Abstract
Diverse stimuli reactivate the Epstein-Barr virus (EBV) lytic cycle in Burkitt lymphoma (BL) cells. In HH514-16 BL cells, two histone deacetylase (HDAC) inhibitors, sodium butyrate (NaB) and trichostatin A (TSA), and the DNA methyltransferase inhibitor azacytidine (AzaCdR) promote lytic reactivation. Valproic acid (VPA), which, like NaB, belongs to the short-chain fatty acid class of HDAC inhibitors, fails to induce the EBV lytic cycle in these cells. Nonetheless, VPA behaves as an HDAC inhibitor; it causes hyperacetylation of histone H3 (J. K. Countryman, L. Gradoville, and G. Miller, J. Virol. 82:4706–4719, 2008). Here we show that VPA blocked the induction of EBV early lytic proteins ZEBRA and EA-D in response to NaB, TSA, or AzaCdR. The block in lytic activation occurred prior to the accumulation of BZLF1 transcripts. Reactivation of EBV in Akata cells, in response to anti-IgG, and in Raji cells, in response to tetradecanoyl phorbol acetate (TPA), was also inhibited by VPA. MS-275 and apicidin, representing two additional classes of HDAC inhibitors, and suberoylanilide hydroxamic acid (SAHA) reactivated EBV in HH514-16 cells; this activity was also inhibited by VPA. Although VPA potently blocked the expression of viral lytic-cycle transcripts, it did not generally block the transcription of cellular genes and was not toxic. The levels and kinetics of specific cellular transcripts, such as Stat3, Frmd6, Mad1, Sepp1, c-fos, c-jun, and egr1, which were activated by NaB and TSA, were similar in HH514-16 cells treated with VPA. When combined with NaB or TSA, VPA did not inhibit the activation of these cellular genes. Changes in cellular gene expression in response to VPA, NaB, or TSA were globally similar as assessed by human genome arrays; however, VPA selectively stimulated the expression of some cellular genes, such as MEF2D, YY1, and ZEB1, that could repress the EBV lytic cycle. We describe a novel example of functional antagonism between HDAC inhibitors.
INTRODUCTION
Epigenetic regulation and chromatin remodeling are critical to the control of gene expression in eukaryotic cells. These complex regulatory systems are equally important in the life cycles of viruses that infect these cells. Although cellular histone proteins are seldom found within virions, the genomes of several classes of eukaryotic viruses, including polyomaviruses, adenoviruses, papillomaviruses, hepatitis B virus, and herpesviruses, are associated with nucleosomes within infected cells (58). The ability of some viruses to establish persistent latent infections is enabled or enhanced via epigenetic mechanisms. The latent reservoir of viruses, such as HIV, that integrate into the cellular genome is thought to be maintained via repressive chromatin modifications, including deacetylation of histone tails and DNA CpG methylation (7, 96). Segregation of latent episomal viral genomes during cell division is dependent on the tethering of viral DNA to cellular metaphase chromosomes by viral factors, such as the human papillomavirus (HPV) E2 and Epstein-Barr virus (EBV) EBNA1 proteins. Recent evidence suggests that cellular chromatin-binding proteins act as “receptors” for viral proteins during this process (57).
