Abstract
Prion sorption to soil is thought to play an important role in the transmission of scrapie and chronic wasting disease (CWD) via the environment. Sorption of PrP to soil and soil minerals is influenced by the strain and species of PrPSc and by soil characteristics. However, the ability of soil-bound prions to convert PrPc to PrPSc under these wide-ranging conditions remains poorly understood. We developed a semiquantitative protein misfolding cyclic amplification (PMCA) protocol to evaluate replication efficiency of soil-bound prions. Binding of the hyper (HY) strain of transmissible mink encephalopathy (TME) (hamster) prions to a silty clay loam soil yielded a greater-than-1-log decrease in PMCA replication efficiency with a corresponding 1.3-log reduction in titer. The increased binding of PrPSc to soil over time corresponded with a decrease in PMCA replication efficiency. The PMCA efficiency of bound prions varied with soil type, where prions bound to clay and organic surfaces exhibited significantly lower replication efficiencies while prions bound to sand exhibited no apparent difference in replication efficiency compared to unbound controls. PMCA results from hamster and CWD agent-infected elk prions yielded similar findings. Given that PrPSc adsorption affinity varies with soil type, the overall balance between prion adsorption affinity and replication efficiency for the dominant soil types of an area may be a significant determinant in the environmental transmission of prion diseases.
INTRODUCTION
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are fatal neurodegenerative diseases that include bovine spongiform encephalopathy (BSE or “mad cow” disease), scrapie (sheep and goats), chronic wasting disease (CWD; deer, elk, and moose), and Creutzfeldt-Jakob disease (CJD; humans) (28, 29). Strong evidence indicates that the infectious agent of prion diseases is solely PrPSc, an abnormally folded isoform of a normal cellular protein, PrPc (12, 14, 43). The misfolded conformation of PrPSc conveys distinct biochemical properties, including resistance to proteolysis and conventional inactivation methods, increased hydrophobicity, and the ability to self-propagate (8, 9, 27, 41).
Prions can enter the environment from both living and dead hosts. Prions are shed from living animals with CWD or scrapie into the environment through saliva, blood, urine, feces, antler velvet, and birthing material (1, 2, 16, 17, 24, 25, 40). Prions also enter the environment via animal mortalities in both captive (e.g., scrapie, BSE, and CWD) and wild (e.g., CWD) populations of prion-infected animals. Once prions enter the environment, they can remain infectious for extended periods of time (10, 31).
Soil and soil minerals may act as significant environmental reservoirs of prion diseases. Scrapie PrPSc has been detected on environmental surfaces on a farm where the disease was endemic (23). Prions are known to sorb (i.e., bind) to a wide range of soils and soil minerals, resist desorption, and remain infectious (20, 31, 33). The potential for CWD or scrapie transmission by exposure to contaminated soil exists since cervids and other ruminants are known to ingest and inhale soil (3, 7), which are known routes of prion infection (6, 18, 19, 22, 37). Environmentally mediated transmission may facilitate a sustained incidence of CWD in free-ranging cervid populations and complicate efforts to eliminate CWD and scrapie in captive herds.
Adsorption of PrP to soil and soil minerals is influenced by the prion strain, species, PrP form (full length versus N-terminally truncated), soil type, and equilibration period (21, 23, 32, 33). The effect of these variables on the ability of soil-bound prions to convert PrPc to PrPSc and initiate infection is poorly understood. To investigate these parameters, we utilized protein misfolding cyclic amplification (PMCA) and animal bioassay to evaluate the ability of prions to replicate and cause disease when bound to a range of soils and soil minerals.
MATERIALS AND METHODS
Prion sources and tissue preparation.
Prion-infected tissue was collected from either hamsters infected with the hyper strain of transmissible mink encephalopathy (HY TME) or from an elk infected with the CWD agent as previously described (32). Prion-infected brain tissue was homogenized to 10% (wt/vol) in Dulbecco's phosphate-buffered saline (DPBS) without Ca2+ or Mg2+ (Mediatech, Herndon, VA) using strain-dedicated Tenbroeck tissue grinders (Kontes, Vineland, NJ). Uninfected Syrian hamster and uninfected Tg(CerPrP)1536+/− mouse brain tissue (11) was homogenized to 10% (wt/vol) in ice-cold conversion buffer (DPBS [pH 7.4] containing 5 mM EDTA, 1% [vol/vol] Triton X-100, and a complete protease inhibitor tablet [Roche Diagnostics, Mannheim, Germany]) and centrifuged at 500 × g for 30 s. The supernatant was collected and stored at −80°C.
Prion adsorption to soil and soil minerals.
