Abstract
Following infection with the hepatitis C virus (HCV), in most cases immunity fails to eradicate the virus, resulting in slowly progressing immunopathology in the HCV-infected liver. We are the first to examine intrahepatic T cells and CD4+ CD25+ FoxP3+ regulatory T cells (Treg) in patients chronically infected with HCV (chronic HCV patients) during and after antiviral therapy by collecting multiple aspiration biopsy samples from the liver at different time points. We found that intrahepatic Treg frequencies were increased upon alpha interferon and ribavirin administration in about 50% of chronic HCV patients, suggesting stronger regulation of intrahepatic immunity by Treg during antiviral therapy. After cessation of antiviral therapy, the frequency of intrahepatic Treg remained above baseline in the large majority of livers of individuals who successfully cleared the virus. The phenotype of those Treg that were retained in the liver months after therapy-induced clearance of HCV RNA indicated a reduced contribution of effector memory cells. Our findings, gathered by multiple samplings of the liver, indicate that successful antiviral therapy of chronic HCV patients does not lead to normalization of the local immune response to a resting state comparable to that for healthy livers. The continuous presence of high numbers of Treg, with a phenotype reflecting a relatively weak suppressive activity, suggests ongoing residual regulation of immunopathology. These findings provide important insight into the dynamics of the immune response to HCV, as well as the effect of therapy on intrahepatic immunity.
INTRODUCTION
Following infection with hepatitis C virus (HCV), immunity fails to successfully eradicate the virus in the majority of individuals (5, 15, 20). As a consequence, an estimated 120 to 170 million individuals are currently chronically infected worldwide. In the long term, these patients are at increased risk of developing cirrhosis and subsequently liver decompensation and hepatocellular carcinoma due to ongoing immunopathology.
The HCV-infected liver displays extensive infiltrates containing mainly CD8+ and CD4+ T cells around the portal tract areas. These cells have not been characterized functionally in detail, but they are generally activated and differentiated and display an effector phenotype (3, 4, 24). Despite the fact that the livers of patients chronically infected with HCV (chronic HCV patients) contain extensive leukocyte infiltrates, liver pathology progresses slowly, and the development of liver fibrosis may take decades. This may be explained by the activity of high numbers of regulatory T cells (Treg), which are present in the livers of chronically HCV-infected patients (11, 18, 24) but absent from healthy livers, and the number of intrahepatic Treg negatively correlates with the development of fibrosis (3).
In chronic HCV infection, disease progression can be impeded only after viral clearance induced by alpha interferon (IFN-α)-based therapy. Unfortunately, the best available treatment, pegylated IFN-α combined with ribavirin, results in sustained clearance of serum HCV RNA in only about 50% of patients. Apart from the direct antiviral effects of therapy (reviewed in reference 6), the impact of IFN-α-based therapy on the size, distribution, and composition of the leukocyte pool may be important in determining the treatment response. While the number of blood leukocytes declines dramatically shortly after start of IFN-α-based treatment, blood lymphocytes seem differently affected. Recently, it was reported that the proportion and phenotype of circulating CD4+ CD25+ Treg remained unchanged during IFN-α and ribavirin therapy of chronic HCV patients (2), whereas IFN-α induced Treg in multiple sclerosis patients in vitro (13).
Although there are studies of HCV-specific immune responses in blood, no studies that examine the kinetics of the immune response in the livers of chronic HCV patients or the effects of antiviral therapy on the intrahepatic immune response have been conducted. This is highly relevant since HCV replication takes place within hepatocytes. Studies using intrahepatic cells are difficult to perform since liver biopsy samples are collected from patients for diagnostic purposes only and are therefore limited to pretreatment assessment of liver cells. The findings of these studies indicate that baseline intrahepatic CD8+ T cell responses in chronic HCV patients are important for successful response to IFN-α-based therapy (7, 9, 12), which is in line with reports on peripheral blood T cells (14, 17). However, it remains unclear how intrahepatic immunity is affected by IFN-α-based therapy and how this contributes to treatment outcome. Also, the role of Treg in the liver during therapy has not been investigated before.
By performing multiple aspiration biopsies of the liver at different time points, we determined the effect of IFN-α-based therapy on recruitment of Treg and other lymphocyte populations to the livers of HCV-infected patients. In addition, we examined the leukocyte composition in the liver after therapy-induced HCV eradication to determine to what extent viral load reduction normalizes the intrahepatic immune system to the state observed in healthy individuals. A better understanding of local immunoregulation in the liver during viral infection and its modulation by antiviral therapy may lead to further improvement of the therapeutic success rate for chronic HCV patients.
MATERIALS AND METHODS
Patients and antiviral therapy.
