Abstract
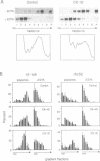
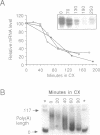
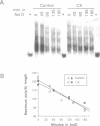
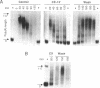
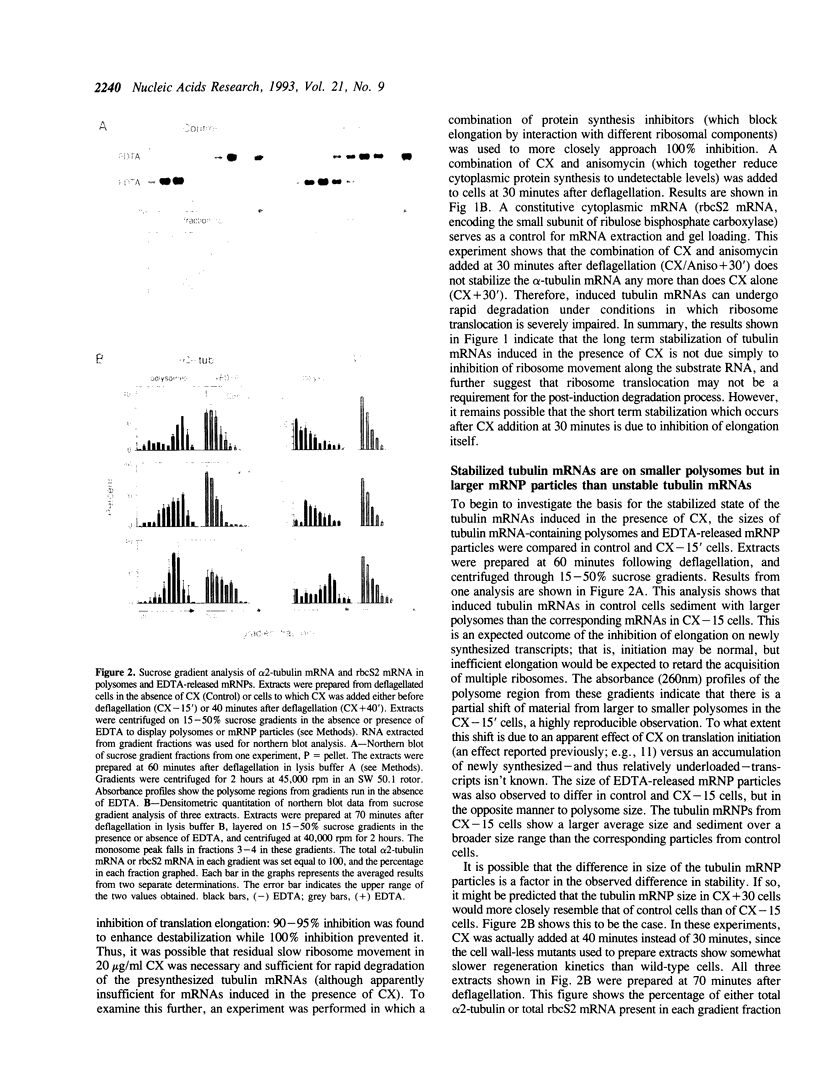
In Chlamydomonas, the usual rapid degradation of tubulin mRNAs induced by flagellar amputation is prevented by inhibition of protein synthesis with cycloheximide. Evidence is presented that the ability of cycloheximide to stabilize alpha-tubulin mRNA depends on the time of addition. Addition of cycloheximide to cells before induction strongly stabilizes the induced mRNAs, while addition after their synthesis stabilizes them only transiently. Moreover, cycloheximide inhibition does not stabilize the same alpha-tubulin mRNA species in uninduced cells. These results suggest that cycloheximide is not acting to stabilize the induced alpha-tubulin mRNAs simply by preventing ribosome translocation. The stabilized state of tubulin mRNA was found to correlate with its occurrence on smaller polysomes but larger EDTA-released mRNP particles than the unstable state. A second effect of cycloheximide on the metabolism of induced tubulin mRNAs is to accelerate complete poly(A) removal. This effect of cycloheximide inhibition, unlike stabilization, occurs whenever cycloheximide is added to cells, and appears unrelated to stabilization. The effect is shown to be mRNA-specific; poly(A)-shortening on the rbcS2 mRNA is not altered in the presence of cycloheximide, nor do completely deadenylated molecules accumulate. Experiments in which cells were released from cycloheximide inhibition suggest that deadenylated alpha-tubulin mRNAs may be less stable than their polyadenylated counterparts during active translation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. S., Jeffery W. R. Poly(adenylic acid) degradation by two distinct processes in the cytoplasmic RNA of Physarum polycephalum. Biochemistry. 1978 Oct 17;17(21):4519–4524. doi: 10.1021/bi00614a025. [DOI] [PubMed] [Google Scholar]
- Altus M. S., Nagamine Y. Protein synthesis inhibition stabilizes urokinase-type plasminogen activator mRNA. Studies in vivo and in cell-free decay reactions. J Biol Chem. 1991 Nov 5;266(31):21190–21196. [PubMed] [Google Scholar]
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Baker E. J., Diener D. R., Rosenbaum J. L. Accelerated poly(A) loss on alpha-tubulin mRNAs during protein synthesis inhibition in Chlamydomonas. J Mol Biol. 1989 Jun 20;207(4):771–781. doi: 10.1016/0022-2836(89)90243-x. [DOI] [PubMed] [Google Scholar]
- Baker E. J., Keller L. R., Schloss J. A., Rosenbaum J. L. Protein synthesis is required for rapid degradation of tubulin mRNA and other deflagellation-induced RNAs in Chlamydomonas reinhardi. Mol Cell Biol. 1986 Jan;6(1):54–61. doi: 10.1128/mcb.6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. J., Schloss J. A., Rosenbaum J. L. Rapid changes in tubulin RNA synthesis and stability induced by deflagellation in Chlamydomonas. J Cell Biol. 1984 Dec;99(6):2074–2081. doi: 10.1083/jcb.99.6.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay R., Coutts M., Krowczynska A., Brawerman G. Nuclease activity associated with mammalian mRNA in its native state: possible basis for selectivity in mRNA decay. Mol Cell Biol. 1990 May;10(5):2060–2069. doi: 10.1128/mcb.10.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. M., Vitek M. P., Morganelli C. M. Transcript length heterogeneity at the small heat shock protein genes of Drosophila. J Mol Biol. 1985 Nov 5;186(1):137–148. doi: 10.1016/0022-2836(85)90264-5. [DOI] [PubMed] [Google Scholar]