PrPSc likely enters the environment as a heterogeneous mixture of full-length and N-terminally truncated forms due to in situ N-terminal cleavage by natural proteases found in prion-infected tissue (34). To simulate this heterogeneity, we incubated (“aged”) 10% HY TME-infected hamster brain homogenate for 2 days at 37°C; this incubation did not significantly decrease the abundance of PrP as measured using the monoclonal antibody (MAb) 3F4 but did decrease the N-terminal MAb 5B2 epitope abundance by approximately 50% (data not shown) (34). This “aged” brain homogenate was used along with “unaged” homogenate. Gamma-irradiated fine white sand (Fisher Scientific, Pittsburgh, PA), Dickinson sandy loam (SL) soil (a Typic Hapludoll), Rinda silty clay loam (SCL) soil (a Vertic Epiaqualf), sodium bentonite clay (CETCO, Arlington Heights, IL), silicon dioxide powder (Sigma Aldrich, St. Louis, MO), and humic acid (HA)-coated silica gel particles (SiO2-HA) were used. The physical and chemical properties of each of these materials have been described previously (33, 35). To obtain soil-bound prions, 10% brain homogenate (aged or unaged) was combined with soil in 1× DPBS and gently rotated at 24 rpm (Mini Labroller; Labnet, Edison, NJ) at 22°C. Incubation time, soil, buffer, and homogenate-to-soil ratios were selected based on previously published results and are detailed in Table 1 (32, 33).
Table 1.
Generation of soil-bound prions
| Soil/mineral | Adsorption time (h) | Soil concn (mg/ml) | % BHa | PMCA seed (mg) |
|---|---|---|---|---|
| Rinda silty clay loam | 24 | 5 | 0.5 | 0.1 |
| Bentonite clay | 24 | 5 | 0.5 | 0.1 |
| SiO2 powder | 24 | 50 | 0.25 | 2 |
| SiO2-humic acid | 168 | 10 | 0.5 | 1 |
| Dickinson sandy loam | 168 | 15 | 0.5 | 3 |
| Fine quartz sand | 168 | 50 | 0.5 | 10 |
BH, brain homogenate.
To achieve maximum or nearly maximum PrPSc adsorption, soil-homogenate mixtures for SiO2-HA, sandy loam soil, and sand required 7-day incubation periods, while silty clay loam soil, bentonite, and SiO2 required a 24-h incubation period (32, 35). Samples were removed after incubation and centrifuged at 100 × g for 5 min. The supernatant was removed, and the pellets were washed twice with DPBS. The original supernatant and washes (i.e., the unbound fraction) as well as the final pellet (bound fraction) were collected and stored at −80°C. The Rinda SCL soil pellet was resuspended in DPBS to 0.1 mg soil/μl.
Animal bioassay of prions.
All procedures involving animals were approved by the Creighton University Institutional Animal Care and Use Committee and comply with the Guide for the Care and Use of Laboratory Animals. Intracerebral inoculations of male Syrian hamsters (Harlan Sprague-Dawley, Indianapolis, IN) were conducted as described previously (22). The incubation period was calculated as the length of time in days between inoculation and the onset of clinical signs that include ataxia and hyperactivity in response to external stimuli. Titer was calculated by the method of Reed and Muench (30).
PMCA.
Protein misfolding cyclic amplification (PMCA) was performed as described previously (36). Briefly, sonication was performed with a Misonix (Farmingdale, NY) 4000 sonicator with amplitude set to level 75, generating an average output of 160 W during each sonication cycle. Before each PMCA round, an aliquot was placed at −80°C as an unsonicated control. After the first round of PMCA, an aliquot of the sonicated sample was added to fresh 10% (wt/vol) uninfected brain homogenate in conversion buffer and subjected to a second round of PMCA. For detection of low levels of PrPSc (see Fig. 2C), the ratio of sonicated sample to uninfected brain homogenate was 1 to 20 and one round consisted of 144 cycles of 5 s of sonication followed by 10 min of incubation at 37°C. Homogenates from round 1 were diluted 1 to 20 for subsequent rounds. For all other experiments, the ratio of sonicated sample to uninfected brain homogenate was 1 to 100 and one round consisted of 144 cycles of 25 s of sonication followed by 10 min of 37°C incubation. Samples containing only uninfected brain homogenate were included with each round of PMCA as negative controls.