Twenty-two treatment-naive chronically HCV-infected patients were included in this study (Table 1); none of them were coinfected with HIV or hepatitis B virus (HBV). Diagnostic core biopsy samples from 18 of 22 patients, obtained within 3 months prior to the start of therapy, were assessed for fibrosis stage by an experienced liver pathologist using the Metavir score. All patients received 24 (genotype 2 or 3) or 48 (genotype 1) weeks of antiviral therapy consisting of orally administered ribavirin and subcutaneous infusions with pegylated IFN-α2b (PegIntron; Schering-Plough, Houten, Netherlands), both weight based. The institutional ethical review board of the Erasmus Medical Center (MC) approved the clinical protocols, and written informed consent was obtained from all individuals.
Table 1.
Individual patient characteristics (n = 22)
| Patient no. | Sex | Age (yr) | Liver fibrosis (Metavir score) | Genotype | HCV RNA(IU/ml) |
Baseline ALT (U/liter) | |
|---|---|---|---|---|---|---|---|
| Baseline | After therapya | ||||||
| 1 | M | 47 | 2 | 1 | 1.41 × 106 | Undetectable | 75 |
| 4 | F | 57 | 3 | 1 | 1.13 × 107 | Undetectable | 150 |
| 5 | F | 37 | NDb | 3 | 3.19 × 103 | Undetectable | 79 |
| 6 | M | 54 | 1 | 1 | 2.72 × 107 | 5.95 × 105 | 49 |
| 9 | F | 27 | 1 | 1 | 3.70 × 102 | Undetectable | 34 |
| 10 | M | 48 | 2 | 3 | 1.09 × 105 | Undetectable | 146 |
| 11 | M | 51 | 2 | 1 | 3.08 × 106 | 1.86 × 106 | 46 |
| 12 | M | 52 | 2 | 3 | 1.46 × 105 | Undetectable | 41 |
| 13 | M | 34 | 2 | 1 | 3.75 × 106 | Undetectable | 103 |
| 14 | M | 56 | 2 | 1 | 6.49 × 106 | 1.57 × 107 | 157 |
| 15 | F | 57 | 4 | 1 | 7.70 × 105 | 1.40 × 106 | 17 |
| 17 | M | 47 | 1 | 1 | 4.46 × 105 | Undetectable | 227 |
| 19 | M | 46 | 3 | 2 | 1.56 × 107 | Undetectable | 47 |
| 20c | F | 41 | 1 | 3 | 8.56 × 105 | Undetectable | 46 |
| 21c | M | 40 | 3 | 3 | 3.23 × 106 | Undetectable | 91 |
| 22 | M | 40 | 2 | 1 | 2.20 × 104 | 4.14 × 103 | 58 |
| 23 | M | 49 | ND | 1 | 2.88 × 105 | Undetectable | 20 |
| 24 | F | 40 | 3 | 1 | 3.14 × 105 | 1.47 × 106 | 120 |
| 25 | M | 45 | ND | 1 | 1.08 × 107 | 1.54 × 107 | 36 |
| 26 | F | 58 | 4 | 1 | 1.56 × 105 | Undetectable | 179 |
| 28 | M | 42 | 1 | 1 | 1.48 × 106 | Undetectable | 65 |
| 30 | F | 42 | ND | 1 | 3.33 × 106 | Undetectable | 107 |
Listed as undetectable if serum HCV RNA levels were negative both at 4 and 24 weeks after end of therapy; otherwise, HCV RNA data from 4 weeks after therapy are shown.
ND, not determined within 3 months before start of therapy.
Patient finished the complete course of antiviral therapy, but aspiration biopsy samples from the liver and blood were not collected at all time points.
Aspiration of liver cells and collection of peripheral blood.
Details of the collection of liver cells as fine-needle aspiration biopsy samples are described elsewhere (21). Briefly, a 25-gauge needle (Braun, Melsungen, Germany) containing a mandrin was used to penetrate into the intercostal space after sonographic localization of the liver and exclusion of vascular or pathological structures. After removal of the mandrin, a syringe filled with RPMI 1640 medium was attached, and liver cells were aspirated by negative syringe pressure. Two aspiration biopsy samples were collected per patient, checked for low erythrocyte contamination, and pooled for further analysis. Aspirate biopsy samples and paired venous blood samples were collected from all patients at the start of therapy (week 0), during therapy (week 4), and after therapy (4 weeks after cessation of treatment). In 3 patients, who successfully cleared the virus, additional aspirate biopsy samples were collected 24 weeks after the end of treatment.
Cell isolation and flow cytometry.
