Abstract
The novel Cav1.3 (α1D) L-type Ca2+ channel plays a significant role in sino-atrial, atrioventricular nodes function and in atrial fibrillation. However, the characterization of α1D Ca2+ channel during heart development is very limited. We used real-time RT-PCR, Western blotting and indirect immunostaining to characterize the developmental expression and localization of α1D Ca2+ channel in rat hearts. Both protein and mRNA levels of α1D Ca2+ channel decreased postnatally. Two forms of α1D Ca2+ channel protein (250 kD and 190 kD) were observed, with the full length (250kD) channel protein being predominant in the prenatal stages. Both Western blots and confocal imaging demonstrated that α1D Ca2+ channel protein was expressed in both atria and ventricles at fetal and neonatal stages but was absent in the adult ventricles. Interestingly, α1D Ca2+ channel was also found at the nucleus/perinucleus of immature, but not adult atrial cells. Furthermore, the nuclear staining was reproduced in adult atrial cell line, HL-1 cells, which possess immature properties. The data are first to show that α1D Ca2+ channel has unique age-dependent expression profile and subcellular localization in the heart, suggesting a developmental stage dependent specific function.
INTRODUCTION
The L-type Ca2+ channel is a heterologomeric complex of α1, β, and α2/δ subunits (1). Four genes encode L-type Ca2+ channel α1 subunits in mammals (α1C, α1S, α1D, α1F) (1, 2). α1C (Cav1.2) represents the most abundant isoform in the cardiovascular system, whereas α1D (Cav1.3) is expressed in neurons and neuroendocrine cells (3, 4). It is therefore believed that the contribution of L-type Ca2+ current (ICa-L) to the physiology/pathophysiology of the heart is mainly mediated through α1C Ca2+ channel.
The functional role of α1D in the heart has been addressed by several published reports (5–7) including from our lab (8). The emerging consensus is that because of the low activation voltage (−60mv to −50mV) and abundant expression in the SA and AV nodes, α1D plays an important role in the pacemaker activity (diastolic depolarization) and action potential conduction at the AV node. This is further supported by data showing that genetic deletion of α1D causes sinus bradycardia and various degrees of AV-block (5–7) and makes the mouse prone to atrial fibrillation (8). Compared with α1C, the α1D Ca2+ has less sensitivity to dihydropyridines (1, 9, 10). Despite these subtle differences, to date, there are no available pharmacological or biophysical approaches to functionally dissect α1D from α1C Ca2+ current in the native tissue.
Developmental change of the α1C Ca2+ channel in the heart has been extensively studied (11, 12). Lower α1C Ca2+ channel expression levels have been demonstrated in immature hearts, followed by an increase with cardiac maturation in both rat and human (11, 13). It is generally believed that a higher expression of the T-type Ca2+ channel and the Na/Ca exchanger compensate for the low levels of the α1C Ca2+ channel and enable the immature heart to maintain functional contractility. However, there is very limited information on the developmental changes of the α1D Ca2+ channel in the heart. This study is the first to characterize the expression levels of the α1D Ca2+ channel and its subcellular localization during development in the rat heart.
METHODS
Use of rats
Fetal (17–20 days gestation), Neonate (1–3 day old), juvenile (4- to 6- weeks old) and adult (6- to 8-months old) Sprague-Dawley rats of either sex used in this study were approved by IACUC at VA New York Harbor Healthcare System.
Real-Time RT-PCR
TaqMan Real-time RT-PCR was performed using 18S ribosomal RNA as internal control. Total RNAs were prepared from the atria at various developmental stages as previously described (14, 15). Predesigned and labeled primer/Taqman probe sets for α1D were purchased (Applied Biosystems, CA). The conditions for real-time RT-PCR was preheating at 50°C for 2 min and at 95°C for 10 min, followed by 40 cycles of shuttle heating at 95°C for 15 s and at 60°C for 1 min. The cycle threshold Ct value for each sample that was proportional to the log of the initial amount of input cDNA was calculated and plotted. 18S rRNA was used as internal control.
Protein extraction and Western blot
Membrane proteins were prepared as previously described (15). Same amount of membrane proteins (50 µg to 100 µg) were loaded for each lane of 4–12% SDS polyacrylamide gels. The immunoblots were incubated overnight at 4 °C with 1:200 primary anti-α1D Ca2+ channel antibodies (Calbiochem, CA), developed with horseradish peroxidase-labeled anti-rabbit antibody, and detected by enhanced chemiluminescence (ELC) (Amersham, NJ). The density of protein bands was quantified by the NIH image software (http://rsbweb.nih.gov/nih-image/). Anti-α1D antibody pre-incubated with its antigenic peptide was included as a negative control.
Isolation of cardiac myocytes
Cardiac myocytes of juvenile and adult rats were obtained from Langendorff-perfused hearts as previously described (16). Hearts were perfused at 37 °C with a HEPES buffered solution containing 1.5 mg/ml collagenase type B (Boehringer Mannheim, Germany) for 8 to 15 minutes and then dispersed then dispersed in a KB solution containing (mmol/L): K glutamate 70, KCl 30, KH2PO4 10, MgCl2 1, taurine 20, glucose 10, HEPES 10. Isolation of fetal and neonatal rat atrial and ventricular myocytes was performed using chopping method with trypsin digestion as described (17) and cells were cultured on cover slips with Dulbecco’s Modified Eagle’s Medium containing 10% calf serum (Gibco, CA), 2% penicillin/streptomycin, before use for immunostainings and transfections.