- Blume J. E., Shapiro D. J. Ribosome loading, but not protein synthesis, is required for estrogen stabilization of Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1989 Nov 25;17(22):9003–9014. doi: 10.1093/nar/17.22.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet P., Paris J., Phillippe M., Osborne H. B. Degradation of a developmentally regulated mRNA in Xenopus embryos is controlled by the 3' region and requires the translation of another maternal mRNA. Mol Cell Biol. 1991 Jun;11(6):3115–3124. doi: 10.1128/mcb.11.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G., Ross J. Regulation of c-myc mRNA stability in vitro by a labile destabilizer with an essential nucleic acid component. Mol Cell Biol. 1989 May;9(5):1996–2006. doi: 10.1128/mcb.9.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. C., Bryan S., Gadson P., Papaconstantinou J. Deadenylation of alpha 1-acid glycoprotein mRNA in cultured hepatic cells during stimulation by dexamethasone. J Biol Chem. 1989 Mar 5;264(7):4112–4119. [PubMed] [Google Scholar]
- Cleveland D. W. Gene regulation through messenger RNA stability. Curr Opin Cell Biol. 1989 Dec;1(6):1148–1153. doi: 10.1016/s0955-0674(89)80065-1. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell K. W. Flagellar regeneration in Chlamydomonas reinhardtii: evidence that cycloheximide pulses induce a delay in morphogenesis. J Cell Sci. 1976 May;20(3):639–654. doi: 10.1242/jcs.20.3.639. [DOI] [PubMed] [Google Scholar]
- Gay D. A., Sisodia S. S., Cleveland D. W. Autoregulatory control of beta-tubulin mRNA stability is linked to translation elongation. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5763–5767. doi: 10.1073/pnas.86.15.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Kelly R., Shaw D. R., Ennis H. L. Role of protein synthesis in decay and accumulation of mRNA during spore germination in the cellular slime mold Dictyostelium discoideum. Mol Cell Biol. 1987 Feb;7(2):799–805. doi: 10.1128/mcb.7.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller D. M., Horowitz J. A., Casey J. L., Klausner R. D., Harford J. B. Translation and the stability of mRNAs encoding the transferrin receptor and c-fos. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7778–7782. doi: 10.1073/pnas.88.17.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird-Offringa I. A., Elfferich P., Knaken H. J., de Ruiter J., van der Eb A. J. Analysis of polyadenylation site usage of the c-myc oncogene. Nucleic Acids Res. 1989 Aug 25;17(16):6499–6514. doi: 10.1093/nar/17.16.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird-Offringa I. A., de Wit C. L., Elfferich P., van der Eb A. J. Poly(A) tail shortening is the translation-dependent step in c-myc mRNA degradation. Mol Cell Biol. 1990 Dec;10(12):6132–6140. doi: 10.1128/mcb.10.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991 Dec;5(12A):2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- Levine B. J., Chodchoy N., Marzluff W. F., Skoultchi A. I. Coupling of replication type histone mRNA levels to DNA synthesis requires the stem-loop sequence at the 3' end of the mRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6189–6193. doi: 10.1073/pnas.84.17.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Lowell J. E., Rudner D. Z., Sachs A. B. 3'-UTR-dependent deadenylation by the yeast poly(A) nuclease. Genes Dev. 1992 Nov;6(11):2088–2099. doi: 10.1101/gad.6.11.2088. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Edwards D. R. Signalling and superinduction. Nature. 1991 Feb 28;349(6312):747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Willis A. C., Barratt M. J. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991 May 31;65(5):775–783. doi: 10.1016/0092-8674(91)90385-c. [DOI] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., Wake S. A. An analysis of the rate of metallothionein mRNA poly(A)-shortening using RNA blot hybridization. Nucleic Acids Res. 1985 Nov 25;13(22):7929–7943. doi: 10.1093/nar/13.22.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D., Parker R. Mutations affecting stability and deadenylation of the yeast MFA2 transcript. Genes Dev. 1992 Nov;6(11):2100–2111. doi: 10.1101/gad.6.11.2100. [DOI] [PubMed] [Google Scholar]
- Parker R., Jacobson A. Translation and a 42-nucleotide segment within the coding region of the mRNA encoded by the MAT alpha 1 gene are involved in promoting rapid mRNA decay in yeast. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2780–2784. doi: 10.1073/pnas.87.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Brewer G., Bernstein P., Hart P. A., Ross J. Regulation of mRNA turnover in eukaryotic cells. Crit Rev Eukaryot Gene Expr. 1991;1(2):99–126. [PubMed] [Google Scholar]
- Petersen R. B., Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989 Nov;1(1):135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A., Silflow C. D., Rosenbaum J. L. mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol Cell Biol. 1984 Mar;4(3):424–434. doi: 10.1128/mcb.4.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu A. B., Belasco J. G., Greenberg M. E. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991 Feb;5(2):221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartwout S. G., Kinniburgh A. J. c-myc RNA degradation in growing and differentiating cells: possible alternate pathways. Mol Cell Biol. 1989 Jan;9(1):288–295. doi: 10.1128/mcb.9.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum S. M., Wormington W. M. Deadenylation of maternal mRNAs during Xenopus oocyte maturation does not require specific cis-sequences: a default mechanism for translational control. Genes Dev. 1990 Dec;4(12B):2278–2286. doi: 10.1101/gad.4.12b.2278. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Baxter R. Specific inhibition of peptide-chain initiation by 2-(4-methyl-2,6-dinitroanilino)-N-methylpropionamide. Biochemistry. 1972 Aug 1;11(16):3060–3064. doi: 10.1021/bi00766a018. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Collis P., Gealt M. A. Control of induction of tubulin synthesis in Chlamydomonas reinhardi. Nature. 1977 Aug 18;268(5621):667–668. doi: 10.1038/268667a0. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Wisdom R., Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991 Feb;5(2):232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]
- Yen T. J., Gay D. A., Pachter J. S., Cleveland D. W. Autoregulated changes in stability of polyribosome-bound beta-tubulin mRNAs are specified by the first 13 translated nucleotides. Mol Cell Biol. 1988 Mar;8(3):1224–1235. doi: 10.1128/mcb.8.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Machlin P. S., Cleveland D. W. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988 Aug 18;334(6183):580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]