Abstract
While T cell-based vaccines have the potential to provide protection against chronic virus infections, they also have the potential to generate immunopathology following subsequent virus infection. We develop a mathematical model to investigate the conditions under which T cells lead to protection versus adverse pathology. The model illustrates how the balance between virus clearance and immune exhaustion may be disrupted when vaccination generates intermediate numbers of specific CD8 T cells. Surprisingly, our model suggests that this adverse effect of vaccination is largely unaffected by the generation of mutant viruses that evade T cell recognition and cannot be avoided by simply increasing the quality (affinity) or diversity of the T cell response. These findings should be taken into account when developing vaccines against persistent infections.
INTRODUCTION
Vaccination expands the numbers of lymphocytes specific for a given pathogen. However, in some circumstances, increasing the number of antigen-specific lymphocytes may fail to provide protection and instead lead to an adverse outcome following exposure to the pathogen. Vaccine-induced pathology can occur for many reasons. For instance, the recent adenovirus 5-based vaccination against human immunodeficiency virus (HIV) led to an increased probability of infection following exposure (18). Alternatively, the severity of infection might increase, as seen with respiratory syncytial virus (RSV) following early attempts at immunization (10, 13, 14) and as potentially occurs with dengue virus, with which previous infection with one serotype may result in more severe pathology following challenge with heterologous serotypes (11, 19). The problem of adverse effects is of current relevance in the context of the development of vaccines against persistent infections. Conventional techniques have not yielded effective vaccines, but a promising approach involves the generation of vaccines that induce T cell responses against such infections. In this paper, we use mathematical models to explore pathology following T cell-based immunization against persistent infections.
Laboratory experiments with mice have demonstrated that increasing the number of specific lymphocytes can lead to increased pathology in chronic diseases, such as those caused by lymphocytic choriomeningitis virus (LCMV) and hepatitis B virus (HBV). HBV-transgenic mice, which are used as a model for chronic human HBV infection, develop acute liver failure due to loss of liver cells after adoptive transfer of HBV-specific CD8 T cells (8). In mice infected with chronic LCMV, the extent of immunopathology depends on the initial dose of virus and initial numbers of naive-phenotype-specific CD8 T cells (6, 9, 22). Since these experiments tested only narrow immune responses, a natural hypothesis was that the increased pathology might be avoided by broadening the immune response (22). However, predicting pathology requires understanding the complex interplay between virus replication, immune exhaustion, and immune escape.
In this paper, we develop a general mathematical model to capture these dynamics and link T cell immunization against persistent viruses to pathology developed after infection. Our model explicitly predicts that increased pathology cannot be avoided simply by broad immunization that induces an immune response against multiple viral epitopes, in contrast to the implication of earlier experiments.
METHODS
We meld a simple model of virus-host interaction together with a framework for quantifying pathology. The model captures the interplay between uninfected target cells (U), virus-infected target cells (V), and the magnitude of the immune response (X). Since the decay of free virus is fast relative to the change in the number of infected cells, the amount of virus is proportional to the number of infected cells (23). Given this simplification, we define
| (1) |
| (2) |
where a is the rate of production of host cells, b is their death rate, β is the rate of infection, α is the rate at which virus-infected cells die due to infection, k is the rate of clearance of infected cells by the antigen-specific CD8 T cell response X, and t is time. The rate of change of the functional immune response, X, is described by
| (3) |
Proliferation (the first term) occurs at a rate dependent on the density of antigen (2), where s is the maximum growth rate and φ is the density of antigen (infected cells) at which growth is half-maximal. The loss of functional cells occurs by exhaustion (the second term). While the mechanistic details of exhaustion are unclear, experimental studies (21, 26, 28) show that exhaustion arises when immune cells receive persistent stimulation and results in a waning of the immune response, either through clonal deletion of antigen-specific cells or by their being rendered nonfunctional. The variable Q tracks the level of exhaustion by integrating over the antigenic stimulus and decaying exponentially, as determined by the formula
| (4) |
where dq is the rate at which the immune response recovers from exhaustion. Functional immune cells, X in equation 3, are lost at maximal rate δ in a manner dependent on the level of exhaustion, Q, which is implemented as a Hill function, with half-maximal constant qc and coefficient n.
