Abstract
Although a major goal of human immunodeficiency virus type 1 (HIV-1) vaccine efforts is to elicit broad and potent neutralizing antibodies (NAbs), there are no data that directly demonstrate a role for such NAbs in protection from HIV-1 infection in exposed humans. The setting of mother-to-child transmission provides an opportunity to examine whether NAbs provide protection from HIV-1 infection because infants acquire passive antibodies from their mothers prior to exposure to HIV-1 through breastfeeding. We evaluated the characteristics of HIV-1-specific NAbs in 100 breast-fed infants of HIV-1-positive mothers who were HIV-1 negative at birth and monitored them until age 2. A panel of eight viruses that included variants representative of those in the study region as well as more diverse strains was used to determine the breadth of the infant NAbs. From their mothers, infants acquired broad and potent NAbs that were capable of recognizing heterologous circulating HIV-1 variants of diverse subtypes, but the presence of NAbs of broad HIV-1 specificity was not associated with transmission risk. There was also no correlation between responses to any particular virus tested, which included a range of diverse variants that demonstrated different neutralization profiles, including recognition by specific antibodies with known epitope targets. The eight viruses tested exhibited neutralization profiles to a variety of monoclonal antibodies (2F5, PG9, and VRC01) similar to those of viruses present in pregnant women in the cohort. These results suggest that the breadth and potency of the heterologous antibody response in exposed infants, measured against a virus panel comprised of variants typical of those circulating in the population, does not predict protection.
INTRODUCTION
At present, there is considerable effort being directed toward identifying human immunodeficiency virus type 1 (HIV-1) vaccine immunogens that elicit broadly neutralizing antibody (NAb) responses (13). This approach is predicated, in part, on the fact that the effective protection afforded by most vaccines is due to antibody responses (21). Moreover, the results of passive immunization studies in the macaque model have demonstrated that antibodies capable of neutralizing an incoming HIV-1 (simian-human immunodeficiency virus [SHIV]) strain can block infection, including through intravenous, vaginal, and oral routes (1, 8, 12, 18, 19, 27–29, 34, 37, 48). Despite the fact that the antibody levels used to achieve protection were very high in these initial studies, the results provided proof of principle that HIV-1-specific antibodies can block HIV-1 infection. In a more recent study, lower antibody levels, closer to those found in natural HIV-1 infection, were shown to delay infection in a low-dose SHIV/macaque challenge model (18). However, in these experiments, the virus used for challenge was one that was exquisitely sensitive to neutralization by the passive antibody tested compared to other strains of HIV-1 (47, 50).
For NAbs to be effective in stopping HIV-1 transmission in humans, it is critical that they have broad specificity and be capable of blocking diverse circulating HIV-1 strains, which can differ by more than 30% in their envelope sequences and by more than 5,000-fold in their neutralization sensitivities (4, 47, 50). Indeed, there are no data that clearly show whether preexisting HIV-specific NAbs in an exposed human will provide protection from infection. In addition, because the animal model studies are limited to analysis of a very few select challenge strains, little is known regarding the antibody specificity that would confer the greatest protection against HIV-1 transmission in humans. Thus, studies that directly test whether NAbs, when present at physiological levels, can reduce the risk of HIV-1 acquisition are critical in helping shape the way forward for HIV-1 vaccine design.
Mother-to-child transmission (MTCT), including transmission by breastfeeding, offers a unique opportunity to explore this question because maternal antibodies are shared with the infant starting in pregnancy and are therefore a potential source of some protection against HIV-1 infection in that setting. Recent studies indicate that the viruses in breast milk and blood are intermingled and are not generally compartmentalized (15, 17), suggesting that breast milk viruses are typical of circulating viruses. Moreover, the probability of infection per exposure during breastfeeding, measured either as per liter of breast milk ingested or by daily exposure, is generally similar to the probability of infection from one unprotected exposure during sexual transmission (38).
To date, MTCT studies have focused on the protective role of antibodies in the infected mother, which does not directly address the situation envisioned in current vaccine approaches—namely, that the antibodies will exert their effects in the exposed, uninfected individual. Nonetheless, studies of maternal antibodies provide some suggestion of a protective role for NAbs. Several early studies, each relatively small, showed that nontransmitting mothers had more frequently detected and/or higher levels of NAb responses than transmitting mothers, suggesting a role for NAb in reducing MTCT (7, 16, 22, 45, 46). A correlation between maternal antibodies and transmission risk was also observed in a larger study of Thai women (n = 90), in which the potency of NAb responses to 2 of 4 viruses tested inversely correlated with transmission (2, 43). Subsequent studies showed that the variants transmitted to infants tended to be those in the mother that were the most resistant to NAbs (10, 51). Together, these studies suggest that the nature and specificity of the NAb response in the mother may influence the risk of HIV-1 acquisition by the infant. However, to date, no one has examined the characteristics of the passively acquired HIV antibodies in the infant and whether they play any role in protecting infants from HIV-1 infection, particularly during the intrapartum and breastfeeding periods, which are times when these antibody levels are expected to be high. Because the infant may have passively acquired HIV-specific antibodies at the time of HIV-1 exposure, infants with broad and potent NAb responses would be expected to be less susceptible to infection if such responses are protective.
At present, the breadth of NAb responses is typically defined against a panel of genetically diverse recently transmitted HIV-1 variants to best assess their potential to neutralize circulating strains (4, 5, 24, 25). NAb breadth, as defined in this manner, is used to identify individuals with potentially desirable NAb responses to mimic in vaccine design, such as responses to conserved epitopes, and is likely to be used to determine which vaccine immunogens have potential for efficacy. Here, we examined whether the breadth of antibody responses, as defined by the ability to neutralize a diverse virus panel, correlates with protection from infection in the setting of MTCT.
MATERIALS AND METHODS
Samples and study population.
Samples were from a randomized clinical trial in Nairobi, Kenya, conducted from 1992 to 1998 to compare HIV-1 transmission in breastfeeding infants to that in formula-feeding infants. Enrollment, counseling, and the specific methods of the study have been described elsewhere (31). Infant infection status, maternal plasma viral load and breast milk viral load, maternal CD4 and CD8 T cell counts, and infant viral loads were available, and the relevant laboratory methods are described elsewhere (20, 32, 42). None of the women or infants in the study received antiretroviral therapy. The ethical review committees of the University of Nairobi, the University of Washington, and the Fred Hutchinson Cancer Center approved the study.
