Abstract
Development of a microbicide that prevents rectal transmission of human immunodeficiency virus (HIV) is a vital component in reducing HIV spread. We recently demonstrated that a formulation of the nonnucleoside reverse transcriptase inhibitor (NNRTI) MIV-150 in carrageenan reduced vaginal infection of macaques with simian immunodeficiency virus SIVmac239 with HIV-1HxB2 reverse transcriptase (SHIV-RT). Herein, we performed the first testing of MIV-150–carrageenan against rectal infection. Rhesus macaques were treated rectally with MIV-150–carrageenan or methyl cellulose (MC) placebo gel up to 4 h prior to rectal challenge with 103 or 104 50% tissue culture infective doses (TCID50) of SHIV-RT. Infection was assessed by measuring plasma virus RNA as well as T and B cell responses. MIV-150–carrageenan protected all animals challenged with 103 TCID50 when gel was applied either 30 min or 4 h prior to challenge, while 100% of the MC-treated animals became infected (n = 4 each; P < 0.03). Partial protection (2 of 4 animals) by MIV-150–carrageenan was observed for rectal challenge with 10-fold more virus applied 4 h after the gel. Sequencing of the RT gene from plasma virus RNA isolated at peak viremia confirmed that both of these animals (like infected MC controls) were infected with wild-type virus. Infection correlated with the development of SIV-specific T and B cell responses. MIV-150 was detected in the rectal fluids and tissues 4 h after gel application but was not detected in the blood at any time (0.5 to 24 h). These data are promising for the development of NNRTI-containing gels to prevent rectal HIV transmission.
INTRODUCTION
The predominant route of human immunodeficiency virus (HIV) transmission is across the genital and rectal epithelia during sexual intercourse (52). Thus, strategies that block mucosal transmission of the virus are urgently needed. Microbicides are substances which are applied topically to the cervicovaginal or rectal mucosa and are designed to block or significantly reduce transmission of HIV and/or other sexually transmitted infections (29). The recent success of the CAPRISA 004 trial (22), which demonstrated that a coitally dependent vaginal microbicide containing an anti-HIV drug can significantly reduce HIV transmission, indicates the potential impact of this approach on the epidemic worldwide. Although these clinical findings are a breakthrough for vaginal microbicides, the differences between the microenvironments of the rectal and cervicovaginal mucosal tissue may require that different formulations be used for the two routes (44). It is possible that formulations that are not effective vaginally may actually be useful as rectal microbicides while others that block vaginal transmission may be inert or even enhance rectal transmission. Furthermore, rectal transmission of HIV is more efficient than vaginal transmission (44), so testing new formulations rectally is crucial and may be considered a more rigorous test of efficacy.
A microbicide must be safe and acceptable for repeated use in men and women. While Carraguard, a 3% carrageenan gel, was found not to be effective in significantly reducing the number of HIV infections in the treatment arm compared to placebo in a phase 3 clinical trial (45), it was shown to be safe and acceptable by the women who used it in the trial (45) as well as by participants in other studies (27, 28, 33, 35, 41, 54). Carrageenan-based gels also have favorable rheological properties (12, 50), rendering them promising candidates for delivering potent antiviral agents.
Using carrageenan as the delivery vehicle, we are developing novel formulations that include the nonnucleoside reverse transcriptase inhibitor (NNRTI) MIV-150. MIV-150 was developed by Medivir AB (Huddinge, Sweden) as an oral antiretroviral drug but was discontinued because of poor systemic absorption and rapid systemic clearance (12). Although these properties rendered MIV-150 a poor candidate for oral therapy, they are not problematic for a topical microbicide that is applied at the site of virus exposure and may need only to be absorbed locally to be effective. MIV-150 is a tight-binding antagonist of reverse transcriptase (RT) and is active against isolates that contain wild-type HIV-1 RT, including the chimeric virus SHIV-RT, which is derived from simian immunodeficiency virus SIVmac239 with the RT of HIV-1HxB2 (50, 51). Preliminary evidence shows that MIV-150 also has activity against HIV-2 (12). In vitro resistance to MIV-150 is associated with the appearance of 3 mutations in RT, while resistance to the NNRTIs nevirapine and efavirenz is detected after only 1 and 2 mutations, respectively (unpublished data). Nevirapine-resistant viruses are still fully sensitive to MIV-150 (unpublished data), which is particularly important given the increase in nevirapine resistance (11) that followed its widespread use for treatment of HIV infection and for prevention of mother-to-child transmission (18).
We have previously demonstrated that MIV-150 possesses strong antiviral and potentially virucidal properties, which are not affected by the presence of seminal fluid, and blocks transmission of both R5- and X4-tropic HIV isolates at nanomolar concentrations in vitro (12, 50). In our first tests of MIV-150–carrageenan gels, we showed that 500 μM MIV-150 in carrageenan was 10 times more active against clinical HIV isolates in vitro than carrageenan alone (12). In vivo, MIV-150-containing gels reduced vaginal SHIV-RT infection by more than 50% when macaques were challenged 4 h after a single gel dose or 8 h after daily gel application for 2 weeks (26).
In the present study, we carried out the first evaluation of the efficacy of a MIV-150–carrageenan gel against rectal SHIV-RT transmission. To test the potency and durability of protection by this microbicide candidate against the rectal route of exposure, we examined its ability to prevent transmission of virus introduced rectally either 30 min or 4 h after application of the gel, challenging with 103 or 104 50% tissue culture infective doses (TCID50) of SHIV-RT.
MATERIALS AND METHODS
Animals, treatments, and virus challenges.
Adult Chinese rhesus macaques (Macaca mulatta) were housed at the Tulane National Primate Research Center (TNPRC; Covington, LA) for these studies. All studies and the use of macaques were approved by the Animal Care and Use Committee of the TNPRC (OLAW assurance A4499-01), which has received continued full accreditation by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC 000594). Animal care procedures were in compliance with the regulations detailed in the Animal Welfare Act (2) and the Guide for the Care and Use of Laboratory Animals (7). The TNPRC Division of Veterinary Medicine has established procedures to minimize pain and distress through several means, in particular anesthesia prior to and during all procedures (8 mg/kg of body weight of tiletimine/zolazepam [CDR2] before treatments and biopsies and 10 mg/kg ketamine-HCl for blood draws). Postprocedural analgesia with buprenorphine (0.01 mg/kg) was administered following biopsies. The animals' average age at the beginning of the study was 8.09 years, and their average weight was 7.41 kg.