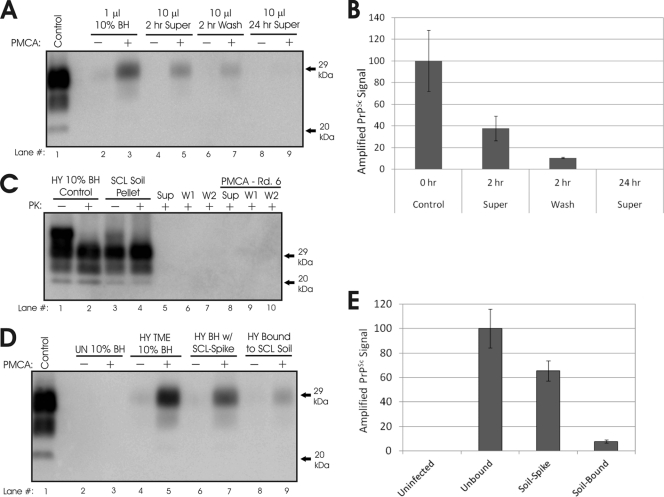
Fig. 2.
Sorption of HY TME to silty clay loam (SCL) soil inhibits PMCA replication. (A and B) Western blot (A) and quantification (B) of PMCA reaction mixtures seeded with supernatants or washes of HY TME incubated with SCL soil for 2 or 24 h. (C) Western blot analysis of SCL-bound aged HY PrPSc pellet, supernatant (Sup), washes (W1, W2), and round 6 of PMCA reaction mixtures seeded with either the supernatant or washes. (D and E) Western blot (D) and quantification (E) of unbound and SCL soil-bound HY TME samples (24 h) prior to (−) and after (+) one round of PMCA.
Western blot analysis.
Western blot analysis was performed as described previously (4, 36) without modification using either 12.5% acrylamide or 4 to 12% bis-Tris–acrylamide gels (NuPage; Invitrogen, Carlsbad, CA). Briefly, samples were incubated at 37°C under constant agitation for 30 min with 25 μg/ml proteinase K (PK) (Roche Diagnostics Corporation, Indianapolis, IN). The PK digestion was terminated by boiling in SDS-PAGE sample buffer. Hamster samples were immunoblotted with MAb 3F4 (Chemicon, Temecula, CA; 1:10,000 dilution) or MAb 5B2 (Santa Cruz Biotechnology; 1:1,000 dilution). Elk/Tg(CerPrP)1536+/− samples were immunoblotted with L42 (R-Biopharm, Marshall, MI; 1:1,000 dilution). Blots were developed with Supersignal West Femto maximum-sensitivity substrate, according to the manufacturer's instructions (Pierce, Rockford, IL), imaged on a 4000R imaging station (Kodak, Rochester, NY), and analyzed using Kodak (New Haven, CT) molecular imaging software, v.5.0.1.27. Sample replicates (n ≥ 3) were normalized to the average of four replicate brain homogenate controls on the same gel to control for intergel variance. For PMCA analysis, sample intensities were also normalized to brain homogenate control samples subjected to PMCA concurrently to control for variance between PMCA treatments. Statistical analysis (two-sample t tests assuming unequal variances) was performed using Prism 4.0 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Semiquantitative PMCA.
There is a relationship between the input titer of prions and the number of serial PMCA rounds required for PrPSc detection (13, 36). We performed a serial dilution of HY TME-infected brain homogenate, with an initial titer of 109.3 intracerebral (i.c.) 50% lethal doses (LD50) per gram (22), in triplicate and evaluated the amplified signal of each sample after a single round of PMCA (Fig. 1). PMCA-generated PrPSc was detected in all samples down to an input dilution of 10−4.3 μg equivalents (103.0 i.c. LD50) of brain homogenate (Fig. 1A). A logarithmic relationship (y = −36.9ln [x] + 97.8) best approximated (R2 = 0.97) the association between the input titer of HY TME agent and the abundance of PMCA-generated PrPSc (Fig. 1B).
Fig. 1.
Single-round semiquantitative PMCA. Western blot (A) and quantification (B) of PMCA reaction mixtures (n ≥ 3) seeded with serial dilutions of HY TME-infected brain following one round of PMCA. Amplified PrPSc abundance was normalized to the PrPSc abundance of PMCA reaction mixtures seeded with 10−1 HY TME-infected brain homogenate (BH). Regression analysis indicated a relationship between input HY TME titer and subsequent PMCA-generated PrPSc (R2 = 0.97).
Sorption of HY TME to SCL soil reduces prion replication in vitro.