Liver specimens were collected in RPMI 1640 medium (Lonza, Verviers, Belgium) and passed through a 70-μm nylon cell strainer (BD Falcon, Bedford, MA). Liver cells and peripheral blood were fixed and erythrocytes were lysed using FixPerm reagent (eBioscience, San Diego, CA). To determine the frequency and phenotype of T cells and Treg cells, multicolor flow cytometry was performed (22). Samples were stained with antibodies against CD25-phycoerythrin (PE)-Cy7 (2A3; BD Biosciences, San Jose, CA), FoxP3-allophycocyanin (APC) (PCH101; eBioscience), CD4-APC-H7 (SK3; BD Biosciences), CD45-Pacific Blue (HI30; eBioscience), or CD45-fluorescein isothiocyanate (FITC) (J33; Beckman, Fullerton, CA) and CD3-AmCyan (SK7; BD-Biosciences). Further phenotyping was performed using antibodies against CD62L-PE-Cy5 (Dreg56; BD Biosciences) and CD45RO-PE (UCHC1; BD Biosciences). For all staining procedures, permeabilization buffer was used (eBioscience). Cells were acquired using the FACSCanto II (BD Biosciences) and analyzed by FACSDiva software (BD Biosciences). On average 19,000 intrahepatic leukocytes could be acquired. Samples from patients were included in the analysis only if at least 50 CD25+ FoxP3+ cells were acquired within the CD45+ CD3+ CD4+ population. On average 238 CD25+ FoxP3+ cells (range, 53 to 826 cells) were acquired within on average 2,764 CD45+ CD3+ CD4+ cells (range, 504 to 9,724 cells). For analysis, gates were set on the basis of isotype antibody controls, where appropriate.
Virological assessment.
Serum HCV RNA levels were determined by quantitative PCR (Cobas Ampliprep/Cobas TaqMan HCV test; limit of detection, <15 IU/ml; Roche Diagnostics, Netherlands). HCV genotypes were determined by an in-house-developed sequence analysis assay (Department of Virology, Erasmus MC).
Statistical analysis.
Cell frequencies in blood and liver samples from different subjects were compared using the Mann-Whitney U test, and paired samples were compared using the Wilcoxon signed-rank test. SPSS17.0 software was used for all analyses. All P values are two-tailed, and values >0.05 were considered not significant.
RESULTS
Fine-needle aspiration biopsies as a method to assess the lymphocyte profile in the liver during the course of antiviral therapy.
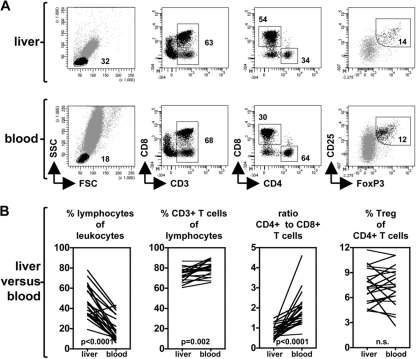
The local immune response in the liver is considered vital in the control of HCV replication in hepatocytes and the persistence of the virus in the livers of infected patients (4, 5). To determine the contribution of conventional T cells and CD4+ CD25+ FoxP3+ Treg to the regulation of immune responses to HCV, multiple minimally invasive and safe liver aspiration biopsy samples were collected and evaluated at baseline, as well as during and after therapy. From the total aspirate, on average 19,000 leukocytes were acquired by flow cytometry at each time point, which allowed us to perform flow cytometric analysis but no another assays. Lymphocytes constituted a major population of liver infiltrating leukocytes in these patients (Fig. 1) (46% on average), the majority being CD3+ T cells. In line with previous reports, in all HCV-infected patients CD4+ to CD8+ T cell ratios in the liver were significantly lower than in blood (respective mean ratios: 0.78 in liver and 1.9 in blood; P < 0.0001), indicating a higher proportion of CD8+ T cells of the total intrahepatic CD3+ T cell population. Sufficient numbers of CD4+ CD25+ FoxP3+ Treg could be detected in aspiration biopsy samples obtained from chronically HCV-infected patients to allow reliable evaluation of their frequency and phenotype by flow cytometry-based analysis (see Fig. S1 in the supplemental material).
Fig. 1.
Assessment of the intrahepatic lymphocyte profile by fine-needle aspiration biopsies. (A) Using flow cytometry, CD45+ leukocytes from the liver (top) and peripheral blood (bottom) from chronic HCV patients were characterized by serial gating on the basis of their forward/side scatter (FSC/SSC) profile and expression of various T cell markers. The dot plots are representative for specimens taken before, during, or after antiviral therapy. (B) Pretreatment frequencies and ratios of various lymphocyte populations from paired liver and blood samples.
Administration of IFN-α and ribavirin does not lead to substantial changes in the general composition of the lymphocyte compartment in the livers of chronic HCV patients.
