Abstract
Antibody-dependent cell-mediated viral inhibition (ADCVI) is an attractive target for vaccination because it takes advantage of both the anamnestic properties of an adaptive immune response and the rapid early response characteristics of an innate immune response. Effective utilization of ADCVI in vaccine strategies will depend on an understanding of the natural history of ADCVI during acute and chronic human immunodeficiency virus type 1 (HIV-1) infection. We used the simian immunodeficiency virus (SIV)-infected rhesus monkey as a model to study the kinetics of ADCVI in early infection, the durability of ADCVI through the course of infection, and the effectiveness of ADCVI against viruses with envelope mutations that are known to confer escape from antibody neutralization. We demonstrate the development of ADCVI, capable of inhibiting viral replication 100-fold, within 3 weeks of infection, preceding the development of a comparable-titer neutralizing antibody response by weeks to months. The emergence of ADCVI was temporally associated with the emergence of gp140-binding antibodies, and in most animals, ADCVI persisted through the course of infection. Highly evolved viral envelopes from viruses isolated at late time points following infection that were resistant to plasma neutralization remained susceptible to ADCVI, suggesting that the epitope determinants of neutralization escape are not shared by antibodies that mediate ADCVI. These findings suggest that despite the ability of SIV to mutate and adapt to multiple immunologic pressures during the course of infection, SIV envelope may not escape the binding of autologous antibodies that mediate ADCVI.
INTRODUCTION
The RV144 Thailand human immunodeficiency virus type 1 (HIV-1) vaccine trial showed that immunogens that elicited nonneutralizing antibody and CD4+ T cell responses but not CD8+ T cell responses reduced HIV-1 acquisition but did not improve control of virus replication after HIV-1 infection. These findings suggest that novel correlates of vaccine-induced HIV-1 protection need to be investigated (4, 9, 34). Interestingly, the ALVAC prime, protein boost regimen used in the RV144 Tai trial has been reported to elicit antibody-dependent cell-mediated cytotoxicity (ADCC) (23). Accruing evidence from human vaccine trials as well as nonhuman primate studies of simian immunodeficiency virus (SIV) vaccination suggests a correlation between effective ADCC and vaccine protection (12, 13, 19). The potential importance of ADCC and antibody-dependent cell-mediated viral inhibition (ADCVI) in HIV-1 infection is further underscored by reports that humans who control HIV-1 replication may have more persistent, potent ADCC than do typical HIV-1 progressors (3, 25).
ADCC and ADCVI are mediated by HIV-1-binding antibodies that interact via their Fc receptors with effector cells, commonly natural killer (NK) cells. The effector cells then destroy infected cells expressing antibody-bound antigen (2, 10). ADCC is a measure of the ability of effector cells to lyse antibody-bound target cells in vitro, while ADCVI describes the ability of virus-specific antibodies and effector cells to inhibit viral replication in a target cell population in vitro. Unlike neutralizing antibody responses, which may take months to develop and are initially highly specific to the individual's infecting virus, binding antibodies that mediate ADCC/ADCVI arise early following infection (15, 43). Despite these characteristics of ADCC/ADCVI, in the vast majority of human HIV-1 infections ADCC/ADCVI is incapable of abolishing virus replication once an infection is established. This may be a consequence of the time lag between infection and the generation of an antibody response. This time lag may allow viral propagation to outpace the ability of ADCC/ADCVI to contain it. NK cell activity increases sharply very early after HIV-1 infection (6), and so the presence of already existing binding antibodies evoked by prior vaccination may facilitate an early interdiction of a viral infection (40).
The remarkable plasticity of the HIV-1 envelope poses a challenge to the success of any antibody-based vaccine strategy. Nonsterilizing protection against infection may become compromised if the envelope of the infecting virus can mutate to evade antibody recognition. Several studies have shown that 3 to 5 months following infection, HIV-1 and SIV evolve mutations in the hypervariable loops of their envelopes and thus escape from recognition by neutralizing antibodies (18, 28, 29, 37, 49). Although there is counterevolution of the host neutralizing antibody response that may be partially effective against these mutated envelopes, sequence evolution allows the virus to anticipate and evade adaptations in neutralizing antibodies. It is unclear whether this extensive evolution of HIV-1 envelope under humoral selective pressure may similarly permit escape from binding of the antibodies that mediate ADCVI.
We have used a nonhuman primate model to characterize the coevolution of SIVmac251 and the nonneutralizing antibody response to this virus. We define the time frame for the emergence of ADCVI in relation to the development of nonneutralizing, gp140-binding antibodies as well as neutralizing antibodies. To investigate the ability of virus in chronically infected animals to evade ADCVI, we have generated infectious SIVmac239 clones bearing envelopes with mutations known to confer neutralization escape. We have characterized the breadth of ADCVI against these chronic viruses and a heterologous strain of SIV.
MATERIALS AND METHODS
Study animals.
Animals were infected as previously described with SIVmac251 (41, 48). All animals were cared for in accordance with the American Association for Accreditation of Laboratory Animal Care guidelines and with approval of the Institutional Animal Care and Use Committee of Harvard Medical School.
Cell culture and purification.
Whole blood from healthy human donors was obtained from a commercial vendor (Research Blood Components). Natural killer cells were isolated using the RosetteSep human NK cell enrichment cocktail (Stem Cell Technologies) and maintained in RPMI supplemented with 10% fetal calf serum and 20 IU/ml interleukin-2 (IL-2). By flow cytometry, we confirmed that ∼50 to 70% of cells expressed CD16 after purification, compared to less than 5% prior to purification; after purification, all cells were CD3 negative. Total peripheral blood mononuclear cells (PBMCs) were simultaneously isolated from the same donor by Ficoll-Hypaque gradient centrifugation and stimulated with 6.25 μg/ml concanavalin A and 20 IU/ml of IL-2. After 1 day of stimulation, CD4+ T cells were isolated from total PBMCs using the EasySep human CD4+ T cell enrichment kit (Stem Cell Technologies). We confirmed by flow cytometry that between 90 and 95% of cells expressed CD4 and CD3 after purification. Purified CD4+ T cells were then infected with SIVmac251 or SIVsmE660 at a multiplicity of infection (MOI) of 0.01.
ADCVI assay.
We measured ADCVI as the ability of NK cells to inhibit viral replication in SIVmac251-infected CD4+ T cells in the presence of plasma from an infected rhesus monkey. The SIVmac251 used in ADCVI assays was derived from the same quasispecies originally used to infect the animals in this study. NK cells and CD4+ T cells were purified and infected as described above. Three days after isolation from whole blood and 2 days after infection of CD4+ T cells, NK cells and infected CD4+ T cells were washed extensively and resuspended in 96-well plates at 5 × 104 CD4+ T cells and 1 × 105 NK cells per well. Heat-inactivated rhesus monkey plasma was added at a final dilution of 1:100 or 1:250. Control wells lacking plasma but containing NK cells (effector control) and viral replication control wells lacking both plasma and NK cells were included on every 96-well plate. Supernatants were collected on days 4 and 6 for quantification of virus.