Sorption of HY PrPSc to silty clay loam (SCL) soil, in a brain homogenate matrix, requires a minimum of 24 h to achieve maximum PrP adsorption (33). To investigate the effect of unbound PrPSc on PMCA, we incubated SCL soil with HY TME-infected brain homogenate for either 2 or 24 h, collected supernatants and washes postincubation, and evaluated these using PMCA for the presence of PrPSc. Detectable PrPSc was amplified from the supernatant and wash of the 2-h sample (Fig. 2A). PrPSc was amplified to a greater level in the 2-h supernatant sample than in the 2-h wash, suggesting that the supernatant contained higher levels of PrPSc (Fig. 2B). In neither the supernatant nor the wash of the 24-h incubation sample was PrPSc amplified (Fig. 2; data not shown). To determine if low levels of PrPSc were present in these samples, serial PMCA was performed using the initial supernatant and washes as the seed. We failed to detect PrPSc after 6 serial rounds of PMCA (Fig. 2C, lanes 8 to 10), which indicates that the sample contains less than 100 LD50 of HY TME agent (36). The SCL soil pellet with adsorbed HY TME and a HY TME-infected brain homogenate control had similar PrPSc abundances and levels of polypeptide migration and glycosylation (Fig. 2C). We subjected the unbound and SCL soil-bound brain homogenates to one round of PMCA and compared the resulting amplified PrPSc signals (Fig. 2D). Compared to that of the unbound aged HY control, sorption of the aged HY TME to SCL resulted in a 92% reduction in the abundance of amplified PrPSc (Fig. 2D, lanes 5 and 9, and 2E). A control containing aged HY TME-infected brain homogenate cospiked with SCL soil was amplified to lower levels than the HY positive control but significantly greater levels than the soil-bound sample (P < 0.01) (Fig. 2E). Comparable results were obtained for unaged and aged HY TME as well as for aged and unaged HY PrPSc bound to SCL soil (Fig. 3B; data not shown). Uninfected brain homogenate negative controls did not contain PK-resistant PrP after PMCA for any experiment (Fig. 2).
Fig. 3.
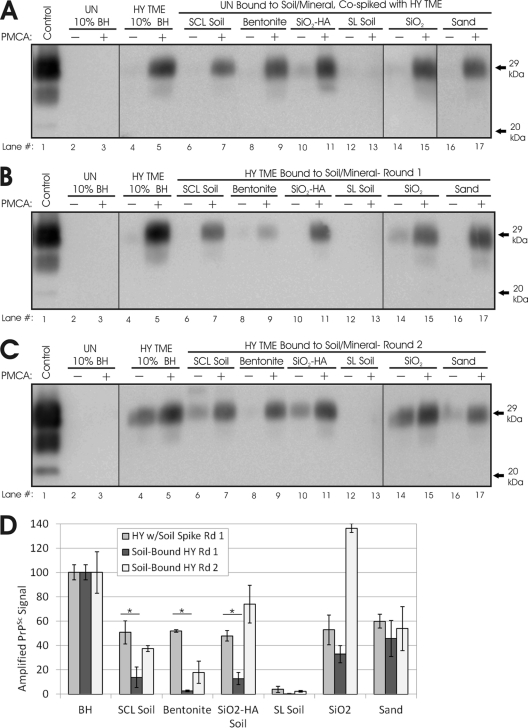
Inhibition of HY TME PMCA replication is dependent on soil type. Shown is Western blot analysis (A to C) and quantification (D) of PMCA reaction mixtures spiked with soil unbound (UN) (A) or bound to HY PrPSc following one (B) or two (C) rounds of amplification. (D) Sample intensities (n ≥ 6) were normalized to 200-μg BH controls (n = 4) and then represented as percentages of PrPSc generated in unbound HY (BH) controls (n = 3) subjected to PMCA concurrently. Asterisks denote significant differences (P < 0.01). SiO2-HA, humic acid-coated silica gel particles.
SCL-bound HY TME is less infectious.
Intracerebral inoculation of groups of 5 hamsters with either the control HY TME, aged HY TME, or SCL-bound aged HY TME resulted in all animals succumbing to disease, with incubation periods of 62 ± 3, 60 ± 3, and 74 ± 3 days, respectively. The incubation period for the HY TME agent-infected animals was similar (P > 0.05) to that for aged HY TME, which is consistent with the failure to detect a reduction in PrPSc abundance following the 2-day aging process (34) (data not shown). To investigate the possibility that the 14-day extension of incubation period in the SCL-bound aged HY TME group was due to a reduction in titer, 10-fold serial dilutions of the aged HY TME or SCL-bound aged HY TME inoculum were intracerebrally inoculated into groups of 5 Syrian hamsters. The titers for the unbound and SCL-bound aged HY TME inoculum were 107.5 and 106.2 i.c. LD50/25 μl, respectively, representing a 1.3-log decrease in HY TME titer upon binding to SCL soil (Table 2). The titer of the aged HY TME was not significantly different from those in our previous studies (22). Brain material from an animal infected with the 10−2 dilution of the SCL-bound HY agent was serially passaged by being intracerebrally inoculated into Syrian hamsters (n = 5). This resulted in all animals succumbing to disease in 61 ± 3 days, which is not statistically different from results for hamsters inoculated with control HY TME agent (P < 0.05). This suggests that SCL binding to HY PrPSc does not permanently alter the HY TME agent. All diseased animals exhibited clinical signs of hyperexcitability and ataxia and PrPSc migration properties that were consistent with HY TME agent-infected animals (data not shown).