The human gammaherpesviruses EBV and Kaposi's sarcoma-associated herpesvirus (KSHV) establish infections that persist for the life of the host. In the latent phase of the life cycle, the genomes of both viruses exist in nuclei as extrachromosomal episomes. The nucleosomal structure of viral episomes is comparable to that of cellular chromatin (41, 58, 79). Several epigenetic mechanisms play a role in maintaining gammaherpesviruses during the latent phase of their life cycle. The DNA methylation status of the promoter regions of EBV genes expressed during latency plays a role in determining latency type (1, 89, 91). Histone modification patterns and binding of the chromatin insulator protein CTCF are also associated with latent gene control (15, 16). Transcriptional suppression of viral lytic switch genes, BZLF1 and BRLF1 for EBV and open reading frame 50 (ORF50) for KSHV, is especially important for the preservation of latency. Expression of the proteins encoded by these transcripts results in reactivation of the entire cascade of lytic gene expression (23, 44, 62, 74, 86, 107). Positioning of a nucleosome over the transcriptional start site prevents the activation of KSHV ORF50; remodeling of chromatin exposes an Sp1 site in the ORF50 promoter that mediates the lytic activation of KSHV by the histone deacetylase (HDAC) inhibitor sodium butyrate (NaB) (60, 102). Acetylation of a single nucleosome positioned over the transcriptional start site of a lytic promoter has also been found to occur during HIV reactivation (93). The cellular protein MEF2D binds to the EBV BZLF1 promoter and recruits HDACs; this results in hypoacetylation of local histones and a repressive chromatin structure (42, 84). Hyperacetylation of histones associated with the EBV BZLF1 and BRLF1 promoters is thought to play a key role in lytic reactivation (13, 51).
Epigenetic modifiers are a broad group of agents that possess the ability to alter the chromatin environment. These include histone deacetylase inhibitors (HDACi), such as NaB, trichostatin A (TSA), and valproic acid (VPA), and DNA methyltransferase inhibitors, such as 5-aza-2′-deoxycytidine (AzaCdR). Hyperacetylation of histone tails following HDACi treatment or demethylation of DNA CpG motifs following treatment with AzaCdR leads to the derepression of silenced promoters and subsequent changes in gene expression (10, 65, 103). Epigenetic modifiers, such as NaB, TSA, and AzaCdR, have been shown to reactivate latent viruses, such as the gammaherpesviruses EBV and KSHV, in a variety of cultured cell lines. However, the response to these agents is often cell line dependent (20, 40, 61, 64, 101).
Efforts to extend the ability to manipulate viral lytic reactivation from cell culture to infected patients are under way. A major problem with viruses that incorporate a latent phase into their life cycles is that suppression of active infection with antiviral therapy does not eliminate the dormant population of viral genomes. Induction of viral reactivation and treatment with antiviral drugs could potentially eradicate this latent reservoir. Initially, the use of this strategy for HIV patients treated with VPA showed promising results (56), although subsequent studies found that VPA treatment had no significant effect in reducing the latent reservoir (78, 81). A second therapeutic avenue for the strategy of reactivation of latent viruses is oncolytic treatment of virus-associated malignancies. The presence of EBV within nearly all tumor cells in many cancers associated with the virus has made this an ideal test case for this approach (49). The use of HDAC inhibitors, such as VPA or NaB, to induce the viral lytic cycle is one possible tactic (33, 95). EBV lytic induction sensitizes tumor cells to chemotherapeutic agents (33). Tumor cells may also be killed by prodrugs, such as ganciclovir or radiolabeled nucleoside analogs, that are activated by viral lytic products, such as the viral thymidine kinase or viral protein kinase (31, 32, 35, 68). In a clinical trial, arginine butyrate in conjunction with ganciclovir produced antitumor responses in EBV-associated lymphoid malignancies (72). However, lytic induction of KSHV by VPA for treatment of Kaposi's sarcoma in HIV-positive patients did not meet the criteria for efficacy (55).
In recent years, there has also been a major focus on the clinical application of epigenetic modifying agents, including HDAC inhibitors and DNA methyltransferase inhibitors, in cancer therapy. A minimum of 80 clinical trials are currently being performed with HDAC inhibitors alone, testing all four classes (short-chain fatty acids [SCFA], hydroxamic acids, benzamides, and cyclic tetrapeptides) for efficacy against a range of malignancies (10, 54, 65, 85, 88, 103). Efforts to improve the effectiveness of these agents have led to the use of drug combinations. The combined use of VPA and AzaCdR treatment for advanced myelodysplastic syndrome (MDS) or acute myelogenous leukemia (AML) has shown promise in early trials (10, 36, 54, 83). Despite a wealth of knowledge regarding the cellular and molecular effects of these compounds, uncertainty remains about how they exert their effects in different types of cancer cells (10, 54). The acquisition of a better understanding of how these agents work alone and in combination will be essential for the design of rational multidrug therapies.