ADCVI and neutralization assays for all monkeys in a given cohort with plasma from all time points were performed simultaneously, using NK and CD4+ T cells from a single donor. Experiments were repeated multiple times in separate donors with representative results demonstrated.
CD4+ T cell neutralization assay.
For every plasma sample tested, neutralization assay mixtures containing infected CD4+ T cells and plasma, without added NK cells, were also generated. These assays were performed in parallel with ADCVI assays, using target cells from the same donor. Briefly, human CD4+ T cells were isolated, stimulated, and infected as described above. Two days after infection, plasma at a 1:100 or 1:250 final dilution was added to 5 × 104 infected CD4+ T cells in 96-well plates. A plasma-free control (viral replication control) was included on every plate, and supernatants were collected on days 4 and 6 for quantification of virus.
Virus quantification and calculation of relative viral replication.
Five microliters of supernatant from ADCVI and neutralization cocultures was added to 2 × 104 TZM-bl cells in 96-well plates. TZM-bl cells were cultured for 48 h, and luminescence (relative luminescence units [RLU]) was quantified on a Perkin-Elmer Victor 3 luminometer. Relative viral replication for neutralization assays was calculated by subtracting background TZM-bl RLU from the RLU of the neutralization supernatant, and this difference was then divided by the RLU of the viral replication control. Relative viral replication for the ADCVI assays was calculated by subtracting background TZM-bl RLU from the RLU of the ADCVI supernatant and then dividing by the RLU of the effector control. RLU values for the effector and viral replication controls in all experiments were between 20,000 and 50,000 and for the background controls were 200 to 400. Normalization of ADCVI to neutralization for a specific plasma sample was calculated by dividing the relative viral replication of the ADCVI for the plasma by the relative viral replication of the neutralization assay.
Generation of chronic envelope viruses.
The dominant circulating envelope variants in each animal at month 16 postinfection were isolated by single genome amplification and cloned as previously described (49). Specifically, the clones PBEΔV1/V4.Mo16.G12, CP1WΔV1/V4.Mo16.39, CR2AΔV1/V4.Mo16.2, and CR53ΔV1/V4.Mo16.14, generated by Yeh et al. (49), were used for our study. These envelopes were extended 20 bp upstream to an SphI site found in SIVmac239 by PCR amplification using the primers 5′-GAAGCATGCTATAACACATGCTATTGTAAAAAGTGTTGCTACCATTGCCAGTTTTGTTTTCTTAAAAAAGG and 3′-CTCACAAGAGAGTGAGCTC.
Full-length envelope PCR products were cloned into TOPO XL PCR (Invitrogen), and their identities were confirmed by sequencing. These month 16 envelopes were subsequently digested with SphI and NheI (NEB) and cloned into the SphI and NheI loci of SIVmac239 (31), using Quick Ligase (NEB), to generate full-length provirus. Proviral DNA was transformed into Stbl3 (Invitrogen) bacterial cells and grown at 30°C. Infectious virus was generated by transfection of 293T cells plated overnight in 25-cm2 flasks until ∼90% confluence was reached with 8 μg of proviral DNA using the Lipofectamine 2000 reagent (Invitrogen) per the manufacturer's protocol. Supernatants were harvested 48 h after transfection and titrated on TZM-bl cells.
gp140 enzyme-linked immunosorbent assay (ELISA).
SIVmac251.30 gp140 was cloned from the challenge stock of SIVmac251, as previously described (8). The SIVmac251.30 gp140 protein was expressed via transient transfection of FreeStyle 293-F cells (Invitrogen). The His-tagged protein was purified over DEAE-Sepharose resin followed by immobilized-metal affinity chromatography (IMAC) using a nickel chelation resin (Sigma). Reacti-Bind enzyme immunoassay (EIA) plates were incubated with 2 μg/ml SIVmac251.30 gp140 overnight at 4°C. Plates were then incubated with serial dilutions of rhesus monkey plasma for 1 h at 37°C. Plates were subsequently incubated with horseradish peroxidase (HRP)-conjugated anti-monkey IgG (Rockland Immunochemicals, Gilbertsville, PA) for 1 h at 37°C. SureBlue TMB substrate was added, and optical density at 450 nm (OD450) was measured. Endpoints were determined with an OD450 cutoff of 0.10 (5-fold above background). Interplate variability was determined to be minimal as assessed with positive and negative controls included on every plate. Positive controls were a chimeric protein made from CD4 and IgG (CD4-Ig) and plasma from a monkey immunized with SIV env DNA. Normal rhesus macaque serum was used as a negative control.
Cytotoxicity assay.
Human CD4+ T cells were purified, stimulated, and infected with an MOI of 1.0 of SIVmac251. Three days after infection, cells were washed and 1 × 104 cells were coincubated with 2 × 104 autologous human NK cells and rhesus monkey plasma at a 1:250 dilution. Control wells for nonspecific lysis contained effector and target cells without plasma. Control wells for background plasma interference with the cytotoxicity assay were also included and did not show evidence of significant background activity. Six hours after coincubation, cytotoxicity was quantified using the Cytotox-glo kit (Promega). Cell lysis was estimated by adding the AAF-Glo reagent to each well and quantifying luminescence. Total cell number per well was then estimated by addition of digitonin-containing buffer and remeasurement of luminescence. RLU from the cell lysis assay were then divided by the total cell number RLU to obtain percent lysis. Percent specific lysis was calculated by subtracting results from the control wells for nonspecific lysis.
Statistical analysis.
Areas under curve (AUCs) for viral load, ADCVI, and neutralization were calculated using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). For this analysis, y = 0 was assumed to be the baseline, and peaks were counted only if they were >10% above baseline. We also used GraphPad Prism 5.00 to calculate Spearman correlations between AUC for viral load and ADCVI, binding antibody, or neutralization, for which we present two-tailed P values.
RESULTS
ADCVI develops in rhesus monkeys within weeks of SIVmac251 infection.
We sought to determine the kinetics of the emergence of ADCVI in SIVmac251-infected monkeys. To do this, we assessed the ability of plasma sampled from a cohort of 6 Mamu-A*01− rhesus monkeys intravenously infected with SIVmac251 to generate ADCVI. Viral loads and CD4+ T cell counts from these animals are summarized in Table 1 (41).
Table 1.
Viral load and CD4+ T cell counts of rhesus monkeys used for characterization of ADCVI kinetics and correlation studiesa
| Animal | No. of plasma viral RNA copies/ml (log10) |
No. of CD4+ T cells/μl |
||
|---|---|---|---|---|
| Day 14 | Day 84 | Day 0 | Day 84 | |
| A2V001 | 7.4 | 6.1 | 798 | 345 |
| A3V005 | 7.1 | 3.6 | 1,007 | 1,087 |
| A3V010 | 7.8 | 7.6 | 1,599 | 751 |
| A3V016 | 7.3 | 4.5 | 1,043 | 248 |
| A3V019 | 7.8 | 6.6 | 1,474 | 918 |
| A3V020 | 7.9 | 8.1 | 1,582 | 935 |
Six Mamu-A*01− rhesus monkeys were infected with SIVmac251 by the intravenous route. Plasma viral RNA was measured by ultrasensitive branched DNA amplification assay, and T cell subsets were enumerated by flow cytometry. Peak (day 14) and set point (day 84) plasma viral RNA levels and preinfection and set point absolute CD4+ T cell counts are summarized.