All animals tested negative for simian type D retroviruses and simian T cell leukemia virus 1 prior to the start of the study. Most of the animals challenged for product efficacy had previously been enrolled in other microbicide studies in which they were exposed to immunodeficiency viruses. However, they were all confirmed negative for SIV and had no evidence of SIV-specific antibody or T cell responses in the periphery at the start of the current study. Animals used for pharmacokinetics (PK) measurements were healthy SHIV-RT-infected macaques also recycled from previous microbicide studies. A complete detailing of the efficacy animals' prior virus exposures is provided here. The only naïve animal was DV78. CF57 was previously exposed once rectally to 3 × 103 TCID50 of SIVmac239 (53). All of the other animals were previously exposed vaginally to SHIV-RT as follows. GJ44, GK05, and GF14 were each exposed once, to 103 (26), 103 (9, 26, 50), and 104 (50) TCID50, respectively. GJ29 was exposed twice, first to 200 and then to 103 TCID50 (9, 26, 50). FI79 and FI84 were each exposed 3 times, the first time to 103 TCID50 and the second and third times to 104 TCID50 (50). GJ34 and GJ79 were both exposed 5 times: to 250, 103, 200, 103, and 103 TCID50 (26, 50). Viral RNA was detected in the plasma samples of two animals, FI79 and FI84, following their third exposure. However, viral load was low in both animals (below 103 RNA copies/ml) and became undetectable after 10 weeks postinfection, remaining below the limit of detection even after CD8 depletion a year later. No SIV-specific antibody or T cell responses were ever mounted in the periphery in these animals. As a part of these earlier studies, many of the animals were also exposed vaginally to MIV-150 containing gels, but at least 1.5 years had passed between their last vaginal exposure to MIV-150 and their first rectal exposure in the current study. A complete list of the animals is provided in Table 1.
Table 1.
Infection and immune parameters for macaques challenged with SHIV-RT
| Virus dose (no. of TCID50) | Gel | Time of application | Animalc | No. of previous exposures to virusa | Infection status | Ab response | IFN-γ status | SHIV-RT infection in vitrod | No. of CD4 cells/μl |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Preinfection | Postinfectionb | |||||||||
| 103 | MC | 30 min | GJ44 | 1 | + | + | + | ND | 1,179 | 1,246 |
| DV78 | 0 | + | + | + | ND | 676 | 381 | |||
| CF57 | 1 | + | + | + | ND | 694 | 751 | |||
| 4 h | GF142 | + | + | + | ND | 654 | 350 | |||
| MIV-150–carrageenan | 30 min | FI791 | 3 | − | − | − | + | 1,103 | 1,262 | |
| GF141 | 1 | − | − | − | + | 784 | 668 | |||
| FI841 | 3 | − | − | − | + | 2,605 | 1,913 | |||
| GK051 | 1 | − | − | − | + | 743 | 521 | |||
| 4 h | GK052 | − | − | − | ND | 500 | 307 | |||
| GJ291 | 2 | − | − | − | ND | 573 | 577 | |||
| FI792 | − | − | − | ND | 1,069 | 759 | ||||
| FI842 | − | − | − | ND | 1,362 | 1,569 | ||||
| 104 | MC | 4 h | FI793 | + | + | + | ND | 1,531 | 789 | |
| GK053 | + | + | + | ND | 466 | 231 | ||||
| MIV-150–carrageenan | GJ292 | − | − | − | + | 990 | 1,141 | |||
| GJ79 | 5 | − | − | − | + | 1,082 | 1,200 | |||
| GJ34 | 5 | + | + | + | ND | 937 | 1,054 | |||
| FI843 | + | + | + | ND | 1,997 | 1,688 | ||||
Previous exposures to virus prior to original enrollment in the current study.
CD4 counts taken on day 112 for GJ44, FI79, GF14, FI84, GK05, DV78, and CF57 and on day 84 for GF14, GK05, GJ29, FI79, and FI84.
Subscript numbers indicate animals that were recycled within the study, with 1, 2, and 3 indicating the challenge that the samples were taken from.
“ND” indicates animals that became infected after challenge, so the in vitro infection was not done.
For efficacy studies, 50 μM MIV-150 in 3% carrageenan (MIV-150–carrageenan) versus the methyl cellulose (MC) placebo gels were applied atraumatically to the rectal epithelium. In the study evaluating protection against 103 TCID50, two gel timings prior to challenge were compared, 30 min (5 ml of either) and 4 h (5 ml of MC versus 3 ml of MIV-150–carrageenan). In the study evaluating protection against 104 TCID50, 3 ml of either gel was applied 4 h prior to challenge. Animals in all efficacy studies were challenged with SHIV-RT (51) in 1 ml of RPMI medium (RPMI 1640, Cellgro; Fisher Scientific, Springfield, NJ) as indicated and under conditions previously described (9). In PK studies, gels were applied rectally exactly as in efficacy studies. Either 4 or 24 h after gel application, rectal swabs were collected, followed by rectal pinch biopsy specimens. Blood samples were collected at 0.5 h, 1 h, 4 h, and 24 h after gel application. Mucosal and blood samples were collected and transported to the laboratory as previously described (9).
Microbicide preparation.
MIV-150–carrageenan (lot numbers 080320A815, 080807A815, 100126A815MR, and 110113A815) comprised 3% (wt/wt) carrageenan, 50 μM MIV-150 (Medivir AB, Sweden), and 1% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS; pH 6.8) (12). MC placebo gel (lot numbers 031908, 080804A2005, 100127A2005MR, and 110110A2005) comprised 2.5% (wt/wt) MC (Fisher, Fair Lawn, NJ) in PBS (pH 6.8) (12, 50). Gels were stored at room temperature and used within 45 days of preparation. The gels were buffered to a pH of 6.8 ± 0.2, and the viscosity and anti-HIV activity of each gel were verified for each lot prior to in vivo use. MIV-150 was developed by Medivir AB and licensed to the Population Council for development as a microbicide.
Virus stock.
