Abstract
This review examines the multiple levels of pre-existing immunity in the upper and lower female reproductive tract. In addition, we highlight the need for further research of innate and adaptive immune protection of mucosal surfaces in the female reproductive tract. Innate mechanisms include the mucus lining, a tight epithelial barrier and the secretion of antimicrobial peptides and cytokines by epithelial and innate immune cells. Stimulation of the innate immune system also serves to bridge the adaptive arm resulting in the generation of pathogen-specific humoral and cell-mediated immunity. Less understood are the multiple components that act in a coordinated way to provide a network of ongoing protection. Innate and adaptive immunity in the human female reproductive tract are influenced by the stage of menstrual cycle and are directly regulated by the sex steroid hormones, progesterone and estradiol. Furthermore, the effect of hormones on immunity is mediated both directly on immune and epithelial cells and indirectly by stimulating growth factor secretion from stromal cells. The goal of this review is to focus on the diverse aspects of the innate and adaptive immune systems that contribute to a unique network of protection throughout the female reproductive tract.
Keywords: female reproductive tract, innate immunity, adaptive immunity, antimicrobials
1. Introduction
The female reproductive tract is a unique immunological site that is required to protect the mucosa from a variety of pathogens without compromising the development of an allogenically distinct fetus. There are more than 30 different types of parasites, bacteria and viruses that infect the female reproductive tract. These include Trichomonas vaginalis (trichomoniasis), Neisseria gonorrhoeae (gonorrhoea), Chlamydia trachomatis (chlamydial infection), Treponema pallidum (syphilis), Herpes simplex virus type 2 (genital herpes), Human papillomavirus (genital warts/cervical cancer), human immunodeficiency virus (AIDS) and Hepatitis B virus (hepatitis). The World Health Organization (WHO) estimates that each day one million people acquire a sexually transmitted infection, which has reached epidemic proportions throughout the world (WHO 2001).
The focus of our research is studying the interactions between HIV infections, sex steroid hormones and innate immune protection in the female reproductive tract. Although the spread of HIV through needles and male-male contact is well recognized, in some areas such as sub-Saharan Africa, HIV is predominantly sexually transmitted with women and girls making up 57% of all people infected. Currently, a striking 76% of young people (aged 15–24 years) living with HIV are female (U.N.A.I.D.S. 2008).
Hormonal changes during the menstrual cycle regulate the immune system throughout the female reproductive tract in a way that optimizes conditions for successful sperm migration, fertilization, implantation and pregnancy (See reviews (Mor and Cardenas 2010, Wira et al. 2010a). Whether by correlation with menstrual cycle stage or through the addition of sex steroid hormones in culture or in vivo studies, estradiol and/or progesterone have been shown to regulate either directly or indirectly all aspects of innate and adaptive immunity. Throughout this review the influences of sex hormones in regulating immunity in the female reproductive tract will be emphasized.
Sexually acquired pathogens initially infect the mucosa of the female reproductive tract from where systemic dissemination can occur. With HIV, for example, cells of the female reproductive tract including macrophages, dendritic cells (DCs) and epithelial cells transfer virus within the mucosa to target CD4+ T cells, leading to viral replication and the subsequent spread throughout the body (Haase 2010). Protecting the female reproductive tract are multiple layers of immune responses that are precisely regulated by sex hormones to confer protection against pathogens. Our goal is to define the presence and function of innate and adaptive immune cells and the mechanisms by which they contribute to immune protection against genital tract pathogens.
2. Barrier protection – Epithelial Cells, Mucus and pH
The mucosal lining of the female reproductive tract, made up of epithelial cells and mucus, provides a robust physical and immunological barrier that prevents the transmission of sexually acquired infections (Figure 1). The upper female reproductive tract, consisting of the endometrium, endocervix and Fallopian tubes is lined with a single layer of columnar epithelial cells, with tight junctions between them. The integrity of the upper female reproductive tract epithelial barrier is directly altered by the presence of estradiol, which reduces tight membrane integrity in vitro (Fahey et al. 2008, Wira et al. 2010a). Furthermore, the underlying stroma directly alters barrier integrity of uterine epithelial cells by secreted cytokines and growth factors from stromal cells, which are also under hormonal control (Grant and Wira 2003). For example, TNFα decreases transepithelial resistance in uterine epithelial cells possibly via altered claudin expression, leading to decreased barrier protection and increased pathogen translocation across the epithelium (Grant-Tschudy and Wira 2005, Nazli et al. 2010). For a recent review on cytokine regulation of tight junctions see (Capaldo and Nusrat 2009).
Figure 1.