We developed a protocol to assay plasma for ADCVI activity based on previously described methods (14), using human blood to derive CD4+ T cell targets and NK cell effectors. The human and rhesus monkey Fcγ receptors share a high degree of homology, and utilization of human lymphocytes to assay rhesus monkey plasma for ADCC and ADCVI has previously been validated (14, 36). We infected human CD4+ T cells with SIVmac251 and subsequently added NK cells from the same healthy human donor at an NK-to-CD4+ T cell ratio of 2:1. We simultaneously added plasma collected from SIVmac251-infected monkeys at various time points after infection, at a 1:250 final dilution. We used this dilution of plasma based on smaller-scale pilot experiments in which we had observed nonspecific inhibition of viral replication by plasma from uninfected animals at dilutions less than 1:50, in the presence of NK cells. We also had determined that ADCVI activity diminished significantly at plasma dilutions greater than 1:1,000.
To control for antibody-independent NK-mediated cytotoxicity, we incubated CD4+ T cell targets with a 2:1 ratio of NK effector cells, in the absence of added plasma (effector control). After 4 to 6 days of coculture, we quantified the amount of replicating virus in culture supernatants using a TZM-bl reporter assay. Results were expressed as relative viral replication by normalization of values obtained in this assay to the plasma-free control samples, with relative viral replication below 1 indicating viral inhibition.
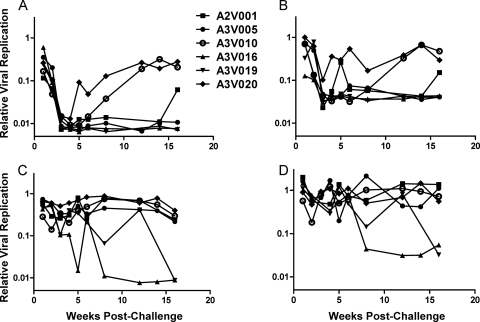
We evaluated plasma samples collected from monkeys between 1 and 16 weeks after infection with SIVmac251 for ADCVI against the infecting virus. By 3 to 4 weeks postinfection, plasma from all animals mediated up to 2-log inhibition of viral replication at a 1:250 dilution of plasma (Fig. 1A and B; results of ADCVI using two separate donors). In 3 of these animals—A3V005, A3V016, and A3V019—plasma sampled at all later time points tested retained potent ADCVI activity. Interestingly, plasma from animals A3V010 and A3V020 appeared to lose the ability to mediate ADCVI over the course of several weeks, with complete loss of activity by 12 weeks. Plasma from A2V001 exhibited an intermediate phenotype, mediating fluctuating ADCVI that retained greater than 10-fold inhibitory activity.
Fig. 1.
Development of ADCVI and neutralizing antibodies following SIVmac251 infection. Heat-inactivated plasma obtained from between 1 and 16 weeks postinfection was diluted 1 to 250 and incubated with SIVmac251-infected human CD4+ T cells. Replicating virus in the culture supernatant was quantified after 5 days of incubation using a TZM-bl reporter assay. Results are shown for ADCVI and neutralization utilizing NK cells and CD4+ T cells isolated from two different human donors. (A) ADCVI using CD4+ T and NK cells from a single donor. Infected CD4+ T cells were incubated with plasma as well as a 2-to-1 ratio of human NK cells. Results of the TZM-bl assay were normalized to infected cells cultured with natural killer cells in the absence of plasma and plotted as relative viral replication (absolute RLU values for viral replication control, 20,000 to 50,000; RLU for no-virus control, 200 to 400). (B) ADCVI using CD4+ T and NK cells from a second donor. (C) Plasma neutralization using CD4+ T cells from the donor in panel A. Infected CD4+ T cells were incubated with plasma alone. Results of the TZM-bl assay were normalized to infected cells cultured in the absence of plasma and plotted as relative viral replication. (D) Plasma neutralization using CD4+ T cells from the donor in panel B.
Neutralizing antibody responses develop later than does ADCVI.
We sought to compare the time frame for the evolution of ADCVI with that of equivalent-titer neutralizing antibodies. To do this, we employed a PBMC neutralization assay to facilitate a comparison between the neutralization and ADCVI assay results. We infected human CD4+ T cells with SIVmac251, incubated them with a 1:250 dilution of the plasmas of interest, and after 4 to 6 days quantified replicating virus in the culture supernatants. Neutralization experiments were performed in parallel with ADCVI experiments, using CD4+ T cells from the same donors.
In contrast to the development of potent ADCVI in all animals by 4 weeks postinfection, plasma samples from all 6 animals displayed little neutralizing activity through the first 7 weeks of infection (Fig. 1C and D). Plasma sampled from 8 weeks postinfection onwards in 2 of the animals—A3V016 and A3V019—mediated 1 to 2 logs of neutralization of virus. Plasma sampled from the remaining 4 animals—A2V001, A3V005, A3V010, and A3V020—mediated only very modest inhibition of a half log at 16 weeks using plasma dilutions of 1:250. Less-dilute plasma did mediate modestly greater neutralization (data not shown), suggesting that while neutralizing antibodies may be present, they were of lower titers than contemporaneous ADCVI plasma activity. The time frame for the development of autologous neutralizing antibodies and the relatively low titer of these antibodies in this PBMC neutralization assay are similar to what has previously been described in SIVmac251-infected rhesus monkeys using a pseudovirion TZM-bl assay (49).
Emergence of envelope-binding antibodies coincides temporally with the development of ADCVI.
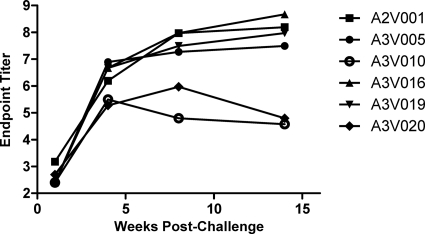
Although neutralizing antibodies arise relatively late in the course of HIV-1 and SIV infections, nonneutralizing envelope-binding antibodies develop more rapidly (1). In acute HIV-1 infection, the first antibodies to gp41 are detectable by 14 days and the first antibodies to gp120 are detectable by 28 days following infection (43). We therefore investigated whether the time frame for the emergence of ADCVI correlated with the development of envelope-binding antibodies (Fig. 2). By ELISA, we were able to detect binding antibodies to gp140 at high titers in all animals by 4 weeks after infection. This coincided closely with the detection of maximal ADCVI activity in the monkeys. Furthermore, in the 2 animals that lost ADCVI activity—A3V010 and A3V020—at 8 and 14 weeks, there was coincident reduction in titer of gp140-binding antibody. These results suggest that, in SIVmac251-infected rhesus monkeys, antibodies capable of generating ADCVI appear in synchrony with the earliest gp140-binding antibodies.
Fig. 2.