The original stock of SHIV-RT was provided by Disa Böttiger, Medivir AB (50). The virus stock for the 103-TCID50 challenge study was grown from the original in human peripheral blood mononuclear cells (PBMCs) activated by 3 days of culture with 5 μg/ml phytohemagglutinin (PHA; Sigma, St. Louis, MO), followed by 3 days of culture with 50 U/ml interleukin 2 (IL-2; Roche, Mannheim, Germany). A subsequent stock was similarly generated and was used for the 104-TCID50 challenge study. The titers of all stocks were determined before in vivo use in the 174xCEM cell line (NIH AIDS Research & Reference Reagent Program), and TCID50 values were calculated using the Reed and Muench formula. Both stocks have been shown to infect with comparable frequencies via the vaginal route in vivo (26).
Cell isolation and sample collection.
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood using Ficoll-Hypaque density gradient centrifugation (GE Healthcare Biosciences, Uppsala, Sweden) as previously described (50). Rectal swabs were collected and processed by the same method previously described for vaginal swabs (9). Rectal pinch biopsy specimens (20 3- by 3-mm biopsy specimens per animal from 6 animals; average total weight of 107 mg for tissues from each animal) were maintained on ice during overnight shipment and were processed immediately upon arrival for a MIV-150 radioimmunoassay (RIA).
In vitro infection.
Freshly isolated PBMCs were resuspended at 106 cells/ml in R10 medium (RPMI 1640) (Cellgro) with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Cellgro), 10 mM HEPES (Gibco-BRL, Life Technologies, Grand Island, NY), 2 mM l-glutamine (Gibco-BRL), and penicillin (100 U/ml)-streptomycin (100 μg/ml) (Gibco-BRL). Following 6 days of activation with phytohemagglutinin (PHA) and IL-2 exactly as described above for expanding the virus stock, the cells were infected with 400 TCID50 of SHIV-RT per 2 × 105 cells/well in 96-well flat-bottomed plates (Falcon, BD, Franklin Lakes, NJ) in 0.1 ml of R10 with 50 U/ml IL-2. Virus production was monitored by testing the SIV p27 gag content of the supernatant by an enzyme-linked immunosorbent assay (ELISA) on days 4, 7, 11, and 14 postinfection in accordance with the protocol provided with the SIV p27 antigen ELISA (ZeptoMetrix, NY).
Virus detection by quantitative PCR.
SIV RNA in plasma separated from whole blood was quantified by centrifugation and stored as previously described (50). Viral RNA copies were measured by quantitative reverse transcription-PCR (RT-PCR) for SIV gag in the plasma samples (6, 31). Quantification of SIV DNA was carried out with minor modifications to the previously published method (14): Genomic DNA was extracted from 5 × 106 dry frozen PBMC pellets by proteinase K digestion in lysis buffer. The primers and probes for SIV were those previously described, while the primers for macaque albumin were AlbF (5′-ATTTTCAGCTTCGCGTCTTTTG-3′) and AlbR (5′-TTCTCGCTTACTGGCGTTTTCT-3′) used with the probe AlbP (6-carboxyfluorescein [FAM]– CCTGTTCTTTAGCTGTCCGTGGTCCTGA–6-carboxytetramethylrhodamine [TAMRA]). Primers and probes were designed using Primer Express software (Applied Biosystems, Foster City, CA) and manufactured by IDT (Coralville, IA).
Measurement of T and B cell responses.
The numbers of gamma interferon (IFN-γ) spot-forming cells (SFCs) in PBMCs were measured by an enzyme-linked immunospot (ELISPOT) assay as previously described (13, 31, 50). Plasma samples were monitored for the presence of SIV-specific antibodies (Abs) using an established ELISA protocol (46).
Cytokine and chemokine analysis.
Cell-free tissue culture supernatants were analyzed for the presence of cytokines and chemokines. Human extracellular protein buffer regent kits (Biosource) and custom multiplex antibody bead kits were used (monkey 14-plex [IL-1β, IL-10, IL-6, CCL5, CCL3, granulocyte-macrophage colony-stimulating factor [GM-CSF], CCL4, CCL2, IFN-γ, tumor necrosis factor alpha {TNF-α}, IL-3, IL-2, IL-4, and CXCL8]) (Invitrogen) with a Luminex 200 instrument (Luminex, Austin, TX). The analysis of the data was performed using STarStation software, version 2.0 (Applied Cytometry, Sacramento, CA).
Detection of MIV-150 by RIA.
Plasma was subjected to extraction of MIV-150 as previously described (26). Rectal swabs and tissue samples were processed and MIV-150 was extracted by the same method used for vaginal swabs and tissues (26). RIA of MIV-150 was an indirect-extraction-based assay adapted from Kumar et al. (26, 30) optimized and validated separately for detection of MIV-150 in macaque plasma, rectal swabs, and rectal tissue (limits of quantitation, 2.7 nM for fluids and 0.01 ng/mg for tissue). MIV-150 concentration in the samples was calculated by interpolation with the standard curve using a curve-fitting procedure (logistic 4-parameter model).
RNA isolation and RT gene sequencing.
The HIV-1 RT gene of SHIV-RT was sequenced from plasma virus RNA using the recently described method (26).
Statistical analysis.
The Fisher exact test was used for statistical comparison of the percentage of SHIV-RT-infected animals in the different groups (GraphPad Prism version 5/02 for Windows; GraphPad) (1).
RESULTS
MIV-150–carrageenan protects against rectal SHIV-RT.
In this study, we evaluated the efficacy of a MIV-150–carrageenan gel against rectal simian/human immunodeficiency virus (SHIV) challenge. SHIV-RT was selected as the challenge virus since it expresses the RT of HIV-1 (the target of MIV-150) in the context of SIV and was shown to be sensitive to MIV-150 (50). Because this was a pilot study, most of the available animals were recycled from previous microbicide studies in which they had been exposed to immunodeficiency viruses (see Materials and Methods). However, prior to the start of this study, all of the animals tested negative for SIV plasma virus RNA and had no SIV-specific antibody or T cell responses in the periphery. Within the current study, animals that did not become infected following their first challenge (verified by negative plasma viremia and lack of systemic adaptive immune responses to SIV) were reenrolled in new groups and rechallenged. Subscripts next to the animal identification numbers in Table 1 and Fig. 1 and 2 are used to designate which challenge in the study is referenced. Animals that were rechallenged were split up into new groups based on their prior MIV-150 exposure status.
