Abstract
The mouse Swiss 3T3-F442A/3T3-C2 cell system is well suited for the isolation of genes involved in commitment to adipogenesis. 3T3-F442A cells convert to adipocytes with high efficiency in response to confluence and insulin. The sister clonal line 3T3-C2 does not respond to these signals, but can convert to adipocytes when transfected with DNA from 3T3-F442A preadipocytes or from human fat. Human fat-tissue biopsy FO46 DNA transfected into 3T3-C2 gave rise to fat foci after two rounds of transfection and selection. A cosmid library of a subclone of secondary transfectant 3T3-C2/FO46-1 was screened for the human repetitive Alu sequence. Five out of eight Alu+ recombinant clones committed 3T3-C2 cells to adipogenesis. The adipose commitment (AC) activity of one cosmid, p18A4, was found to reside in two small, non-identical, subcloned sequences 1.2kb and 2.0kb in length, each separately able to commit 3T3-C2, precrisis mouse and rat fibroblasts and the multipotential C3H10T1/2 cell line to adipogenesis. We conclude that commitment to adipogenesis can be effected in vitro with high efficiency by transfection of specific sequences into a variety of host cells.
Full text
PDF



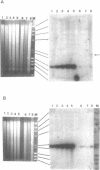
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates P. Double cos site vectors: simplified cosmid cloning. Methods Enzymol. 1987;153:82–94. doi: 10.1016/0076-6879(87)53049-x. [DOI] [PubMed] [Google Scholar]
- Blanck G., Li D., Pomert E., Pollack R., Chen S. Multiple insertions and tandem repeats of origin-minus simian virus 40 DNA in transformed rat and mouse cells. J Virol. 1988 May;62(5):1520–1523. doi: 10.1128/jvi.62.5.1520-1523.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Grass D. S., Blanck G., Hoganson N., Manley J. L., Pollack R. E. A functional simian virus 40 origin of replication is required for the generation of a super T antigen with a molecular weight of 100,000 in transformed mouse cells. J Virol. 1983 Nov;48(2):492–502. doi: 10.1128/jvi.48.2.492-502.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Teicher L. C., Kazim D., Pollack R. E., Wise L. S. Commitment of mouse fibroblasts to adipocyte differentiation by DNA transfection. Science. 1989 May 5;244(4904):582–585. doi: 10.1126/science.2470149. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukasawa K., Sakoulas G., Pollack R. E., Chen S. Excess wild-type p53 blocks initiation and maintenance of simian virus 40 transformation. Mol Cell Biol. 1991 Jul;11(7):3472–3483. doi: 10.1128/mcb.11.7.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination. Cell. 1984 Oct;38(3):791–800. doi: 10.1016/0092-8674(84)90274-5. [DOI] [PubMed] [Google Scholar]
- Miner J. H., Wold B. J. c-myc inhibition of MyoD and myogenin-initiated myogenic differentiation. Mol Cell Biol. 1991 May;11(5):2842–2851. doi: 10.1128/mcb.11.5.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson C. P., Jr 10T1/2 cells: an in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ Health Perspect. 1989 Mar;80:221–227. doi: 10.1289/ehp.8980221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]