Longitudinal gp140-binding antibody titers. EIA plates were coated with purified SIVmac251 gp140 and then incubated with serial dilutions of plasma from infected animals.
Lysis of SIVmac-infected cells by plasma that mediates ADCVI.
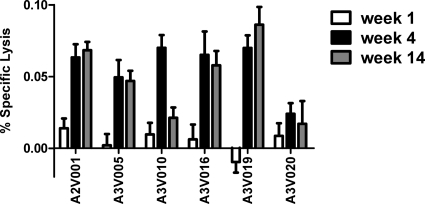
It has previously been shown that one mechanism by which plasma antibodies and effector cells produce ADCVI is through direct lysis of infected cells (15). To explore this as a possible mechanism of ADCVI in these monkeys, we infected human CD4+ T cells with SIVmac251 and then coincubated the infected cells with NK cells from the same human donor and plasma from the infected animals. We used a nonradioactive cytotoxicity assay (Promega) to measure cell lysis (Fig. 3). Plasma from week 1 after infection was unable to induce lysis of infected cells; plasma from weeks 4 and 14 did, however, mediate modest specific lysis. Plasma from animals A3V010 and A3V020, which mediated reduced ADCVI in vitro, also demonstrated poor specific lysis of infected cells. In the absence of NK cells, incubation of the infected cells with plasma alone did not result in their lysis (data not shown).
Fig. 3.
Specific lysis of SIVmac251-infected cells by plasma from infected animals. Human CD4+ T cells were infected with an MOI of 1.0 of SIVmac239, incubated for 3 days, and then cocultured in the presence of autologous NK cells at a ratio of 2 to 1 and plasma from the noted infected monkeys. After 4 h, cytotoxicity and total cell viability were measured utilizing the Cytotox-glo system (Promega). Infected cells in the presence of NK cells but without plasma were used to calculate percent specific lysis.
Correlation between ADCVI and virus control in infected monkeys.
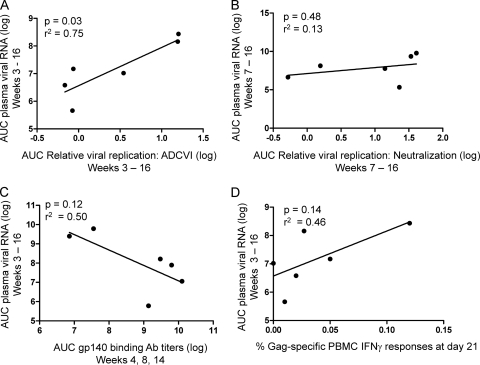
ADCVI reached maximal magnitude between days 21 and 28 after infection, at approximately the same time that plasma viral RNA levels reached set point. Additionally, animals A3V010 and A3V020, which exhibited a diminution in the magnitude of ADCVI after day 28 postinfection, also had the highest viral loads in this cohort of monkeys at day 84 postinfection and thereafter. These findings raised the possibility that ADCVI and viral control may be linked. First, we queried whether there was a statistically significant relationship between the magnitude of the ADCVI response and viral load during the early set point period in this cohort of monkeys. We expressed ADCVI for each animal as area under curve for relative viral replication in the assay between week 3, when ADCVI is first consistently detected, and week 16. We similarly calculated area under curve for viral RNA copy number per milliliter of plasma over that same time frame. We found a statistically significant negative correlation between average relative viral replication in the ADCVI assay and plasma viral RNA copy number in these monkeys (Fig. 4A). Thus, stronger and more sustained ADCVI activity (i.e., lower relative viral replication) was associated with a lower average early set point viral load.
Fig. 4.
Correlation of plasma viral RNA with ADCVI, neutralizing antibody, and Gag-specific CD8+ T cell responses. Area under curve (AUC) was calculated for plasma viral RNA levels for each animal between either day 21 or day 49 and day 112 postinfection. These values were plotted against AUC calculations for relative viral replication for ADCVI (A) or neutralizing antibody responses (B) for the corresponding animals. Aggregate binding antibody (Ab) titers over the time points tested were plotted against AUC viral copy number during that time frame (C). Finally, we also plotted AUC plasma viral RNA levels against previously obtained data on the frequencies of CD8+ T cell Gag-specific IFN-γ responses following exposure to a pool of 15-mer Gag peptides (D).
We then examined whether the magnitude of other antiviral responses correlated with viral control in these monkeys. We explored whether there was a correlation between plasma neutralization and viral load. Area under curve was determined as a measure of relative viral replication in the neutralization assay from the onset of measurable neutralization at week 7 until week 16 (Fig. 4B). Consistent with findings in a prior study, we found no significant association between plasma neutralization and viral load in the monkeys (16).
Although there did not appear to be a relationship between neutralization and ADCVI (Fig. 1), there did appear to be a temporal association between the emergence of ADCVI and gp140-binding antibodies (Fig. 2). Both ADCVI and binding antibodies were first detected between weeks 3 and 4 postinfection. Additionally, animals A3V010 and A3V020, which exhibit a decline in ADCVI activity at later time points, also demonstrated a reduction in gp140-binding antibody titers over time. We therefore explored a correlation between average binding antibody titer and viral load over the same time period when we had identified a correlation between ADCVI activity and viral load (Fig. 4C). Although there appeared to be a trend toward an association of higher binding antibody titers and lower viral load, this did not achieve statistical significance. However, when we correlated binding antibody titer at specific time points following infection with viral load or ADCVI activity, we found that binding antibody titers correlated well with both viral load and ADCVI activity at week 4 as well as later time points (P value for various comparisons ranged from 0.015 to 0.030). The association in these monkeys between binding antibody titers from weeks 8 and 14 and either viral load or ADCVI activity did not achieve statistical significance.
Finally, we assessed the possible association of the CD8+ T cell responses with viral load after peak in these monkeys. The magnitude of Gag-specific CD8+ T cell responses has been shown to associate strongly with suppression of early set point viremia in Mamu-A*01+ rhesus monkeys (30, 32). The animals used in this study were Mamu-A*01−, and therefore the magnitude of Gag-specific CD8+ T cell responses would be expected to be lower than that in Mamu-A*01+ animals (26). Indeed, we did not find a substantial number of gamma interferon (IFN-γ)-producing CD8+ T cells after stimulation of PBMCs with pooled Gag peptides (41). The lack of a significant Gag-specific CD8+ T cell response in the animals from this cohort likely accounts for our inability to detect an association between the CD8+ T cell response and viral load (Fig. 4D).
Generation of replication-competent SIVmac239 expressing envelopes isolated from chronically infected monkeys.
We have demonstrated that antibodies capable of mediating ADCVI against the infecting virus strain arise relatively early after infection in comparison to comparable-titer neutralizing antibodies and that in a substantial fraction of infected animals, ADCVI against the infecting virus persists for at least several months after infection. In HIV-1 infection, although autologous neutralizing antibodies develop, the virus evolves to escape neutralization (35, 44). We sought to determine whether envelope mutations arising during the course of infection permit viral escape from ADCVI and whether counterevolution of the host antibody response occurs to effectively reestablish ADCVI.