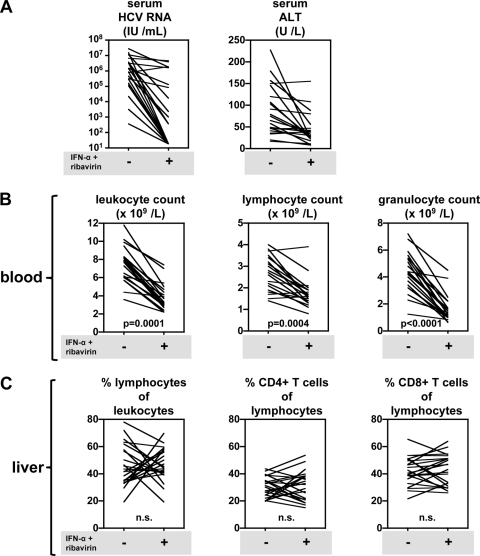
As expected, 4 weeks after the start of administration of IFN-α and ribavirin, reduction of the viral load and alanine transaminase (ALT) levels, accompanied by major losses in circulating leukocytes, was observed in most patients (Fig. 2A). In peripheral blood, the decrease in blood leukocyte counts was due to a drop in the absolute numbers of circulating granulocytes as well as lymphocytes within the first weeks of treatment (Fig. 2B).
Fig. 2.
Administration of IFN-α and ribavirin does not lead to dramatic changes in the composition of the intrahepatic lymphocyte compartment in chronic HCV patients. (A) Serum HCV RNA and ALT levels in blood from 22 chronic HCV patients prior to treatment (−) and 4 weeks after starting therapy (+). (B) Absolute numbers of leukocytes, lymphocytes, and granulocytes in blood. (C) Effect of antiviral therapy on the frequencies of intrahepatic leukocytes, as determined by flow cytometry on aspirate biopsy samples from the liver.
We now show that the frequency of intrahepatic lymphocytes relative to leukocytes was not significantly changed at the group level when the compositions of the liver at baseline and during IFN-α-based therapy were compared (Fig. 2C). Also, the relative contributions of CD4+ and CD8+ T cells to the lymphocyte population in the liver were not significantly affected at the group level. However, individual patients showed considerable shifts in the frequency of intrahepatic lymphocytes. Out of 22 patients, 13 showed an increased frequency of lymphocytes, while in 9 patients the frequency of intrahepatic lymphocytes was reduced as a consequence of treatment. Similarly, highly heterogeneous changes in the CD4+ and CD8+ T cell frequencies in the liver as a consequence of therapy were observed in these patients (Fig. 2C). Changes in liver lymphocyte and CD4+ and CD8+ T cell frequencies were not associated with the magnitude of serum HCV RNA or ALT reductions during IFN-α and ribavirin therapy. Due to the small volume of the aspiration biopsy samples, accurate enumeration of the absolute leukocyte and lymphocyte numbers in HCV-infected livers was unreliable for individual patients.
The frequency of intrahepatic CD4+ CD25+ FoxP3+ Treg increased during IFN-α and ribavirin therapy in half of the HCV-infected patients.
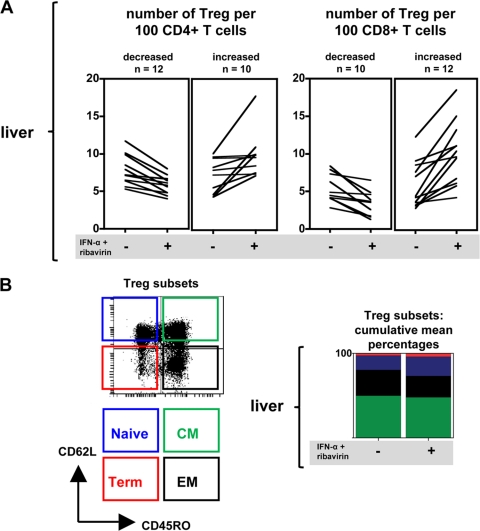
We previously showed that CD4+ CD25+ FoxP3+ Treg are present at high numbers in the livers of chronically HCV-infected patients but virtually absent in livers from healthy controls (3). To determine the effect of IFN-α-based therapy on immunoregulatory mechanisms in the livers of chronic HCV patients, the frequencies of Treg were determined before and 4 weeks after start of treatment. As shown in Fig. 3A, during antiviral treatment the number of intrahepatic CD4+ CD25+ FoxP3+ Treg increased in 10 out of 22 patients relative to CD4+ T cells and in 12 out of 22 patients relative to CD8+ T cells. Augmented Treg proportions may be the result of either an increase in the number of Treg or a reduction of the number of effector cells. At the group level, these ratios were significantly enhanced, with mean increases of 1.3 Treg per 100 CD4+ T cells (P = 0.0018) and 4.3 Treg per 100 CD8+ T cells (P = 0.0086). In some, but not all, cases enhancement of CD4+ CD25+ FoxP3+ Treg numbers in the liver by IFN-α-based therapy was also observed in peripheral blood (data not shown). However, these changes were not significant at the group level for Treg relative to CD4+ and CD8+ T cells (P = 0.39 and P = 0.91, respectively). Therefore, antiviral therapy appears to modulate the capacity to regulate HCV-specific immune responses by CD4+ CD25+ FoxP3+ Treg to a greater extent locally than systemically in HCV-infected livers.
Fig. 3.
