Abstract
Purpose
We assessed the effect of ovariectomy and estrogen replacement on nociceptive responses to bladder distention in a rat model.
Materials and Methods
Female Sprague-Dawley rats (Harlan™) underwent ovariectomy or sham surgery. Visceromotor responses (abdominal contractions) to bladder distention were determined 3 to 4 weeks later under isoflurane anesthesia. In rat subsets estrogen was chronically replaced with a subcutaneous estrogen pellet vs a placebo pellet or acutely replaced by subcutaneous injection 24 hours before testing. Effects of estrogen withdrawal were examined in another group of rats by implanting a pellet and explanting the pellet 24 hours before testing. Uterine weight was measured to assess the estrogen dose.
Results
Visceromotor responses to bladder distention were significantly less vigorous in ovariectomized rats vs controls. Acute estrogen replacement increased visceromotor responses in these rats but chronic estrogen replacement did not. Sudden chronic estrogen withdrawal resulted in increased visceromotor responses. Uterine weight was consistent with the physiological estrogen dose.
Conclusions
Estrogen alone was not sufficient to produce increased nociceptive responses but an acute decrease in estrogen resulted in increased visceromotor responses. These data suggest that the pronociceptive effects of estrogen may be due to a mismatch between peripheral vs central and/or genomic vs nongenomic effects of the hormone, which occur during rapidly decreasing estrogen levels.
Keywords: estrogens, ovary, pain, ovariectomy, rats, Sprague-Dawley
Clinical pain, such as that associated with the painful bladder disorder interstitial cystitis, varies as a function of the menstrual cycle.1 The variable responsible for this inconsistency is uncertain but there is obvious potential for the involvement of cyclical ovarian hormones such as estrogen. Gender differences in human pain perception are well documented and laboratory studies in healthy individuals show that women often respond to pain differently than men.2–5 Estrogen was postulated as the source of some of these differences.6–8 Further supporting a role of estrogen in pain related processes is the observation that a number of other chronic pain conditions, such as migraine headache, fibromyalgia and irritable bowel syndrome, are more prevalent in women than in men, occur primarily during the reproductive years and have menstrual cycle variations. To complement these findings in humans numerous laboratory studies in animals clearly indicate a role for estrogen in nociceptive processing.3,9 Given the clinical use of estrogen as hormonal therapy for fertility, menopausal symptoms and gynecological pains it is important to define the role of this hormone in urological nociceptive systems.
We determined the role of estrogen specific to bladder nociceptive sensation in an animal model previously shown to be affected by the estrous cycle.10 This model uses the stimulus of phasic UBD at 10 to 60 mm Hg intensity to evoke reliable, reproducible spinobul-bospinal cardiovascular (pressor) and visceromotor (abdominal contractions) reflex responses that are useful for within and between animal studies. Although this stimulus differs from normal slow physiological bladder filling by fluid and so has limited relevance to micturition studies, it is useful to characterize bladder nociception since evoked responses are graded with stimulus intensity in the noxious range, inhibited by analgesics in a dose dependent manner10–12 and accentuated by sensitizing stimuli such as bladder inflammation.13 Reflex responses to UBD were measured after ovariectomy combined with chronic or acute estrogen administration.
Materials and Methods
Animals
Female Sprague-Dawley rats at ages 11 to 13 weeks were used. All protocols were approved by the University of Alabama at Birmingham institutional animal care and use committee and adhered to International Association for the Study of Pain Committee for Research and Ethical Issues guidelines.
Experimental Protocols
Four experiments were done to assess the impact of manipulations on VMRs evoked by UBD, including 1) OVX, 2) acute estrogen replacement after OVX, 3) chronic estrogen replacement after OVX and 4) sudden withdrawal of chronic estrogen replacement after OVX. All rats underwent initial survival surgery while anesthetized with isoflurane (2% to 5% in oxygen). Sterile technique was used. Perioperatively ampicillin (50 mg/kg) was administered subcutaneously and midline laparotomy was done to remove the 2 ovaries. Rats were recovered for 3 to 4 weeks and then underwent additional procedures as part of experiments 1 to 4.