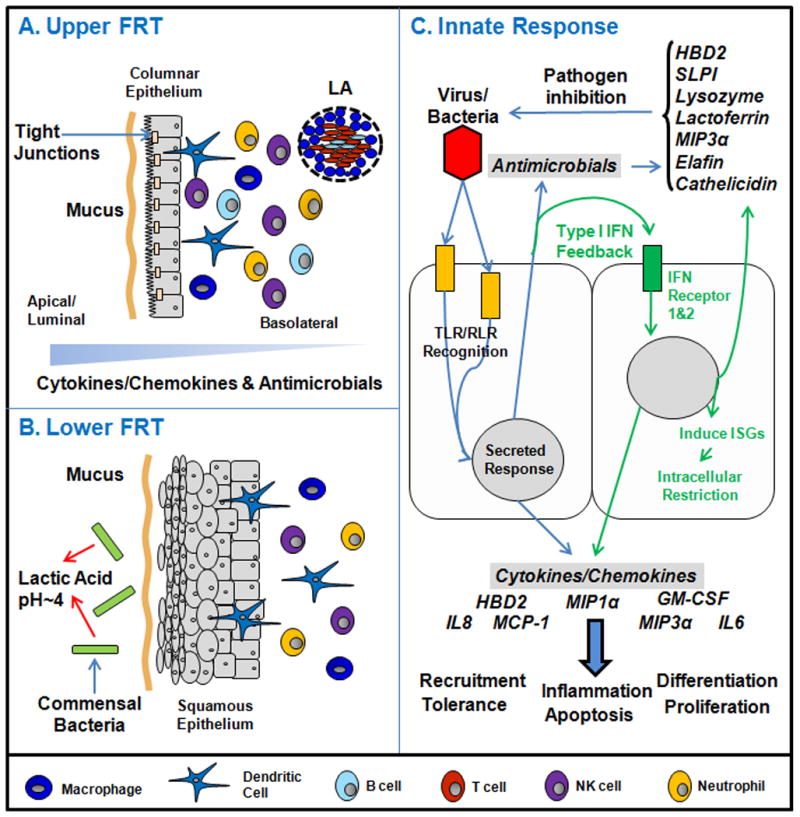
Schematic of the major components of the mucosal innate immune system in the human female reproductive tract (female reproductive tract). Panel A: The upper female reproductive tract, consisting of the Fallopian tubes, uterine endometrium and endocervix is lined by a single layer of columnar epithelial cells linked by tight junctions. Overlying the cells is a protective mucus layer (Section 2). Secretion is generally preferential towards the apical/luminal compartment with a gradient across the epithelial layer from lumen to tissue. Underlying the epithelial cells are innate and adaptive immune cells (Section 3). Also shown are lymphoid aggregates (LA) that are unique to the uterus. Panel B: The lower female reproductive tract, consisting of the ectocervix and vagina is covered by a layer of stratified squamous epithelial cells (Sections 2 and 3). Similar to the upper female reproductive tract, epithelial cells are protected by a mucus layer. The lower female reproductive tract has a resident commensal bacterial population that produces lactic acid thus lowering vaginal pH (Section 2). Below the epithelial layer are innate and adaptive immune cells. Panel C: The innate immune response of upper female reproductive tract epithelial cells to an invading pathogen. Epithelial cells express a panel of Toll-like receptors (TLRs) and RIG-like receptors (RLRs) that allow them to recognize and respond to bacteria or viruses. The Type I Interferon (IFN) response (green arrows, middle section of panel C) is a potent defense system in female reproductive tract cells (Sections 4 and 5). Additionally, in response to pathogens and sex hormones, antimicrobials and cytokines/chemokines are secreted to confer broad spectrum protection.
The lower female reproductive tract, consisting of the vagina and ectocervix, is lined with multiple layers of non-keratinized stratified squamous epithelium attached to a basement membrane. The outer squamous layer effectively protects the underlying tissue from abrasions during intercourse. A lack of tight junctions in the squamous epithelial layers permits the movement of small molecules within epithelial spaces between cells. This may lead to intra-epithelial transport of pathogens such as HIV, which in turn leads to pathogen contact with potential cellular targets including Langerhans cells and CD4+ T cells dispersed throughout the basal vaginal epithelium (Hladik et al. 2007). Not only forming a physical barrier, uterine and vaginal epithelial cells in vitro express pattern recognition receptors (PRRs) including Toll-like receptors (TLRs) and NOD-like receptors (NLRs) that recognize conserved pathogen-associated molecular patterns (PAMPs) on microorganisms. Upon stimulation, PPRs mediate the secretion of cytokines, chemokines and antimicrobial peptides.
Protecting vaginal and uterine epithelial cells from direct contact with pathogens is a layer of mucus. Mucus is composed of a family of glycosylated proteins, known as mucins that physically trap pathogens in a thick gel phase. Encoded by the MUC gene family, at least 13 mucins are present and are differentially expressed by epithelial cells with respect to both location and menstrual status (Gipson et al. 1997, Vigil et al. 2009). A recent study has shown that mucins adhere directly to microorganisms, including Candida albicans, through fucosylated glycans (Domino et al. 2009). Interspersed within mucin complexes is an aqueous phase containing immunoglobulins and antimicrobials (see Section 4), which prevent viable pathogens from infecting the epithelial mucosa (Ming et al. 2007).
Secreted by cervical crypts, human cervico-vaginal mucus is the most extensively studied mucus in the female reproductive tract and is an important barrier protecting the upper female reproductive tract from ascending sexually transmitted infections. Two types of human cervico-vaginal mucus are present within the female reproductive tract depending on the stage of the menstrual cycle. Estrogenic mucus is thin and watery with a low viscosity that permits sperm movement. Estrogenic mucus is present at the proliferative stage and increases at ovulation. Estrogenic mucus is sub-divided into three types: S, L and P depending on its crystalization pattern. Progestational mucus is thick, sticky and blocks the passage of spermatozoa. Progestational mucus is present at high concentrations following ovulation during the secretory phase but is also present at low levels during the menstrual and early proliferative phases (Elstein 1978, Vigil et al. 2009).
An important component of cervico-vaginal mucus that affects the transmission of pathogenic organisms is pH. Normally the cervico-vaginal mucus is acidified to a pH of 4-5 (Ravel et al. 2010). The acidic mucus environment of the vagina has been shown to slow the rate of HIV diffusion by abolishing the negative surface charge of the virus. In the presence of semen, which has a pH closer to 8, HIV retains its negative surface charge resulting in a higher rate of diffusion (Lai et al. 2009).
The acidic microenvironment of the vagina is maintained by lactic acid producing commensal bacteria. The most common of these found throughout the reproductive cycle of normal pre-menopausal healthy women is Lactobacillus (Witkin et al. 2007). Although Lactobacillus is most common, bacterial microdomes differ between ethnic groups. When comparing Asian, White, Black and Hispanic North American women, a higher pH of 4.7-5 was observed in Hispanic and Black women compared with a lower pH of 4.2- 4.4 for White and Asian groups (Ravel et al. 2010). A higher pH in some ethnic groups correlates with a higher proportion of anaerobic bacteria present in the vagina. Currently, vaginal pH and an increased prevalence in anaerobic bacteria are used for the clinical diagnosis for a number of infections including bacterial vaginosis caused by the bacterium Gardnerella (pH>4.5) and the parasite Trichomonas vaginalis (pH 5.0-6.0)(Wilson 2004). Recent findings by Ravel et al imply that the normal vaginal microdome of individuals needs to be taken into account when determining the level of risk for infection and the diagnosis of disease (Ravel et al. 2010).
In addition to regulating vaginal pH, a recent study by Ahmed et al reported that specific commensal microdomes protect against HIV infection (Ahmed et al. 2010). This study showed that Escherichia coli, Veillonella parvula and Neisseria mucosa suppressed HIV-1 infection through TLR-4 activation. In contrast, TLR-2 activation by Lactobacillus acidophilus, Prevotella melaninogenica, Prevotella bivia and Mycobacterium smegmatis enhanced infection (Ahmed et al. 2010). This concept requires further research but is an important observation on how commensal bacteria may directly alter the vaginal environment thus mediating infection.