We have studied extensively the effects of the HDAC inhibitors NaB and TSA and the DNA methyltransferase inhibitor AzaCdR on viral reactivation (22, 24, 26, 41, 64, 102). We use the Burkitt lymphoma (BL) cell line HH514-16 for these studies, since these cells have a very low level of spontaneous reactivation and are readily induced by NaB, TSA, or AzaCdR. Recently we discovered that VPA, which, like NaB, belongs to the SCFA class of HDAC inhibitors, fails to activate EBV in this cell line, despite inducing equivalent levels of global acetylation of histone H3 (AcH3) (24). Chromatin immunoprecipitation (ChIP) analysis indicated that VPA also promotes histone H3 hyperacetylation directly at the promoters of three different early lytic genes: BZLF1, BRLF1, and BMRF1. This result suggested that either hyperacetylation of histone tails was not sufficient to activate the lytic cycle or VPA counteracted other events required for lytic activation.
The purpose of the current study was to investigate further the basis for the failure of VPA to activate the EBV lytic cycle. We sought to determine whether VPA simply failed to activate the lytic cycle in HH514-16 cells or whether it generally blocked EBV reactivation in response to diverse stimuli in several BL cell lines. We also investigated whether all classes of HDAC inhibitors are competent to activate EBV in HH514-16 cells or whether other HDAC inhibitors exhibit effects similar to that of VPA. Lastly, we compared cellular gene expression changes induced by NaB, TSA, and VPA in order to determine whether the differences in EBV lytic-cycle activation could be attributed to differences in the capacities of TSA, NaB, and VPA to alter cellular gene expression.
MATERIALS AND METHODS
Cell lines.
The EBV-infected HH514-16 cell line is a subclone of the P3J-HR1K Burkitt lymphoma cell line (45). The Akata Burkitt lymphoma cell line was generously supplied by Kenzo Takada (87). Raji is a Burkitt lymphoma-derived cell line (30). Cells were cultured in RPMI 1640 containing 8% fetal bovine serum, penicillin (50 U/ml), streptomycin (50 U/ml), and amphotericin B (1 μg/ml). Cells were grown at 37°C under 5% CO2.
Chemical treatment of cell lines.
Cells were subcultured at 3 × 105 ml−1; 48 h later, they were treated with chemical stimuli. NaB (catalog no. B5887; Sigma) was used at 3 mM. TSA (catalog no. 204-11991; Wako) and AzaCdR (catalog no. 3656; Sigma) were used at 5 μM. VPA (catalog no. P4543; Sigma) was used at 10 mM or as indicated in Fig. 4. MS-275 (catalog no. 13284; Cayman Chemicals), apicidin (catalog no. GR-340; Enzo Life Sciences), and suberoylanilide hydroxamic acid (SAHA) (catalog no. 10009929; Cayman Chemicals) were used at the concentrations indicated in Fig. 5 and 6. Tetradecanoyl phorbol acetate (TPA) (catalog no. 524400; Calbiochem) was used at 20 ng/ml. Rabbit anti-human IgG (anti-IgG) (catalog no. A042301-2; Dako) was used at 7.5 μg/ml. Dimethyl sulfoxide (DMSO) was obtained from J.T. Baker (no. 9224-01).
Fig. 4.