Maintenance of adult atrial HL-1 cells
The HL-1 cells were cultured in Claycomb medium supplemented with 10% fetal bovine serum (Invitrogen, CA), 2 mM L-glutamine, 100 µM norepinephrine, 100 U/ml penicillin and 100 µg/ml streptomycin on pre-coated flasks with fibronectin (18).
Indirect immunofluorescence staining
Briefly, immunofluorescent stainings were performed as previously described (14). Cardiac cells were permeabilized, blocked, incubated overnight at 4°C with primary anti-α1D antibodies against a peptide [(KY)DNKVTIDDYQEEADKD)] corresponding to residues 809–825 of rat brain α1D subunit (1:200, Calbiochem, CA and Sigma, MO), and detected with FITC-conjugated anti-rabbit antibody (1:200, Johnson Immunol, PA) and viewed with a confocal scanning laser microscope (MRC-600; Bio-Rad, CA) using XYZ scan. Surface plot was used to illustrate anti-α1D antibody staining. Secondary antibody alone and anti-α1D antibody pre-incubated with its antigenic peptide were included as negative controls.
Statistic analysis
Statistical comparisons were evaluated using unpaired student t-test, and one-way ANOVA as appropriate. Data are presented as mean ± SEM. A value of P<0.05 is considered significant.
RESULTS
Perinatal and postnatal expression level of α1D mRNA
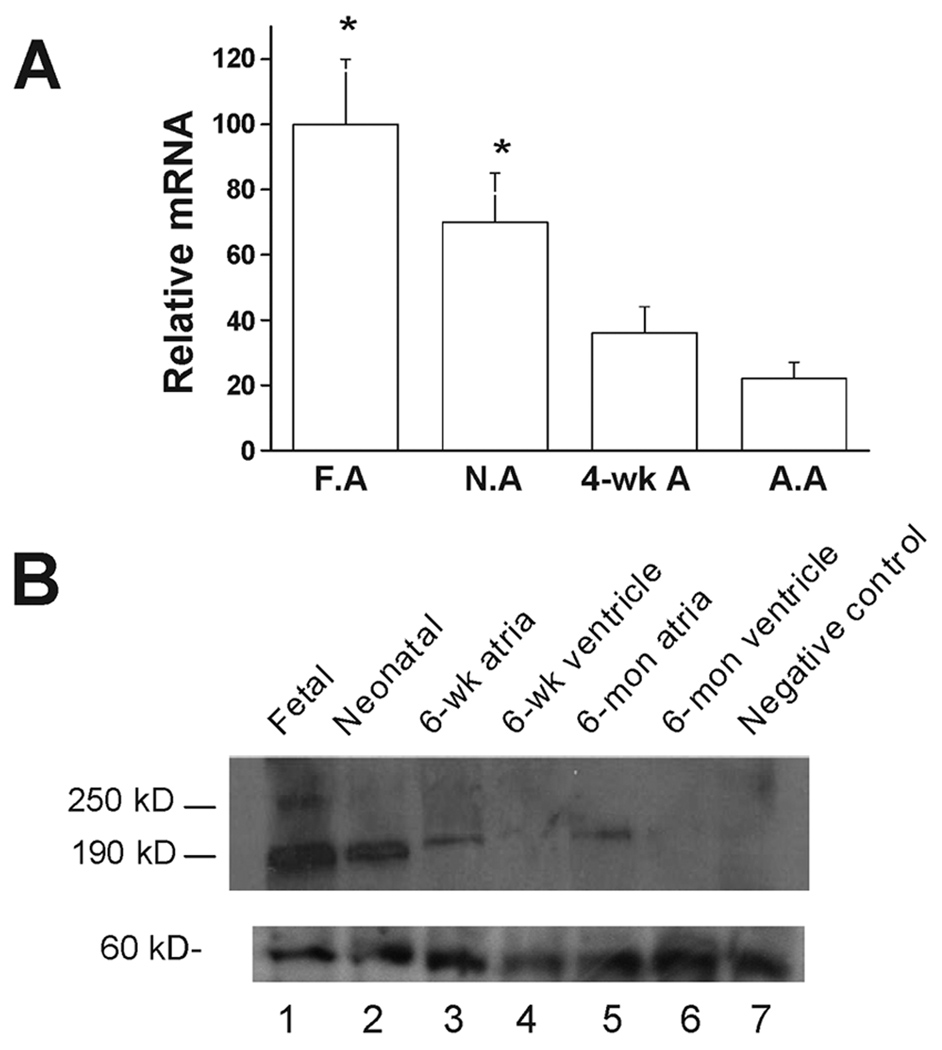
The α1D Ca2+ channel mRNA level during heart development was carried out by real time RT-PCR using total RNA isolated from fetal, neonatal, 4–6 weeks and 6–8-months old rat atrial tissue using 18sRNA as an internal control. For easy comparison, the value of the α1D Ca2+ channel mRNA at different developmental stages was normalized to that of the fetal stage. α1D Ca2+ channel mRNA level decreased to 0.70 at neonatal stage, to 0.36 at 4-week and reached the lowest level of 0.22 (n=3, P<0.05) at the adult stage. The results from three different experiments are summarized in Fig 1A.
Figure 1.