IFN-α and ribavirin therapy rapidly increases the frequency of Treg in the livers of many HCV-infected patients. (A) Liver CD4+ CD25+ FoxP3+ Treg frequencies relative to CD4+ and CD8+ T cells at baseline and 4 weeks after starting antiviral therapy (n = 22). The findings are grouped as “decreased” or “increased” on the basis of the difference between the Treg frequencies before and during therapy, irrespective of the magnitude of the shifts of liver Treg relative to CD4+ or CD8+ T cells. (B) Treg subsets from 22 chronic HCV patients phenotyped by flow cytometry and classified as naive, central memory (CM), effector memory (EM), or terminally differentiated (Term) based on CD45RO and CD62L expression. All changes in the frequencies of specific memory populations determined prior to and during therapy were not significant.
We confirmed our previous findings that liver CD4+ CD25+ FoxP3+ Treg from chronic HCV patients generally express an effector or central-memory phenotype prior to treatment (Fig. 3B; see Fig. S1 in the supplemental material) (mixed CD45RO+ CD62L− and CD45RO+ CD62L+) (3). Following exposure to IFN-α and ribavirin no significant changes of the memory phenotype were observed for intrahepatic CD4+ CD25+ FoxP3+ Treg (P > 0.05). Similarly, comparison of the phenotypes of conventional CD4+ CD25− FoxP3− T cells in the liver did not demonstrate any effects of therapy (data not shown). These findings suggest that the relative increase in intrahepatic CD4+ CD25+ FoxP3+ Treg in chronic HCV patients as a consequence of antiviral therapy is unlikely to be the result of de novo generation of Treg.
CD4+ CD25+ FoxP3+ Treg remain present in the livers of subjects after therapy-induced clearance of HCV.
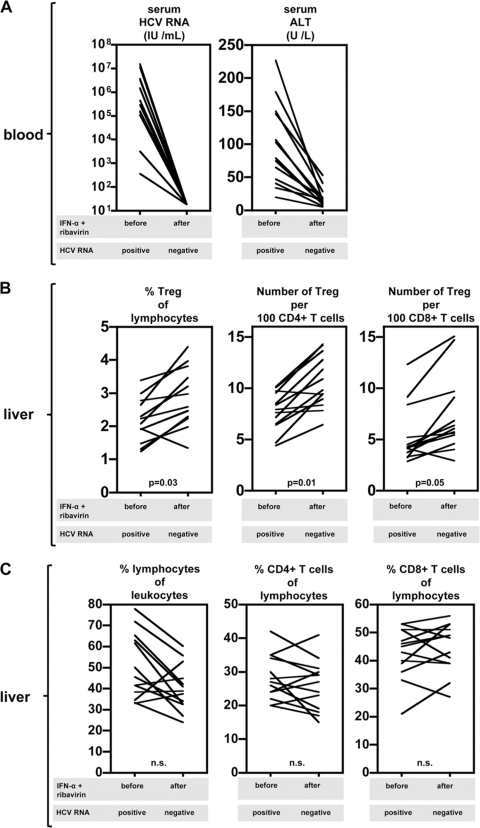
Next, we determined the modulation of intrahepatic Treg frequency after ending antiviral therapy. Out of 20 patients with a complete follow-up, 13 patients responded successfully to antiviral therapy and remained negative for serum HCV RNA after termination of therapy (Fig. 4A). All these individuals were negative early during the 24- to 48-week treatment period, so HCV RNA was absent or undetectable in serum for at least 6 months. Despite the absence of virus, enhanced intrahepatic CD4+ CD25+ FoxP3+ Treg frequencies were observed in the majority of these individuals, compared to the Treg frequencies prior to treatment. Four weeks after cessation of treatment, intrahepatic Treg were increased relative to total liver lymphocytes and CD4+ and CD8+ T cells in all but one patient (Fig. 4B). Additional sampling of liver cells 24 weeks after successful antiviral therapy in 3 patients demonstrated that intrahepatic CD4+ CD25+ FoxP3+ Treg remained present (Treg frequencies relative to CD4+ T cell frequencies: 6.9, 11.2, and 4.4); in some patients, their levels were lower than those 4 weeks after treatment but, more importantly, substantially higher than those in healthy livers, which were almost without Treg (3).
Fig. 4.
Therapy-induced clearance of HCV RNA does not lead to reduced Treg frequencies in the liver. (A) Serum HCV RNA and ALT levels prior to and 4 weeks after termination of IFN-α and ribavirin treatment. Only data for patients that were HCV RNA negative after therapy (n = 13) are depicted. (B) Liver CD4+ CD25+ FoxP3+ Treg frequencies relative to lymphocytes and CD4+ and CD8+ T cells before and 4 weeks after stopping IFN-α-based therapy. (C) Frequencies of liver lymphocytes relative to leukocytes and CD4+ and CD8+ T cells relative to lymphocytes before and 4 weeks after ending IFN-α-based therapy.