We have previously described the evolution of SIV envelope over the course of infection using a cohort of 4 rhesus monkeys infected by intrarectal inoculation with SIVmac251. We have shown that predictable deletions in the V1-V2 and V4 variable loops resulted in attenuated envelope sensitivity to neutralization (48). We used this same cohort of SIVmac251-infected animals to investigate whether these variable-loop deletions also permitted escape from ADCVI. Viral load and CD4+ T cell data for these animals are summarized in Table 2.
Table 2.
Viral load and CD4+ T cell counts of rhesus monkeys used for characterization of ADCVI to envelopes from chronically infected monkeysa
| Animal | No. of plasma viral RNA copies/ml (log10) |
No. of CD4+ T cells/ml |
||
|---|---|---|---|---|
| Peak | Set point | Preinfection | Day ∼100 | |
| PBE | 7.0 | 5.1 | 745 | 295 |
| CR2A | 6.6 | 4.7 | 1,134 | 690 |
| CP1W | 7.3 | 4.1 | 2,285 | 1,707 |
| CR53 | 6.8 | 3.1 | 2,578 | 1,253 |
Four rhesus monkeys were infected with SIVmac251 by repeat weekly intrarectal exposures. After 18 exposures, uninfected animals were inoculated via the intravenous route. Periodic plasma viral RNA levels were measured by ultrasensitive branched DNA amplification assay, and T cell subsets were enumerated by flow cytometry. Peak and set point plasma viral RNA levels and set point absolute CD4+ T cell counts are summarized.
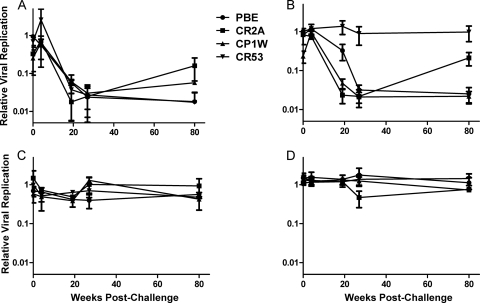
We initially tested the ability of plasma from the animals in this second cohort to induce ADCVI activity against the inoculated stock of SIVmac251 (Fig. 5A). These animals were challenged by repeat intrarectal inoculation and were not infected simultaneously after the first challenge but rather became infected over the course of several challenges. Data are presented with day 0 as the day of the first intrarectal challenge of all the animals. We did not have plasma available from these animals at early time points postchallenge.
Fig. 5.
ADCVI responses to SIVmac251 infection. Human CD4+ T cells were infected with an MOI of 0.01 of the same SIVmac251 stock used to challenge the animals in this study. These target cells were incubated with plasma from infected animals obtained at various times postchallenge in the presence of autologous NK cells. After 5 days of coincubation, virus was quantified by TZM-bl assay and normalized to a plasma-free control to derive a relative viral replication value. Relative viral replication is plotted as a function of time after challenge for the 4 animals in this study. Samples were assayed in duplicate in each of two different donors for effector and target cells, and the means and standard errors of the quadruplicate results are plotted. (A) ADCVI assay with 1:100 dilution plasma. (B) ADCVI assay with 1:250 dilution of plasma. (C) Neutralization assay with 1:100 dilution of plasma. (D) Neutralization assay with 1:250 dilution of plasma.
By 16 weeks after initial challenge, plasma from all animals in this cohort mediated ADCVI, with a 2-log reduction in viral replication at a 1:100 dilution of plasma. At a 1:250 dilution of plasma, one animal, CR53, demonstrated diminution of ADCVI activity against the inoculating virus. None of the animals in this cohort showed a substantial attenuation of ADCVI by 22 months postinfection at the 1:100 dilution of plasma. The ability of plasma at both 1:100 and 1:250 dilutions to mediate ADCVI was substantially greater than the plasma neutralizing activity at these dilutions, even at late time points (Fig. 5B). These data are consistent with the low-titer neutralization observed in prior investigation of these animals (49).
Having confirmed in this second cohort of monkeys the observations on the relative kinetics of ADCVI and neutralization activity made in our first cohort of animals, we sought to define the effects of neutralization escape on the susceptibility of virus to ADCVI. To do this, we generated replication-competent viruses expressing mutant envelopes that evolved at late time points following infection and are resistant to neutralizing antibodies. The major circulating envelope variants from month 16 postinfection were cloned from the plasma of the chronically infected rhesus monkeys by single-genome amplification and have been described extensively, previously (49). Month 16 envelopes from the different animals shared approximately 96% amino acid identity with each other and approximately 93% amino acid identity with envelope sequence from the inoculating stock, with the major difference between the inoculum and the chronic viruses being the V1 and V4 deletions in the latter.
We subcloned the SphI-to-NheI restriction digest fragments of these month 16 envelopes into the full-length SIVmac239 plasmid (gift of Heinrich Gottlinger) (47), an infectious molecular clone very similar to the SIVmac251 inoculum stock. This fragment extended from upstream of the envelope start codon to the end of gp140 and included regions with mutations in V1-V2 and V4. We cloned envelopes from month 16 from all 4 animals in this study into SIVmac239 and verified that they replicated in vitro comparably to SIVmac251 (data not shown).
SIVmac239 viruses constructed with chronic envelopes are susceptible to ADCVI mediated by plasma from monkeys early after infection.
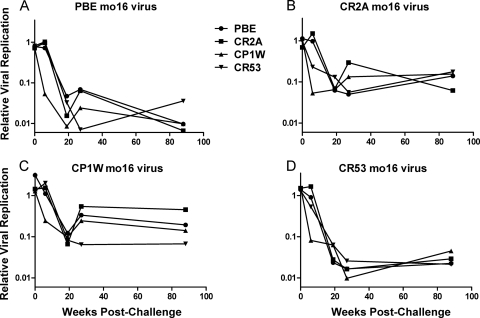
We began our investigation of the effects of envelope neutralization escape on the susceptibility of SIVmac251 to ADCVI using highly mutated viruses isolated from monkeys relatively late in the course of infection. We reasoned that month 16 envelopes had accumulated the major mutations that conferred escape from neutralizing antibodies and therefore used those envelopes in this study. We infected human CD4+ T cells with mutant SIVmac239 containing representative month 16 envelopes from each of the animals PBE, CR2A, CP1W, and CR53 (Fig. 6A, B, C, and D, respectively) and measured ADCVI activity of plasma at a 1:250 dilution against each of these viruses. We tested each month 16 virus for ADCVI sensitivity using plasma from the animal of origin of the virus as well as plasma from the other animals in this cohort sampled at selected time points following infection (Fig. 6).
Fig. 6.
ADCVI responses to chronic viruses. SIV molecular clones expressing month 16 envelopes were used to infect human CD4+ T cells. Four molecular clones were used, constructed with envelopes representing the dominant circulating viruses isolated from each of 4 chronically infected animals. These viruses were assayed against plasma from all 4 animals at all time points postinfection, from week 0 to week 22. ADCVI results were corrected for plasma neutralizing activity. Each panel, A to D, represents the ADCVI sensitivity of the noted month 16 virus to plasma from all of the animals.