Table 2.
Reduced titer of soil-bound HY TME
| Agent dilution | Mean incubation period (days) ± SEM (no. affected/no. inoculated) for inoculuma of: |
|
|---|---|---|
| HY TME | HY TME sorbed to SCL | |
| 10−2 | 62 ± 3 (5/5)a | 74 ± 3 (5/5) |
| 10−3 | NDb | 78 ± 3 (5/5) |
| 10−4 | 74 ± 4 (5/5) | 84 ± 3 (5/5) |
| 10−5 | ND | 96 ± 3 (5/5) |
| 10−6 | 98 ± 3 (5/5) | 120 ± 25 (3/5) |
| 10−7 | 127 ± 18 (5/5) | >275 (0/5) |
| 10−8 | >275 (0/5) | >275 (0/5) |
| 10−9 | >275 (0/5) | >275 (0/5) |
| Mock | >275 (0/5) | >275 (0/5) |
Titers: HY TME agent, 107.5 i.c. LD50/25μl; HY TME agent sorbed to SCL, 106.2 i.c. LD50/25μl.
ND, not done.
A range of soils and soil minerals selectively inhibits HY TME amplification.
To compare levels of bound prion replication across a range of soil types, brain homogenates were incubated with a range of whole soils and soil minerals as detailed in Table 1. Given that we observed no difference in PMCA efficiency between aged and unaged SCL soil samples, we used only unaged HY TME for these experiments. We subjected HY TME bound to each soil type to two rounds of serial PMCA to compare the amplification efficiencies across soil types and between bound and unbound agents. The amounts of soil used as a PMCA seed are indicated in Table 1. Equal amounts of bound HY were used as PMCA seeds in the case of SCL soil, bentonite, SiO2, and the unbound (brain homogenate) control. HY TME bound to three of the seven soil types evaluated exhibited a significantly (P < 0.01) reduced efficiency compared to spiked controls (Fig. 3). In addition to the reduced efficiency observed for SCL soil, HY TME bound to the organic SiO2-HA surface also had a significantly reduced ability for amplification (87%) (Fig. 3B, lane 11, and D), while HY binding to bentonite clay nearly completely inhibited PrPSc replication after one round of PMCA (97%) (Fig. 3B, lane 9, and D). HY PrPSc bound to Dickinson sandy loam soil failed to amplify through at least three rounds (Fig. 3B, lane 13, and D; data not shown). In contrast, binding of HY PrPSc to sand and SiO2 powder did not inhibit HY PrPSc amplification compared to that for seeded controls (Fig. 3B, lanes 15 and 17, and D). A second serial round of PMCA led to greater PrPSc amplification for all soils except SL soil compared with unbound controls (Fig. 3C and D). All amplified PrPSc products showed migration patterns similar to those for HY TME controls (Fig. 3). To evaluate if soil could induce PrPSc formation either during PrPc soil adsorption or during the PMCA process, uninfected hamster brain homogenate was incubated with the soils and soil minerals in place of infectious brain homogenate. Following incubation, soil pellets were washed in the same manner as HY TME soil samples and then subjected to PMCA. PK-resistant PrP was not detected after three rounds of serial PMCA in any soil or uninfected brain homogenate samples (data not shown). This suggests that soil-bound PrPc or other bound brain constituents do not readily induce PrPSc formation.
Inhibition of soil-bound CWD agent amplification.
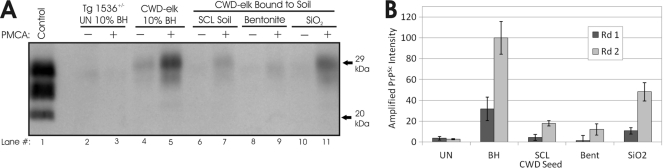
Given that we have observed differences in PrP adsorption and resistance to degradation between HY TME and the CWD agent infecting elk (CWD-elk) (33–35), we also performed PMCA experiments on infectious CWD-elk prions bound to soil. CWD-elk prions were adsorbed to SCL soil, bentonite, and SiO2 powder using the sample protocol used for HY TME and then subjected to PMCA using a brain homogenate substrate from transgenic mouse brains expressing deer PrPc [Tg(CerPrP)1536+/−] (11). The levels of inhibition of PMCA replication using the CWD-elk and Tg1536+/− substrate were similar to HY TME results (Fig. 4). After two rounds of PMCA, unbound CWD prion-infected brain homogenate exhibited significantly greater PrPSc amplification (P < 0.01) than the reaction mixtures seeded with CWD prions bound to SCL soil (82% reduction), bentonite (96% reduction), or SiO2 powder (50% reduction) (Fig. 4A, lanes 7 and 9, and B). Binding of CWD PrPSc to SiO2 powder inhibited CWD PrPSc amplification less than binding to SCL soil and bentonite samples, consistent with HY TME results (Fig. 3).