α1D Ca2+ channel mRNA and proteins levels during heart development using real-time PCR and Western blot respectively. A: α1D Ca2+ channel mRNA during rat heart development by real-time RT-PCR using 18sRNA as internal control. Value of the fetal stage was set at 100 for easy comparison. * indicates p<0.05 compared to adult stage. F.A: fetal atria; N.A: neonatal atria; A.A: adult atria. B: Representative Western Blot experiment using anti-α1D Ca2+ channel antibody. Total membrane protein (100 µg) was loaded in each lane. Lane 1, fetal stage; Lane 2, neonatal stage; lane 3, 6-weeks atria; lane 4, 6-week ventricles; lane 5, 6-months atria; lane 6, 6-months ventricle and lane 7, negative control (Anti-α1D Ca2+ channel antibody preincubated with antigenic peptide using membrane protein from fetal stage). Panel B, lower part, shows non-specific protein bands observed in each lane, indicating that absence of α1D protein in the adult stage is not secondary to loading error.
Perinatal and postnatal expression of α1D Ca2+ channel protein
The α1D Ca2+ channel protein level at various stages was assessed by Western blot and shown in Fig 1B. At the fetal stage (lane 1), a full size (250 kD) and a short form (190 kD) of the α1D Ca2+ channel protein were observed. At 6-week (lane 3) and 6-month (lane 5) old atria, only the short form band was observed. However, none of the α1D Ca2+ channel protein bands (250 kD and 190 kD) were observed in the 6-week (lane 4) and 6-month ventricles (lane 6). Both the full size and the short form bands were absent when pre-incubating the anti-α1D antibody with its antigenic peptide (lane 7). The lower panel of Fig 1B shows a band corresponding to non-specific proteins in each lane demonstrating that the absence of the α1D protein in the adult stage is not due to a loading error. The α1D Ca2+ channel protein level decreased postnatally and reached steady level at 6-week. The value of α1D Ca2+ channel protein at different developmental stages was normalized to that of the 6-week and 6-month old atria. The α1D Ca2+ channel protein level was 12±2.5 and 4.5±0.6 fold higher at fetal and neonatal stage respectively (n=3, P<0.05).