Infants were selected for study on the basis of the following criteria.
The mother was randomized to breastfeeding in the clinical trial (31), the infant was HIV-1 DNA PCR negative at the time of birth, an infant serum sample was available from either cord or peripheral blood from the first week of life, and subsequent HIV-1 DNA test results were available to determine infection status and to estimate the time of infection. One hundred mother-infant pairs who met these criteria were identified.
Pseudovirus particles were generated in 293T cells by cotransfecting the full-length envelope clone with a subtype A proviral clone lacking an envelope (Q23Denv) as described previously (5, 51). Virus was harvested at 48 h posttransfection, the supernatant was filtered through a 0.22-micron-pore-size filter, and the infectious titer was determined by infecting TZM-bl cells (obtained through the NIH AIDS Research and Reference Reagent Program; kindly supplied by John Kappes) and visually counting infected cells.
Eight envelope variants representing recently transmitted HIV-1s from different subtypes were used to generate the virus panel (Table 1). The panel was weighted toward viruses from the study region, as they best represent variants circulating in the cohort under study (n = 6), but it also included two viruses from other geographic areas to optimally probe for broad NAb responses. The eight viruses spanned a range of neutralization sensitivities, including tier 1B and tier 2 viruses (Table 1). The virus panel included four clones from Kenyan women infected through sexual contact (Q461d1, Q168b23, Q842d16, and QD435.100ENV.A4; subtypes A and D [4, 5, 26]), two from Kenyan infants infected during the postpartum period (BJ613.E1 and BF535.A1; subtypes A and A/D [51]), one from a U.S. man infected by another man (THRO4146.18/SVPB15; subtype B [24]), and one from a South African woman infected through heterosexual contact (DU156.12/SVPC3m; subtype C [25]) (Table 1).
Table 1.
Neutralization sensitivities of eight recently transmitted HIV-1 envelope variants of different subtypes
| Pseudovirus | Subject/transmission routea | Time postinfectionb (days) | Subtype | Tierc | IC50 for pooled plasmad | IC50 (μg/ml) for indicated MAb |
Source or reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b12 | 4E10 | 2F5 | PG9 | VRC01 | |||||||
| BJ613.E1 | Infant/vertical | 42 | A | 2 | 151 | >50 | >50 | 37.5 | 0.03 | 0.08 | 51 |
| BF535.A1 | Infant/vertical | 42 | A/D | 2 | 99 | >50 | 21.4 | >50 | 0.04 | 0.2 | 51 |
| Q461d1 | Female/heterosexual | 17 | A | 1B | 469 | 1 | 0.11 | 0.18 | 1 | 0.29 | 5 |
| Q168b23 | Female/heterosexual | 23 | A | 1B | 372 | >20 | 0.01 | 0.41 | 0.04 | 0.15 | 5 |
| Q842d16 | Female/heterosexual | 49 | A | 2 | 136 | >20 | 14.6 | >20 | 0.08 | 0.51 | 5 |
| QD435.100 M.ENV.A4 | Female/heterosexual | 100 | D | 2 | 69 | 5.74 | 2.58 | 0.76 | >10 | 0.36 | 4 |
| THRO4156.18 | Male/MSM | 17 | B | 2 | 69 | 0.5 | 0.3 | >50 | >10 | >10 | 24 |
| Du156.12 | Female/heterosexual | 28 | C | 2 | 155 | 0.8 | 0.2 | >50 | 0.16 | 0.29 | 25 |
MSM, men who have sex with men.
For adults, this estimate is based on published data and methods for estimating time of infection as described in reference 23. For infants, the time postinfection is the first time point after birth at which the infant was HIV DNA positive.
Tier designation is based on available data (47) or, in some cases, on extrapolation using the IC50 values shown here.
The IC50 is the reciprocal dilution. Pooled plasma was from 30 HIV-positive individuals and would be predicted to include infections with subtypes A, C, and D (5).
Envelope variants representing maternal viruses were described previously (51). To represent the spectrum of neutralization sensitivities of maternal variants, we chose two variants, the one that was most sensitive and the one that was most resistant to autologous maternal antibodies based on previous studies (51).
Neutralization assay.
The TZM-bl neutralization assay has been described in detail elsewhere (5, 51). Briefly, 500 50% tissue culture infective doses (TCID50) were mixed with infant plasmas in triplicate at 37°C. Six 2-fold plasma dilutions were tested, starting at 1:50 for Q461d1 and Q168b23, the two variants known to be the most neutralization sensitive, and 1:25 for all other variants (4, 5, 26). In the case of monoclonal antibodies (MAbs), six 2-fold dilutions were also tested, starting at an initial concentration that ranged from 1 μg/ml to 25 μg/ml, depending on the sensitivity of the virus being tested to a given MAb. After 60 min, 10,000 TZM-bl cells in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and l-glutamine were added to each well. β-Galactosidase levels were measured using the Galacto-Light system (Applied Biosystems, Foster City, CA) at 48 h postinfection (51). The concentration of plasma that resulted in 50% inhibition of virus infection (IC50) was determined as described previously (51).
Breadth and potency scores.
Data from all eight pseudoviruses were used to define a breadth and potency neutralization score for each infant plasma sample, as described previously (9, 11, 36, 37, 40, 44). This scoring system accounts for the differences in neutralization sensitivity of the different viruses tested. To define breadth, each plasma-virus combination was scored as 0 or 1 by comparing the IC50 value for that plasma sample to those of all other plasma samples tested with the same virus. A 1 was given when the IC50 was above the median for all plasma samples for that virus and a zero when it was below the median. Potency was defined by taking the IC50 value for a given plasma-virus combination and dividing it by the median IC50 value for that virus across all plasma samples.
Statistical analyses.
NAb data used for analyses included the median IC50 values for each infant plasma-virus combination determined independently as well as the calculated breadth and potency. For plasma that did not neutralize at the highest concentrations, the midpoint value between the lowest dilution and 0 was used for analysis. Continuous data were compared using the Mann-Whitney U test. Maternal viral load, NAb breadth and potency, and individual IC50 responses were tested for correlation using the Spearman rank method. The time to the first HIV-1 DNA PCR positive test from birth to 24 months was used for Kaplan-Meier and Cox regression analyses.
Heat map.