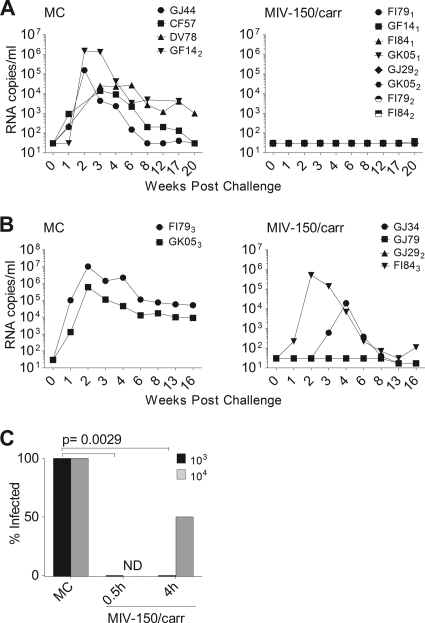
Fig. 1.
MIV-150–carrageenan (50 μM) applied up to 4 h prior to challenge protects against rectal SHIV-RT infection. MC or MIV-150–carrageenan (MIV-150/carr) gel was applied to the rectum before challenge with 103 (A) or 104 (B) TCID50 of SHIV-RT. Plasma viral loads are shown as numbers of RNA copies/ml over time for MC control animals (A, n = 4; B, n = 2) in the left panels and for animals that received MIV-150–carrageenan (A, 30 min [n = 4] and 4 h [n = 4]; B, 4 h [n = 4]) in the right panels. (C) Percent infection in each gel group is shown for each challenge. MIV-150–carrageenan-mediated protection was significant at both 30 min and 4 h in the 103-TCID50 challenge groups. ND, not done.
We first evaluated protection against 103 TCID50 of SHIV-RT, which we previously demonstrated infects about 50% of Depo-Provera-treated naïve animals and 100% of herpes simplex virus 2 (HSV-2)-infected animals upon vaginal challenge (9, 50). MIV-150–carrageenan or MC control gels were applied atraumatically to the animals' rectal epithelia, followed by challenge either 30 min or 4 h later. The treatment details for each animal are provided in Table 1. Plasma virus RNA testing revealed that all MC-treated animals became infected with SHIV-RT (4 of 4 animals infected) and experienced typical viremia, with the peak occurring around 2 weeks postinfection (Fig. 1A, left panel). Due to unavailability of samples from 2 of these animals, DV78 and GF14, we cannot be sure that their viremia peaked at 2 weeks postinfection (Fig. 1A). However, their plasma viral loads at weeks 3 and 4 were similar to those of the other animals that were decreasing after peaking at 2 weeks. Viral loads decreased to an average set point of 1,483 copies/ml by week 12 postinfection (ranging from 30 to 4,500 copies/ml) that was maintained for the duration of the study. In contrast, all animals receiving MIV-150–carrageenan 30 min or 4 h prior to challenge remained virus free throughout the observation period (0/4 animals infected in each group; 0/8 animals infected total) (Fig. 1A, right panel).
To determine a level at which the gel no longer protected, we evaluated whether MIV-150–carrageenan could prevent infection with a 10-fold-higher dose of SHIV-RT. Animals were treated with MIV-150–carrageenan (n = 4) or MC (n = 2) and then rectally challenged 4 h later with 104 TCID50 of SHIV-RT (Table 1). As expected, both MC control animals became infected after challenge with the higher inoculum, with peak viremia (average, 5.3 × 106 copies/ml) occurring at 2 weeks postinfection and resolving to an average set point of 3.4 × 104 copies/ml by week 13 (Fig. 1B, left panel). MIV-150–carrageenan still afforded 50% protection against the higher challenge dose, and one of the 2 infected animals, GJ34, showed markedly reduced and delayed peak viremia compared to the controls (1.9 × 104 copies/ml at week 4) (Fig. 1B, right panel). Although the MIV-150–carrageenan gel reduced infection by 50% even when animals were challenged with 104 TCID50, we could not test if this was significant due to the small number of monkeys in each group. However, protection against the 103 TCID50 challenge was statistically significant (Fig. 1C).
Because the animals used for these studies had been challenged in previous studies and also recycled and rechallenged within the current study (Table 1), we evaluated the possibility that animals remaining uninfected after multiple challenges might be inherently resistant to SHIV-RT infection. To address this, we tested the susceptibility of PBMCs from these animals to SHIV-RT infection in vitro. Activated PBMCs were inoculated with the same virus stock as that used for in vivo challenge, and culture supernatants were sampled periodically for 2 weeks to determine the p27 content. PBMCs from all of the animals that did not become infected in vivo became infected with SHIV-RT in vitro (Table 1), indicating that the MIV-150–carrageenan gel, and not a natural resistance to the virus, was responsible for the in vivo protection.
Impact of MIV-150–carrageenan on innate and adaptive immunity and the rectal environment.
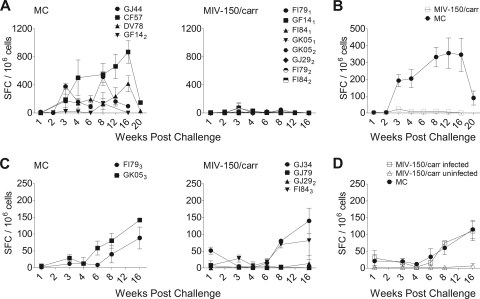
Adaptive immune responses were measured throughout the 16-week observation period. SIV-specific T cell responses in PBMCs were measured by the production of IFN-γ in response to AT-2 SIV stimulation, which has been shown to reliably detect SIV-specific T cells (13, 50). As expected, all animals developed SIV-specific antibodies and T cell responses following infection (Table 1 and Fig. 2). FI79 and FI84 had both displayed transient low-level SIV RNA in the plasma in a prior study (50) following SHIV-RT challenge but subsequently controlled viremia below the limit of detection even after CD8 depletion and never seroconverted or mounted an ELISPOT assay response. In this study, both animals seroconverted and developed ELISPOT assay responses following infection. Animals with stronger ELIPSOT assay responses tended to have lower viral loads overall, regardless of the challenge dose (Fig. 1 and 2). Animals infected with the 103-TCID50 inoculum (Fig. 2A and B) exhibited earlier and higher-level T cell responses than animals infected with 104 TCID50 (Fig. 2C and D). T cell responses in animals that became infected following exposure to MIV-150–carrageenan gel were indistinguishable from the responses in infected animals treated with MC (Fig. 2D). GJ34, which experienced attenuated viremia, had a T cell response at 1 week postinfection (Fig. 2C, right panel).
Fig. 2.