Developmental-dependent subcellular localization of the α1D Ca2+ channel
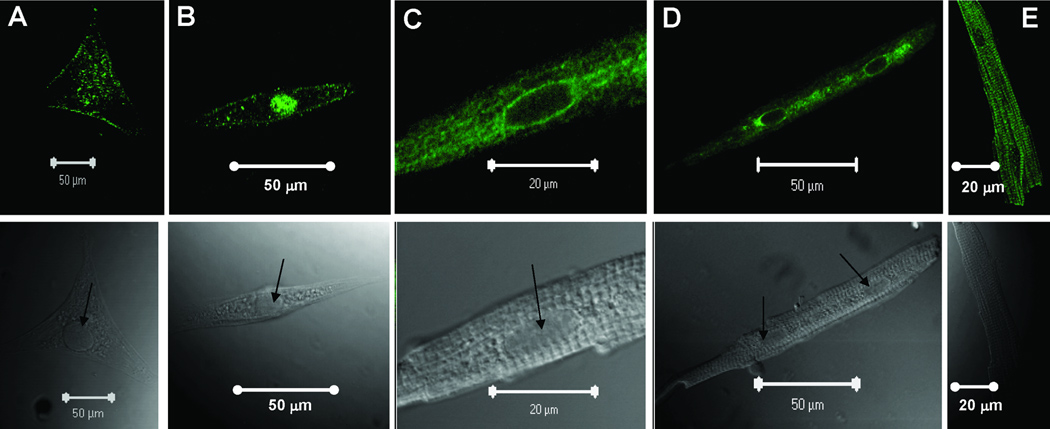
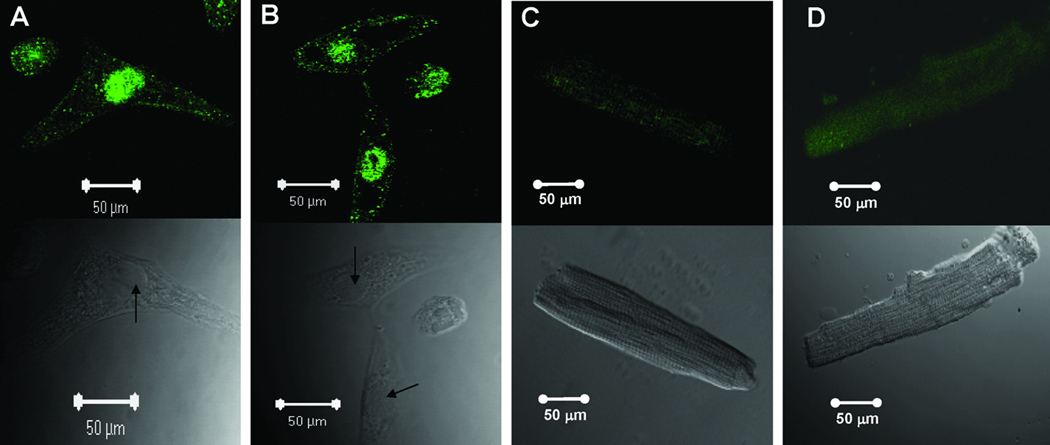
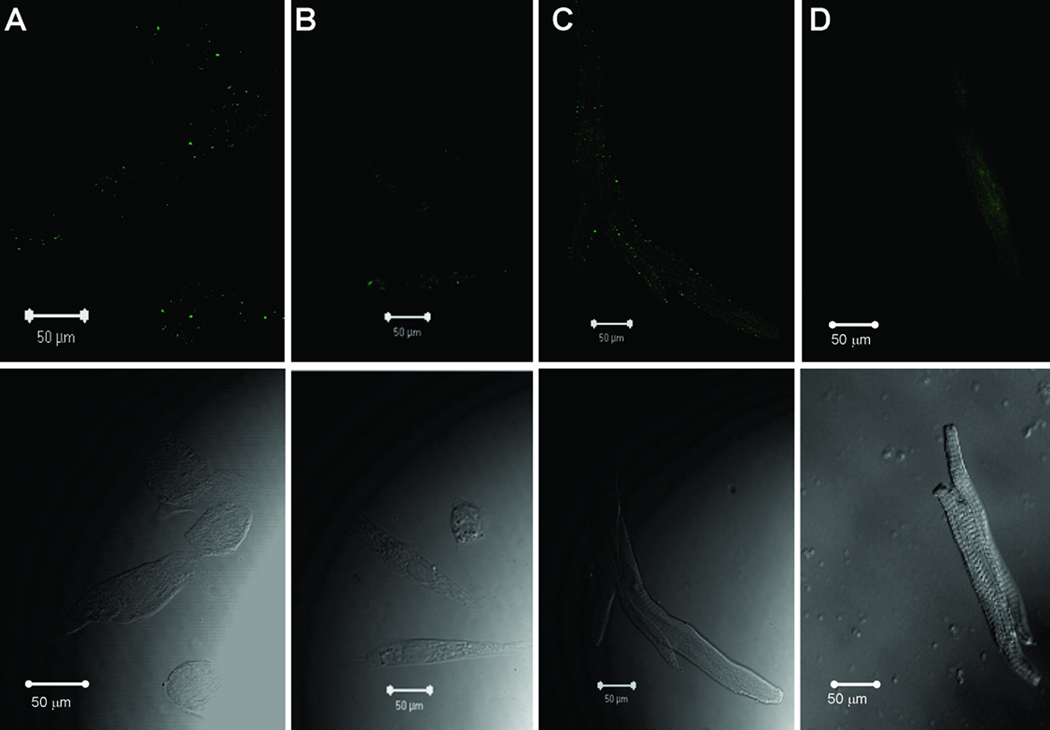
Subcellular localization of the α1D Ca2+ channel was carried out by confocal indirect immunostaining using the anti-α1D antibody. Sarcolemmal staining was observed in the fetal (Fig 2A), neonatal (Fig 2B), 6-week (Fig 2C and D) and 6-month (Fig 2E) atrial cells.Ventricular cells at both the fetal (Fig 3A) and neonatal (Fig 3B) stages showed similar staining pattern to atrial cells (Fig 2A and 2B), indicating that the α1D Ca2+ channel protein is expressed in both the atria and the ventricles of the immature hearts. Interestingly, no staining was observed in the 6-week and 8-month old ventricular cells (Fig 3C and 3D). The staining pattern of the α1D Ca2+ channel in the atrial cells was not observed when using the secondary antibody alone (data not shown) or using the anti-α1D antibody preincubated with its antigenic peptide at the fetal (Fig 4A), the neonatal (Fig 4B), 6-week (Fig 4C) and 6-month (Fig 4D) stages, indicating the specificity of the anti-α1D antibody.
Figure 2.
Representative confocal images of isolated atrial myocytes using anti-α1D Ca2+ channel antibody. Upper panels show the staining pattern of the α1D Ca2+ channel protein using FITC-labeled secondary antibody. The lower panels show the phase controls of the cells at different developmental stages. A: fetal, B: neonatal, C: 6-weeks (amplified from panel D), D: 6-weeks, E: 6-months. Arrows indicate the nucleus.
Figure 3.
Representative confocal images using anti-α1D Ca2+ channel antibody on ventricular cells from various developmental stages: A: fetal ventricular cells, B: neonatal ventricular cells, C: 6-weeks ventricular cell, D: 8-months ventricular cell.
Figure 4.
Representative confocal images of negative controls using anti-α1D Ca2+ channel antibody preincubated with its antigenic peptid: A: fetal atrial cells, B: neonatal atrial cells, C: 6-weeks atrial cells, D: 6-months atrial cell. Arrows indicate the nucleus.