We keep the model as simple as possible because, in the absence of detailed information on the terms and parameters, simpler models frequently generate more robust qualitative results than complex models (12, 17). Our model captures the dynamics of the virus and immune system using 4 variables and 11 parameters, which contrasts with previous more complex models incorporating immune exhaustion (7, 25) (10 variables and 13 parameters or 4 variables and 18 parameters) that sought to address questions different from those considered in this paper.
We take two approaches to quantifying pathology: first, we determine the loss of host target cells, and second, we measure the secretion of cytokines by activated immune cells. The loss of target cells is typically determined by the decrease in total target cell numbers (U + V) from homeostatic equilibrium in the absence of infection (U = a/b) (15). If infected cells are only partly functional, then the effective number of target cells would be (U + fV), where f is the functionality of infected cells on a scale from 0 to 1. The secretion of cytokines by activated immune cells can be modeled by including a variable for the concentration of cytokines. As the qualitative results for cytokine-induced pathology are similar to those for the decrease in total target cell numbers, we focus on target cell loss in this paper and present the results for cytokine-induced pathology in the supplemental material. Pathology changes over time, and we focus on the maximum pathology that occurs over the course of the infection.
RESULTS
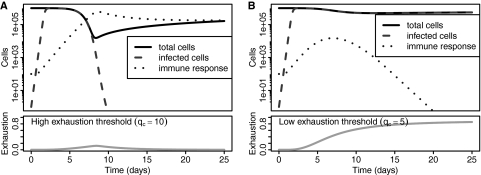
The model captures the basic features of acute and chronic infections with noncytopathic viruses such as LCMV (Fig. 1). If the threshold for exhaustion is high (e.g., qc = 10), then the immune response controls the virus prior to exhaustion to yield an acute infection with a functional response (Fig. 1A). If the threshold is low (e.g., qc = 5), then exhaustion occurs to yield a chronic infection (Fig. 1B). If we compare the losses of host cells (the minimum of the black line in Fig. 1A versus B), we find an order of magnitude less pathology in the chronic infection. Thus, while exhaustion prevents clearance of the virus, it reduces immunopathology, suggesting that exhaustion may be an adaptive feature of the CD8 T cell response.
Fig. 1.
Dynamics of host cells and virus during the course of acute and chronic infections. (A) A high exhaustion threshold (qc = 10) leads to acute infection with clearance of the virus and long-term immunity. (B) A low exhaustion threshold (qc = 5) leads to persistent infection with loss of immune cells. Pathology measured by the maximal loss of host target cells (black line) is an order of magnitude lower in chronic (B) than in acute (A) infections. Other parameters: a = 104 target cells/day, b = 0.01/(target cells · day), α = 0/(target cells · day), β = 8 × 10−6/[(target cells)2 · day], k = 1.0 × 10−5/(target cells · T cells · day), s = 1.3/(T cells · day), φ = 103 target cells, δ = 3/(T cells · day), dq = 0.1, n = 3, f = 1. Initial values were as follows: the number of uninfected cells at steady-state (U) was equal to a/b, the number of infected cells (V) was 1, and the number of naive CD8 T cells (X) that were fully functional (Q = 0) was 100.
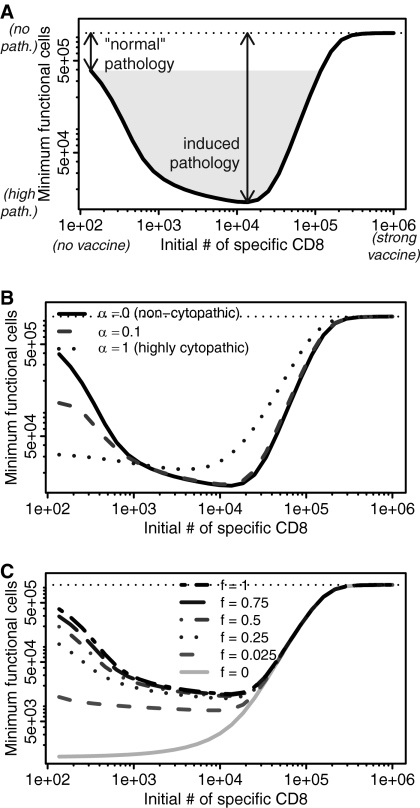
From here onward, we focus on viruses that normally give rise to a persistent infection (e.g., Fig. 1B). We examine how increasing the number of virus-specific CD8 T cells by vaccination affects pathology during a subsequent infection. As seen in Fig. 2A, a vaccinated individual with intermediate numbers of CD8 cells could experience more pathology than an unvaccinated individual when pathology is defined by loss of target cells. Intuitively, when we start with a few specific CD8 T cells, they eventually become exhausted, and during the period prior to exhaustion, their numbers are insufficient to kill many virus-infected cells. At the other extreme, when we start with many specific CD8 T cells, they quickly control the infection prior to the virus infecting many cells, and once again relatively few cells are killed. However, when we start with intermediate numbers of specific CD8 T cells, we have both large numbers of infected cells and a large functional immune response, leading to greater pathology.