To assess the effect of OVX alone there were 2 groups of rats in experiment 1, including those with OVX and a SHAM comparison group that underwent identical surgery except a piece of abdominal fat was removed rather than the ovaries (OVX vs SHAM, comparison 1). These 2 groups received subcutaneous safflower oil (100 μl) injection 48 hours before testing. Rats in experiment 2 underwent OVX and received subcutaneous injection of safflower oil (100 μl) vehicle (group V-24) or 17-β-estradiol-3 benzoate (Sigma-Aldrich®) (50 μg dissolved in safflower oil) 24 (group E-24) or 48 (group E-48) hours before bladder nociceptive testing (V-24 vs E-24 and E-48, respectively, comparison 2). Rats in experiment 3 received a 60-day release estrogen pellet (Innovative Research of America, Sarasota, Florida) (1 mg per pellet) (E-chronic group) or a placebo pellet (P-chronic group) placed subcutaneously in mid scapular subcutaneous tissue at OVX, which remained in place during bladder nociceptive testing (E-chronic vs P-chronic, comparison 3). In experiment 4 rats received similar estrogen (E-withdrawal group) and placebo (P-withdrawal group) pellets at OVX and the pellet was explanted under isoflurane anesthesia 24 hours before bladder nociceptive testing (E-withdrawal vs P-withdrawal, comparison 4). Estrogen dose and pellet type used were based on literature values indicating that they produce normal to high physiological hormone levels.14–16
Bladder Nociceptive Testing
Using mask isoflurane anesthesia (1% to 3% isoflurane in oxygen) a 22 gauge angiocatheter was placed in the bladder via the urethra and held in place by a tight suture around the distal urethral orifice. Silver wire electrodes were inserted in the external oblique musculature immediately superior to the inguinal ligament. After surgery isoflurane anesthesia was decreased (1% to 1.25% isoflurane) until flexion reflexes were present in the hind limbs but spontaneous escape behavior was absent. UBD 20 seconds in duration was produced using compressed air and a distention control device, as previously described.12 Intravesical pressure was monitored using an in-line, low volume pressure transducer. VMRs, recorded as EMG activity, were measured via the electrodes using standard differential amplification and rectification, and saved on a computer using Spike2 software and associated Micro 1401 data acquisition hardware (Cambridge Electronic Design, Cambridge, United Kingdom). EMG activity was quantified using the equation, (rectified EMG activity during UBD – rectified baseline EMG before UBD)/rectified baseline EMG.12 Approximately 15 minutes after initial anesthesia induction EMG responses to 3 presentations of 60 mm Hg UBD at 3-minute intervals were recorded to overcome the bladder sensitization period that occurs before vigorous and reliable VMRs. Responses to graded stimuli (10 to 60 mm Hg at 1-minute intertrial intervals) were determined. After UBD testing the animals were exsanguinated by cardiac transection under anesthesia. The uterus was removed and weighed to assess hormone treatment effectiveness.
Statistical Analysis
All data are shown as the group mean ± SEM. Data were analyzed by repeated measures ANOVA. The post hoc t test was done and Holm's procedure17 was used to correct for multiple comparisons. In all analyses p <0.05 was considered statistically significant.