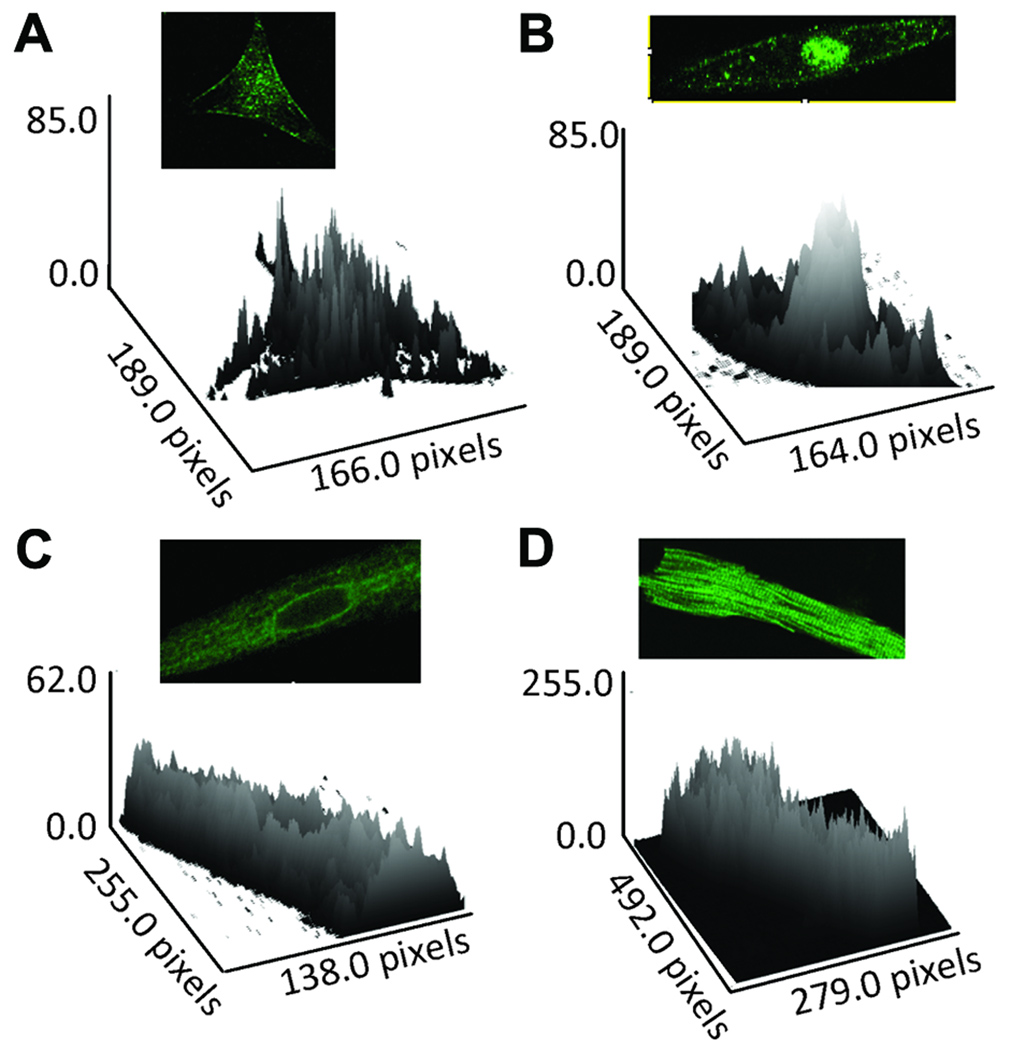
Interestingly, in addition to the sarcolemmal staining, a unique nuclear staining was observed in immature hearts (Fig 2A and 2B, Fig 3A and 3B). This staining pattern was not seen in 6-week and 6-month old rat atrial cells (Fig 2C, 2D and 2E). The specificity of the nuclear staining was demonstrated by the use of anti-α1D antibodies from two commercial vendors which showed the same staining pattern (data not shown). In addition, the nuclear staining was not observed in the negative control experiments in which the anti-α1D antibody was preincubated with its antigenic peptide (Fig 4A and 4B), and with the secondary antibody alone (data not shown). Immunostaining intensity across the cells was assessed by surface plot and shows that the α1D protein is present at the sarcolemma, but is more prominent at the nuclear region of fetal and neonatal stages (Fig 5A and 5B), compared to adult stages (Fig 5C and 5D).
Figure 5.
Surface plots corresponding to Figure 2 showing staining intensity of the α1D protein across the atrial cells including sarcolemma and nucleus at different developmental stages. A: fetal, B: neonatal, C: 6-weeks, D: 6-weeks, E: 6-months. Note prominent nuclear staining at both fetal and neonatal stages compared with adult stages. Note that nuclear staining is more prominent that sarcolemmal staining in the fetal and neonatal atria (panels A and B) compared to 6 weeks and 6 months old atria (panels D and E).
Expression of the α1D Ca2+ channel in adult atrial HL-1 cells
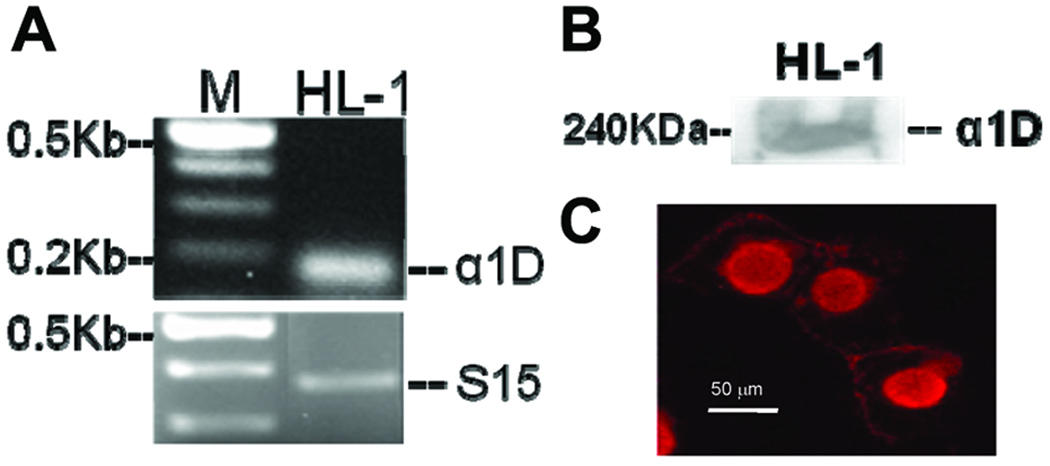
To confirm that the nuclear staining was unique only to the immature cells, we took advantage of a tumor derived mouse atrial cell line, HL-1 cells, to perform immunostaining using the anti-α1D antibody. The unique feature of the HL-1 cells is that they retain the properties of the normal adult atrial cells, but ultrastructurally resemble immature mitotic mouse atrial myocytes. The expression of α1D Ca2+ channel in HL-1 cells was demonstrated by RT-PCR using the α1D specific primer (Fig 6A) and by Western blot (Fig 6B) using the anti-α1D antibody. Interestingly, in these HL-1 cells, the α1D Ca2+ channel was found to be on the sarcolemma and also in the nucleus, a staining pattern that is indistinguishable from that of the fetal and neonatal cardiac myocytes (Fig 6C) suggesting that the α1D Ca2+ channel might play unique roles in the immature cardiac cells.