SIV-specific T cell responses are detected in infected but not in uninfected animals. SIV-specific T cell responses in PBMCs over time were measured by IFN-γ ELISPOT and expressed as numbers of SIV-specific spot-forming cells (SFC) per 106 cells (mean ± SEM). (A) The responses elicited in animals challenged with 103 TCID50 of SHIV-RT are shown. (B) Mean ± SEM responses mounted in infected animals challenged with 103 TCID50 of SHIV-RT. Data from animals receiving the MIV-150–carrageenan gel at either 30 min or 4 h prior to challenge are pooled (since they are all negative). (C) SIV-specific SFC elicited in animals challenged with 104 TCID50 of SHIV-RT are shown for the MC- versus the MIV-150–carrageenan-treated animals. (D) Mean ± SEM responses in infected versus uninfected animals challenged with 104 TCID50 of SHIV-RT.
We examined whether or not MIV-150–carrageenan elicited local innate responses that might contribute to its activity in a separate group of animals, as we have done previously following vaginal application of the gel (26). Rectal swabs were collected at 4 h, 24 h, or 1 week after administration of the gel. At the 4-h time point, when MIV-150–carrageenan completely blocked infection in challenged animals, the swabs were too viscous from residual gel for cytokines and chemokines to be looked at by the Luminex multiplex bead assay. Swabs from both the 24-h and the 1-week time points showed no difference in cytokines and chemokines compared to the pretreatment samples (data not shown; n = 6 per group).
There were no significant changes in rectal pH 4 h or 24 h after treatment with MIV-150–carrageenan or MC relative to the level for pretreatment samples (average baselines, 7.50 ± 0.27 versus 7.50 ± 0.58 at 4 h and 8.50 ± 0.50 at 24 h for MC-treated animals and 7.31 ± 0.35 versus 7.88 ± 0.18 at 4 h and 7.88 ± 0.52 at 24 h for MIV-150–carrageenan-treated animals).
Local but not systemic absorption of MIV-150 and lack of selection of drug-resistant viruses after rectal MIV-150–carrageenan treatment.
Since MIV-150–carrageenan effectively prevented rectal SHIV-RT infection in the absence of innate immune responses, we were interested to determine if MIV-150 was absorbed into the tissues and/or bloodstream at the time of challenge and whether this correlated with protection. Blood, rectal swab fluid, and rectal tissue samples were collected before and 4 h after rectal application of MIV-150–carrageenan in an additional group of animals (n = 6) for PK measurements. MIV-150 was undetectable in the plasma samples of all animals by RIA at the time points tested (0.5 to 24 h; data not shown). However, MIV-150 was detected both in the rectal swab fluid samples and associated with the tissue samples of most of the animals at 4 h after gel application (Fig. 3). Drug was detected in the rectal tissue samples of 5 of the 6 animals, and in 4 of these, drug was also found in the fluid samples. In only one animal was there no MIV-150 detected at all (Fig. 3, circles). The amounts of MIV-150 both in the swab fluid samples and associated with the tissue samples differed substantially between animals (ranges, 8.91 nM to 1,275.62 nM for swab samples and 0.02 ng/mg to 0.16 ng/mg for tissue-associated drug), but animals with more drug in the fluid also tended to have more drug associated with the tissue, with the exception of one animal with a high level of tissue-associated drug but nothing in the swab (Fig. 3, diamonds). This suggests that MIV-150 was retained in the recta of some animals better than others, and retention was generally associated with local absorption.
Fig. 3.
MIV-150 is detected locally but not systemically after single application. Animals were treated with a 3-ml single application of 50 μM MIV-150–carrageenan rectally. Rectal fluids and tissue biopsy specimens were collected 4 h later, and the levels of MIV-150 were measured by RIA. Individual symbols mark each animal. The horizontal line denotes the mean.
The two infections that occurred in the presence of MIV-150–carrageenan gel (animals challenged with 104 TCID50) could reflect the selection of drug-resistant variants or failure of the drug to prevent wild-type virus infection across the rectal mucosa. To verify that the MIV-150–carrageenan-treated animals that became infected were not carrying viral variants encoding drug resistance mutations in RT, we isolated virus RNA from the plasma at peak viremia and sequenced the RT gene. In all of the clones tested, there were no amino acid changes at positions that confer resistance to NNRTIs (21, 32, 36) (see Table S1 in the supplemental material). Therefore, the microbicide was less protective against a higher-level challenge but not by inducing drug resistance.
DISCUSSION
Microbicide research has focused largely on the development of products for vaginal use, as HIV acquisition occurs predominantly through heterosexual intercourse (52). For the first time, a coitally dependent vaginal microbicide gel has been shown to exert significant protection from HIV acquisition (22). Nonetheless, anal intercourse occurs in both homosexual and heterosexual couples (3, 17, 43), and topical microbicides that are developed for vaginal use are likely to be used rectally. It is important to verify that protection against this more efficient route of transmission will be maintained without causing inflammation or damage to the epithelium.
Carrageenan gel is an appealing delivery vehicle for rectal microbicides because it has been shown to be safe and acceptable for vaginal use in humans (10, 27, 28, 33, 35, 45, 54), safe when applied to the rectal epithelia of mice (49), and both safe and acceptable rectally in a small human trial (41). Carrageenan also has favorable rheological properties for delivering antiretroviral drugs (12, 50). We have focused on developing carrageenan-based gels that contain μM doses of the potent NNRTI MIV-150, with the aim of using the lowest drug concentration possible to achieve maximal protection. In a recent study, a single dose of a MIV-150–carrageenan gel containing 500 μM (185 μg/ml) MIV-150 provided partial protection when applied 4 h prior to vaginal challenge with 103 TCID50 of SHIV-RT (1 of 7 animals infected [14%] versus 9 of 16 animals infected [56%] in the MC control group [26]). Repeated application (daily for 2 weeks) of a gel containing only 50 μM MIV-150 still afforded partial protection against vaginal infection with the same viral inoculum for up to 8 h (2 of 7 animals infected [28%] versus 9 of 14 animals infected [64%] in the carrageenan control group [26]). Strikingly, we demonstrate in this study that the lower dose 50 μM MIV-150–carrageenan gel completely prevented rectal infection with 103 TCID50 of SHIV-RT (0 of 8 animals infected) when gel was applied up to 4 h prior to challenge, in stark contrast to the 100% infection of control animals treated with MC (4 of 4 animals infected).