Results
Experiment 1 (OVX)
VMRs to graded UBD in female rats with OVX were significantly less vigorous than in SHAM female rats (part A of figure). Baseline EMG activity did not differ for OVX vs SHAM (14.1 ± 0.7 vs 14.7 ± 0.8 μV rectified), suggesting that decreased evoked activity was not due to intrinsic changes in muscular function. Uterine weight in OVX rats was significantly less than in SHAM rats (150 ± 6 vs 534 ± 23 mg, p <0.01). These data indicate that hormones released from ovaries had a uterine trophic effect. A similar decrease in the vigor of cardiovascular responses to UBD in OVX vs SHAM rats was previously noted.10
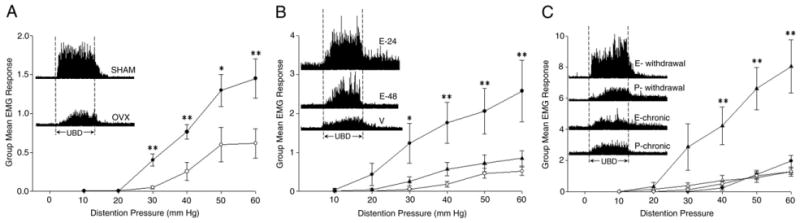
VMRs to UBD in rats. A, experiment 1 in 8 rats with bilateral OVX (open circles) and 6 with SHAM (filled circles) 3 to 4 weeks before testing. VMRs were significantly lower in OVX vs SHAM rats (F = 10.10, p <0.01). Single asterisk indicates statistically significant difference (p <0.05). Double asterisks indicate statistically significant difference (p <0.01). Inset, histogram of electromyographic activity shows VMR responses to 60 mm Hg 20-second UBD in 2 typical rats. B, experiment 2 in 7 to 9 rats per group with bilateral OVX 3 to 4 weeks before testing and 1 subcutaneous injection of 100 μl safflower oil vehicle (V, open circles) or 50 μg 17-β-estradiol-3 benzoate 24 (filled circles) or 48 (triangles) hours before testing. E-24 rats had enhanced VMRs vs vehicle treated rats (F = 8.418, p <0.05). E-48 rats were not statistically different from vehicle treated rats. Single asterisk indicates significantly different vs vehicle (p <0.05). Double asterisks indicate significantly different vs vehicle (p <0.01). Inset, histogram of electromyographic activity shows VMR responses to 60 mm Hg 20-second UBD in 3 typical rats. C, experiments 3 and 4 in 7 to 9 rats per group with bilateral OVX 3 weeks before testing and estrogen or placebo pellet implanted subcutaneously. E-chronic (open triangles) and P-chronic (open circle) rats had pellets in place but in E-withdrawal (filled triangles) and P-withdrawal (filled circles) rats pellets were removed 24 hours before testing. There was no statistically significant difference between E-chronic and P-chronic rats (F = 0.159, p = 0.694). E-withdrawal rats had enhanced VMRs vs P-withdrawal rats (F = 12.96, p <0.01). Asterisks indicate significantly different vs P-withdrawal (p <0.01). Inset, histogram of electromyographic activity shows VMR responses to 60 mm Hg 20-second UBD in 4 typical rats.
Experiment 2 (Acute Estrogen Replacement)
Acute estradiol administration produced an increase in the vigor of VMRs evoked by UBD in OVX rats when given 24 hours before testing vs that in controls but this effect was decreased 48 hours after administration (part B of figure). There was also an effect of acute 24 and 48-hour treatment vs V-24 (254 ± 19 and 245 ± 25 mg, respectively, vs 154 ±11, each p <0.01) on increasing uterine weight. There were no statistically significant differences in baseline EMG activity between the groups.
Experiment 3 (Chronic Estrogen Replacement)
A surprising finding was that chronic estrogen administration by pellet implantation failed to produce a change in the vigor of VMRs evoked by UBD in OVX rats vs that in placebo pellet controls (part C of figure). This treatment affected uterine weight in the E-chronic and P-chronic groups (624 ± 31 and 150 ± 9 mg, p <0.01). Baseline EMG activity was slightly greater in the estrogen pellet group (15.7 ± 0.7 vs 14.2 + 0.8 μV rectified) but this difference was not statistically significant (p = 0.2). Uterine weight in estrogen pellet implanted rats did not statistically differ from that in SHAM rats, suggesting that the estrogen dose administered by the pellet was at physiological levels.