Figure 6. Expression and localization of α1D Ca channel in HL- cells.
A. Transcript of 180 bp corresponding to the α1D Ca2+ channel was amplified from HL-1 cells. A 361-bp housekeeping gene S15 confirms accuracy of cDNA integrity and gel loading techniques. M indicates markers. B: Expression of the α1D Ca2+ channel protein in HL-1 cells by Western blot using anti- α1D Ca2+ channel antibody. C: Confocal immunostaining of the α1D Ca2+ channels in HL-1 cells using anti- α1D Ca2+ channel antibody.
DISCUSSION
Although the developmental expression profile of the α1C Ca2+ channel has been extensively studied previously (11, 13, 19), to date, no such information is available for the α1D Ca2+ channel. This study is the first to characterize the expression and subcellular localization of α1D Ca2+ channel during heart development. The striking findings of this study are the abundant level, the universal expression, the unique nuclear localization and the existence of the full length (250 kD) α1D Ca2+ channel in the immature heart. In contrast, only the short form (190 kD) of the α1D Ca2+ channel was expressed in the adult stage and its expression is restricted to the atria, but not to the ventricles. Interestingly, the nuclear localization of the α1D Ca2+ channel was not observed in the adult atrial cells. Altogether, the data suggest that the α1D Ca2+ channel may play unique roles in the immature heart. However, because of the unavailable pharmacological or biophysical approaches to separate the α1D from the α1C channel currents, the functional relevance of the α1D Ca2+ channel during heart development was not sought.
Distinct subcellular localization of the α1D Ca2+ channel at different developmental stages
An intriguing observation of this study is the age-dependent subcellular localization of the α1D Ca2+ channel. While in the fetal and neonatal stages, the α1D Ca2+ channel is highly expressed in the nucleus in addition to the sarcolemma, it gradually moves to the perinuclear region and eventually resolves from the perinuclear areas at the adult stage. The specificity of the nuclear/perinuclear staining was confirmed with different experimental approaches. First, the anti-α1D Ca2+ channel antibodies from two commercial resources yielded the same staining patterns. Second, the nuclear staining was not observed when the anti-α1D antibody was preincubated with its antigenic peptide. Third, using the same antibody, the nuclear staining was observed in only the fetal and the neonatal stages but not in the adult stage. Forth, the α1D Ca2+ channel was observed in the nucleus of adult atrial HL-1 cells which possess properties of the immature cardiac cells. Lastly, consist with the present results, Zhang et al., using the same anti-α1D antibody (Calbiochem, San Diego, CA), showed similar staining pattern for the adult atrial cells, in which the nuclear staining was not observed (7). Furthermore, this anti-α1D antibody did not show any specific staining in atrial cells from the α1D Ca2+ channel knockout mice. We have also ruled out the possibility that the observed nuclear staining may be an artifact associated with the cultures of fetal and neonatal atrial and ventricular cells since similar staining patterns of the α1D Ca2+ channel were also observed in freshly isolated human fetal cardiac cells (15). Altogether, these data demonstrate that the nuclear staining in fetal and neonatal cardiac myocytes is specific. Further support for this nuclear/perinuclear localization of the α1D Ca2+ channel is that the expression of ion channels in the nucleus has been reported for Cl channels, Ca-ATPases, InsP3 receptors, and K channels (20–24). The exact role of the α1D Ca2+ channel in the nucleus is not yet known and warrant further investigations.
It is also noteworthy that the α1D Ca2+ channel was not expressed in the adult rat ventricles. This observation is supported by studies from Takemura et al., which showed similar result and (25) and by Zhang et al., who showed a significantly lesser expression levels of the α1D Ca2+ channel in the adult mice ventricles (7). This developmental expression pattern for the α1D Ca2+ channel is strikingly similar to that of the T-type Ca2+ channel (11) with the exception that the T-type Ca2+ channel is re-expressed in the adult ventricles under pathological conditions (26, 27). Whether the α1D Ca2+ channel is re-expressed in the adult ventricles under pathological conditions remains to be determined.
Expression levels of the α1D Ca2+ channel in the heart at different stages
Studies involving with developmental changes have faced the challenge of choosing an internal control with invariant expression. Among the commonly used internal control housekeeping genes, such as glyceraldehydes phosphate dehydrogenase (GAPDH), β-actin and ribosomal RNA (18S and 28S), 18sRNA appears to be the better standard as it shows constant levels throughout gestation and its expression does not appear to vary between individuals (28, 29). On the other hand, the expression of GAPDH and β-actin varies across tissues during cell proliferation (28, 30). Significant variation in GAPDH expression has also been reported between individuals and during development (28, 29, 31). In this study, α1D Ca2+ channel mRNA level, determined by real time RT-PCR using 18sRNA as an internal control, decreased after birth. Postnatal decrease of the α1D Ca2+ channel mRNA was paralleled by the protein level determined by Western blot. Physiologically, the more abundant expression of the α1D Ca2+ channel in the fetal cardiac stage is likely beneficial because of the low levels of the α1C Ca2+ channel expression (19, 32) and for the less abundant sarcoplasmic reticulum in the fetal heart compared with the adult heart (33, 34).