Fig. 2.
Pathology is maximum for intermediate initial numbers of virus-specific CD8 T cells. The lower the minimum number of functional host target cells (U + fV) was at any point during infection, the greater the pathology. The number of host target cells in the absence of infection is shown by the horizontal dotted line. (A) An intermediate response to vaccination can induce pathology beyond that experienced by an unvaccinated individual. (B) Noncytopathic viruses (α = 0, solid line) in particular show greatly increased pathology. As we increase the cytopathicity of the virus (α), the pathology at low numbers of CD8 cells becomes more similar to the pathology at intermediate numbers of CD8 T cells. (C) This effect holds as long as infected cells retain some degree of functionality, f (f > 0). For all panels, other parameters are the same as described for Fig. 1B.
Virus cytopathicity and the functionality of infected cells.
Viruses vary greatly in cytopathicity: some budding viruses, such as LCMV, cause minimal damage to the cell; lytic viruses, such as herpesviruses, kill cells in which they proliferate. Cytopathic viruses cause more pathology in unimmunized individuals with few CD8 T cells than noncytopathic viruses, but this pathology increases only slightly in vaccinated individuals with intermediate numbers of CD8 T cells (Fig. 2B). Vaccinated individuals with high numbers of CD8 T cells have low pathology with both cytopathic and noncytopathic viruses. These results are consistent with those of earlier studies (15, 22).
If infected cells have only limited functionality, then the extent of increased pathology for intermediate initial numbers of CD8 cells becomes more limited (Fig. 2C). However, provided infected cells retain any functionality, maximum pathology will occur at intermediate initial numbers of CD8 cells.
Sensitivity of the immune response.
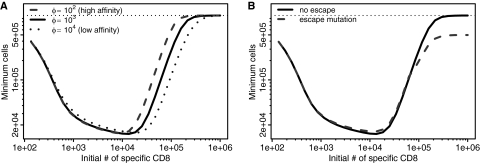
We next investigated whether the sensitivity of the vaccine-induced T cell response affected pathology during subsequent infection. We expect the sensitivity, φ, to be inversely proportional to the affinity of T cells for antigen. Our results suggest that pathology will be largely unaffected by affinity (Fig. 3A), although increasing affinity (a lower φ) results in maximum pathology occurring at a slightly lower initial number of CD8 T cells.
Fig. 3.
Pathology results are robust to parameter changes. (A) Pathology is largely independent of altering T cell affinity/sensitivity by manipulating φ, where a lower φ corresponds to increased sensitivity and a higher φ corresponds to decreased sensitivity. (B) Pathology is also minimally affected by immune escape in which a mutant virus that does not display the primary epitope but can still be targeted by a secondary immune response (details for this modified model are in the supplemental material). The dotted horizontal line indicates the number of target cells in the absence of infection. Other parameters are the same as described for Fig. 1B.
Breadth of immunity and virus escape.
Previous experimental studies (6, 22) have implied that increasing the breadth of the immune response might prevent immunopathology. We test this hypothesis by examining how pathology changes if we divide the total response into multiple responses to multiple epitopes. For simplicity of presentation, consider the situation in which the virus does not change (there is no antigenic escape) and the parameters for all responses are identical. More rigorously, in the case of m responses to m epitopes, equation 3 would be split into m different equations for X1, …, Xn, in which each Xi proliferates, kills, and dies at the same rate as every other Xj≠i. If , the dynamics will be exactly the same as the narrow (single) response model and pathology will not change. A more complicated model of multiple responses in which each has different parameters does not lend itself to such straightforward analytic analysis. Fundamentally, however, given a virus that normally causes immune exhaustion, increasing the initial numbers of any lineage of virus-specific CD8 cells has the potential to lead to increased pathology.