Both autologous and heterologous plasma were able to inhibit month 16 virus from animal PBE, although with variable efficiency (Fig. 6A). Autologous plasma from 16 weeks postinfection inhibited the virus by 1 log, and this inhibition increased to 2 logs with the use of plasma from later in infection. Heterologous plasma from early infection inhibited the PBE month 16 virus by 2 logs, and this did not significantly wane with the use of plasma from later time points. Thus, the PBE month 16 virus, which we have previously shown escapes neutralization by early plasma in a pseudovirion-based TZM-bl assay, was susceptible to ADCVI with the use of plasma from both early and late time points.
The month 16 virus from animal CR53 also exhibited broad sensitivity to ADCVI (Fig. 6D). Plasma sampled from all animals in the cohort, from both acute-phase and late time points, was able to inhibit the chronic CR53 virus by more than 2 logs. Although the month 16 viruses from animals CR2A and CP1W (Fig. 6B and C) exhibited kinetics in the development and persistence of susceptibility to ADCVI similar to those of the CR53 and PBE viruses from month 16, the CR2A and CP1W month 16 viruses were less sensitive to ADCVI, with plasma inhibiting the replication of these two viruses by approximately 1 log (Fig. 6B and C). However, neither of the mutant viruses isolated from CR2A and CP1W was significantly more resistant to early plasma than to late plasma. In fact, at late time points, there was a relative attenuation of ADCVI activity in plasma from some animals for these viruses. However, this was a consequence of the contribution of plasma neutralizing activity, which emerged in some animals later following infection. Thus, we found no evidence of escape from autologous ADCVI activity in any of the animals that we studied.
These chronic viruses were not more sensitive to ADCVI mediated by autologous plasma than to ADCVI mediated by heterologous plasma. Viruses from animals PBE, CR2A, and CR53 exhibited comparable sensitivities to plasma from all animals in the cohort. Virus from CP1W, which was the least sensitive to ADCVI, exhibited as much as a half log of variability in susceptibility to ADCVI mediated by plasma from the different animals; its sensitivity to autologous plasma did not, however, fall at either extreme. None of the month 16 viruses manifested reduced sensitivity to ADCVI from plasma sampled at early time points postinfection compared to plasma sampled at late time points, suggesting that mutations that conferred escape from neutralization did not confer escape from ADCVI.
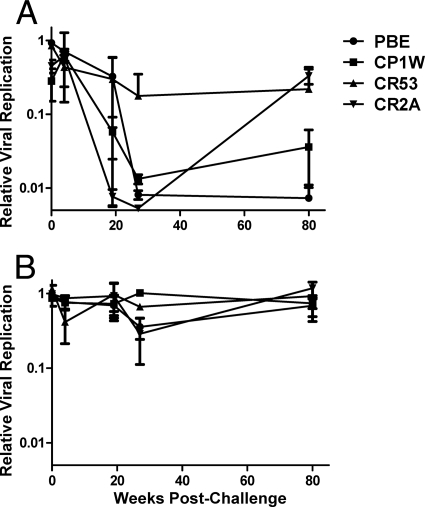
Plasma from SIVmac251-infected monkeys mediates ADCVI against SIVsmE660.
Given the absence of an effect of envelope evolution on the sensitivity of a virus to ADCVI, we queried whether plasma from an SIV-infected monkey mediated viral inhibitory activity against divergent strains of this virus. We therefore tested the ADCVI activity of plasma from SIVmac251-infected animals against SIVsmE660. SIVsmE660 and SIVmac251 envelopes are approximately 83% similar at the amino acid level, comparable to the difference between two different HIV-1 isolates belonging to the same clade (48). Cross neutralization in vitro of SIVsmE660 by plasma from animals vaccinated against SIVmac251 has been observed elsewhere (N. L. Letvin, S. S. Rao, D. C. Montefiori, M. S. Seaman, Y. Sun, W. W. Yeh, M. Asmal, R. S. Gelman, S. Lim, L. Shen, J. B. Whitney, C. Seoighe, M. Lacerda, S. Keating, J. P. Todd, A. Dodson, J. R. Mascola, and G. J. Nabel, unpublished data). We infected CD4+ T cells with SIVsmE660 and assessed plasma from our cohort of four intrarectally infected animals for ADCVI against the SIVsmE660-infected lymphocytes. At a 1:250 dilution, plasma from 2 of 4 animals in the cohort exhibited 2 logs of sustained inhibitory activity against SIVsmE660 (Fig. 7A). Animal CP1W exhibited transient potent ADCVI that diminished by week 88. Plasma from animal CR53 did not exhibit ADCVI against SIVsmE660 at any time point tested. Normalization of ADCVI for plasma neutralization did not alter these results, suggesting that cross-strain inhibition by neutralizing antibodies was minimal at this dilution of plasma and that the magnitude of viral inhibition by ADCVI activity is substantially greater than that by neutralizing antibody (Fig. 7B).
Fig. 7.
ADCVI to the heterologous virus SIVsmE660. Human CD4+ T cells were infected with SIVsmE660 and then incubated with plasma from SIVmac251-infected monkeys and human NK cells. (A) ADCVI at 1:250 dilution of plasma. (B) Neutralization at 1:250 dilution of plasma. Means and standard errors for ADCVI activity and neutralization from two different human donors are presented.
DISCUSSION
Antibodies that neutralize a diversity of HIV-1 strains isolated from infected individuals have been difficult to identify and cannot be generated by existing vaccine modalities (11, 18). In contrast, nonneutralizing antibodies arise in all HIV-1-infected patients and can be readily induced by existing vaccine strategies (34, 42). However, the mechanisms by which nonneutralizing antibodies may be able to inhibit HIV-1 replication are incompletely understood.
We present data from an SIV model of HIV-1 infection suggesting that ADCVI produced by the interaction of NK cells with nonneutralizing antibodies can mediate extremely potent viral inhibition in vitro. Although we have not quantitatively determined the titer of plasma ADCVI activity in this study and have not rigorously defined the maximal dilution of plasma at which ADCVI activity can be detected, we do show that at plasma dilutions at which neutralizing activity has previously been shown to be minimal in our cohort of animals (49), ADCVI activity is substantial. We have defined the early kinetics of ADCVI development in rhesus monkeys and found them to be similar to what has been observed in acute HIV infection of humans (15, 43), suggesting that rhesus monkeys may be a valid model for studying ADCVI responses to vaccines. Furthermore, we found no evidence of escape from autologous ADCVI activity in viruses from chronic SIV infection that have escaped autologous neutralization. Finally, antibodies capable of inducing potent ADCVI in an infected animal are effective both against viruses that have evolved in other hosts and against a heterologous virus.
Given the potency of ADCVI and its activity against viruses that have accrued envelope mutations, it is important to determine whether this response has an effect in vivo in the setting of infection. It is unlikely that ADCVI mediated by antibodies in the plasma plays a role in modulating the early phase of exponential viral expansion, since by the time that significant plasma ADCVI activity is detectable—21 to 28 days postinfection—peak viremia has already been reached. It is interesting that viral load begins to decline during primary SIVmac251 infection coincident with the development of maximal ADCVI activity, suggesting that either ADCVI or another host immune response that evolves within a similar time frame contributes to the containment of early virus replication.