An IC50 heat map was generated using R software (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2008) and the heatmap.2 (Andy Liaw, original; R. Gentleman, M. Maechler, W. Huber, G. Warnes, revisions) and RColorBrewer version 1.0-2 (Erich Neuwirth) packages. All IC50 values were log transformed prior to analysis.
RESULTS
Clinical characteristics and correlates of the mother-infant pairs.
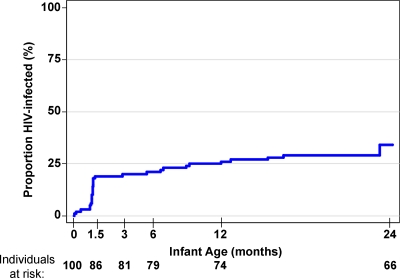
Mother-infant pairs from the Nairobi Breastfeeding Trial were included in the study if they met the following criteria: the mothers were randomized to breastfeeding in the original study (31), the infant was HIV-1 DNA PCR negative at the time of birth, and an infant serum sample was available from either cord or peripheral blood from the first week of life. One hundred mother-infant pairs meeting these criteria were included in the present study. The mean duration of breastfeeding among these women was 21.3 months, ranging from <1 month to ≥24 months (the end of the follow-up period). Of the 100 infants, 32 (32%) infants who were HIV-1 DNA negative at birth became positive before the end of the follow-up period at 2 years (Fig. 1). The median maternal CD4 T-cell count was 410 cells/μl (n = 93; range, 10 to 1,165 cells/μl), and the median maternal plasma viral load was 4.67 log10 HIV-1 RNA copies/ml (n = 96; range, 1.77 to 6.93 log10 HIV-1 RNA copies/ml) at or near 32 weeks gestation (Table 2). Most women (59%) had CD4 T-cell counts between 200 and 500, 33% had CD4 T-cell counts of >500, and only 8% had CD4 T-cell counts of <200. The median breast milk viral load obtained from samples during the first postnatal week was 2.59 log10 HIV-1 RNA copies/ml (n = 55; range, 1.18 to 5.29 HIV-1 RNA copies/ml). Of 89 women for whom HIV-1 subtype information was available (32), 59 were infected with subtype A, 21 with subtype D, 8 with subtype C, and 1 with an A/D intersubtype recombinant.
Fig. 1.
Kaplan-Meier estimates of HIV-1 incidence in infants during 24-month follow-up. The proportion of HIV-infected infants is shown on the y axis relative to infant age on the x axis. Infection status was determined by HIV-1 DNA PCR amplification of infant blood samples taken in the first week of life and at 1.5, 3, and 6 months and quarterly thereafter until 2 years of age, as previously described (31). The number of infants who contributed data at each time point is indicated at the bottom of the graph as individuals at risk.
Table 2.
Characteristics of infants in this study
| Characteristica | Medianb (range) for all infants | Medianb (range) for HIV+ infants | Medianb (range) for HIV− infant | P valuec |
|---|---|---|---|---|
| Maternal CD4 count | 410 (10–1,165) | 329 (104–690) | 451 (10–1,165) | 0.016 |
| Maternal viral load | 4.67 (1.77–6.93) | 5.00 (4.01–6.50) | 4.51 (1.77–6.93) | <0.001 |
| Breast milk viral load | 2.59 (1.18–5.29) | 2.72 (1.18–5.29) | 2.57 (1.18–4.03) | 0.47 |
| Infant viral load | 6.25 (4.09–7.54) | |||
| IC50 for Q461d1 | 278 (25–1,573) | 287 (42–1,487) | 263 (25–1,573) | 0.63 |
| IC50 for Q168b23 | 863 (25–1,700) | 677 (92–1,700) | 863 (25–1,700) | 0.9 |
| IC50 for Q842d16 | 47 (12.5–585) | 55 (12.5–416) | 46 (12.5–585) | 0.38 |
| IC50 for BF535.A1 | 229 (12.5–900) | 228 (19–640) | 254 (12.5–900) | 0.43 |
| IC50 for BJ613.E1 | 56 (12.5–751) | 68 (12.5–303) | 54 (12.5–751) | 0.58 |
| IC50 for QD435.A4 | 102 (12.5–741) | 161 (12.5–547) | 82 (12.5–741) | 0.46 |
| IC50 for DU156.12 | 68 (12.5–805) | 79 (12.5–327) | 65 (12.5–805) | 0.88 |
| IC50 for THRO4156.18 | 40 (12.5–333) | 46 (12.5–198) | 35 (12.5–333) | 0.7 |
| Breadth score | 4 (0–8) | 4.5 (0–8) | 4 (0–8) | 0.41 |
| Potency score | 10.0 (1.3–41.3) | 10.8 (1.7–23.8) | 8.7 (1.3–41.3) | 0.68 |
Maternal CD4 counts were taken at the third-trimester visit, maternal and breast milk viral load measurements were taken at time points closest to delivery, and infant viral loads were measured at the first available time point after infection.
There were 100 infants in the study; 32 infants were HIV positive (HIV+), and 68 infants were HIV negative (HIV−). Viral loads are expressed as log10 values, and IC50 values are given for the eight variant viruses. The IC50 values represent the averages of six results from duplicate experiments performed in triplicate. For experiments where there was no detectable neutralization above 50%, values were assigned at the midpoint between zero and the lowest dilution tested.
Determined by the Mann-Whitney U test.
Mothers that transmitted HIV-1 to their infants had lower CD4 counts (329 cells/ml) and higher viral loads (5.0 log10 HIV-1 RNA copies/ml) than nontransmitting mothers (CD4 count, 451 cells/μl, P = 0.02; viral load, 4.51 HIV-1 log10 copies/ml, P = <0.001) (Table 2). The median viral load in the infected infants was 6.25 log10 HIV-1 RNA copies/ml (n = 29; range, 4.09 to 7.54 log10 HIV-1 RNA copies/ml).
Characteristics of the HIV-specific NAbs in infants.