Fig. 4.
CWD PrPSc replication is selectively inhibited by sorption to soil. (A) Western blot analysis of soil-bound CWD agent-infected samples prior to (−) and after (+) two rounds of PMCA. (B) Quantification (n = 3) of PrPSc abundance following one or two rounds of PMCA of samples seeded with either uninfected brain, CWD agent-infected elk (CWD-elk) brain homogenate, or CWD-elk brain homogenate bound to soil. Amplified PrPSc abundance was normalized to the second-round unbound (BH) sample. Bent, bentonite.
DISCUSSION
The degree to which PrPSc is adsorbed to soil corresponds with a decrease in the capacity of PrPSc to replicate. We have previously shown that complete adsorption of HY PrPSc in a brain homogenate matrix to SCL takes at least 24 h to complete (33). An increase in the amount of HY PrPSc bound to SCL over time corresponded with a reduction in PMCA conversion activity (Fig. 2), suggesting that unbound PrPSc is the favored template for PMCA replication compared to SCL-bound PrPSc. The observed decrease in the amplification of prions upon binding to SCL could be due to a number of factors. First, conformational changes in PrPSc structure, including changes in aggregation state, may occur upon binding to soil (31, 33). Since aggregate size and PrPSc structure (i.e., different prion strains) can correspond with differences in titer (5, 38), changes in these properties due to soil adsorption could explain the decrease in PrPSc amplification. Alternatively, avid PrPSc binding to soil may also “inactivate” a portion of the total PrPSc by occupying, shielding, or distorting active sites, either through direct PrPSc-soil surface interactions or through interactions between PrPSc and other bound brain constituents. Evidence for this mechanism is found in the inefficient desorption of PrP from clay and organic surfaces, even under harsh denaturing conditions (35). Consistent with this observation, PrP desorption from SiO2 and sand was near 100% (data not shown) and binding to these soils did not significantly inhibit PMCA replication of HY PrPSc (Fig. 3). Given the lower affinity of PrP for SiO2-HA, SL soil, and sand (33, 35), smaller amounts of HY may have been bound to these soils (20 to 50% compared with the unbound control) (data not shown). However, our PMCA results indicate that changes in PrPSc seed abundance in this range do not correspond with significant changes in PMCA efficiency (Fig. 1); therefore, the large differences in PMCA efficiency observed with respect to soil type (Fig. 3) are not attributable to the relatively small differences in initial spiked amounts. Therefore, soil types that support PrPSc desorption correspond with an increased ability of soil-bound PrPSc to serve as a template for PMCA conversion relative to soil types yielding inefficient PrPSc desorption. We hypothesize that the population of PrPSc that is irreversibly bound to the soil surface is unable serve as a template for conversion and suggest that two populations of PrPSc bound to soil exist: one that is both desorbable and infectious and one that is neither. This hypothesis predicts that desorption of PrPSc is required for conversion of PrPc to PrPSc. Alternatively, PrPSc bound to soil may facilitate conversion of PrPc to PrPSc at a reduced efficiency or a subpopulation of soil-bound PrPSc may be replication competent. Additional experimentation is needed to evaluate this hypothesis and the mechanisms behind the observed decrease in prion titer and ability to replicate upon binding to certain soil surfaces.
Inhibition of HY PrPSc PMCA replication by sorption to SCL corresponds with an increase the incubation period of disease and a reduction of HY TME agent titer (Table 2). The incubation periods of HY TME- and aged HY TME-infected brain homogenate were similar, consistent with previous studies indicating that the PK-sensitive N-terminal region of PrPSc is not required for prion infectivity (5). Sorption of SCL to HY PrPSc resulted in a 12-day extension in the incubation period in the group of animals inoculated with the 1% (wt/vol) brain homogenate. This extension in the incubation period is consistent with the 1.3-log reduction in titer determined by endpoint dilution, suggesting that sorption of HY PrPSc to soil does not alter the relationship between incubation period and titer, which has been observed following heat or chemical treatment of the prion agent (15, 26, 39, 42). Serial passage in hamsters resulted in a reduction of the incubation period to 61 ± 3 days, similar to that for our starting HY TME agent, indicating that sorption of PrPSc to SCL does not permanently alter the HY TME agent. The observed reduction in titer differs from results in previous reports that demonstrate that infectivity is enhanced via binding to soil (20). Several reasons may explain this discrepancy. Methodological differences such as different adsorption buffers (1× DPBS versus 10 mM NaCl), soil types (SCL soil versus pure montmorillonite clay), soil-to-PrP ratios, routes of inoculation, source of PrPSc, and adsorption times (24 h versus 2 h) may influence the results. The last point may be an important factor since unabsorbed PrPSc could mask the inhibition of replication of the sorbed material. Overall, comparison of the results here with other studies indicates that the differences in titer, either increased or decreased, are relatively small compared to the vast amount of remaining infectious agent. Perhaps more importantly, these studies consistently demonstrate that prions sorbed to soil remain infectious.