Because this was a pilot study, we did not include a carrageenan-alone control group, and we have not yet tested carrageenan rectally against SHIV-RT in macaques. Thus, we cannot rule out the possibility that carrageenan contributed to protection. When animals were challenged vaginally 30 min after gel application with either 103 or 104 TCID50 of SHIV-RT (50), carrageenan reduced infection compared to MC (1 of 4 animals infected [25%] compared to 6 of 13 animals infected [46%] at 103 TCID50; 0 of 7 animals infected [0%] compared to 5 of 6 animals infected [83.3%] at 104 TCID50). However, this barrier effect was no longer evident 8 h after repeated vaginal gel dosing (26) and so would be expected to contribute minimally 4 h following rectal administration. It is possible that carrageenan is more protective rectally than vaginally, but it seems unlikely that any barrier effect would be more pronounced in the open-ended anatomy of the rectum, particularly given the greater efficiency of rectal than of vaginal transmission. Comparative rheological studies have not yet been conducted to determine whether there are differences in carrageenan flow and spreading in the rectum versus the vagina. Future studies are planned to clarify whether/how carrageenan contributes to protection from rectal SHIV transmission.
We tested the protective effect of MIV-150–carrageenan gel against rectal transmission of SHIV-RT at two doses, 103 and 104 TCID50. These inocula contained >1.6 × 106 to 1.6 × 107 RNA copies, which is at least 100- to 1,000-fold greater than the median virus RNA levels in human semen (5, 19, 20, 40). Although the relevance of the single high-dose challenge model to human HIV exposure has been questioned (25, 34), infecting with a high inoculum highlights the stringency of the test model, pushing the limits of the microbicide to block infection. Importantly, viral kinetics and the development of immune responses are similar in animals infected following single high-level or repeated low-level rectal challenge with SIV (34). Intrarectal challenge with SIV doses in the range of our 103-TCID50 inoculum (6 × 105 to 6 × 106 RNA copies) has also been shown to mimic human mucosal HIV infection in that only a single or a very limited number of viral envelope variants are transmitted and establish infection (25, 48), while SIV doses upwards of our 104-TCID50 inoculum (6 × 107 RNA copies) can lead to the transmission of multiple variants (25).
Envelope variation in transmitted variants was beyond the scope of this study; however, we were interested in whether the presence of an NNRTI resulted in the transmission or emergence of viral variants resistant to the drug and whether this was affected by increasing the viral inoculum. All of the animals that became infected in this study were infected with viruses carrying the wild-type RT gene whether they were challenged with the low or the high dose of SHIV-RT. The virus stocks were grown for a limited number of passages in vitro, and sequencing confirmed that they were clonal and wild type in RT at the time of challenge (unpublished data) as expected (47, 51). Thus, it is not surprising that drug-resistant viruses were not transmitted. Nonetheless, drug resistance may emerge if an infected individual being treated with an antiretroviral drug-containing therapy uses a microbicide that contains a drug in the same class. Similarly, the use of a microbicide may increase the likelihood of transmitting drug-resistant variants that are already present in the donor semen. In a preexposure prophylaxis study, emtricitabine-associated resistance mutations were identified in 2 of 6 macaques treated orally with this nucleoside reverse transcriptase inhibitor (NRTI) prior to challenge with SHIV (16). Even though there was no effect of tenofovir on drug resistance in people who became infected with HIV in the CAPRISA 004 trial (22), tenofovir is used to treat HIV-infected people, posing a risk for the emergence and transmission of resistance. MIV-150 is not used to treat infection, and resistance in vitro takes longer to develop and requires more mutations than resistance to other NNRTIs currently in use for therapy and the prevention of mother-to-child transmission. Additionally, if MIV-150 is absorbed, it is rapidly cleared from the body. This reduces the likelihood that topical MIV-150 will select for viruses resistant to other NNRTIs. Studies are nonetheless ongoing to assess the in vivo emergence of drug-resistant viruses in SHIV-RT-infected animals that are treated chronically with MIV-150. An even better microbicide candidate would combine anti-HIV activities from multiple drug sources with different modes of action or from both drug and nondrug sources to further improve the resistance profile. Our studies on such formulations are ongoing.
In this study, MIV-150–carrageenan still provided 50% protection when the higher viral inoculum of 104 TCID50 was used, and 1 of the 2 animals with breakthrough infection, GJ34, had markedly attenuated viremia compared to the controls. GJ34 also had a PBMC ELISPOT response at week 1 postinfection, which is earlier than responses are normally detected. This animal had been challenged vaginally several times as part of previous microbicide studies in which it neither became infected nor developed detectable adaptive immune responses in the periphery (26, 50). Studies of highly exposed seronegative individuals have documented the presence of virus-specific CD8 T cell responses in the periphery and mucosa that may contribute to protection in persistently exposed individuals (23, 24, 42). It is possible that a previous low-level peripheral/mucosal immune response in GJ34 was boosted by infection across an inductive site, the rectal mucosa, and that this blunted viremia despite breakthrough infection. In two other animals, FI79 and FI84, previous repeated viral exposure and transient viremia did not impact on their infection or immune responses in this study. Both animals exhibited normal viremia, generated normal ELISPOT responses, and seroconverted. Blunted viremia in breakthrough infections was observed when macaques were administered preexposure prophylaxis consisting of a combination of the nucleoside reverse transcriptase inhibitors (NRTIs) tenofovir and emtricitabine prior to repeated low-dose (7.6 × 105-RNA-copy) rectal SHIV challenge (16). Significant but not complete protection was also achieved in a preclinical rectal study of the 1% (1-mg/ml) tenofovir gel that went on to be effective vaginally in the recent CAPRISA 004 clinical trial (8, 22). Tenofovir gel protected 89% of animals from normal viremia following rectal challenge with a single high dose of SIVmac251 2 h after gel application (6 of 9 macaques were completely protected, and 2 of 9 macaques developed highly attenuated viremia) (8). Interestingly, some of the protected animals treated with 1% tenofovir gel developed SIV-specific T cell responses (8, 39). While the development of adaptive immunity signals a low-level abortive infection with responses that may contribute to durable protection, activated T cells could also be targets for infection and might therefore ultimately undermine the gel's efficacy. Notably, the presence of considerably less drug in the MIV-150–carrageenan gel tested here than in the tenofovir gel coincided with complete protection for up to 4 h after gel application. It is thus possible that the 50 μM MIV-150–carrageenan gel would also perform well in humans.