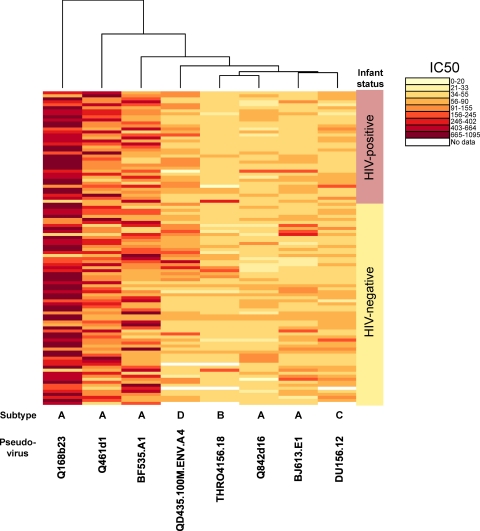
Of the 100 infant plasma samples examined for the presence of HIV-specific NAbs, 99 had antibodies capable of neutralizing at least one of the eight HIV-1 variants tested (Fig. 2). This panel included variants isolated soon after infection for both infants and adults and included subtype A, B, C, and D variants (Table 1). Importantly, this panel, which included primarily tier 2 variants, also included variants sensitive to MAbs that recognize the CD4 binding site (b12) and the membrane proximal region of the transmembrane domain (2F5 and 4E10). Forty-nine of the infant plasma samples had detectable NAb activity against all eight viruses, and 74 neutralized more than half of the variants tested.
Fig. 2.
Neutralization by infant plasma of heterologous HIV-1 variants representing transmitted strains. A heat map of the log-transformed IC50 value for each infant plasma sample (right) and each pseudoviral variant (bottom) is shown. Each rectangle in this heat map grid shows a color-coded IC50 value for the specific virus-plasma combination tested. The IC50 range denoted by each color is shown to the right of the heat map. The lightest color indicates that there was no detectable neutralization above 50% with the highest amount of plasma tested. The IC50 values represent the averages of 6 values derived from 2 independent assays performed in triplicate with at least two different virus preparations. The branches at the top and to the left of the heat map show the clustering of the data from viruses and plasma, respectively.
The mean IC50 values for the eight viruses tested spanned a median of 863 for a highly sensitive subtype A variant (Q168b23; range, ≤50 to 1,700) to 40 for the subtype B variant, B15 (range, ≤25 to 333) (Table 2). The IC50 values for the uninfected infants were in general very similar to those for 70 chronically infected Kenyan women in a prior study (36). Of the three viruses that were tested in common in both studies, the median IC50 values for infants who had passive antibodies from their mothers were within 3-fold of those for chronically infected women (Q168b23, 863 versus 312; Q461d1, 278 versus 441; Q842d16, 47 versus 140 for the infants and women, respectively).
Infant NAbs in relation to maternal clinical measures.
Because prior studies demonstrated a positive correlation between plasma viral load and NAb levels within an individual (9, 11, 35, 36, 40, 44), we examined the relationship between passive antibody measures for infants and viral load in their mothers. Maternal viral load did not correlate with the IC50 values for any of the eight variants individually, although one variant, Q168b23, showed a statistical trend (P value range, 0.07 to 0.99). The breadth and potency of the NAb response across the virus panel were also examined, using a method that accounts for the variable neutralization sensitivity of the viruses tested, the same method used in a prior study that examined NAb breadth and its relation to viral load in chronically infected women (36). Maternal viral loads were not correlated with either the breadth (P = 0.6) or the overall potency (P = 0.7) of the neutralizing antibody response in the infants. Similarly, neither maternal CD4 count nor breast milk HIV-1 RNA viral load was correlated with the breadth or potency of HIV-specific NAbs in the infants (data not shown).
Relationship of passively acquired HIV-specific NAbs and infant infection.
The relationship between infant passively acquired HIV-specific NAbs and the infant's risk of HIV-1 acquisition was examined. For each of the eight viral variants tested here, there was no significant difference in the distribution of the IC50 values between the HIV-positive and HIV-negative infants (Table 2). Composite scores representing the breadth of antibody neutralization or the potency of individual responses also did not differ between groups.
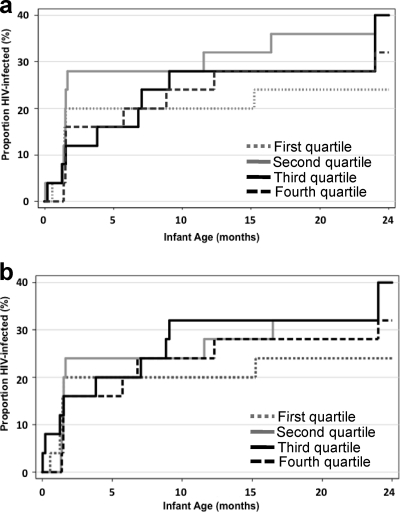
Another method used to examine the relationship between NAbs and infant infection was Kaplan Meier analysis incorporating either neutralization potency or breadth with time to HIV-1 as the outcome. In these models, there was no difference in time to infection between the quartile of infants with the highest neutralization potency scores and those with the lowest scores (Fig. 3a). Similar results were observed when the breadth scores (Fig. 3b) and the IC50 values for individual viruses (data not shown) were tested in this model. Results were similar with Cox proportional hazard models that adjusted for maternal viral load (data not shown).
Fig. 3.
Kaplan-Meier estimates of HIV-1 infection for all infants by quartiles of NAb potency (a) and breadth (b). The proportion of HIV-1-infected infants is shown on the y axis relative to infant age on the x axis. The quartiles of potency (a) and breadth (b) are indicated in the legends on the graphs; the dashed black lines represent the infants whose potency (a) or breadth (b) scores were in the highest quartile.
NAb decay.
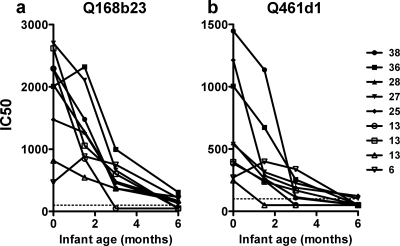
The above analyses compared NAb levels within the first week of life with the subsequent transmission risk due to breastfeeding through 2 years of life. To address whether such antibodies persisted during the period of breast milk exposure, we examined NAb levels over time in infants who had the highest potency scores (>25) and had available samples at multiple times, including 0 and 6 weeks as well as 3 and 6 months after birth (n = 5) (Fig. 4). We also examined four infants with moderate potency scores but with antibodies capable of neutralizing the most sensitive viruses, Q168b23 and Q461.d1, which were used to examine NAb decay. In all cases, there was a decline in IC50 values over each time point tested. There was an ∼20-fold reduction in titer against Q168b23 (range, 2- to 54-fold), but NAbs could be detected through 6 months of life in most cases. Only two of the infant plasma samples showed detectable neutralization against the Q461d1 pseudovirus 6 months after birth.