In this study we have semiquantitatively correlated the amplified sample PrPSc Western blot intensities following a single round of PMCA with the initial sample titer (Fig. 1). The relationship between PMCA amplification efficiency and prion infectivity is supported by the correlation between the reduced HY TME titer upon adsorption to SCL soil and the reduced PMCA efficiency of the SCL-bound HY TME, as well as the correlation between the equal titers of aged and unaged HY TME agents and their equal PMCA efficiencies. We can conclude here that (i) there is a distinct relationship between initial HY titer and the amplified, single-round PMCA signal, (ii) the decrease in PMCA efficiency observed for SCL-bound HY compared to unbound HY recapitulates the measured decrease in HY TME titer upon binding to SCL soil, and, therefore, (iii) similar differences in PMCA efficiency observed for other soil minerals (clay and organic matter) suggest similar decreases in infectious titer.
The results of this study suggest that soil-bound prion replication, and thereby the potential for prion transmission, may vary with soil type and therefore influence the ecology of prion disease. Our results predict that PrPSc bound to clay soils as well as soils with high organic contents exhibits a reduced ability to initiate PrPc conversion, while PrPSc bound to sand surfaces exhibits no such reduction. Conversely, previous studies have observed that soil adsorption, which may protect PrPSc from environmental degradation or transport, is known to be greater for clay and organic surfaces and lower for sand and sandy soils (21, 33). Thus, the overall balance between prion adsorption affinity and replication efficiency for the dominant soil types of an area may be a key determinant in the risk of CWD or scrapie transmission. In this study and others, the effect, if any, of the relatively small changes in titer upon binding to soil has yet to be established. The results presented here, coupled with our previous findings (32–35), provide further evidence that the exact circumstances of prion entry into the soil environment, including N-terminal truncation of PrPSc, the time of PrPSc and soil contact, the biological matrix containing PrPSc, the soil-to-PrPSc ratio, and the soil type can influence prion fate and transmission.
ACKNOWLEDGMENTS
We thank Jacob Ayers, Maria Christensen, and Qi Yuan for excellent technical assistance, Glenn Telling for the Tg(CerPrP)1536+/− mouse brains, Ken Clinkenbeard for the CWD-elk brain, and Robert Bulman for the humic acid-coated silica gel particles. We thank Michael Beard at Beckon-Dickenson, Columbus, NE, for gamma irradiation of soils.
This research was supported in part by the UNL Research Council, UNL Othmer and Milton Mohr Fellowships, a Creighton University New Initiatives Award, and the National Center for Research Resources (P20 RR0115635-6 and C06 RR17417-01).