Despite the abundant expression of the α1D Ca2+ channel in the fetal stage, the knockout of the α1C Ca2+ channel in mice is embryonic lethal at day 14.5 postcoitum (35), while the α1D−/− mice survives (5, 7, 36). This suggests that the α1D Ca2+ channel in the immature heart may be important to cellular functions other than excitation-coupling. Indeed, the unique nuclear localization of the α1D channel in the fetal and neonatal stages also points to other unique functions of the α1D in the immature hearts.
Two forms of the α1D Ca2+ channel are present in the immature hearts
Two forms of the α1D Ca2+ channel protein, a full length (250 kD) and a short form (190 kD) were observed in the immature heart. A splicing site at the C-terminal region of α1D has been reported (37, 38). A 58 base pair deletion results in a frame shift that produces a premature termination codon giving rise to the α1D subunit that differs in length by 535 amino acid, with a corresponding molecular weight of 190KD (37, 38). On the other hand, Experiments in the cardiac myocytes and neurons indicate that the C terminus of the α1C channel is proteolytically cleaved, yielding a truncated channel (39, 40), with the full length observed in only the fetal heart (13, 41), indicating an increased proteolytic activity in the adult heart. The authors also reported a novel mechanism of the α1C channel associated transcription regulation in which the proteolytic C-terminal fragment of the α1C Ca2+ channel, translocates to the nucleus and regulates gene transcriptions (41). It is unclear whether these two sizes of α1D protein observed in this study are the products of proteolytic activity or splicing variant. Whether these spliced variants are developmentally regulated and whether the observed differential localization of the protein is related to the expression of different splice variants is unknown and warrant further investigations.
In conclusion, the α1D Ca2+ channel is a newly discovered L-type Ca2+ channel isoform in the heart. It has distinct age dependent expression and subcellular localization, suggesting unique function during heart development.
ACKNOWLEDGEMENT
We thank Dr. Seino and Dr. Striessnig for providing the plasmids used in this study.
Financial Support: This study is supported by National Institutes of Health [R01 HL-077494] and Veterans Affairs MERIT grants [to M.B.]; Veteran Affairs MREP grant [to Y.Q.].
ABBREVIATIONS
- ICa-L
L-type Ca2+ current
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 2.Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 3.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson JH, Bell GI. Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci USA. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthes J, Yildirim L, Wietzorrek G, Reimer D, Striessnig J, Herzig S. Disturbed atrio-ventricular conduction and normal contractile function in isolated hearts from Cav1.3-knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:554–562. doi: 10.1007/s00210-004-0940-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]
- 8.Mancarella S, Yue Y, Karnabi E, Qu Y, El Sherif N, Boutjdir M. Impaired Calcium Homeostasis is Associated with Atrial Fibrillation in the {alpha}1D L-Type Ca2+ Channel KO Mouse. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.00537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nurnberg B, Dolphin AC. Biophysical properties, pharmacology, and modulation of human, neuronal L-type (alpha(1D), Ca(V)1.3) voltage-dependent calcium currents. J Neurophysiol. 2001;85:816–827. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- 10.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 11.Qu Y, Boutjdir M. Gene expression of SERCA2a and L- and T-type Ca channels during human heart development. Pediatr Res. 2001;50:569–574. doi: 10.1203/00006450-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Brillantes AM, Bezprozvannaya S, Marks AR. Developmental and tissue-specific regulation of rabbit skeletal and cardiac muscle calcium channels involved in excitation-contraction coupling. Circ Res. 1994;75:503–510. doi: 10.1161/01.res.75.3.503. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, O'Hara DS, Cala SE, Poornima I, Hines RN, Marsh JD. Developmental regulation of the L-type calcium channel alpha1C subunit expression in heart. Mol Cell Biochem. 2000;205:101–109. doi: 10.1023/a:1007013900827. [DOI] [PubMed] [Google Scholar]
- 14.Qu Y, Karnabi E, Chahine M, Vassalle M, Boutjdir M. Expression of skeletal muscle Na(V)1.4 Na channel isoform in canine cardiac Purkinje myocytes. Biochem Biophys Res Commun. 2007;355:28–33. doi: 10.1016/j.bbrc.2007.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005;111:3034–3041. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- 16.Boutjdir M, Chen L, Zhang ZH, Tseng CE, El-Sherif N, Buyon JP. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr Res. 1998;44:11–19. doi: 10.1203/00006450-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa E, Saito Y, Harada M, Kamitani S, Kuwahara K, Miyamoto Y, Ishikawa M, Hamanaka I, Kajiyama N, Takahashi N, Nakagawa O, Masuda I, Kishimoto I, Nakao K. Outside-in signalling of fibronectin stimulates cardiomyocyte hypertrophy in cultured neonatal rat ventricular myocytes. J Mol Cell Cardiol. 2000;32:765–776. doi: 10.1006/jmcc.2000.1119. [DOI] [PubMed] [Google Scholar]