Fig. 4.
Decay of HIV-1-specific neutralizing antibodies in nine HIV-uninfected, breastfeeding infants. Neutralization of the Q168b23 (a) and Q461d1 (b) pseudoviruses by longitudinal plasma from birth through 6 months of monitoring is displayed for nine infants. The IC50 values for the babies' plasma samples against the pseudoviruses are displayed on the y axis, and the infant ages at the time the plasma samples were tested are shown on the x axis, with zero indicating birth. Each infant's plasma is indicated by a different symbol, and in the key to the right of the figure, that infant's corresponding overall potency score against the entire virus panel is indicated next to the symbol. The limit of detection was an IC50 value of 100, as shown by the dotted line.
Because passively acquired NAbs decayed over the first 6 month of life, their potential role in blocking HIV-1 infection could be limited to this early breastfeeding period. Therefore, we performed the analyses described above, restricting infected infants to those who were HIV positive at 6 weeks or 6 months of life. For this purpose, we compared all measures of neutralization (the IC50 values for each variant as well as the breadth and potency scores) between infants infected in the first 6 weeks of life (n = 14) and all other infants (n = 86) (Table 3) as well as between infants infected during the first 6 months of life (n = 21) and all others (n = 79) (Mann-Whitney U test) (Table 4). In this analysis, there were no differences in the individual mean IC50 values, breadth scores, or potency scores between infants who became infected and those who were not infected in the defined interval for either of these comparisons (Tables 3 and 4).
Table 3.
Characteristics of infants HIV positive within 6 weeks of birth
| Characteristica | Medianb (range) for infants HIV+ within 6 wk of birth | Medianb (range) for all other infants | P valuec |
|---|---|---|---|
| Maternal CD4 count | 410 (136–690) | 412 (10–1,165) | 0.74 |
| Maternal viral load | 4.95 (4.35–6.50) | 4.58 (1.77–6.93) | 0.02 |
| Breast milk viral load | 2.98 (1.60–5.29) | 2.57 (1.18–4.25) | 0.15 |
| Infant viral load | 6.27 (4.09–6.92) | ||
| IC50 for Q461d1 | 235 (46–1084) | 293 (25–1,573) | 0.42 |
| IC50 for Q168b23 | 395 (92–1700) | 863 (25–1,700) | 0.34 |
| IC50 for Q842d16 | 38 (12.5–416) | 49 (12.5–585) | 0.78 |
| IC50 for BF535.A1 | 198 (19–592) | 230 (12.5–900) | 0.46 |
| IC50 for BJ613.E1 | 72 (12.5–303) | 56 (12.5–751) | 0.93 |
| IC50 for QD435.A4 | 51 (12.5–464) | 108 (12.5–741) | 0.17 |
| IC50 for DU156.12 | 61 (12.5–327) | 71 (12.5–805) | 0.22 |
| IC50 for THRO4156.18 | 54 (12.5–180) | 36 (12.5–333) | 0.96 |
| Breadth score | 3 (0–8) | 4 (0–8) | 0.57 |
| Potency score | 8.9 (1.7–23.8) | 11.2 (1.3–41.3) | 0.42 |
Maternal CD4 counts were taken at the third-trimester visit, maternal and breast milk viral load measurements were taken at time points closest to delivery, and infant viral loads were measured at the first available time point after infection.
There were 100 infants in the study; 14 infants were HIV positive (HIV+) within 6 weeks of birth, and 86 infants were HIV negative at 6 weeks of age. Viral loads are expressed as log10 values, and IC50 values are given for the eight virus variants. IC50 values represent the averages of six results from duplicate experiments performed in triplicate. For experiments where there was no detectable neutralization above 50%, values were assigned at the midpoint between zero and the lowest dilution tested.
Determined by the Mann-Whitney U test.
Table 4.
Characteristics of infants HIV positive within 6 months of birth
| Characteristica | Medianb (range) for infants HIV+ within 6 mo of birth | Medianb (range) for all other infants | P valuec |
|---|---|---|---|
| Maternal CD4 count | 385 (104–690) | 416 (10–1,165) | 0.17 |
| Maternal viral load | 5.06 (4.35–6.50) | 4.52 (1.77–6.93) | <0.001 |
| Breast milk viral load | 2.95 (1.60–5.29) | 2.55 (1.18–4.25) | 0.16 |
| Infant viral load | 6.32 (4.09–7.46) | ||
| IC50 for Q461d1 | 297 (46–1,260) | 246 (25–1,573) | 0.96 |
| IC50 for Q168b23 | 444 (92–1,700) | 863 (25–1,700) | 0.56 |
| IC50 for Q842d16 | 47 (12.5–416) | 47 (12.5–585) | 0.87 |
| IC50 for BF535.A1 | 165 (19–592) | 233 (12.5–900) | 0.32 |
| IC50 for BJ613.E1 | 65 (12.5–303) | 62 (12.5–751) | 0.99 |
| IC50 for QD435.A4 | 165 (12.5–464) | 101 (12.5–741) | 0.71 |
| IC50 for DU156.12 | 65 (12.5–327) | 70 (12.5–805) | 0.37 |
| IC50 for THRO4156.18 | 45 (12.5–180) | 38 (12.5–333) | 0.78 |
| Breadth score | 3 (0–8) | 4 (0–8) | 0.93 |
| Potency score | 9.0 (1.7–23.8) | 10.3 (1.3–41.3) | 0.63 |
Maternal CD4 counts were taken at the third-trimester visit, maternal and breast milk viral load measurements were taken at time points closest to delivery, and infant viral loads were measured at the first available time point after infection.
There were 100 infants in the study; 21 infants were HIV positive (HIV+) within 6 months of birth, and 79 infants were HIV negative at 6 months of age. Viral loads are expressed as log10 values, and IC50 values are given for the eight virus variants. IC50 values represent the averages of six results from duplicate experiments performed in triplicate. For experiments where there was no detectable neutralization above 50%, values were assigned at the midpoint between zero and the lowest dilution tested.
Determined by the Mann-Whitney U test.
Defining the neutralization properties of maternal viruses in relation to the panel viruses used to examine the breadth of the infant NAbs.