Experiment 4 (Chronic Estrogen Withdrawal)
Others reported that acute subcutaneous administration of estrogen leads to a brief increase (peak at 4 to 10 hours) and then a decrease in systemic estrogen.14,16 Thus, we assessed whether the increased vigor of VMRs evoked by UBD may be due to abrupt estrogen loss rather than to the presence of estrogen by implanting a chronic estrogen pellet at OVX and removing it 24 hours before nociceptive testing. Acute loss of chronically administered estrogen produced a robust increase in the vigor of VMRs evoked by UBD in E-withdrawal compared with that in P-withdrawal controls (part C of figure). There was a residual effect of chronic hormonal treatment on uterine weights in the E-withdrawal vs P-withdrawal groups (414 ± 13 vs 121 ± 8 mg, p <0.01). Baseline EMG activity was virtually identical in the 2 groups (11.4 ± 0.8 and 11.4 ± 0.07 μV rectified, respectively).
Discussion
We report evidence of a complex effect of estrogen on bladder nociceptive processing, consistent with clinical observations, which may provide insight into potential therapeutic interventions for painful urological disorders. Powell-Boone et al studied pain sensation in female subjects diagnosed with interstitial cystitis and reported that the clinical pain of this disorder, as measured by daily pain diaries, is worst in the perimenstrual period, that is the period when blood estrogen decreases with the resultant onset of menses.1 They also found that patients with interstitial cystitis had an increased micturition rate during the perimenstrual period. Others noted that urinary symptoms generally increase in the perimenstrual period.18 Thus, clinically the effect of decreasing estrogen may not be limited to nociception.
The mechanisms of estrogen effects on pain perception are complex and the existing scientific literature is confusing with specific results varying depending on the stimulus and measurement timing.9 There is a general lack of agreement on which stage of the menstrual (human) or estrous (rodents) cycle is associated with higher or lower nociceptive responses to experimental pain and incongruity related to the effects of particular ovarian hormones. A number of differences in estrogen effect studies may contribute to the discrepant results, including tissue type (visceral or somatic), nociceptive model type (chronic or acute, somatic or visceral, or inflammatory or neuropathic) and nociceptive response measure (motor, cardiovascular, complex behavioral or neuronal). Thus, it is notable that our results are consistent with those in preclinical studies showing that maximal sensitivity (decreased threshold for response) to UBD in rats is in estrous cycle periods, when dynamic alterations occur in estrogen (proestrus/estrus).10,19,20 To our knowledge similar alterations in the vigor of motor responses to bladder stimulation to suprathreshold stimuli during periods of rapid hormonal change have not yet been reported. Interpreting alterations in responses by the hormonal changes in our study must be limited to those evoked by mechanical rather than by chemical or other stimuli. The acute estrogen effects on VMRs evoked by UBD in our series are consistent with those in a parallel study by Ji et al, who observed similar effects of identical doses of estrogen on VMRs evoked by another visceral mechanical stimulus (colorectal distention) in rats.14
Unfortunately the presence or absence of estrogen did not explain all of our observations. The lack of estrogen (OVX) and the continuous estrogen dose (chronic pellet implantation) had the similar effect of low vigor VMRs. In contrast, acute estrogen administration and chronic estrogen withdrawal resulted in increased VMR vigor. The former conditions have a steady hormonal state in common. What the latter conditions have in common is that they represent a dynamic alteration in circulating estrogen and each was measured during an abrupt decrease in blood estrogen. Estrogen blood levels peak 4 to 10 hours after acute subcutaneous administration of 50 μg of estradiol and then rapidly decrease.14,16 A similar estrogen decrease would be expected after pellet explantation. An abrupt decrease in circulating estrogen is clinically relevant in humans since such a decrease occurs in the perimenstrual period. Increases in clinical pain associated with a dynamic decrease in blood estrogen have been noted in cases of multiple chronic pain disorders, such as perimenstrual migraine.21
In addition to effects on bladder sensation, ovarian hormones may have trophic effects, such that deprivation of these hormones may alter the motor components of bladder function22 and the motor functions of skeletal muscle23,24 with potential secondary effects on the magnitude of VMRs to UBD. We recognize the potential for such effects but they are unlikely to have been the source of the current observations given the minimal baseline EMG differences between groups and the virtually identical VMRs in OVX rats with sustained estrogen doses by pellet (E-chronic group) and OVX rats without estrogen replacement (P-chronic group). This observation does not completely rule out complex effects of estrogen administration and withdrawal on motor function but suggests they would reflect potential sensory effects.