Together, epithelial cells, mucus lining and acid producing commensal bacteria form a dynamic physiological structure that interacts with microorganisms to prevent infection.
3. Innate Immune Cells - Presence and Function
Macrophages and Dendritic cells
Macrophages and DCs are important cells that phagocytose and subsequently kill pathogens through acidic and enzymatic digestion (Table 1). Macrophages account for approximately 10% of the leukocytes present in the female reproductive tract with their numbers highest in the endometrial stroma and myometrial connective tissue (Wira et al. 2005). The movement of macrophages into endometrial tissue is regulated by estradiol and progesterone, with the number of macrophages in the endometrium greatest prior to menstruation (Starkey et al. 1991). In contrast, vaginal tissue macrophage numbers remain stable throughout the menstrual cycle (Wira et al. 2005). Phenotypically, vaginal macrophages are distinct from their gastrointestinal counterpart. For example, vaginal macrophages express higher levels of the HIV-1 receptor CD4, and co-receptors CCR5 and CXCR4 than intestinal macrophages (Shen et al. 2009, Cassol et al. 2010). This unique phenotype correlates with a greater susceptibility towards HIV-1 infection than intestinal macrophages (Shen et al. 2009).
Table 1. Immune cells of the female reproductive tract.
Summary of the function and distribution of cells in the upper female reproductive tract (Fallopian tubes, uterine and endocervical mucosa) and the lower female reproductive tract (ectocervical and vaginal mucosa). Distribution of cells (upper vs lower) is expressed relative to each other.
| Cell | Role | Function | Upper | Lower |
|---|---|---|---|---|
| Epithelial Cells | Innate | Physical Barrier Mucin production Broad spectrum antimicrobials (uterine) pIgR mediated IgA transport (uterine) |
Single Cuboidal | Stratified Squamous |
| Neutrophils | Innate | Phagocytosis Antimicrobial α defensins |
High | Low |
| Dendritic/Lange rhans | Innate | Antigen presentation | High | Low |
| Macrophages | Innate | Phagocytosis (includes antibody bound pathogens) Antigen presentation |
High | low |
| Natural Killer | Innate | Apoptosis of infected cell | Low | High |
| CD4+ T cells | Adaptive | TH-1 Cell Mediated Responses IFN secretion – antiviral activity |
High | Low |
| CD8+ cells | Adaptive | TH-1 Cell Mediated Responses Apoptosis of infected cells |
High | Low |
| B Cells | Adaptive | TH2 Humoral responses Maturation into IgA/IgG secreting plasma cells |
lgG<?>lgA | lgG>lgA |
For adaptive responses, cell distribution is dependant on inflammation site in FRT
DCs are localized to the sub-epithelial stroma of the endometrium. In contrast, vaginal DCs are present within the epithelial layer (Iijima et al. 2008). Recently we found that uterine epithelial cells secrete soluble mediators basolaterally towards the stroma. These mediators induce a tolerogenic phenotype in local dendritic cell populations that is characterized by decreased expression of the co-stimulatory molecules CD83 and CD86, as well as decreased sensitivity to TLR3 and 4 stimulation (Ochiel et al. 2010a). However, the identity of these mediators is undetermined and whether epithelial cell secretions directly affect the phenotype of other immune cells remains an intriguing question.
As professional antigen presenting cells (APCs), macrophages and dendritic cells (DCs) are important for the generation of adaptive immune responses during infection. Pathogen exposure and phagocytosis induces macrophage and DC maturation and antigen presentation on type I or type II major histocompatibility complexes (MHC). Antigen presentation to naive T cells results in the expansion of pathogen-specific adaptive immunity (Sallusto and Lanzavecchia 2002) (see Section 6). Although the roles of macrophages and DCs are to prevent infection by direct inactivation or the stimulation of adaptive immunity, recent studies have implicated these cells as facilitators of HIV transmission. The expression of DC-SIGN by these cells has been shown to support HIV infections and contributes to the formation of a T cell synapse (Chehimi et al. 2003, Gringhuis et al. 2010).
Natural Killer (NK) Cells
NK cells possess cytotoxic activity and constitute approximately 10% of systemic leukocytes but up to 70% of mucosal leukocytes in the endometrium. NK cells are involved in several processes including host defense, implantation and pregnancy (Wira et al. 2005, Mselle et al. 2007). Blood NK cells generally possess a distinct phenotype from female reproductive tract NK cells. For example, female reproductive tract NK cells express CD9, a marker absent from blood NK cells (Mselle et al. 2007). Within the female reproductive tract, NK cells demonstrate differing phenotypic characteristics. CD69 and CD94 are both expressed by NK cells in the endocervix and endometrium but not in the ectocervix (Mselle et al. 2007). The number of NK cells varies in the endometrium across the menstrual cycle, reaching a peak of approximately 70% of the total leukocyte population in the secretory phase (Wira et al. 2005). However, NK cell numbers in other regions of the female reproductive tract are not affected during the menstrual cycle and account for 10-30% of leukocytes at these locations. Overall, the lowest number of NK cells is found in the Fallopian tubes. The mechanisms controlling the specific localization of NK cells within the female reproductive tract are unknown and may reflect the different functional responsibilities between different tissues in the female reproductive tract.
Similar to blood NK cells, uterine NK cells produce pro-inflammatory cytokines such as GM-CSF, IL-10, IL-8 and IFNγ and thus promote the inflammatory response, induce macrophage activation and cytotoxic T cell generation. Uterine NK cells, but not blood NK cells, also produce angiogenic growth factors and leukemia inhibitory factors, both of which are essential for blood vessel development. Their important role in female reproductive tract innate defense is highlighted by the increased rate of herpes viruses in patients with defects in NK cell function (Bloomfield and Lopez 1980). Furthermore, unlike blood NK cells, uterine NK cells can inhibit the infection of target cells by HIV X4 but not R5 strains via the secretion of CXCL12 (Mselle et al. 2009).