Many viruses that establish chronic infections do so by mutation of epitopes and evasion of T cell responses. We incorporate virus evolution by introducing escape variants which are not recognized by the response to the dominant epitope boosted by the vaccination (see the supplemental material for details). We find that the generation of escape variants has a minimal effect on pathology (Fig. 3B). However, the generation of escape can change the dynamics of infection. In particular, when the initial numbers of specific T cells are high, the generation of escape viruses changes the long-term outcome from virus clearance to a persistent infection, although the maximal loss of target cells is largely unchanged.
Experimental data versus model data.
Experimental studies using persistent LCMV infections (6, 9, 20, 22) have shown that vaccination could lead to adverse effects upon subsequent exposure to virus. Blattman et al. (6) systematically varied the numbers of transgenic P14 CD8 T cells specific for the GP33-41 epitope of LCMV in CD4-depleted C57BL/6 mice, a step that mimics different levels of a narrow vaccination that focuses on a single virus epitope. Then they challenged these mice with the clone 13 strain of LCMV, which usually gives rise to exhaustion and chronic infection, and monitored how the vaccination altered the dynamics of virus and pathology (Fig. 4, left panels). We now describe how our model explains the results of Blattman et al. (6).
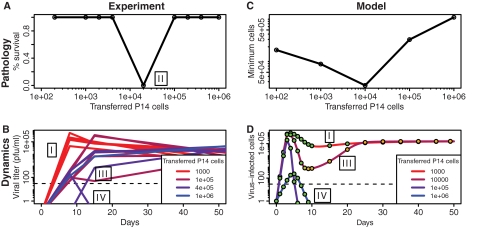
Fig. 4.
Pathology and dynamics of clone 13 LCMV infections. Experimental results of Blattman et al. (6) (A, B) can be qualitatively reproduced by our model (C, D). Mice were given adoptive transfers of different numbers of P14 cells (specific for the GP33 epitope) and subsequently infected with the clone 13 strain of LCMV, which typically gives rise to a persistent infection. Low initial numbers of P14 cells lead to chronic infection (outcome I). Intermediate initial numbers of P14 cells lead to the death of the mice around day 10 (outcome II). High numbers of transferred P14 cells lead to two possible outcomes: either the generation of an escape variant which forms a persistent infection (outcome III) or clearance of the virus (outcome IV). (D) In model time series, filled circles illustrate the relative amounts of wild-type to mutant virus, where green indicates that 100% of the cells were infected with the wild type and orange indicates that 100% were infected with the escape mutant. “Mutation” from a wild-type to an escape virus arises deterministically by starting at a low initial value (10−4) for a simulation using 104 transferred cells. We assume a 10% take of the transferred cells (5) in order to compare simulations to the experiment. Other parameters are the same as described for Fig. 1B except that α = 0.05/(target cells · day), β = 5 × 10−6/[(target cells)2 · day], k = 3.5 × 10−5 · day/(target cells · T cells · day), and s = 1.2/(T cells · day). Initial values were as follows: U = a/b, V1 = 1, V2 = 10−3 for simulation with 104 transferred cells (V2 = 0 otherwise), X1 = 10 × specified number of transferred cells, X2 = 102, and Q1 = Q2 = 0.
Blattman et al. (6) observed four outcomes. Transfer of relatively few P14 cells resulted in a typical chronic infection with exhaustion of both P14 and endogenous T cells and no host mortality (outcome I in Fig. 4). Transfer of an intermediate number of P14 cells resulted in the rapid death of all animals 9 to 10 days after infection (outcome II). Transfer of very large numbers of P14 cells resulted in either chronic infection (outcome III) or clearance (outcome IV), each of which occurred approximately half the time. The equal likelihoods of either outcome suggest that the initial inoculum effectively contains an escape mutant half of the time. Outcome III corresponds to the generation of an escape variant of the virus having a GP35 V→A mutation that prevents binding to P14 CD8 T cells. In this case, functional P14 responses were generated, but responses to other epitopes were exhausted. Outcome IV corresponds to clearance of the infection and the generation of functional immune responses to all virus epitopes. Only outcome IV corresponds to effective vaccination.