In the other 7 patients, treatment failed and chronic infection with HCV persisted. Also in these patients, intrahepatic CD4+ CD25+ FoxP3+ Treg frequencies were increased after cessation of therapy compared to baseline (data not shown). Changes in the frequencies of lymphocytes in responders and nonresponders to therapy, both in liver and blood (Fig. 4C and data not shown, respectively), did not show a clear pattern and were not related to treatment outcome. We did however find that pretreatment intrahepatic CD8+ T cell frequencies as a proportion of lymphocytes were higher in patients with therapy-induced clearance of HCV than in therapy nonresponders (43 versus 34%; P = 0.03). This is in line with previous reports suggesting that intrahepatic CD8+ T cells may be important for viral clearance (7, 12, 14). This difference in CD8+ T cells between responders and nonresponders was not affected by IFN-α and ribavirin and remained unchanged 4 weeks after therapy (45 versus 35%; P = 0.03).
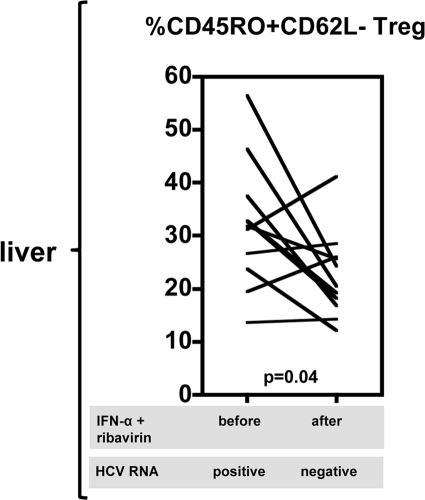
During the course of treatment, the differentiation status of intrahepatic Treg was not affected at the group level (Fig. 3B). However, as shown in Fig. 5, we evaluated the differentiation status of Treg after stopping IFN-α and ribavirin administration in 11 out of 13 individuals who responded to therapy and became negative for HCV RNA. Of these 11 patients, 7 showed a decline of the percentage of intrahepatic CD45RO+ CD62L− effector memory Treg when the differentiation status at baseline and that at 4 weeks after the end of treatment were compared. In sum, these findings suggest that, irrespective of the outcome of therapy, intrahepatic Treg are still present in the livers of individuals even after prolonged negativity for serum HCV RNA. However, although their regulatory function could not be assessed directly because of the limitation in obtaining sufficient material, differences in effector memory phenotypes suggest that liver CD4+ CD25+ FoxP3+ Treg in responders after therapy are weaker than those prior to therapy, when there is still virus present in the liver.
Fig. 5.
Effector CD45RO+ CD62L− phenotype of liver Treg is decreased in patients after IFN-α- and ribavirin-induced viral clearance. The proportions of intrahepatic Treg expressing a CD45RO+ CD62L− effector phenotype are shown for 11 patients before and 4 weeks after termination of antiviral therapy for patients that were HCV RNA negative after therapy.
DISCUSSION
The local immune response in the liver is important for the outcome of HCV infection and the persistence of the virus in the liver, since replication takes place in hepatocytes. However, the dynamics of the immune response in the livers of chronic HCV patients is largely unknown. Repeated aspiration biopsies during the course of IFN-α-based therapy enabled us to study this for the first time. We demonstrated that the consequence of antiviral therapy is an increased contribution of CD4+ CD25+ FoxP3+ Treg in the inflamed liver without alterations in the distribution of intrahepatic CD4+ and CD8+ T cells. In addition, we showed that the livers of individuals who became negative for HCV RNA as a result of treatment were still inflamed, and in fact Treg remained above baseline after cessation of therapy in the large majority of patients. However, these subjects showed a smaller contribution of intrahepatic CD45RO+ CD62L− effector memory Treg, whereas in patients with persistent infections, the phenotype of Treg was more skewed toward effector memory cells, suggesting important differences in the magnitudes of suppressive abilities of intrahepatic CD4+ CD25+ FoxP3+ Treg.
Longitudinal assessment of intrahepatic immunity by core liver biopsies is generally considered unethical for research purposes due to the risk of complications and patient discomfort. In addition, experimental animal models to examine the intrahepatic immune system during chronic HCV infections are not readily available (1). However, using fine-needle aspiration biopsies we were able to perform such a study without these constraints. To our knowledge, in only one study were liver biopsy samples collected before and 4 h after the start of IFN-based treatment in the same patient to examine the early effects of therapy on intrahepatic miR-122 expression, and effects on intrahepatic immunity during therapy were not investigated (19).