To determine if the viruses in the panel were representative of those found in mothers in the cohort, we examined the neutralization sensitivities of maternal viruses generated from envelope variants cloned directly from blood taken at delivery from six transmitting mothers in the Nairobi Breastfeeding Trial (51). For this purpose, we used three MAbs with broad specificities targeted to distinct regions of the envelope protein: 2F5, which targets an epitope in the membrane proximal region of gp41 (30), VRC01, which targets the CD4 binding site (52), and PG9, which targets a quaternary epitope formed by conserved residues in V2 and V3 (50). The maternal variants had a range of neutralization sensitivities to these MAbs (Table 5). About half of the maternal viruses showed no detectable neutralization with 2F5 at the highest concentration tested, which was similar to what was observed for viruses from Kenya in general (4, 5), including the virus panel (Table 1). The majority of maternal viruses were neutralized by VRC01 and PG9, but there was a range of IC50 values, from 0.03 to >10 μg/ml. An identical range of IC50 values with these MAbs, from 0.03 to 10 μg/ml, was observed across the 8-virus panel (Table 1). Some mothers had two variants that were both sensitive to a particular MAb (e.g., ML274 for PG9 and VRC01), others had a mixture of sensitive and resistant viruses (e.g., MK184 for PG9 and VRC01), while others had two viruses that were both resistant to a particular MAb (e.g., MJ613 and 2F5). There was no apparent relationship between sensitivity to any particular MAb and sensitivity to autologous plasma. For example, in some cases the virus was sensitive to plasma and resistant to PG9 (e.g., MF535.B1), in other cases the converse was true (e.g., MJ613.C7), and in still others, the virus was sensitive to both (e.g., MK184.G3) or resistant to both (e.g., MK184.E4).
Table 5.
Neutralization sensitivities of maternal variants to the MAbs and plasma
| Maternal pseudovirusa | Subtype | IC50 for autologous plasmab | IC50 (μg/ml) for indicated MAb |
||
|---|---|---|---|---|---|
| 2F5 | PG9 | VRC01 | |||
| MF535.B1 | D/A | 1,297.3 | 0.03 | >10 | 9.95 |
| MF535.E2 | D/A | 12.5 | >10 | 0.63 | 0.99 |
| MG505.C2 | A | 246.8 | 2.51 | 0.12 | 0.66 |
| MG505.A2 | A | 12.5 | >10 | 0.32 | 0.27 |
| MJ412.C1 | C | 77.2 | 1.71 | 0.03 | 7.63 |
| MJ412.F2 | C | 12.5 | 3.02 | 0.03 | 6.47 |
| MJ613.A2 | A | 88.8 | >10 | >10 | 1.37 |
| MJ613.C7 | A | 12.5 | >10 | 0.03 | 0.36 |
| MK184.G3 | C/D | 536 | 5.07 | 0.14 | 0.71 |
| MK184.E4 | C/D | 12.5 | 4.42 | >10 | >10 |
| ML274.A1 | A | 41 | >10 | 0.16 | 0.4 |
| ML274.B1 | A | 12.5 | 9.3 | 0.16 | 0.26 |
DISCUSSION
Infants of HIV-1-positive mothers have passively acquired HIV-specific NAbs at the time of virus exposure, and thus represent a unique setting to examine the potential for such antibodies to impact the risk of HIV-1 acquisition. Here we found that infants who remained uninfected with HIV had NAb breadth and potency at birth similar to those who became HIV infected. Thus, despite the presence of these HIV-specific antibodies, there is no evidence that they provide any protection from infection in exposed infants. There was no correlation between the infant infection status at 6 weeks, 6 months, or 24 months of life and the breadth and potency of passively acquired HIV-specific NAbs in infants.
Virtually all infants (99%) had antibodies capable of neutralizing at least one of the viruses that were generated from a panel of eight envelope variants representing both vertically and sexually transmitted HIV-1s of diverse subtypes (4, 5, 51). Importantly, this virus panel included variants that were sensitive to CD4 binding site antibodies (VRC01 and b12), antibodies that recognized a quaternary epitope on the surface unit (PG9), and antibodies directed to the membrane proximal domain of the transmembrane protein (4E10 and 2F5). Thus, the selected viruses provided a surrogate measure of whether antibodies directed to the epitopes recognized by broadly neutralizing antibodies, which are presumably accessible on variants that are neutralized by the MAbs, may play a role in protection. However, there was no association between the infants' ability to neutralize any of these viruses individually and subsequent acquisition, even when controlling for maternal viral load.
In order to determine whether unique characteristics of the maternal viruses could make them less sensitive to NAbs targeted to conserved epitopes, we examined whether viruses from women in this cohort were recognized by HIV-1-specific MAbs with broad specificities. The maternal viruses exhibited a range of neutralization sensitivities to 2F5, VRC01, and PG9 similar to what has been reported for a large panel of diverse HIV-1 variants (4, 50, 52), suggesting that maternal viruses are not unusual in their susceptibilities to broad and potent MAbs. The neutralization sensitivities of the maternal variants were also similar to those of the viruses used to examine the breadth of the antibodies present in infant plasma. Thus, the virus panel was representative of the viruses that an infant encounters with respect to susceptibility to NAbs targeted to epitopes that are recognized by broad and potent HIV-specific MAbs.
The breadth and potency of the NAb responses acquired by the infants through maternal passive transfer were comparable to those observed in chronically infected women who were screened with a subset of the same viruses (36). This suggests efficient transfer of NAb antibodies to infants and allowed us to examine whether protection was afforded by HIV-specific NAbs at levels similar to those found in natural HIV-1 infection. Our finding that passively acquired NAbs at these levels are not broadly protective in the setting of MTCT is consistent with studies of passive antibody protection in macaques, including a recent study showing protection using a low-dose challenge model designed to more closely mirror antibody levels in natural HIV-1 infection (18). In this study and several other studies of passive transfer of HIV-1 antibodies, the macaques were exposed to a challenge virus that encodes a highly sensitive HIV-1 envelope variant (SHIVSF162) that is highly sensitive to the MAb tested (Ig-Gb12; IC50, 0.29 μg/ml [19]). Indeed, based on in vitro studies (50), more than half of all circulating viral variants studied to date would be predicted to require at least ∼100-fold-more b12 to achieve a similar potency of neutralization. Thus, NAbs at this level would not be expected to protect against most of the diverse variants to which high-risk humans are exposed. Highly neutralization-sensitive variants of the type used in the macaque studies may be even more rare in the setting of MTCT, where viral evolution in the mother in response to continued antibody pressure may further increase the fraction of variants that are more resistant to maternal antibodies.