Like other NNRTIs, MIV-150 blocks reverse transcription in cells which virus has entered but not yet established productive infection. However, some NNRTIs also possess virucidal properties in vitro. Efavirenz and UC781 can both inactivate free virus particles through their tight-binding mode of inhibition of the RT (4, 37). Thus, at higher challenge doses, more drug is required to block infection (37). MIV-150 similarly has been shown to inactivate free virus in vitro (12), although in vivo virucidal activity has not been confirmed. It is possible that at high viral doses in the context of a cell-free virus challenge, MIV-150 is soaked up by free virus, rendering the drug a limiting factor to block infection. Loss of protection at higher viral challenge doses is also consistent with a previous study of MIV-150–carrageenan gels from our group (50).
Although our studies, like other microbicide studies, aim to correlate antiviral drug levels in the fluids and tissues with protection (8, 15, 16, 38, 39), it is often not possible to collect the relevant samples without perturbing the environment and affecting infection, thereby compromising the efficacy study. Thus, we could measure MIV-150 levels only in the blood samples of challenged animals and had to use a separate set of identically treated animals to examine MIV-150 levels in rectal fluids and tissues. For rats, we have shown that (i) MIV-150 is absorbed after vaginal application, (ii) detection in the blood requires 10 to 100 times more MIV-150 than used herein, and (iii) MIV-150 has a very short plasma half life, becoming undetectable within 24 h (unpublished data). Not surprisingly, MIV-150 was not detected in the plasma after rectal application of 50 μM MIV-150–carrageenan formulation (even as early as 30 min after dosing), but it was detected in the rectal fluids and associated with the tissues (in 4 of the 6 and in 5 of the 6 animals treated, respectively) 4 h after the gel was administered (the time at which animals were challenged in the efficacy study). It is unclear from this study whether fluid- or tissue-associated drug is more predictive of protection since the PK measurements were not taken from challenged animals; however, the nM to low-μM fluid levels combined with the low-ng/mg tissue levels observed at 4 h after gel application were sufficient to completely protect animals from challenge with 103 TCID50 of SHIV-RT. Notably, the amount of MIV-150 per mg of rectal tissue at 4 h after gel application was smaller than that in either the vagina or the cervix at 8 h after gel application following repeated vaginal application of the same MIV-150–carrageenan formulation in a setting which resulted in a lower level of protection in efficacy studies (0.09 ± 0.08 ng/mg for rectum versus 0.41 ± 0.21 or 0.24 ± 0.21 ng/mg for vagina or cervix, respectively [26]). It is possible that lower concentrations of tissue-associated MIV-150 are required to block rectal infection than vaginal infection, although this would be unexpected due to the difference in transmission efficiency. Alternatively, MIV-150–carrageenan gel may be better absorbed across the rectal epithelium and/or may penetrate deeper into the rectal than vaginal tissue. Deeper biopsy specimens than those obtained under the survival surgeries performed for this study would be required to evaluate this. We are not aware of any prior reports of rectal topically applied antiretroviral drug containing microbicides that include rectal PK measurements. However, in PK studies of orally administered tenofovir or tenofovir with emtricitabine, the drugs were detected in plasma and PBMCs as well as in the rectal fluids and tissues of the treated animals up to 24 h after dosing when administered at a level that protected most of the animals in the efficacy study from rectal SHIV challenge (15, 16). Systemic and widespread tissue absorption of the drugs was associated with a lower level of protection than we observed with a regimen in which no systemic MIV-150 was found and fluid and tissue-associated levels were transient. This further highlights the potent antiviral activity of the formulation in the rectum despite its low uptake and retention.
Recent clinical trials of microbicides have raised concerns with adherence for coitally dependent vaginal gels (45). Importantly, most acts of anal intercourse already involve lubrication (17), suggesting that individuals engaging in anal intercourse (i) may be willing to choose a microbicide gel and (ii) may be more likely to use it with every sex act, which is critical for success of a microbicide for rectal use. Taken together, our data suggest that MIV-150–carrageenan-based gels are promising candidates for prevention of rectal HIV transmission.
Supplementary Material
ACKNOWLEDGMENTS
We thank the veterinary staff at the TNPRC for continued support as well as Julian Bess, William Bohn, Jeremy Miller, Terra Schaden-Ireland, Rodman Smith, and Elena Chertova at NCI—Frederick for generating and characterizing AT-2 SIV and microvesicle preparations. Special thanks are due to David Phillips and Robin Maguire for scientific discussions on the concept and design of the gels.
This work was supported by the Swedish Ministry for Foreign Affairs and the Swedish International Development Cooperation Agency, with additional support provided by the United States Agency for International Development (USAID) Cooperative Agreement. This research is made possible by the generous support of the American people through the USAID and was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. M.R. is a 2002 Elizabeth Glaser Scientist.
The contents of this article are the sole responsibility of the Population Council and do not necessarily reflect the views of our funders or the U.S. Government.