Supporting the hypothesis that ADCVI and viral load are linked in the small cohort of Mamu-A*01− animals that we studied, we have shown a statistically significant correlation between the aggregate of ADCVI activity from the time that it first emerges at 21 days until 100 days following infection and plasma virus copy number during that same time period. This correlation is stronger than the correlation of Gag-specific cytotoxic T cell responses or titers of neutralizing antibody to plasma virus. One possible explanation for the observed inverse correlation between magnitude of ADCVI activity and viral load is that the production of nonneutralizing antibodies capable of mediating ADCVI is dependent on preserved CD4+ T cell function. SIV-infected monkeys that cannot control viral replication and thus lose CD4+ T cell help may therefore lose the ability to produce ADCVI-mediating antibodies (20).
Alternatively, the inverse correlation between the development of sustained ADCVI and the magnitude of plasma viral copy number after peak may suggest a role for ADCVI in suppression of viral replication in SIV-infected monkeys. This hypothesis is supported by prior reports implying that anti-SIV antibodies may be important for control of viremia beyond 4 weeks of infection. Acutely infected monkeys treated with anti-CD20 that were unable to mount an anti-SIV immunoglobulin response maintained higher levels of viremia following peak virus replication than did animals that received inoculations of anti-SIV immunoglobulin (27). Since neutralizing antibodies develop only weeks to months after the establishment of early set point viral load, responses mediated by nonneutralizing antibodies, such as ADCVI, may be responsible for the sensitivity of plasma viremia to B cell depletion (35, 44). Additionally, in studies of acute HIV-1 infection of humans, detailed single-genome sequencing of envelope has revealed early envelope evolution that cannot be ascribed to either cytotoxic T cell or neutralizing antibody pressure (17, 45); ADCVI may be the driving force behind early envelope selection.
While ADCVI may contribute to some suppression of viral replication after peak viremia, nonneutralizing antibodies are incapable of completely suppressing viral replication during chronic infection. Despite the ability of highly dilute plasma from multiple chronically infected animals to inhibit viral replication 100-fold in vitro, all of these animals have sustained chronic plasma viremia. We have shown that the inability of ADCVI to control virus in chronic infection is not due to the accumulation of envelope mutations that permit evasion from nonneutralizing antibodies, since viruses with late envelopes are suppressed in vitro by ADCVI activity in plasma sampled from monkeys early after infection. Furthermore, since plasma from chronically infected monkeys can mediate effective ADCVI activity in the presence of NK cells from healthy donors, there does not appear to be a loss of intrinsic ability of antibody from chronically infected animals to engage effector cells. Rather, ADCVI may be attenuated in vivo because of a reduction in the number of circulating effector cells in the setting of chronic infection or because of a decline in functionality of these effector cells during chronic infection, through either effector cell fatigue or a downregulation of CD16 expression (2, 10, 21).
The ability of plasma from SIVmac251-infected animals to mediate ADCVI activity against viruses that have evolved over many months and against the divergent SIVsmE660 strain suggests either that the antibodies that mediate ADCVI target regions of envelope are highly conserved or that these antibodies are polyclonal, targeting a breadth of epitopes such that even multiple mutations do not allow escape from ADCVI. It is possible that the epitope specificities of the numerous antibodies comprising the nonneutralizing response may change during the course of infection as the virus evolves (29, 38). However, even if epitope specificity of ADCVI is dynamic, this does not appear to result in a loss of plasma ADCVI activity in the animals that we have studied.
Two animals in our first cohort did, however, demonstrate a diminution of ADCVI activity over time. Attenuation of ADCVI activity in these animals coincided with a reduction in anti-gp140 binding titers. Spontaneous loss of antienvelope antibodies in SIVmac251-infected rhesus monkeys has previously been described (20, 22). The mechanism for this decline in anti-SIV immunoglobulins in a subset of SIVmac251-infected animals is unclear, but it has been posited to be due to a loss of CD4+ T cell help caused by rapid disease progression in some animals.
In all of the animals in the cohort that we studied, the timing of the development of measurable gp140-binding antibody appears to coincide closely with the emergence of ADCVI activity. However, the titer of antibodies to gp140 beyond 4 weeks, as measured by ELISA, does not correlate with the efficacy of ADCVI. This finding suggests that while binding antibodies may be the mediators of ADCVI, the gp140 ELISA titer may not be the optimal surrogate marker for ADCVI efficacy. One possible explanation for this discrepancy is that the affinity of plasma antibody for plate-bound gp140 may differ from its affinity for envelope expressed on the cell surface. Additionally, it is likely that antibodies capable of attaching to cell surface envelope and subsequently engaging the Fc receptor of NK cells comprise a distinct subset of total anti-gp140 antibodies, such that for a given animal the gross quantification of anti-gp140 antibodies does not necessarily reflect the proportion of these antibodies that mediate ADCVI activity.
It is unclear what might distinguish gp140-binding antibodies that are capable of mediating ADCVI from those that cannot. While the results of the present study suggest that the epitope specificity of ADCVI-mediating antibodies is probably broad, it is possible that some epitopes are better targets for ADCVI than are others (5, 24, 40, 46). Epitopes that are masked by envelope glycosylation or that are more prone to mutation may, for example, be poorer targets for ADCVI. Alternatively, it has been suggested that Fc modifications may be important for regulating the capacity of antibodies to bind effector cell receptors, and differences in these modifications between circulating antibodies may account for variation in ADCVI capability. In particular, Fc glycosylation has been shown to influence immunoglobulin interactions with the Fc receptor, and heterogeneity in glycosylation among subpopulations of gp140-binding antibodies might account for discrepancies between the magnitude of measured gp140 binding titers and the effectiveness of ADCVI (7, 33).
Just as the epitope determinants of ADCVI are unclear, so too are the effects of ADCVI pressure on envelope evolution. Mutations in HIV-1 envelope accrue early after infection, substantially antedating the development of a detectable autologous neutralizing response (39). While some of these mutations may occur in envelope epitopes targeted by cytotoxic T cells, some of them do not (17, 45). Thus, it is feasible that early envelope evolution may be influenced by ADCC or ADCVI. In this study, we specifically have focused our attention on envelopes that emerge late after infection, at a time when we hypothesize that the pressure exerted by ADCVI is likely to be severely compromised by a disadvantageous effector-to-target cell ratio. In addition, these late envelope sequences are likely to be heavily shaped by selection applied by neutralizing antibodies (49). Thus, the envelopes that we have studied are ill suited for investigating the effects that ADCVI may have on early envelope evolution. Future investigation of ADCVI activity of plasma versus contemporaneous virus in the period between the emergence of binding antibodies at 3 weeks and the emergence of neutralizing antibodies at 2 to 3 months may be interesting in this regard.
