in mammary tissue from 4–5 week old Prom1+/+ and Prom1−/− mice, with a Gapdh control. (d) Quantification of the area of individual colonies from Prom1+/+ and Prom1−/− cells collected from virgin (n=2) and parous (n=4) mice. *p<0.05, **p<0.01, bars=SD.
Immunofluorescence labeling for cilia (acetylated α-tubulin, red) in (a) ovarian follicles, and (b) mammary ducts, from Prom1+/+ and Prom1−/− mice; scale bars: (a) 25 μm, (b) 50 μm. O=oocyte, fc=follicle cells.
Acknowledgments
Grants:
(L.H.A., C.A.B, and G.H.S.) Grant Sponsor: NCI, NIH; Intramural Research Program of the Center for Cancer Research.
(P.C.) Grant Sponsor: Flemish Government; Methusalem funding and grant number FWO G.0209.07.
The authors thank Anna Kuchnio and Ann Bouché for collection of blood samples.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions:
Lisa H. Anderson—conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Corinne A. Boulanger—collection of data, critical reading of manuscript and edits
Gilbert H. Smith—financial support, provision of study materials, critical reading of manuscript and edits
Peter Carmeliet—provision of study materials, critical reading of manuscript and edits
Christine J. Watson—conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript
References
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M, Podsypanina K, Varmus H. Regulation of transgenes in three-dimensional cultures of primary mouse mammary cells demonstrates oncogene dependence and identifies cells that survive deinduction. Genes Dev. 2009;23:1677–1688. doi: 10.1101/gad.1801809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ET, Nicola T, Roarty K, Yoder BK, Haycraft CJ, Serra R. Role for primary cilia in the regulation of mouse ovarian function. Dev Dyn. 2008;237:2053–2060. doi: 10.1002/dvdy.21612. [DOI] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote P, Clarke C, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Karbanova J, Missol-Kolka E, Fonseca AV, Lorra C, Janich P, Hollerova H, Jaszai J, Ehrmann J, Kolar Z, Liebers C, Arl S, Subrtova D, Freund D, Mokry J, Huttner WB, Corbeil D. The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J Histochem Cytochem. 2008;56:977–993. doi: 10.1369/jhc.2008.951897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryan J, Howlin J, Kenny PA, Shioda T, Martin F. ERalpha-CITED1 co-regulated genes expressed during pubertal mammary gland development: implications for breast cancer prognosis. Oncogene. 2007;26:6406–6419. doi: 10.1038/sj.onc.1210468. [DOI] [PubMed] [Google Scholar]
- McDermott KM, Liu BY, Tlsty TD, Pazour GJ. Primary Cilia Regulate Branching Morphogenesis during Mammary Gland Development. Curr Biol. 2010;20:731–737. doi: 10.1016/j.cub.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ. Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13:13–28. doi: 10.1007/s10911-008-9069-5. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Naylor M, Harris J, Robertson F, Horseman ND, Lindeman GJ, Visvader J, Kelly PA. Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Recent Prog Horm Res. 2003;58:297–323. doi: 10.1210/rp.58.1.297. [DOI] [PubMed] [Google Scholar]
- Piazza TM, Lu JC, Carver KC, Schuler LA. SRC family kinases accelerate prolactin receptor internalization, modulating trafficking and signaling in breast cancer cells. Mol Endocrinol. 2009;23:202–212. doi: 10.1210/me.2008-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine SR, Ryan BM, Varticovski L, Robles AI, Harris CC. Microenvironmental modulation of asymmetric cell division in human lung cancer cells. Proc Natl Acad Sci U S A. 2010;107:2195–2200. doi: 10.1073/pnas.0909390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- Sekhri KK, Pitelka DR, DeOme KB. Studies of mouse mammary glands. I. Cytomorphology of the normal mammary gland. J Natl Cancer Inst. 1967;39:459–490. [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, Clevers H. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187–2194. e2181. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Oh H, Wilsch-Brauninger M, Missol-Kolka E, Jaszai J, Jansen S, Tanimoto N, Tonagel F, Seeliger M, Huttner WB, Corbeil D, Dewerchin M, Vinckier S, Moons L, Carmeliet P. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29:2297–2308. doi: 10.1523/JNEUROSCI.2034-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organoids from Prom1+/+ and Prom1−/− mammary tissue grown in Matrigel for 7 days, with or without bFGF supplementation; scale bars: 100 μm.
Tissue sections from Prom1+/+ and Prom1−/− mice labeled with antibodies against (a) cytokeratin 18 (K18, red) and cytokeratin 14 (K14, green), and (b) ZO1 (red) and K14 (green), showing normal distribution in the mammary ducts of both genotypes; scale bars: 50 μm.
Immunofluorescence of Prom1 (red) expression in virgin mammary tissue and throughout pregnancy; scale bars: 50 μm.
(a) Individual colonies established in 2D culture, labeled for K18 (red) and smooth muscle actin (SMA, green); scale bars: 100 μm. (b) Western blot analysis of ERK activation (phosphorylation) in mammary tissue from 4–5 week old Prom1+/+ and Prom1−/− mice, with a β-actin loading control. (c) RT-PCR analysis of cyclin D1 (Ccnd1) cDNA levels in mammary tissue from 4–5 week old Prom1+/+ and Prom1−/− mice, with a Gapdh control. (d) Quantification of the area of individual colonies from Prom1+/+ and Prom1−/− cells collected from virgin (n=2) and parous (n=4) mice. *p<0.05, **p<0.01, bars=SD.
Immunofluorescence labeling for cilia (acetylated α-tubulin, red) in (a) ovarian follicles, and (b) mammary ducts, from Prom1+/+ and Prom1−/− mice; scale bars: (a) 25 μm, (b) 50 μm. O=oocyte, fc=follicle cells.