- 18.Qu Y, Baroudi G, Yue Y, El-Sherif N, Boutjdir M. Localization and modulation of {alpha}1D (Cav1.3) L-type Ca channel by protein kinase A. Am J Physiol Heart Circ Physiol. 2005;288:H2123–H2130. doi: 10.1152/ajpheart.01023.2004. [DOI] [PubMed] [Google Scholar]
- 19.Huynh TV, Chen F, Wetzel GT, Friedman WF, Klitzner TS. Developmental changes in membrane Ca2+ and K+ currents in fetal, neonatal, and adult rabbit ventricular myocytes. Circ Res. 1992;70:508–515. doi: 10.1161/01.res.70.3.508. [DOI] [PubMed] [Google Scholar]
- 20.Draguhn A, Borner G, Beckmann R, Buchner K, Heinemann U, Hucho F. Large-conductance cation channels in the envelope of nuclei from rat cerebral cortex. J Membr Biol. 1997;158:159–166. doi: 10.1007/s002329900253. [DOI] [PubMed] [Google Scholar]
- 21.Franco-Obregón A, Wang HW, Clapham DE. Distinct ion channel classes are expressed on the outer nuclear envelope of T- and B-lymphocyte cell lines. Biophys J. 2000;79:202–214. doi: 10.1016/S0006-3495(00)76284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stehno-Bittel L, Perez-Terzic C, Luckhoff A, Clapham DE. Nuclear ion channels and regulation of the nuclear pore. Soc Gen Physiol Ser. 1996;51:195–207. [PubMed] [Google Scholar]
- 23.Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama Y, Shimada H, Taniguchi J. Ca(2+)-activated K(+)-channels in the nuclear envelope isolated from single pancreatic acinar cells. Pflugers Arch. 1995;430:148–150. doi: 10.1007/BF00373851. [DOI] [PubMed] [Google Scholar]
- 25.Takemura H, Yasui K, Opthof T, Niwa N, Horiba M, Shimizu A, Lee JK, Honjo H, Kamiya K, Ueda Y, Kodama I. Subtype switching of L-Type Ca 2+ channel from Cav1.3 to Cav1.2 in embryonic murine ventricle. Circ J. 2005;69:1405–1411. doi: 10.1253/circj.69.1405. [DOI] [PubMed] [Google Scholar]
- 26.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–782. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 27.Martínez ML, Heredia MP, Delgado C. Expression of T-type Ca(2+) channels in ventricular cells from hypertrophied rat hearts. J Mol Cell Cardiol. 1999;31:1617–1625. doi: 10.1006/jmcc.1999.0998. [DOI] [PubMed] [Google Scholar]
- 28.Bas A, Forsberg G, Hammarstrom S, Hammarstrom ML. Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol. 2004;59:566–573. doi: 10.1111/j.0300-9475.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 29.Patel P, Boyd CA, Johnston DG, Williamson C. Analysis of GAPDH as a standard for gene expression quantification in human placenta. Placenta. 2002;23:697–698. doi: 10.1053/plac.2002.0859. [DOI] [PubMed] [Google Scholar]
- 30.Mansur NR, Meyer-Siegler K, Wurzer JC, Sirover MA. Cell cycle regulation of the glyceraldehyde-3-phosphate dehydrogenase/uracil DNA glycosylase gene in normal human cells. Nucleic Acids Res. 1993;21:993–998. doi: 10.1093/nar/21.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oikarinen A, Makela J, Vuorio T, Vuorio E. Comparison on collagen gene expression in the developing chick embryo tendon and heart. Tissue and development time-dependent action of dexamethasone. Biochim Biophys Acta. 1991;1089:40–46. doi: 10.1016/0167-4781(91)90082-w. [DOI] [PubMed] [Google Scholar]
- 32.Boucek RJ, Jr, Shelton M, Artman M, Mushlin PS, Starnes VA, Olson RD. Comparative effects of verapamil, nifedipine, and diltiazem on contractile function in the isolated immature and adult rabbit heart. Pediatr Res. 1984;18:948–952. doi: 10.1203/00006450-198410000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Hoerter J, Mazet F, Vassort G. Perinatal growth of the rabbit cardiac cell: possible implications for the mechanism of relaxation. J Mol Cell Cardiol. 1981;13:725–740. doi: 10.1016/0022-2828(81)90255-8. [DOI] [PubMed] [Google Scholar]
- 34.Maylie JG. Excitation-contraction coupling in neonatal and adult myocardium of cat. Am J Physiol. 1982;242:H834–H843. doi: 10.1152/ajpheart.1982.242.5.H834. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Welling A, Paparisto S, Hofmann F, Klugbauer N. Enhanced expression of L-type Cav1.3 calcium channels in murine embryonic hearts from Cav1.2-deficient mice. J Biol Chem. 2003;278:40837–40841. doi: 10.1074/jbc.M307598200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 37.Safa P, Boulter J, Hales TG. Functional properties of Cav1.3 (alpha1D) L-type Ca2+ channel splice variants expressed by rat brain and neuroendocrine GH3 cells. J Biol Chem. 2001;276:38727–38737. doi: 10.1074/jbc.M103724200. [DOI] [PubMed] [Google Scholar]
- 38.Scholze A, Plant TD, Dolphin AC, Nurnberg B. Functional expression and characterization of a voltage-gated CaV1.3 (alpha1D) calcium channel subunit from an insulin-secreting cell line. Mol Endocrinol. 2001;15:1211–1221. doi: 10.1210/mend.15.7.0666. [DOI] [PubMed] [Google Scholar]
- 39.De Jongh KS, Colvin AA, Wang KK, Catterall WA. Differential proteolysis of the full-length form of the L-type calcium channel alpha 1 subunit by calpain. J Neurochem. 1994;63:1558–1564. doi: 10.1046/j.1471-4159.1994.63041558.x. [DOI] [PubMed] [Google Scholar]
- 40.Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the alpha(1C) subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]