The livers of patients with chronic HCV infections show extensive leukocyte infiltration, with substantial numbers of FoxP3+ Treg around the portal tract areas (3, 24). We found that, in response to exposure to IFN-α and ribavirin, CD4+ CD25+ FoxP3+ Treg frequencies increased in the livers of chronically HCV-infected patients. The possibility that FoxP3 is transiently upregulated on a small subset of intrahepatic CD4+ T cells upon activation, as demonstrated by in vitro studies (reviewed in reference 16), cannot be completely ruled out. However, to our knowledge there is no information that this occurs in vivo on human T cells. Importantly, similar to Burton et al., we also found that Treg frequencies measured in blood remained unchanged during IFN-α-based therapy (2). These findings suggest that the intrahepatic CD4+ CD25+ FoxP3+ Treg compartment may play a more important role than blood Treg in regulating a curative immune response to HCV during IFN-α-based therapy. Evaluation of intrahepatic cells obtained from aspirate biopsy samples does not allow us to draw conclusions on the change in absolute numbers of these Treg over time. However, in experimental autoimmune encephalomyelitis in mice, it has been shown that the ratio of effector T cells to Treg, and not the absolute numbers, is of crucial importance in controlling disease severity caused by immunopathology (8). Similarly, in our experimental setup, it is likely that a high ratio of intrahepatic CD4+ CD25+ FoxP3+ Treg to effector T cells results in strong negative regulation of HCV-specific immune responses. In line with this, 4 of our patients with the highest Treg to CD8+ T cell ratios all failed to clear the HCV infection (data not shown), and it is tempting to speculate that the delicate balance between intrahepatic CD4+ CD25+ FoxP3+ Treg and effector T cells explains the response to IFN-α-based treatment in a subset of chronic HCV patients.
We showed that therapy-induced HCV eradication did not lead to normalization of the intrahepatic immune system after therapy. Levels of intrahepatic CD4+ CD25+ FoxP3+ Treg remained above baseline after cessation of therapy in the large majority of patients, including subjects that showed a therapy-induced viral clearance and who became negative for HCV RNA early during the course of treatment. Importantly, in a follow-up evaluation of 3 patients who successfully cleared the virus and who had undetectable virus levels 24 weeks after ending antiviral therapy, the intrahepatic CD4+ CD25+ FoxP3+ Treg still remained above baseline. These findings were unexpected, since we reported before that Treg were virtually absent from healthy livers not previously exposed to HCV antigens (3), and suggest that inflammation and regulation by intrahepatic CD4+ CD25+ FoxP3+ Treg are ongoing in patients who successfully clear the virus and in whom HCV antigens are not longer detectable. However, we cannot rule out the possibility that these Treg will resolve later. Recently, it was reported that in plasma samples from 15% of sustained virologic response patients trace amounts of HCV RNA were detected years after successful therapy (23). Importantly, the reappearance of HCV RNA was found to induce HCV-specific T cell responses. Therefore, it may be speculated that the intrahepatic Treg that remain for the first 6 months after successful IFN-α therapy control residual HCV replication in the liver. Furthermore, processes other than HCV-specific immunity, such as bystander T cell activation and ongoing fibrogenesis, may also be controlled. The latter is in line with our previous findings suggesting a role for Treg in limiting the extent of immunopathology in patients still infected with HCV (3). In addition, other inflammatory processes in the liver, for example, alcoholic or nonalcoholic steatohepatitis (10), may be modulated by Treg present in the liver after clearance of HCV as well. Despite our finding that Treg frequencies did not differ between nonviremic patients and patients with no response to therapy, the continued regulation by Treg in nonviremic patients may be less potent, since their lower proportion of CD45RO+ CD62L− Treg hints at a less immediate effector activity. Further characterization of Treg in liver aspiration biopsy samples in only two patients showed that the expression of the activation marker HLA-DR was not changed during and after therapy, and we observed that the high level of HLA-DR expression on intrahepatic Treg was maintained (data not shown).
In conclusion, we show for the first time that intrahepatic CD4+ CD25+ FoxP3+ Treg frequencies increase during IFN-α-based therapy and that intrahepatic Treg ratios remain above baseline even 6 months after successful clearance of HCV RNA from serum. These findings indicate that immunoregulation by CD4+ CD25+ FoxP3+ Treg is not only important during chronic HCV infection and therapy but also pivotal in controlling liver immunity in previously HCV-infected patients.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Foundation for Liver and Gastrointestinal Research (SLO), Rotterdam, Netherlands, and further supported by Schering-Plough (now MSD) and a ZonMW VIDI grant (NWO 016-56-329) for H. L. A. Janssen.
We thank the clinical research bureau and research nurses of our department, especially Heleen van Santen-van der Stel, for their dedicated assistance throughout the study. Furthermore, we are grateful to Duygu Turgut and Anthonie Groothuismink for their excellent technical assistance.