None of the authors has a conflict of interest with this research. None of the material in this article has been published or is under consideration elsewhere.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Agresti A. 1992. A survey of exact inference for contingency tables. Stat. Sci. 7:131–153 [Google Scholar]
- 2. Animal Welfare Act and Regulation of 2001 Code of Federal Regulations, title 9, chapter 1, subchapter A: animals and animal products. U.S. Department of Agriculture, Beltsville, MD [Google Scholar]
- 3. Baldwin J. I., Baldwin J. D. 2000. Heterosexual anal intercourse: an understudied, high-risk sexual behavior. Arch. Sex. Behav. 29:357–373 [DOI] [PubMed] [Google Scholar]
- 4. Borkow G., et al. 1997. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71:3023–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler D. M., et al. 2010. The origins of sexually transmitted HIV among men who have sex with men. Sci. Transl. Med. 2:18re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cline A. N., Bess J. W., Piatak M., Jr., Lifson J. D. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312 [DOI] [PubMed] [Google Scholar]
- 7. Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources 1985. Guide for the care and use of laboratory animals. Publication no. 85-23, 1985:1-83 U. S. Department of Health and Human Services, National Institutes of Health, Bethesda, MD [Google Scholar]
- 8. Cranage M., et al. 2008. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crostarosa F., et al. 2009. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One 4:e8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummins J. E., Jr., et al. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother. 51:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eshleman S. H., Jackson J. B. 2002. Nevirapine resistance after single dose prophylaxis. AIDS Rev. 4:59–63 [PubMed] [Google Scholar]
- 12. Fernández-Romero J. A., et al. 2007. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 34:9–14 [DOI] [PubMed] [Google Scholar]
- 13. Frank I., et al. 2003. Presentation of exogenous whole inactivated simian immunodeficiency virus by mature dendritic cells induces CD4+ and CD8+ T cell responses. J. Acquir. Immune Defic. Syndr. 34:7–19 [DOI] [PubMed] [Google Scholar]
- 14. Frank I., et al. 2008. A fusion inhibitor prevents dendritic cell (DC) spread of immunodeficiency viruses but not DC activation of virus-specific T cells. J. Virol. 82:5329–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Lerma J. G., et al. 2010. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4. [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Lerma J. G., et al. 2008. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gross M., et al. 2000. Anal. sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team. J. Acquir. Immune Defic. Syndr. 24:393–398 [DOI] [PubMed] [Google Scholar]
- 18. Guay L. A., et al. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795–802 [DOI] [PubMed] [Google Scholar]
- 19. Gupta P., et al. 1997. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J. Virol. 71:6271–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halfon P., et al. 2010. Semen may harbor HIV despite effective HAART: another piece in the puzzle. PLos ONE 5:e10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halvas E. K., et al. 2010. Low frequency nonnucleoside reverse-transcriptase inhibitor-resistant variants contribute to failure of efavirenz-containing regimens in treatment-experienced patients. J. Infect. Dis. 201:672–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karim Q. A., et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaul R., et al. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602–1611 [DOI] [PubMed] [Google Scholar]
- 24. Kaul R., et al. 2001. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Invest. 107:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keele B. F., et al. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kenney J., et al. 2011. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLos ONE 6:e15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilmarx P. H., et al. 2008. A randomized, placebo-controlled trial to assess the safety and acceptability of use of carraguard vaginal gel by heterosexual couples in Thailand. Sex. Transm. Dis. 35:226–232 [DOI] [PubMed] [Google Scholar]
- 28. Kilmarx P. H., et al. 2006. Safety and acceptability of the candidate microbicide Carraguard in Thai women: findings from a phase II clinical trial. J. Acquir. Immune Defic. Syndr. 43:327–334 [DOI] [PubMed] [Google Scholar]
- 29. Klasse P. J., Shattock R., Moore J. P. 2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455–471 [DOI] [PubMed] [Google Scholar]
- 30. Kumar N., et al. 1990. Radioimmunoassay of 7 alpha-methyl-19-nortestosterone and investigation of its pharmacokinetics in animals. J. Steroid Biochem. Mol. Biol. 37:587–591 [DOI] [PubMed] [Google Scholar]
- 31. Lifson J. D., et al. 2001. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llibre J. M., Schapiro J. M., Clotet B. 2010. Clinical implications of genotypic resistance to the newer antiretroviral drugs in HIV-1-infected patients with virological failure. Clin. Infect. Dis. 50:872–881 [DOI] [PubMed] [Google Scholar]
- 33. Martin S., et al. 2010. Carragaurd acceptability among men and women in a couples study in Thailand. J. Women's Health 19:1–7 [DOI] [PubMed] [Google Scholar]
- 34. McDermott A. B., et al. 2004. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. J. Virol. 78:3140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McLean C. A., et al. 2010. HIV genital shedding and safety of Carraguard use by HIV-infected women: a crossover trial in Thailand. AIDS 24:717–722 [DOI] [PubMed] [Google Scholar]
- 36. Metzner K. J., et al. 2009. Minority quasispecies of drug-resistant HIV-1 that lead to early therapy failure in treatment-naive and -adherent patients. Clin. Infect. Dis. 48:239–247 [DOI] [PubMed] [Google Scholar]
- 37. Motakis D., Parniak M. A. 2002. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nuttall J. P., et al. 2008. Concentrations of dapivirine in the rhesus macaque and rabbit following once daily intravaginal administration of a gel formulation of [14C]dapivirine for 7 days. Antimicrob. Agents Chemother. 52:909–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parikh U. M., et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pasquier C., et al. 2009. Determining seminal plasma human immunodeficiency virus type 1 load in the context of efficient highly active antiretroviral therapy. J. Clin. Microbiol. 47:2883–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phillips D. M., Taylor C. L., Zacharopoulos V. R., Maguire R. A. 2000. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception 62:149–154 [DOI] [PubMed] [Google Scholar]
- 42. Rowland-Jones S., et al. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59–64 [DOI] [PubMed] [Google Scholar]
- 43. Schwandt M., Morris C., Ferguson A., Ngugi E., Moses S. 2006. Anal and dry sex in commercial sex work, and relation to risk for sexually transmitted infections and HIV in Meru, Kenya. Sex. Transm. Infect. 82:392–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shattock R. J., Moore J. P. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25–34 [DOI] [PubMed] [Google Scholar]
- 45. Skoler-Karpoff S., et al. 2008. Efficacy of Carraguard for prevention of HIV infection among women in South Africa: a randomized, double-blind, placebo-controlled trial. Lancet 372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 46. Smith S. M., et al. 1999. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res. Hum. Retroviruses 15:1691–1701 [DOI] [PubMed] [Google Scholar]
- 47. Soderberg K., et al. 2002. A nucleotide substitution in the tRNAlys primer binding site dramatically increases replication of recombinant simian immunodeficiency virus containing a human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 76:5803–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stone M., et al. 2010. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J. Virol. 84:7083–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sudol K. M., Phillips D. M. 2004. Relative safety of sexual lubricants for rectal intercourse. Sex. Transm. Dis. 31:346–349 [DOI] [PubMed] [Google Scholar]
- 50. Turville S. G., et al. 2008. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLos ONE 3:e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uberla K., et al. 1995. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 92:8210–8214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. UNAIDS 2008. Report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland: http://data.unaids.org/pub/GlobalReport/2008/jc1511_gr08_executivesummary_en.pdf [Google Scholar]
- 53. Vagenas P., et al. 2009. Tonsillar application of AT-2 SIV affords partial protection against rectal challenge with SIVmac239. J. Acquir. Immune Defic. Syndr. 52:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Whitehead S. J., et al. 2006. Acceptability of Carraguard vaginal gel use among Thai couples. AIDS 20:2141–2148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.