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Andreoletti O., et al. 2002. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J. Gen. Virol. 83:2607–2616 [DOI] [PubMed] [Google Scholar]
- 2. Angers R. C., et al. 2009. Chronic wasting disease prions in elk antler velvet. Emerg. Infect. Dis. 15:696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arthur W. J., Alldredge A. W. 1979. Soil ingestion by mule deer in north central Colorado. J. Range Manage. 32:67–71 [Google Scholar]
- 4. Bartz J. C., et al. 2007. Prion interference is due to a reduction in strain-specific PrPSc levels. J. Virol. 81:689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bessen R. A., Marsh R. F. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 68:7859–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bessen R. A., Martinka S., Kelly J., Gonzalez D. 2009. Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. J. Virol. 83:6435–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beyer W. N., Connor E. E., Gerould S. 1994. Estimates of soil ingestion by wildlife. J. Wildlife Manage. 58:375–382 [Google Scholar]
- 8. Bolton D. C., McKinley M. P., Prusiner S. B. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309–1311 [DOI] [PubMed] [Google Scholar]
- 9. Bolton D. C., McKinley M. P., Prusiner S. B. 1984. Molecular characteristics of the major scrapie prion protein. Biochemistry 23:5898–5906 [DOI] [PubMed] [Google Scholar]
- 10. Brown P., Gajdusek D. C. 1991. Survival of scrapie virus after 3 years' interment. Lancet 337:269–270 [DOI] [PubMed] [Google Scholar]
- 11. Browning S. R., et al. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 78:13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castilla J., Saa P., Hetz C., Soto C. 2005. In vitro generation of infectious scrapie prions. Cell 121:195–206 [DOI] [PubMed] [Google Scholar]
- 13. Chen B., Morales R., Barria M. A., Soto C. 2010. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat. Methods 7:519–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deleault N. R., Harris B. T., Rees J. R., Supattapone S. 2007. Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. U. S. A. 104:9741–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dickinson A. G., Fraser H. 1969. Modification of the pathogenesis of scrapie in mice by treatment of the agent. Nature 222:892–893 [DOI] [PubMed] [Google Scholar]
- 16. Haley N. J., Mathiason C. K., Zabel M. D., Telling G. C., Hoover E. A. 2009. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 4:e7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haley N. J., Seelig D. M., Zabel M. D., Telling G. C., Hoover E. A. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamir A. N., et al. 2008. Experimental transmission of scrapie agent to susceptible sheep by intralingual or intracerebral inoculation. Can. J. Vet. Res. 72:63–67 [PMC free article] [PubMed] [Google Scholar]
- 19. Haybaeck J., et al. 2011. Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathog. 7:e1001257. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Johnson C. J., Pedersen J. A., Chappell R. J., McKenzie D., Aiken J. M. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 3:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson C. J., et al. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kincaid A. E., Bartz J. C. 2007. The nasal cavity is a route for prion infection in hamsters. J. Virol. 81:4482–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maddison B. C., et al. 2010. Environmental sources of scrapie prions. J. Virol. 84:11560–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathiason C. K., et al. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathiason C. K., et al. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136 [DOI] [PubMed] [Google Scholar]
- 26. Mould D. L., Dawson A. M. 1970. The response in mice to heat treated scrapie agent. J. Comp. Pathol. 80:595–600 [DOI] [PubMed] [Google Scholar]
- 27. Pan K. M., et al. 1993. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. U. S. A. 90:10962–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prusiner S. B. 1991. Molecular biology of prion diseases. Science 252:1515–1522 [DOI] [PubMed] [Google Scholar]
- 29. Prusiner S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144 [DOI] [PubMed] [Google Scholar]
- 30. Reed L. J., Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27:493–497 [Google Scholar]
- 31. Saunders S. E., Bartelt-Hunt S. L., Bartz J. C. 2008. Prions in the environment: occurrence, fate and mitigation. Prion 2:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saunders S. E., Bartz J. C., Bartelt-Hunt S. L. 2009. Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ. Sci. Technol. 43:5242–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saunders S. E., Bartz J. C., Bartelt-Hunt S. L. 2009. Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ. Sci. Technol. 43:7728–7733 [DOI] [PubMed] [Google Scholar]
- 34. Saunders S. E., Bartz J. C., Telling G. C., Bartelt-Hunt S. L. 2008. Environmentally-relevant forms of the prion protein. Environ. Sci. Technol. 42:6573–6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saunders S. E., Bartz J. C., Vercauteren K. C., Bartelt-Hunt S. L. 2010. Enzymatic digestion of chronic wasting disease prions bound to soil. Environ. Sci. Technol. 44:4129–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shikiya R. A., Ayers J. I., Schutt C. R., Kincaid A. E., Bartz J. C. 2010. Coinfecting prion strains compete for a limiting cellular resource. J. Virol. 84:5706–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sigurdson C. J., et al. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 80:2757–2764 [DOI] [PubMed] [Google Scholar]
- 38. Silveira J. R., et al. 2005. The most infectious prion protein particles. Nature 437:257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Somerville R. A., Carp R. I. 1983. Altered scrapie infectivity estimates by titration and incubation period in the presence of detergents. J. Gen. Virol. 64:2045–2050 [DOI] [PubMed] [Google Scholar]
- 40. Tamguney G., et al. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor D. M. 2000. Inactivation of transmissible degenerative encephalopathy agents: a review. Vet. J. 159:10–17 [DOI] [PubMed] [Google Scholar]
- 42. Taylor D. M., Fernie K. 1996. Exposure to autoclaving or sodium hydroxide extends the dose-response curve of the 263K strain of scrapie agent in hamsters. J. Gen. Virol. 77:811–813 [DOI] [PubMed] [Google Scholar]
- 43. Wang F., Wang X., Yuan C. G., Ma J. 2010. Generating a prion with bacterially expressed recombinant prion protein. Science 327:1132–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]