We disclose no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Boonstra A., van der Laan L. J., Vanwolleghem T., Janssen H. L. 2009. Experimental models for hepatitis C viral infection. Hepatology 50:1646–1655 [DOI] [PubMed] [Google Scholar]
- 2. Burton J. R., Jr., et al. 2008. Prospective analysis of effector and regulatory CD4+ T cells in chronic HCV patients undergoing combination antiviral therapy. J. Hepatol. 49:329–338 [DOI] [PubMed] [Google Scholar]
- 3. Claassen M. A., de Knegt R. J., Tilanus H. W., Janssen H. L., Boonstra A. 2010. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J. Hepatol. 52:315–321 [DOI] [PubMed] [Google Scholar]
- 4. Crispe I. N. 2009. The liver as a lymphoid organ. Annu. Rev. Immunol. 27:147–163 [DOI] [PubMed] [Google Scholar]
- 5. Dustin L. B., Rice C. M. 2007. Flying under the radar: the immunobiology of hepatitis C. Annu. Rev. Immunol. 25:71–79 [DOI] [PubMed] [Google Scholar]
- 6. Feld J. J., Hoofnagle J. H. 2005. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 436:967–972 [DOI] [PubMed] [Google Scholar]
- 7. Freeman A. J., Marinos G., Ffrench R. A., Lloyd A. R. 2005. Intrahepatic and peripheral blood virus-specific cytotoxic T lymphocyte activity is associated with a response to combination IFN-alpha and ribavirin treatment among patients with chronic hepatitis C virus infection. J. Viral Hepat. 12:125–129 [DOI] [PubMed] [Google Scholar]
- 8. Korn T., et al. 2007. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lohr H. F., et al. 1999. The viral clearance in interferon-treated chronic hepatitis C is associated with increased cytotoxic T cell frequencies. J. Hepatol. 31:407–415 [DOI] [PubMed] [Google Scholar]
- 10. Ma X., et al. 2007. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology 46:1519–1529 [DOI] [PubMed] [Google Scholar]
- 11. Miyaaki H., et al. 2009. Study of liver-targeted regulatory T cells in hepatitis B and C virus in chronically infected patients. Liver Int. 29:702–707 [DOI] [PubMed] [Google Scholar]
- 12. Nelson D. R., Marousis C. G., Ohno T., Davis G. L., Lau J. Y. 1998. Intrahepatic hepatitis C virus-specific cytotoxic T lymphocyte activity and response to interferon alfa therapy in chronic hepatitis C. Hepatology 28:225–230 [DOI] [PubMed] [Google Scholar]
- 13. Penton-Rol G., et al. 2008. Treatment with type I interferons induces a regulatory T cell subset in peripheral blood mononuclear cells from multiple sclerosis patients. Int. Immunopharmacol. 8:881–886 [DOI] [PubMed] [Google Scholar]
- 14. Pilli M., et al. 2007. HCV-specific T-cell response in relation to viral kinetics and treatment outcome (DITTO-HCV project). Gastroenterology 133:1132–1143 [DOI] [PubMed] [Google Scholar]
- 15. Rehermann B., Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229 [DOI] [PubMed] [Google Scholar]
- 16. Roncarolo M. G., Gregori S. 2008. Is FOXP3 a bona fide marker for human regulatory T cells? Eur. J. Immunol. 38:925–927 [DOI] [PubMed] [Google Scholar]
- 17. Rosen H. R., et al. 2007. Selective decrease in hepatitis C virus-specific immunity among African Americans and outcome of antiviral therapy. Hepatology 46:350–358 [DOI] [PubMed] [Google Scholar]
- 18. Sakaki M., et al. 2008. Intrahepatic status of regulatory T cells in autoimmune liver diseases and chronic viral hepatitis. Hepatol. Res. 38:354–361 [DOI] [PubMed] [Google Scholar]
- 19. Sarasin-Filipowicz M., Krol J., Markiewicz I., Heim M. H., Filipowicz W. 2009. Decreased levels of microRNA miR-122 in individuals with hepatitis C responding poorly to interferon therapy. Nat. Med. 15:31–33 [DOI] [PubMed] [Google Scholar]
- 20. Shoukry N. H., Cawthon A. G., Walker C. M. 2004. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu. Rev. Microbiol. 58:391–424 [DOI] [PubMed] [Google Scholar]
- 21. Sprengers D., et al. 2005. Flow cytometry of fine-needle-aspiration biopsies: a new method to monitor the intrahepatic immunological environment in chronic viral hepatitis. J. Viral Hepat. 12:507–512 [DOI] [PubMed] [Google Scholar]
- 22. Stoop J. N., et al. 2008. Intrahepatic regulatory T cells are phenotypically distinct from their peripheral counterparts in chronic HBV patients. Clin. Immunol. 129:419–427 [DOI] [PubMed] [Google Scholar]
- 23. Veerapu N. S., Raghuraman S., Liang T. J., Heller T., Rehermann B. 2011. Sporadic reappearance of minute amounts of hepatitis C virus RNA after successful therapy stimulates cellular immune responses. Gastroenterology 140:676–685, e671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward S. M., et al. 2007. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J. Hepatol. 47:316–324 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.