Neutrophils
Neutrophils are present throughout the female reproductive tract where their numbers are highest in the Fallopian tubes and progressively decrease from the upper female reproductive tract into the vagina (Wira et al. 2005). Their levels are relatively constant across the menstrual cycle. However, in the endometrium there is a significant increase in neutrophils at menses that is preceded by a surge in IL-8. The presence of neutrophils may serve two purposes at menses: first, to aid in the breakdown of endometrial tissue via the release of elastase which subsequently activates matrix-metalloproteinases; second, to increase innate immune defense as the epithelial barrier is disrupted. Neutrophils express TLRs 1-9 and respond to pathogens through phagocytosis, production of oxidative compounds, and release of antimicrobial peptides. Of note, neutrophils produce protease inhibitors (Trappin-2/Elafin), α-defensins known as human neutrophil peptides (HNPs), phospholipases and cytokines (Selsted and Ouellette 1995).
4. Antimicrobials Peptides
Secretions isolated from the female reproductive tract contain a number of broad-spectrum antimicrobial peptides. These include defensins (α and β), chemokines, anti-proteases and enzymes. Antimicrobials prevent and/or reduce infection by killing or preventing the growth of microorganisms by direct or indirect mechanisms (See review (Ganz 2003). For example human β defensin 2 (hBD2) directly kills bacteria through membrane pore formation, whereas the chemokines CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES and CXCL12/SDF-1α block HIV-1 binding to co-receptors CCR5 and CXCR4 on host cells (see reviews (Verani and Lusso 2002, Ganz 2003)). In addition to directly interfering with the infectivity of the pathogen, many antimicrobial factors are chemo-attractants recruiting innate and adaptive immune cells to female reproductive tract sites. These are examples of the multifaceted capabilities of these factors, as well as the multi-layered immune defenses that exist to protect the female reproductive tract.
Antimicrobial peptides present in the female reproductive tract are effective at inhibiting Gram-positive and Gram-negative bacteria (ex. S. aureus, N. gonorrhoeae, C. trachomatis), fungi (ex. C. albicans) and enveloped viruses (ex. HIV-1) (Wira et al. 2010b). Indeed, some endogenous female reproductive tract antimicrobials are broad-spectrum in that one antimicrobial can inhibit bacterial, fungal and viral female reproductive tract infections. For example, secretory leukocyte protease inhibitor (SLPI) has been shown to inhibit S.aureus, C.albicans and HIV-1 (Tomee et al. 1997, Hocini et al. 2000, Fahey and Wira 2002).
Antimicrobials are produced both constitutively and following microbial stimulation by epithelial and immune cells. In vitro, upper female reproductive tract epithelial cells secrete several types of antimicrobial peptides, which include a range of human β-defensins (hBDs), SLPI, lysozyme, tracheal anti-microbial peptide, MIP3α/CCL20, Trappin-2/Elafin and cathelicidin (Wira and Fahey 2004, Wira et al. 2005). Furthermore, our work has shown that apical uterine epithelial cell secretions prevent and/or minimize pathogen infectivity of sexually transmitted infection prior to contact with epithelial barrier (Fahey et al. 2005, Wira et al. 2010b). Vaginal epithelial cell lines, upon stimulation, have been shown to secrete hBD2 and chemokines (Pivarcsi et al. 2005). Some antimicrobials however, are present in cervico-vaginal lavage fluid that cannot be accounted for by vaginal cell production. This suggests movement of upper tract antimicrobial secretions down into the lower tract and/or the production of antimicrobials by vaginal leukocytes.
Like most immune functions in the female reproductive tract, the relative concentration of molecules is mediated by hormonal fluctuation during the menstrual cycle. At mid-cycle (day 13), following estradiol stimulation, a suppression of SLPI, hBD2, HNP1-3, and lactoferrin in cervico-vaginal lavage fluids occurs and remains depressed for 7-10 days (Keller et al. 2007). In contrast, protein levels remained unchanged over the course of the menstrual cycle. It has been shown that cervico-vaginal lavage fluids from healthy women have an intrinsic ability to inhibit HIV-1 and HSV-2 (Keller et al. 2006). Anti-HSV-2 activity correlated with the concentrations of α-defensins in cervico-vaginal lavage fluids. These secretions have also been shown to contain the antimicrobials SLPI, MIP3α/CCL20, Elafin, and hBD2, some of which correlate with the inhibition of HIV by cervico-vaginal lavage fluid in vitro and therefore might potentially inhibit viral infection in vivo (Ghosh et al. 2010). The suppression of antimicrobial production during the normal menstrual cycle can have a significant impact on female reproductive tract vulnerability to sexually transmitted pathogens, specifically HIV.
Although broad-spectrum, antimicrobial peptides do not inhibit colonization by normal protective commensal bacteria. Our laboratory has shown that secretions from primary cultures of uterine or Fallopian tube epithelial cells directly inhibit N. gonorrhoeae, C. albicans, and HIV-1, all potential pathogens of the female reproductive tract (Wira et al. 2010b). Significantly, these same secretions had no effect on the commensal Lactobacillus crispatus, suggesting that commensals and antimicrobials have co-evolved to enhance protection in the lower female reproductive tract (Wira et al. 2010b). The multitude of microbial factors in the female reproductive tract shows possibilities of being exploited to develop novel interventions and represents a promising and exciting advance in the field of reproductive immunology.
5. Communication - Cytokines and Chemokines
Cytokines and chemokines are chemical messengers that maintain the normal homeostatic environment and mediate endometrial proliferation, menstruation and implantation (Kayisli et al. 2002). Moreover, cytokines and chemokines regulate many innate and immune functions in the female reproductive tract.
In most cases the secretion of cytokines from uterine epithelial cells occurs preferentially towards the apical/luminal compartment resulting in a gradient that is important for attracting immune cells to the epithelial surface (Fahey et al. 2005) (Figure 1). For example IL-8, produced by uterine epithelial cells both constitutively and in the presence of specific TLR agonists, is secreted at higher levels into the apical compartment of transwell inserts in vitro (Fahey et al. 2005, Schaefer et al. 2005). Apically secreted IL-8 induces neutrophil migration across the epithelium suggesting that, in the absence of a chemokine gradient, neutrophils would be less likely to cross the epithelial barrier, potentially reducing the level of protective α-defensins in luminal secretions (Carolan et al. 1997). Other cytokines and chemokines such as TGFβ are secreted into the basolateral/sub-epithelial compartment where they influence the development and function of resident immune cells (Eriksson et al. 2006, Ochiel et al. 2010b).