Overall, our findings suggest that a vaccine capable of inducing antibodies which can mediate ADCVI may be able to provide protection against both phylogenetically divergent and neutralization-resistant viruses. Ultimately, understanding what makes an antibody mediate ADCVI will be helpful in the prospective evaluation of envelope-targeted vaccines. The SIV-infected rhesus monkey should be a useful model for probing the epitope determinants of ADCVI and for assessing the ability of different envelope immunogens to elicit ADCVI-mediating antibodies. Additionally, this model should also allow us to investigate the relative efficiencies of different vaccine modalities at stimulating antibodies capable of mediating ADCVI.
ACKNOWLEDGMENTS
We thank Galit Alter, Heinrich Gottlinger, and Mike Seaman for gifts of reagents and helpful discussions.
The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.
This research was supported by grants from the NIH AI060354-06 and AI-067854 and the Center for HIV/AIDS Vaccine Immunology and Harvard University Center for AIDS Research.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Aasa-Chapman M. M., et al. 2004. Development of the antibody response in acute HIV-1 infection. AIDS 18:371–381 [DOI] [PubMed] [Google Scholar]
- 2. Ahmad A., Menezes J. 1996. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10:258–266 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad R., et al. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21:227–233 [DOI] [PubMed] [Google Scholar]
- 4. AIDS Vaccine Evaluation Group 022 Protocol Team 2001. Cellular and humoral immune responses to a canarypox vaccine containing human immunodeficiency virus type 1 Env, Gag, and Pro in combination with rgp120. J. Infect. Dis. 183:563–570 [DOI] [PubMed] [Google Scholar]
- 5. Alsmadi O., Tilley S. A. 1998. Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope of primary or laboratory-adapted strains by human and chimpanzee monoclonal antibodies of different epitope specificities. J. Virol. 72:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alter G., Altfeld M. 2009. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 265:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anthony R. M., Ravetch J. V. 2010. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J. Clin. Immunol. 30(Suppl. 1):S9–S14 [DOI] [PubMed] [Google Scholar]
- 8. Basavapathruni A., et al. 2010. Envelope vaccination shapes viral envelope evolution following simian immunodeficiency virus infection in rhesus monkeys. J. Virol. 84:953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belshe R. B., et al. 2001. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J. Infect. Dis. 183:1343–1352 [DOI] [PubMed] [Google Scholar]
- 10. Chung A., Rollman E., Johansson S., Kent S. J., Stratov I. 2008. The utility of ADCC responses in HIV infection. Curr. HIV Res. 6:515–519 [DOI] [PubMed] [Google Scholar]
- 11. Doria-Rose N. A., et al. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 84:1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Florese R. H., et al. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 182:3718–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forthal D. N., Gilbert P. B., Landucci G., Phan T. 2007. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J. Immunol. 178:6596–6603 [DOI] [PubMed] [Google Scholar]
- 14. Forthal D. N., et al. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 80:9217–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forthal D. N., Landucci G., Daar E. S. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fultz P. N., et al. 1990. Humoral response to SIV/SMM infection in macaque and mangabey monkeys. J. Acquir. Immune Defic. Syndr. 3:319–329 [PubMed] [Google Scholar]
- 17. Goonetilleke N., et al. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 206:1253–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray E. S., et al. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hidajat R., et al. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J. Virol. 83:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirsch V. M., et al. 2004. Immune failure in the absence of profound CD4+ T-lymphocyte depletion in simian immunodeficiency virus-infected rapid progressor macaques. J. Virol. 78:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iannello A., Debbeche O., Samarani S., Ahmad A. 2008. Antiviral NK cell responses in HIV infection. II. Viral strategies for evasion and lessons for immunotherapy and vaccination. J. Leukoc. Biol. 84:27–49 [DOI] [PubMed] [Google Scholar]
- 22. Kannagi M., et al. 1986. Humoral immune responses to T cell tropic retrovirus simian T lymphotropic virus type III in monkeys with experimentally induced acquired immune deficiency-like syndrome. J. Clin. Invest. 78:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karnasuta C., et al. 2005. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine 23:2522–2529 [DOI] [PubMed] [Google Scholar]
- 24. Kmieciak D., et al. 1998. The effect of deletion of the V3 loop of gp120 on cytotoxic T cell responses and HIV gp120-mediated pathogenesis. J. Immunol. 160:5676–5683 [PubMed] [Google Scholar]
- 25. Lambotte O., et al. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J., et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller C. J., et al. 2007. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J. Virol. 81:5024–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore P. L., et al. 2008. The c3-v4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J. Virol. 82:1860–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore P. L., et al. 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 5:e1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mothe B. R., et al. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naidu Y. M., et al. 1988. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J. Virol. 62:4691–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peyerl F. W., et al. 2003. Simian-human immunodeficiency virus escape from cytotoxic T-lymphocyte recognition at a structurally constrained epitope. J. Virol. 77:12572–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raju T. S. 2008. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 20:471–478 [DOI] [PubMed] [Google Scholar]
- 34. Rerks-Ngarm S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 35. Richman D. D., Wrin T., Little S. J., Petropoulos C. J. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 100:4144–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogers K. A., Scinicariello F., Attanasio R. 2006. IgG Fc receptor III homologues in nonhuman primate species: genetic characterization and ligand interactions. J. Immunol. 177:3848–3856 [DOI] [PubMed] [Google Scholar]
- 37. Rong R., et al. 2007. Unique mutational patterns in the envelope alpha 2 amphipathic helix and acquisition of length in gp120 hypervariable domains are associated with resistance to autologous neutralization of subtype C human immunodeficiency virus type 1. J. Virol. 81:5658–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rong R., et al. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salazar-Gonzalez J. F., et al. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stratov I., Chung A., Kent S. J. 2008. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J. Virol. 82:5450–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun Y., Permar S. R., Buzby A. P., Letvin N. L. 2007. Memory CD4+ T-lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection. J. Virol. 81:8009–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomaras G. D., et al. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tyler D. S., et al. 1990. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. Humoral and cellular defects. J. Immunol. 144:3375–3384 [PubMed] [Google Scholar]
- 44. Wei X., et al. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312 [DOI] [PubMed] [Google Scholar]
- 45. Wood N., et al. 2009. HIV evolution in early infection: selection pressures, patterns of insertion and deletion, and the impact of APOBEC. PLoS Pathog. 5:e1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamada T., Watanabe N., Nakamura T., Iwamoto A. 2004. Antibody-dependent cellular cytotoxicity via humoral immune epitope of Nef protein expressed on cell surface. J. Immunol. 172:2401–2406 [DOI] [PubMed] [Google Scholar]
- 47. Yeh W. W., et al. 2006. Compensatory substitutions restore normal core assembly in simian immunodeficiency virus isolates with Gag epitope cytotoxic T-lymphocyte escape mutations. J. Virol. 80:8168–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yeh W. W., et al. 2009. Partial protection of simian immunodeficiency virus (SIV)-infected rhesus monkeys against superinfection with a heterologous SIV isolate. J. Virol. 83:2686–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeh W. W., et al. 2010. Autologous neutralizing antibodies to the transmitted/founder viruses emerge late after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys. J. Virol. 84:6018–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]