The model can qualitatively reproduce the four outcomes seen in the experimental results (Fig. 4, right panels). In particular, the model generates increased pathology when one starts with an intermediate number of specific CD8 T cells (outcome II). This outcome holds when pathology is quantified both by loss of target cells and by number of cytokines (Fig. 2 and see Fig. S2 in the supplemental material).
Note that we do not formally fit model parameters to the data, nor do we attach specific meaning to the parameters used to generate the model results presented in Fig. 4. With such simple data, many model parameters would be difficult to identify; thus, we focus on qualitative results over quantitative results (17).
DISCUSSION
Our model shows that immunization against persistent viral infections can, under some circumstances, lead to an increase in pathology following infection. This increased pathology is greatest for noncytopathic viruses and occurs at intermediate levels of T cell memory. This pathology is only minimally affected by T cell affinity, the breadth of the T cell response, or virus mutations leading to T cell escape.
We build on a quantitative framework for pathology (15) with the addition of immune exhaustion, measuring transient rather than steady-state pathology and considering cytokine-induced pathology (in addition to the loss of target cells). While we get the same basic results when pathology is due to target cell loss and cytokines, their relative contributions depend on the system being studied: cytokines have been shown to play a role in LCMV-induced pathology (3, 4, 16), and target cell loss has been shown to contribute to HBV-induced pathology (1, 8).
The model is consistent with experimental studies using persistent LCMV infections (6, 9, 20, 22) that have shown that vaccination can lead to adverse effects upon subsequent exposure to a virus. We might expect that these adverse effects of T cell vaccination arise from virus escape following narrow vaccination (as Oehen et al. [22] point out, this result “happens usually not with whole virus vaccines … but may be induced when only one or few of the virus epitopes are used for vaccination.”). Our model suggests that this is not the case; we predict that these adverse effects could occur even with a broad, vaccine-induced response.
Our model makes testable predictions and suggests key experiments. First, our expectation for the robustness of the intermediate pathology result in the face of viral escape mutations can be tested by adding small numbers of escape variants to the virus inoculum to simulate the occurrence of a mutation that modifies an epitope. Second, we predict that this observation holds regardless of the breadth of the immune response. This prediction could be tested by transferring multiple types of CD8 cells specific to a variety of epitopes rather than monoclonal GP33-specific cells. Finally, we note that it may be worth considering how the outcome depends on CD8 T cells of different phenotypes and proliferative abilities. This would be best done by repeating the experiments of Blattman et al. (6) using cells with naive, effector memory, and central memory phenotypes.
Models can play an important role in exploring adverse outcomes resulting from vaccination against chronic infections. The first step, which we take in this paper, is to investigate pathology in a simple model system: chronic LCMV infections. The next step is to extend these models to consider the dynamics of other, more complex persistent infections. In the case of persistent mycobacterium tuberculosis infections, we would need to meld a model of granuloma formation (27) and a model of exhaustion of CD4 cell responses. In this case, it will be interesting to understand the dynamics of the proliferation, differentiation, and function of PD-1-expressing CD4 cells in spite of continual antigenic stimulation (24). In the cases of pathogens that use antigenic variation to persist (HIV, malaria agent, hepatitis C virus), we would need to model pathogen variation and the generation of responses to the different variants. For the cases of simian immunodeficiency virus and HIV, we would also have to account for the virus infecting CD4 cells and thus interfering with the immune response. In the cases of specific infections, we would also need to incorporate the interaction of CD8 T cells with other components of the specific response, including CD4 T cell and antibody responses as well as the interaction between specific and innate responses.
Our current study suggests that vaccines that tip the balance away from immune exhaustion and toward viral clearance may inadvertently increase transient immunopathology, even when there is virus escape. This immunopathology is greatest for intermediate initial numbers of CD8 cells, which may arise immediately after vaccination or following the gradual decay of immunological memory to the virus over time. Research into the design and testing of vaccines against chronic infections should be aware of the possibility of the generation of vaccine-induced pathology.