Our laboratory and others have demonstrated that changes in cytokine levels in cervico-vaginal lavage fluids occur during the menstrual cycle (Keller et al. 2007). Secretion of these cytokines may be under direct control of estradiol and/or progesterone or indirect, through the actions of sex hormones on underlying stromal cells. For example, progesterone withdrawal from human endometrium leads to the up-regulation of IL-8, MCP-1 and COX-2 (Critchley et al. 2003). In contrast, the production of some cytokines and chemokines from uterine epithelial cells are regulated indirectly by stromal cell secretion of growth factors. For example, following estradiol treatment, uterine stromal cells up-regulate the production of hepatocyte growth factor (HGF) that in turn regulates TNFα and MIP3α/CCL20 secretion by uterine epithelial cells (Grant-Tschudy and Wira 2005, Coleman et al. 2009, Haddad and Wira 2010).
An important cytokine family involved in female reproductive tract immunity, particularly against viruses, are the Type I interferons (IFNs). These consist of multiple IFNα subtypes, IFNβ, IFNε, IFNω and IFNκ in humans (Le Bon and Tough 2002). IFNs are rapidly induced in the presence of viral and bacterial pathogens (Schaefer et al. 2005, Trinchieri 2010), which up-regulate the production of hundreds of interferon-stimulated genes (ISGs) via autocrine and paracrine actions. Given their large number, ISGs are involved in a range of cellular processes including pathogen defense and cell death. A unique characteristic of this system is the near ubiquitous expression of IFNs and their receptors, thus allowing for a single cell or small group of cells to induce a widespread innate antiviral/antibacterial state across multiple cell types (Trinchieri 2010).
The predominant secreted IFN in the female reproductive tract is unknown and whether it varies with location (upper and lower female reproductive tract) remains an intriguing question. Our laboratory has shown that IFNβ is induced in uterine epithelial cells by the double-stranded viral agonist polyriboinosinic polyribocytidylic acid (poly(I:C)) (Patel et al. unpublished data). IFNβ stimulation of uterine epithelial cells leads to the rapid up-regulation of the intracellular antiviral genes Myxovirus A (MxA), 2′- 5′ oligoadenylate synthetase (2′-5′ OAS) and protein kinase R (PKR). Blockade of IFNβ signaling with an anti-IFNβ antibody only partially abrogates ISG induction upon poly(I:C) stimulation, suggesting that other Type I IFNs are required for maximal ISG up-regulation. Furthermore, poly(I:C)-induced ISG up-regulation is not affected by the presence of estradiol. IFNβ also induces expression of the anti-HIV molecules MIP3α/CCL20 and hBD2, indicating that it may be protective against HIV-1 infection in the female reproductive tract (Patel et al, unpublished data). IFN production has been detected in the vaginal epithelial cell lines but not in primary human vaginal cells (Trifonova et al. 2009).
While the role of Type I IFNs in innate immune defense is well defined, the role of sex hormones in the modulation of the IFN response in humans is relatively unknown. Progesterone induces ISG expression in the luminal and germinal epithelium of the ovine uterus (Bazer et al. 2009). However, it also inhibits the TLR9-mediated up-regulation of IFNα in plasmacytoid DCs so its overall contribution to mucosal defense is unresolved (Hughes et al. 2008).
The secretion of cytokines/chemokines leads to rapid communication between the different cell types present in the female reproductive tract. Furthermore, these molecules elicit a potent innate immune response, which creates an environment hostile to pathogen survival. As mentioned in the previous section, many chemokines are also active antimicrobial compounds directly interfering with microbial pathogenesis. While some induced responses are ubiquitous, many are unique to each cell type, allowing for a finely tuned response towards a specific pathogen. Overall, cytokines and chemokines are regulators of innate and adaptive immunity, barrier integrity of uterine epithelial cells as well as having an important role in normal endometrial physiology.
6. Adaptive Immunity
Adaptive immunity is a pathogen-specific driven response following presentation and stimulation of T cells by APCs. A number of cells in the female reproductive tract have been shown to present antigens on MHC molecules. These include classical APCs including macrophages, dendritic cells and Langerhans cells, but also epithelial cells of the cervix and endometrium. This suggests that antigen presentation is mediated by migratory and resident APCs in the female reproductive tract. Adaptive immunity includes Th1 (cell-mediated), Th2 (humoral), T regulatory and Th17 responses.
As illustrated in Figure 2, Th1 cell-mediated immunity involves the destruction of intracellular pathogens and is driven primarily by T lymphocytes. CD8+ cytotoxic effector T cells target pathogen infected cells through peptide-bound MHC class I molecules expressed on the cell surface, inducing apoptosis through perforin- and granzyme-mediated cytolysis (Lieberman 2003). Additionally, CD4+ T cells secrete high levels of IFNγ that mediate cytotoxic T cells as well as directly block viral replication (Iijima et al. 2008, Nakanishi et al. 2009). T cells are located in the stroma of the vagina, cervix and uterus both below the epithelium and also dispersed within epithelial cells where they are known as intraepithelial lymphocytes (Johansson et al. 1999).
Figure 2. The effector arm of adaptive (TH1: cell-mediated and TH2: humoral) immune responses.