Although the complexity of estrogen signaling is not completely understood, estrogens have genomic (direct control of gene expression) and nongenomic (regulation of cell signaling/phosphorylation cascades) effects that control critical cell signaling pathways. These effects have differing temporal characteristics with nongenomic effects tending to be rapid in onset and of limited duration while genomic effects are generally slower in onset with a more prolonged duration.25 In addition to differing cellular mechanisms of estrogens, there can be differences in sites of action. Bennett et al reported clear evidence of ERs on neuropeptide containing peripheral nervous system structures innervating the bladder26 and central nervous system actions of estrogens were also reported.9 Adding to the complexity of this system is the existence of multiple ER subtypes (ER-α and β) and recently identified GPR30. These receptors are colocalized in many tissues but when activated they may produce opposing effects. For example, an ER-α selective agonist increases anxiety related behaviors and an ER-β selective agonist has anxiolytic effects.27 Estrogen also has complex effects on bladder structure and contractility,22,28,29 such that subtle alterations in symptoms due to estrogen presence or absence become difficult to interpret.
As a consequence of the intricacy of the estrogen signaling system, a potential exists for a mismatch of estrogen effects and the consequences of this mismatch could serve as the mechanism of augmented nociceptive responses. The overall effect of estrogen depends on the balance between genomic and nongenomic signaling, central and peripheral effects, and receptor density. Perimenstrual migraine is associated with a dynamic decrease in blood estrogen and it was postulated that a mismatch in the genomic vs the nongenomic signaling cascade leads to an imbalance in the pronociceptive vs antinociceptive effects of the hormone,30 increasing migraine severity and incidence. Apparently the complex effects of estrogen should perhaps be expected during states of dynamic circulating hormonal levels and so similar phenomena may occur in urological nociceptive systems.
The clinical implication of these studies is that estrogen replacement alone should not be an exacerbating factor in relation to bladder pain since stable, continuous doses of estrogen were not associated with increased nociception. However, an abrupt alteration in estrogen produced increased nociception, such that variable dose formulations or those that lead to rapid changes after ingestion may be more problematic, particularly in individuals with baseline painful bladder conditions such as interstitial cystitis. Clinical trials comparing different hormonal formulations, including those using transdermal (stable blood levels) and oral (variable blood levels) administration routes would be of value. To our knowledge the roles of other gonadal hormones, such as progesterone and testosterone, are yet to be determined.
Conclusions
The removal of all ovarian hormones combined with no hormonal replacement or continuous replacement of one of the hormones (estrogen) led to decreased bladder nociception. This effect was reversed by acute estrogen administration and by the abrupt withdrawal of continuous estrogen. These seemingly opposite treatments have the similarity of rapid changes in hormonal levels, which can result in a mismatch of modulatory actions of estrogen. Thus, decreased hormonal fluctuations via pharmacological or surgical manipulations would be expected to have beneficial effects on bladder pain.
Acknowledgments
Supported by DK51413, DK080981 and an unrestricted gift from the Henderson Family.
Abbreviations and Acronyms
- E-24
24 hours before testing
- E-48
48 hours before testing
- E-chronic
estrogen pellet
- ER
estrogen receptor
- E-withdrawal
estrogen implantation and explantation
- OVX
ovariectomy
- P-chronic
placebo pellet
- P-withdrawal
placebo implantation and explantation
- SHAM
sham surgery
- UBD
bladder distention
- V-24
OVX plus vehicle
- VMR
visceromotor response
Footnotes
Study received University of Alabama at Birmingham institutional animal care and use committee approval.