Indeed, one caveat to these experiments is the fact that there are likely to be viruses that were already selected in the mother to be less susceptible to her specific repertoire of NAbs. Because HIV-1 continually evolves to escape NAb pressure (39), most individuals generally harbor a mixture of variants that range in neutralization sensitivity. Thus, even if the mother has a broad antibody response, the more resistant variants could be transmitted despite the presence of a strong NAb response. This model is supported by studies of autologous NAb responses in the setting of MTCT, showing that viruses that are transmitted are among the more neutralization-resistant viruses in the mother (10, 51, 53). However, while the aggregate data indicate selection for more resistant variants in MTCT, there are cases where the viruses transmitted to infants are still relatively sensitive to maternal NAbs (53). Collectively, these findings suggest that there may be a threshold of NAbs required for protection, perhaps one that may also be influenced by the presence of other cofactors that affect MTCT. In some cases, the levels of HIV-1-specific NAbs found may be adequate to provide some protection, but only against highly neutralization-sensitive variants. In this context, it is perhaps not surprising that a broad and potent NAb response would not predict protection because breadth and potency against a panel of viruses do not measure the neutralization sensitivity against the most neutralization-resistant virus in the population.
Despite the unique aspects of MTCT noted above, the finding that the breadth and potency of the NAb response are not correlates of protection from HIV-1 acquisition is not specific to this setting. Similar results were observed among a small number of cases of HIV-1 superinfection (3). As with infants vertically infected with HIV-1, individuals who became superinfected did not exhibit any deficits in the breadth or potency of NAb responses compared to controls, and in some cases, they had NAbs specific for the superinfecting strain near the time of exposure (3). Collectively, these studies strongly suggest that the levels of HIV-specific NAbs found in natural HIV-1 infection, while potentially capable of providing partial protection against the most sensitive HIV-1 envelope variant, are not adequate to provide protection against HIV-1 infection with diverse, circulating strains of HIV-1 that span a range of neutralization sensitivities.
The focus of this study was on antibodies present in the infant at the time of exposure to HIV, as this is most relevant for considering the potential of antibodies elicited through immunization to protect exposed individuals. Even when the analyses were restricted to infants infected at a time when the antibody levels were highest (within 6 weeks or 6 months of life), there was no correlation between infant infection and NAb breadth and potency. However, these studies did not address the potential roles of maternal antibodies in blood, genital secretions, or breast milk or infant antibodies in the gut in impacting infant infection. It is unknown whether the repertoire of maternal antibodies is precisely reflected in the antibodies transferred to her infant. Indeed, several studies suggest that antibodies in the mother, the index case, may provide some protection (2, 7, 10, 16, 22, 45, 46, 51) even if antibodies in the infant, the exposed individual, do not protect, as suggested by this study.
We cannot rule out that there could be viruses that would predict the ability of NAbs to protect that were not included in the virus panel used here. In a Thai study of maternal antibodies and MTCT, there was evidence that the neutralizing potential against one particular regional HIV-1 strain was correlated with infant infection (2, 43). It is difficult to compare these studies directly because the prior study examined antibodies in the maternal index case, while ours focused on antibodies in the exposed infant. But these studies collectively raise the possibility that breadth and potency as currently measured using virus panels may not be ideal for predicting protective benefit; rather, there may be particular HIV-1 variants that provide a better surrogate measure of protective antibodies that remain to be identified. These studies may also suggest that our current NAb assays are not detecting responses that are relevant in transmission. Such assays measure the potential of antibodies to neutralize cell-free virus, and they do not address whether antibodies capable of neutralizing cell-associated virus may play a role. Such antibodies may be particularly important in protection in the setting of MTCT, where there is a stronger association with the levels of cell-associated virus and risk of infant infection than with cell-free virus levels (41).
Current vaccine efforts to prevent HIV-1 infections are focused on immunization of an uninfected population, and that is why the studies described here examined NAbs in the infant. One notable difference between passively transferred antibodies and those found in vaccinees is that the latter would be elicited in an immunocompetent host who had the potential to develop more potent NAb responses, particularly to a highly immunogenic vaccine. There is currently considerable interest in whether NAbs elicited by vaccination can protect exposed humans from HIV-1, but there is not yet data demonstrating that such protection is possible. In this first detailed study of the protective effect of neutralizing antibodies in infants, no benefit was observed, at least when NAb responses were measured against a virus panel. As noted above, this does not preclude a protective effect of NAbs in the index case. However, it is important to distinguish these two situations when drawing inferences for vaccine strategies, given that current efforts are not directed at immunizing an infected population to prevent viral spread. Indeed, MTCT offers a unique setting to compare the role of NAbs in protection in the index case versus in the exposed partner.
While our studies suggest that a broad and potent HIV-1 response at levels found in natural HIV-1 infection may not be adequate to provide protection from infection, they do not rule out the possibility that NAbs, if present at higher levels and/or of different specificities, could provide protection from infection. Indeed, other protective viral vaccines, such as those for hepatitis B virus (HBV) and human papillomavirus (HPV), induce NAb levels that are similar to, or even significantly higher than, those found in natural infection (6, 33, 49). At present, the NAb responses induced by current HIV-1 vaccine immunogens are at a considerably lower level than those found in chronic HIV-1 infection, where there is continual antigenic stimulation by an evolving viral quasi-species (for examples, see references 14 and 24). Thus, a major unmet challenge is to identify HIV-1 immunogens that can elicit broad NAb responses of higher potency than those elicited during natural infection.
There are promising new HIV-specific broad antibodies that have been isolated from infected individuals (50, 52), and there is evidence that some MAbs are more efficacious in passive-transfer studies than would be predicted based on in vitro neutralization measures (19). However, until there are SHIV/macaque models that better mirror the neutralization properties of circulating strains of HIV, it is hard to define the levels of antibodies needed for protection in these models. Thus, it is critical that we continue to evaluate immune correlates of protection in exposed human populations, such as infants of HIV-1-positive mothers, to define the potential for specific immune responses to protect against diverse circulating strains of HIV.