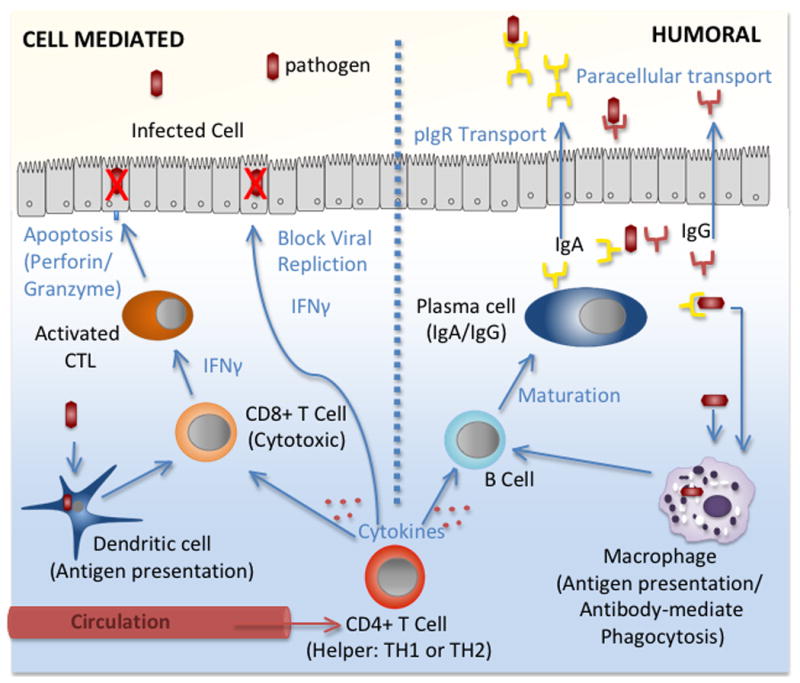
During infection, pathogen specific adaptive responses are driven by antigen presentation to T and B cells directly by dendritic cells, macrophages and epithelial cells in the mucosa or following activation by CD4+ T cell migration from circulation. Once activated through cytokine stimulation, T and B cells proliferate and differentiate. The cell-mediated response (left) is characterized by the production of IFNγ and the apoptosis of infected cells by cytotoxic CD8+ T cells. IFNγ also stimulates the production of intracellular antiviral genes that block viral replication. The humoral response (right) is mediated by B cell differentiation into antibody-secreting plasma cells. Both IgG and IgA are produced in the female reproductive tract and are secreted into the mucosa. Antibodies bind to pathogens, blocking infection by mediating phagocytosis or complement pathways.
In the female reproductive tract, CD8+ T cells (35-50%) predominate over CD4+ T cells (25%) (Kamat and Isaacson 1987, Givan et al. 1997). Unique to the uterine mucosa, lymphoid aggregates are made up of predominately CD8+ T cells that surround a central B cell core and encapsulated by macrophages. Lymphocyte aggregates develop during the proliferative phase and are largest during the secretory phase of the menstrual cycle (Yeaman et al. 1997, Yeaman et al. 2001). An absence of lymphocyte aggregates in post-menopausal women provides further evidence that estradiol and progesterone regulate aggregate formation and size. Although unclear, the development of lymphocyte aggregates suggests a role in the suppression of cell-mediated immunity that occurs in the uterus during the secretory phase of the cycle, when ovulation and implantation is most likely to occur (White et al. 1997).
Humoral immunity is characterized by the production of antibodies that bind to free as well as cell associated antigens, thereby inhibiting cell entry and/or neutralizing the biological activity of a pathogen (Figure 2). Antibody binding further mediates pathogen elimination through subsequent phagocytosis by macrophages or by the complement system. CD4+ helper T cells play a role in humoral immunity by driving B cell maturation to antibody secreting plasma cells (Kutteh and Mestecky 1994). Unlike other mucosal surfaces such as gastrointestinal and respiratory surfaces where IgA secretion is the dominant isotype, both IgG and secretory-IgA (SIgA) are expressed in genital secretions.
Studies of IgG to IgA ratios in human lower female reproductive tract secretions indicate that levels of IgG are two-fold to six-fold higher than that of IgA in cervical mucus and cervico-vaginal lavage fluid (for review see (Schumacher 1973, Kaushic and Wira 2008)). Unlike the lower female reproductive tract, ratios in uterine secretions are unknown owing to low baseline levels of secretion during the menstrual cycle. In contrast to other studies of cervical mucus, endocervical secretions reportedly have IgA levels that are higher than IgG (Quesnel et al. 1997). Levels of IgA most likely correspond to the presence of IgA secreting plasma cells in the endocervix. In the human uterus, levels of polymeric immunoglobulin receptor (pIgR), the epithelial cell receptor responsible for transporting IgA from tissue to lumen, varies with stage of the menstrual cycle (Sullivan et al. 1984). When expressed as the percentage of total protein, luminal uterine secretory component levels, the cleaved portion of membrane bound pIgR, were highest during the secretory phase, significantly reduced during the proliferative phase and lowest during menstruation. Using the rat model, previous studies from our laboratory have shown that IgA, IgG and pIgR are elevated in the uterus by estradiol during the reproductive cycle and following estradiol treatment of ovariectomized rats (Sullivan et al. 1983, Sullivan and Wira 1984, Wira and Sullivan 1985). In contrast, all three are inhibited in the vagina with hormone treatment (Kaushic et al. 1995, Kaushic et al. 1997). Unlike IgA which is transported from tissue to lumen against a concentration gradient, IgG moves down a gradient from blood to tissue to lumen in response to estradiol. Whether IgG moves passively by paracellular diffusion or is transported into luminal secretions in the female reproductive tract by neonatal Fc receptor (FcRn), which is expressed at other mucosal sites including the male reproductive duct, remains to be determined (Knee et al. 2005).
Although IgA antibody secretion is hormonally controlled during the menstrual cycle, changes in local production are further mediated by antigenic stimulation. Others have reported a marked increase of specific antibodies in cervico-vaginal lavage fluids compared to serum following HIV infection (Bélec and Pillot 1995). Furthermore, the route of antigen administration, as shown through vaccination, mediates the localization of the humoral response. For example, vaginal immunization induces the homing of antibody secreting cells to the cervix, while nasal vaccination stimulates homing to the vaginal mucosa (Johansson et al. 1998). Differences in the type, intensity and anatomical localization of antibodies in the female reproductive tract suggest a compartmentalization of hormonal and antigen-mediated humoral responses.
At the time of fertilization, the immune system throughout the female reproductive tract is dampened to optimize conditions for implantation and pregnancy. During the proliferative (estradiol-dominant) phase of the menstrual cycle leading up to ovulation, expansion of CD4+ CD25+ Foxp3+ regulatory T cells occurs in the human uterus (Arruvito et al. 2007). This coincides with a decrease in cytolytic activity by uterine CD8+ cells after ovulation in the uterus (White et al. 1997). Furthermore, a unique subset of intraepithelial lymphocytes, designated by a γδT cell phenotype, is present in the uterus and suppresses the maternal anti-fetal immune responses through TGFβ (Nishimura et al. 2004). These γδT cells are hypothesized to bridge innate and adaptive immunity by driving an adaptive T helper type 1 (Th1) response through the secretion of IFN that occurs following genital infection with HSV-2 (Nishimura et al. 2004, Holtmeier and Kabelitz 2005).