ACKNOWLEDGMENTS
This research was supported in part by NIH grant R01 AI049334 to R. Antia, Swiss National Science Foundation grant 315200-114148 to R. R. Regoes, Gates Foundation grant 37902 and NIH/NIAID grant P01 AI27757-17 to J. N. Blattman, and NIH grant R01 AI30048 to R. Ahmed. B. F. Kochin was supported by the Fannie and John Hertz Foundation.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Ando K., et al. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152:3245–3253 [PubMed] [Google Scholar]
- 2. Antia R., Levin B. R., May R. M. 1994. Within-host population-dynamics and the evolution and maintenance of microparasite virulence. Am. Nat. 144:457–472 [Google Scholar]
- 3. Araki K., et al. 2010. Pathogenic virus-specific T cells cause disease during treatment with the calcineurin inhibitor FK506: implications for transplantation. J. Exp. Med. 207:2355–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badovinac V. P., Hamilton S. E., Harty J. T. 2003. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18:463–474 [DOI] [PubMed] [Google Scholar]
- 5. Blattman J. N., et al. 2002. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 195:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blattman J. N., et al. 2009. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83:4386–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bocharov G. A. 1998. Modelling the dynamics of LCMV infection in mice: conventional and exhaustive CTL responses. J. Theor. Biol. 192:283–308 [DOI] [PubMed] [Google Scholar]
- 8. Chisari F. V., Ferrari C. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29–60 [DOI] [PubMed] [Google Scholar]
- 9. Ehl S., Klenerman P., Zinkernagel R. M., Bocharov G. 1998. The impact of variation in the number of CD8(+) T-cell precursors on the outcome of virus infection. Cell. Immunol. 189:67–73 [DOI] [PubMed] [Google Scholar]
- 10. Fulginiti V. A., et al. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 89:435–448 [DOI] [PubMed] [Google Scholar]
- 11. Halstead S. B., O'Rourke E. J. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741 [DOI] [PubMed] [Google Scholar]
- 12. Hilborn R., Mangel M. 1997. The ecological detective: confronting models with data. Monographs in population biology 28. Princeton University Press, Princeton, NJ [Google Scholar]
- 13. Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405–421 [DOI] [PubMed] [Google Scholar]
- 14. Kim H. W., et al. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422–434 [DOI] [PubMed] [Google Scholar]
- 15. Krakauer D. C., Nowak M. 1999. T-cell induced pathogenesis in HIV: bystander effects and latent infection. Proc. Biol. Sci. 266:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu F., Feuer R., Hassett D. E., Whitton J. L. 2006. Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8 T cell-mediated, TNF-dependent immunopathology. J. Clin. Invest. 116:465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. May R. M. 2004. Uses and abuses of mathematics in biology. Science 303:790–793 [DOI] [PubMed] [Google Scholar]
- 18. McElrath M. J., et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept step study: a case-cohort analysis. Lancet 372:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morens D. M. 1994. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis. 19:500–512 [DOI] [PubMed] [Google Scholar]
- 20. Moskophidis D., Laine E., Zinkernagel R. M. 1993. Peripheral clonal deletion of antiviral memory CD8+ T cells. Eur. J. Immunol. 23:3306–3311 [DOI] [PubMed] [Google Scholar]
- 21. Moskophidis D., Lechner F., Pircher H., Zinkernagel R. M. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758–761 [DOI] [PubMed] [Google Scholar]
- 22. Oehen S., Hengartner H., Zinkernagel R. M. 1991. Vaccination for disease. Science 251:195–198 [DOI] [PubMed] [Google Scholar]
- 23. Perelson A. S. 2002. Modelling viral and immune system dynamics. Nat. Rev. Immunol. 2:28–36 [DOI] [PubMed] [Google Scholar]
- 24. Reiley W. W., et al. 2010. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 107:19408–19413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rouzine I. M., Murali-Krishna K., Ahmed R. 2005. Generals die in friendly fire, or modeling immune response to HIV. J. Comput. Appl. Math. 184:258–274 [Google Scholar]
- 26. Wherry E. J., Ahmed R. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young D., Stark J., Kirschner D. 2008. Systems biology of persistent infection: tuberculosis as a case study. Nat. Rev. Microbiol. 6:520–528 [DOI] [PubMed] [Google Scholar]
- 28. Zajac A. J., et al. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]