ACKNOWLEDGMENTS
This work was supported by NIH grant R01 AI076105 to J.O. J.B.L. and C.A.B. were supported in part by NIH mentored awards K08 AI081546 and K08 AI068484, respectively.
We thank Joan Kreiss and Dorothy Mbori-Ngacha for all their efforts in the original Nairobi Breastfeeding Clinical Trial, as these were critical to the success of future derivative studies. We thank John Mascola, Xueling Wu, and the VRC team for providing the VRC01 antibody and Dennis Burton and the IAVI NAC team for providing the PG9 antibody. We thank Nicholas Provine for help and input on the neutralization assays. We especially acknowledge the women and infants who participated in the Nairobi Breastfeeding Clinical Trial and all of the study support team.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Baba T. W., et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 [DOI] [PubMed] [Google Scholar]
- 2. Barin F., et al. 2006. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J. Infect. Dis. 193:1504–1511 [DOI] [PubMed] [Google Scholar]
- 3. Blish C. A., et al. 2008. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J. Virol. 82:12094–12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blish C. A., et al. 2009. Cross-subtype neutralization sensitivity despite monoclonal antibody resistance among early subtype A, C, and D envelope variants of human immunodeficiency virus type 1. J. Virol. 83:7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blish C. A., Nedellec R., Mandaliya K., Mosier D. E., Overbaugh J. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 21:693–702 [DOI] [PubMed] [Google Scholar]
- 6. Böcher W. O., et al. 1996. Regulation of the neutralizing anti-hepatitis B surface (HBs) antibody response in vitro in HBs vaccine recipients and patients with acute or chronic hepatitis B virus (HBV) infection. Clin. Exp. Immunol. 105:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bongertz V., et al. 2002. Neutralization titres and vertical HIV-1 transmission. Scand. J. Immunol. 56:642–644 [DOI] [PubMed] [Google Scholar]
- 8. Conley A. J., et al. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70:6751–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deeks S. G., et al. 2006. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J. Virol. 80:6155–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickover R., et al. 2006. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J. Virol. 80:6525–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doria-Rose N. A., et al. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emini E. A., et al. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728–730 [DOI] [PubMed] [Google Scholar]
- 13. Fauci A. S., et al. 2008. HIV vaccine research: the way forward. Science 321:530–532 [DOI] [PubMed] [Google Scholar]
- 14. Gilbert P., et al. 2010. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J. Infect. Dis. 202:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray R. R., et al. 2011. Multiple independent lineages of HIV-1 persist in breast milk and plasma. AIDS 25:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guevara H., et al. 2002. Maternal HIV-1 antibody and vertical transmission in subtype C virus infection. J. Acquir. Immune Defic. Syndr. 29:435–440 [DOI] [PubMed] [Google Scholar]
- 17. Heath L., et al. 2010. Restriction of HIV-1 genotypes in breast milk does not account for the population transmission genetic bottleneck that occurs following transmission. PLoS One 5:e10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hessell A. J., et al. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hessell A. J., et al. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. John G. C., et al. 1997. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J. Infect. Dis. 175:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karlsson Hedestam G. B., et al. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6:143–155 [DOI] [PubMed] [Google Scholar]
- 22. Lathey J. L., et al. 1999. Lack of autologous neutralizing antibody to human immunodeficiency virus type 1 (HIV-1) and macrophage tropism are associated with mother-to-infant transmission. J. Infect. Dis. 180:344–350 [DOI] [PubMed] [Google Scholar]
- 23. Lavreys L., et al. 2004. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS 18:695–697 [DOI] [PubMed] [Google Scholar]
- 24. Li M., et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li M., et al. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 80:11776–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long E. M., Rainwater S. M. J., Lavreys L., Mandaliya K., Overbaugh J. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retroviruses 18:567–576 [DOI] [PubMed] [Google Scholar]
- 27. Mascola J. R., et al. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascola J. R., et al. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 29. Mc Cann C. M., Song R. J., Ruprecht R. M. 2005. Antibodies: can they protect against HIV infection? Curr. Drug Targets Infect. Disord. 5:95–111 [DOI] [PubMed] [Google Scholar]
- 30. Muster T., et al. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nduati R., et al. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167–1174 [DOI] [PubMed] [Google Scholar]
- 32. Neilson J., et al. 1999. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J. Virol. 73:4393–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olsson S. E., et al. 2007. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 25:4931–4939 [DOI] [PubMed] [Google Scholar]
- 34. Parren P. W., et al. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereyra F., et al. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571 [DOI] [PubMed] [Google Scholar]
- 36. Piantadosi A., et al. 2009. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression J. Virol. 83:10269–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prince A. M., et al. 1991. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res. Hum. Retroviruses 7:971–973 [DOI] [PubMed] [Google Scholar]
- 38. Richardson B. A., et al. 2003. Breast-milk infectivity in human immunodeficiency virus type 1-infected mothers. J. Infect. Dis. 187:736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richman D. D., Wrin T., Little S. J., Petropoulos C. J. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 100:4144–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodriguez S. K., et al. 2007. Comparison of heterologous neutralizing antibody responses of human immunodeficiency virus type 1 (HIV-1)- and HIV-2-infected Senegalese patients: distinct patterns of breadth and magnitude distinguish HIV-1 and HIV-2 infections. J. Virol. 81:5331–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rousseau C. M., et al. 2004. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J. Infect. Dis. 190:1880–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rousseau C. M., et al. 2003. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J. Infect. Dis. 187:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samleerat T., et al. 2009. Maternal neutralizing antibodies against a CRF01_AE primary isolate are associated with a low rate of intrapartum HIV-1 transmission. Virology 387:388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sather D. N., et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scarlatti G., et al. 1993. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc. Natl. Acad. Sci. U. S. A. 90:1721–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scarlatti G., et al. 1993. Neutralizing antibodies and viral characteristics in mother-to-child transmission of HIV-1. AIDS 7(Suppl. 2):S45–S48 [DOI] [PubMed] [Google Scholar]
- 47. Seaman M. S., et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shibata R., et al. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204–210 [DOI] [PubMed] [Google Scholar]
- 49. Villa L. L., et al. 2006. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24:5571–5583 [DOI] [PubMed] [Google Scholar]
- 50. Walker L. M., et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu X., et al. 2006. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J. Virol. 80:835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu X., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang H., et al. 2010. Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission. Virology 400:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]