Understanding the generation of hormone-regulated tolerance is important for the development of vaccines that protect the female reproductive tract. Studies in mice show that, unlike antigen administered at diestrus, antigen given at estrus, when estradiol levels are high, results in the induction of a tolerance phenotype (Black et al. 2000, Kaushic et al. 2000, Gockel et al. 2003). Understanding cyclic mediated tolerance is important for the generation of a robust adaptive response against sexually transmitted pathogens including HIV.
In the presence of pro-inflammatory cytokines such as IL-6, regulatory T cells differentiate into a distinct Th17 subset that is involved in inflammation. Th17 cells are associated with neutrophil recruitment to the female reproductive tract and are critical for the clearance of N. gonorrhoeae during infection (Feinen et al. 2010). Th17 cells have also been shown to regulate neutrophil migration in mouse chlamydial infections and are thought to play a role in late stage spontaneous abortion (Nakashima et al. 2010, Scurlock et al. 2010). The generation of protective Th17 responses specifically for pathogen clearance and/or pathology is unclear and requires further study.
Overall, the development of Th1, Th2, T regulatory and Th17 adaptive immune responses can be mediated directly by hormones and/or indirectly by a hormonally regulated cytokine environment during antigen stimulation. A number of animal studies have shown that with vaccination or infection, the generation of cellular and humoral adaptive immune responses is influenced by the stage of the reproductive cycle (Kaushic et al. 2000, Gockel et al. 2003). The mechanisms through which adaptive responses are balanced to induce pathogen specific protection and memory while supporting pregnancy remains to be elucidated.
8. Conclusions
In conclusion, this review demonstrates the presence of a complex network of immune protection that resides throughout the female reproductive tract. Analysis of the epithelial mucus barrier, innate immune cells, and their secretions in the female reproductive tract, indicate the presence of multiple levels of protection that minimize the risk of infection by potential pathogens. Superimposed on innate immune protection is the adaptive immune system, which protects the female reproductive tract through cell-mediated and humoral responses on a pathogen-specific basis. Both systems work in tandem and through integrated interactions to provide protection in the female reproductive tract. Under the influence of sex hormones, growth factors and commensal organisms, cells of the innate and adaptive immune systems are regulated to protect the female reproductive tract against potential pathogens.
Acknowledgments
This work was supported by AI51877 and AI071761 (awarded to Dr. Charles Wira) from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed N, et al. Suppression of human immunodeficiency virus type 1 replication in macrophages by commensal bacteria preferentially stimulating Toll-like receptor 4. J Gen Virol. 2010;91:2804–2813. doi: 10.1099/vir.0.022442-0. [DOI] [PubMed] [Google Scholar]
- Arruvito L, et al. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- Bazer FW, et al. Interferons and uterine receptivity. Semin Reprod Med. 2009;27:90–102. doi: 10.1055/s-0028-1108013. [DOI] [PubMed] [Google Scholar]
- Bélec L, Pillot J. Raped women and HIV infection. J Forensic Sci. 1995;40:925–926. [PubMed] [Google Scholar]
- Black CA, et al. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- Bloomfield SE, Lopez C. Herpes infections in the immunosuppressed host. Ophthalmology. 1980;87:1226–1235. doi: 10.1016/s0161-6420(80)35098-7. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolan EJ, et al. Cytokine-induced neutrophil transepithelial migration is dependent upon epithelial orientation. Am J Respir Cell Mol Biol. 1997;17:727–732. doi: 10.1165/ajrcmb.17.6.2745. [DOI] [PubMed] [Google Scholar]
- Cassol E, et al. Macrophage polarization and HIV-1 infection. J Leukoc Biol. 2010;87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- Chehimi J, et al. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J Leukoc Biol. 2003;74:757–763. doi: 10.1189/jlb.0503231. [DOI] [PubMed] [Google Scholar]
- Coleman KD, et al. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril. 2009;92:1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HOD, et al. Antiprogestins as a model for progesterone withdrawal. Steroids. 2003;68:1061–1068. doi: 10.1016/j.steroids.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Domino SE, et al. Cervical mucins carry alpha(1,2)fucosylated glycans that partly protect from experimental vaginal candidiasis. Glycoconj J. 2009;26:1125–1134. doi: 10.1007/s10719-009-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstein M. Functions and physical properties of mucus in the female genital tract. Br Med Bull. 1978;34:83–88. doi: 10.1093/oxfordjournals.bmb.a071464. [DOI] [PubMed] [Google Scholar]
- Eriksson M, et al. Endogenous transforming growth factor-beta inhibits toll-like receptor mediated activation of human uterine natural killer cells. Am J Reprod Immunol. 2006;56:321–328. doi: 10.1111/j.1600-0897.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Fahey JV, et al. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Human Reproduction. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- Fahey JV, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinen B, et al. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal immunology. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Ghosh M, et al. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, et al. Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997;56:999–1011. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- Givan AL, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Gockel CM, et al. Influence of the murine oestrous cycle on the induction of mucosal immunity. Am J Reprod Immunol. 2003;50:369–379. doi: 10.1034/j.1600-0897.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Grant KS, Wira CR. Effect of mouse uterine stromal cells on epithelial cell transepithelial resistance (TER) and TNF alpha and TGF beta release in culture. Biol Reprod. 2003;69:1091–1098. doi: 10.1095/biolreprod.103.015495. [DOI] [PubMed] [Google Scholar]
- Grant-Tschudy KS, Wira CR. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J Reprod Immunol. 2005;67:1–12. doi: 10.1016/j.jri.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, et al. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Haddad SN, Wira CR. Keratinocyte growth factor stimulates Macrophage Inflammatory Protein 3alpha and Keratinocyte-derived Chemokine secretion by mouse uterine epithelial cells. Am J Reprod Immunol. 2010;64:197–211. doi: 10.1111/j.1600-0897.2010.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocini H, et al. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin Diagn Lab Immunol. 2000;7:515–518. doi: 10.1128/cdli.7.3.515-518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmeier W, Kabelitz D. gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- Hughes GC, et al. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E, et al. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson EL, et al. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat BR, Isaacson PG. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol. 1987;127:66–73. [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, et al. Polymeric immunoglobulin A receptor in the rodent female reproductive tract: influence of estradiol in the vagina and differential expression of messenger ribonucleic acid during estrous cycle. Biol Reprod. 1997;57:958–966. doi: 10.1095/biolreprod57.5.958. [DOI] [PubMed] [Google Scholar]