References
- 1.Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832. doi: 10.1097/01.ju.0000176747.40242.3d. [DOI] [PubMed] [Google Scholar]
- 2.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 2003;19:168. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic response. Neurosci Biobehav Rev. 2000;24:485. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 5.Hurley RW, Adams MC. Sex, gender and pain: an overview of a complex field. Anesth Analg. 2008;107:309. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannino CA, South SM, Quinones-Jenab V, et al. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Spooner MF, Robichaud P, Carrier JC, et al. Endogenous pain modulation during the formalin test in estrogen receptor beta knockout mice. Neuroscience. 2007;150:675. doi: 10.1016/j.neuroscience.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117:433. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Craft RM. Modulation of pain by estrogens. Pain. 2007;132:S3. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distension in rats: sources of variability and effect of analgesics. J Urol. 2001;165:968. [PubMed] [Google Scholar]
- 11.Blatt LK, Lashinger ESR, Laping NJ, et al. Evaluation of pressor and visceromotor reflex responses to bladder distension in urethane anesthetized rats. Neurourol Urodyn. 2008;28:442. doi: 10.1002/nau.20650. [DOI] [PubMed] [Google Scholar]
- 12.Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Letts. 2001;306:97. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- 13.Randich A, Uzzell T, Cannon R, et al. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal neurons to colorectal stimulation in the rat. J Neurosci. 2003;23:3908. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z, Shen X, Capodanno I, et al. Validation of rat endometriosis model by using raloxifene as a positive control for the evaluation of novel SERM compounds. J Invest Surg. 2005;18:177. doi: 10.1080/08941930591004412. [DOI] [PubMed] [Google Scholar]
- 16.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of β–preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Mol Brain Res. 1995;28:61. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- 17.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hextall A, Bidmead J, Cardozo L, et al. The impact of the menstrual cycle on urinary symptoms and the results of urodynamic investigation. Br J Obstet Gynaecol. 2001;108:1193. doi: 10.1111/j.1471-0528.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 19.Shea VK, Cai R, Crepps B, et al. Sensory fibers of the pelvic nerve innervating the rat's urinary bladder. J Neurophysiol. 2000;84:1924. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 20.Johnson OL, Berkley KJ. Estrous influences on micturition thresholds of the female rat before and after bladder inflammation. Am J Physiol. 2002;282:R289. doi: 10.1152/ajpregu.2002.282.1.R289. [DOI] [PubMed] [Google Scholar]
- 21.MacGregor EA, Frith A, Ellis J, et al. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67:2154. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 22.Fleischmann N, Christ G, Sclafani T, et al. The effect of ovariectomy and long-term estrogen replacement on bladder structure and function in the rat. J Urol. 2002;168:1265. doi: 10.1016/S0022-5347(05)64637-X. [DOI] [PubMed] [Google Scholar]
- 23.Moran AL, Nelson SA, Landisch RM, et al. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102:1387. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 24.Korobi M, Yamamuro T. Effects of gonadectomy and estrogen administration on rat skeletal muscle. Clin Ortho Rel Res. 1989;243:306. [PubMed] [Google Scholar]
- 25.McEwen BS, Coirini H, Schumacher M. Steroid effects on neuronal activity: when is the genome involved? Ciba Found Symp. 1990;153:3. doi: 10.1002/9780470513989.ch2. [DOI] [PubMed] [Google Scholar]
- 26.Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auto Neurosci Basic Clin. 2003;105:90. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 27.Lund TD, Rovis T, Chung WC, et al. Novel actions of estrogen receptor-beta on. anxiety-related behaviors. Endocrinology. 2005;146:797. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 28.Seidlova-Wuttke D, Schultens A, Jarry H, et al. Urodynamic effects of estradiol (E2) in ovariectomized (ovx) rats. Endocrine. 2004;23:25. doi: 10.1385/ENDO:23:1:25. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida J, Aikawa K, Yoshimura Y, et al. The effects of ovariectomy and estrogen replacement on acetylcholine release from nerve fibres and passive stretch-induced acetylcholine release in female rat bladder. Neurourol Urodyn. 2007;26:1050. doi: 10.1002/nau.20438. [DOI] [PubMed] [Google Scholar]
- 30.Welch KMA, Brandes JL, Berman NEJ. Mismatch in how oestrogen modulates molecular and neuronal function may explain menstrual headache. Neurol Sci. 2006;27:S190. doi: 10.1007/s10072-006-0599-6. [DOI] [PubMed] [Google Scholar]