- Kaushic C, et al. Regulation of polymeric immunoglobulin A receptor messenger ribonucleic acid expression in rodent uteri: effect of sex hormones. Endocrinology. 1995;136:2836–2844. doi: 10.1210/endo.136.7.7789308. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Wira CR. IgA and Reproductive Tract Immunity. In: Kaetzel C, editor. Mucosal Immune Defense: Immunoglobulin A. Kluwer Academic/ Plenum Publisher; New York: 2008. pp. 291–320. [Google Scholar]
- Kaushic C, et al. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000;68:4207–4216. doi: 10.1128/iai.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayisli UA, et al. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–221. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- Keller MJ, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- Keller MJ, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- Knee RA, et al. Transport of IgG across the blood-luminal barrier of the male reproductive tract of the rat and the effect of estradiol administration on reabsorption of fluid and IgG by the epididymal ducts. Biol Reprod. 2005;73:688–694. doi: 10.1095/biolreprod.105.041079. [DOI] [PubMed] [Google Scholar]
- Kutteh WH, Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Lai SK, et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Cur Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- Ming L, et al. Purification of antimicrobial factors from human cervical mucus. Hum Reprod. 2007;22:1810–1815. doi: 10.1093/humrep/dem128. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mselle TF, et al. Human uterine natural killer cells but not blood natural killer cells inhibit human immunodeficiency virus type 1 infection by secretion of CXCL12. J Virol. 2009;83:11188–11195. doi: 10.1128/JVI.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mselle TF, et al. Unique characteristics of NK cells throughout the human female reproductive tract. Clinical Immunology. 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, et al. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, et al. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol. 2010;64:4–11. doi: 10.1111/j.1600-0897.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- Nazli A, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, et al. Intraepithelial gamma/delta T cells may bridge a gap between innate immunity and acquired immunity to herpes simplex virus type 2. J Virol. 2004;78:4927–4930. doi: 10.1128/JVI.78.9.4927-4930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiel DO, et al. Human uterine epithelial cell secretions regulate dendritic cell differentiation and responses to TLR ligands. J Leukoc Biol. 2010a;88:435–444. doi: 10.1189/jlb.1009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiel DO, et al. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS One. 2010b;5:e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivarcsi A, et al. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005;7:1117–1127. doi: 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Quesnel A, et al. Comparative analysis of methods for collection and measurement of immunoglobulins in cervical and vaginal secretions of women. J Immunol Methods. 1997;202:153–161. doi: 10.1016/s0022-1759(97)00003-3. [DOI] [PubMed] [Google Scholar]
- Ravel J, et al. Microbes and Health Sackler Colloquium: Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1002611107. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002;4 3:S127–132. doi: 10.1186/ar567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TM, et al. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist Poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- Schumacher GFB. Soluble proteins in cervical mucus. In: Blandau RJ, Moghissi K, editors. The Biology of the Cervix. The University of Chicago Press; Chicago: 1973. pp. 201–233. [Google Scholar]
- Scurlock AM, et al. IL-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infection and Immunity. 2010 doi: 10.1128/IAI.00984-10. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Defensins in granules of phagocytic and non-phagocytic cells. Trends Cell Biol. 1995;5:114–119. doi: 10.1016/s0962-8924(00)88961-8. [DOI] [PubMed] [Google Scholar]
- Shen R, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 Infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey PM, et al. Variation during the menstrual cycle of immune cell populations in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1991;39:203–207. doi: 10.1016/0028-2243(91)90058-s. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, et al. Variations in the levels of secretory component in human uterine fluid during the menstrual cycle. J Steroid Biochem. 1984;20:509–513. doi: 10.1016/0022-4731(84)90263-2. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, et al. Steroid hormone regulation of free secretory component in the rat uterus. Immunology. 1983;49:379–386. [PMC free article] [PubMed] [Google Scholar]
- Sullivan DA, Wira CR. Hormonal regulation of immunoglobulins in the rat uterus: Uterine response to multiple estradiol treatments. Endocrinology. 1984;114:650–658. doi: 10.1210/endo-114-2-650. [DOI] [PubMed] [Google Scholar]
- Tomee JF, et al. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–747. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- Trifonova RT, et al. Polyanionic microbicides modify Toll-like receptor-mediated cervicovaginal immune responses. Antimicrob Agents Chemother. 2009;53:1490–1500. doi: 10.1128/AAC.01152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.N.A.I.D.S. AIDS epidemic update. Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO); Geneva, Switzerland: 2008. [Google Scholar]
- Verani A, Lusso P. Chemokines as natural HIV antagonists. Curr Mol Med. 2002;2:691–702. doi: 10.2174/1566524023361862. [DOI] [PubMed] [Google Scholar]
- Vigil P, et al. Scanning electron and light microscopy study of the cervical mucus in women with polycystic ovary syndrome. J Electron Microsc (Tokyo) 2009;58:21–27. doi: 10.1093/jmicro/dfn032. [DOI] [PubMed] [Google Scholar]
- W.H.O. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva: World Health Organisation (WHO); 2001. [Google Scholar]
- White HD, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- Wilson J. Managing recurrent bacterial vaginosis. Sexually transmitted infections. 2004;80:8–11. doi: 10.1136/sti.2002.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, et al. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Rep Immunol. 2010a doi: 10.1111/j.1600-0897.2010.00842.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, et al. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Wira CR, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal immunology. 2010b doi: 10.1038/mi.2010.72. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Sullivan DA. Estradiol and progesterone regulation of IgA, IgG and secretory component in cervico-vaginal secretions of the rat. Biol Reprod. 1985;32:90–95. doi: 10.1095/biolreprod32.1.90. [DOI] [PubMed] [Google Scholar]
- Witkin SS, et al. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol. 2007;21:347–354. doi: 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Yeaman GR, et al. CD8+ T cells in human uterine endometrial lymphoid aggregates: evidence for accumulation of cells by trafficking. Immunology. 2001;102:434–440. doi: 10.1046/j.1365-2567.2001.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman GR, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]