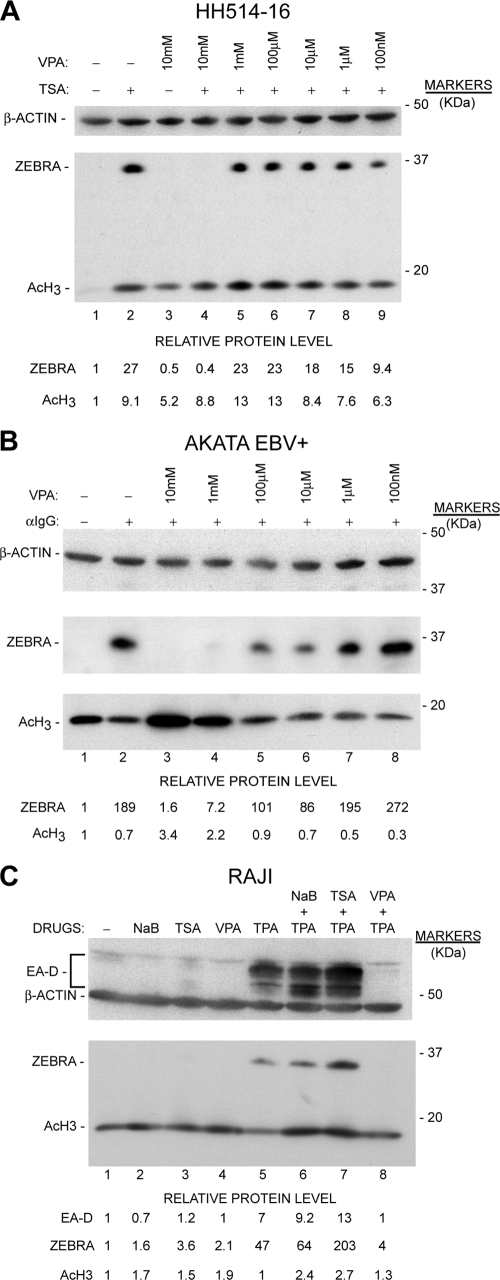
Inhibition of EBV lytic-cycle activation by VPA is dose dependent in HH514-16 cells and is also observed in the Akata and Raji Burkitt lymphoma cell lines. (A) HH514-16 cells were treated with TSA, VPA, or TSA plus varying concentrations of VPA (10-fold dilutions from 10 mM to 100 nM). Cells were harvested at 12 h and were analyzed for ZEBRA, AcH3, and β-actin expression as for Fig. 3. (B) EBV-positive Akata cells either were left untreated or were treated with anti-IgG alone or anti-IgG plus varying concentrations of VPA. Cells were harvested at 16 h and were analyzed as for panel A. (C) Raji cells were treated either with TPA, NaB, TSA, or VPA alone or with TPA plus NaB, TSA, or VPA. TPA was removed after 30 min by washing. NaB, TSA, and VPA were present for 24 h, after which cells were harvested and analyzed as for panel A.
Fig. 5.
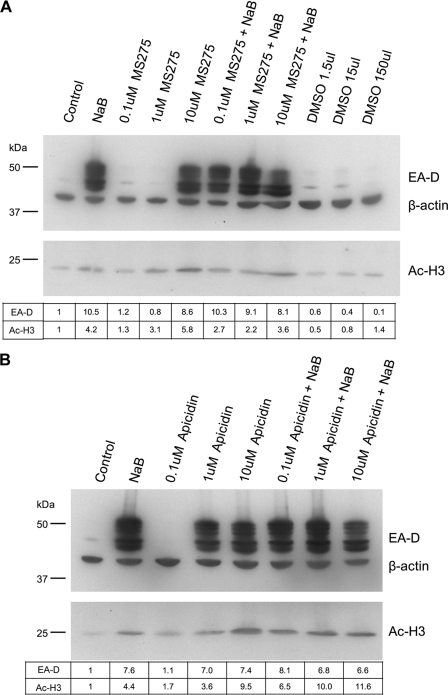
Individual members of the benzamide and cyclic tetrapeptide classes of HDAC inhibitors activate the EBV lytic cycle in HH514-16 cells and do not block activation by NaB. (A) HH514-16 cells were treated with NaB, varying concentrations of MS-275, or both NaB and MS-275. Cells were also treated with the MS-275 solvent DMSO in volumes equivalent to those used for MS-275-treated samples. Cells were harvested and analyzed as for Fig. 3. (B) Cells were treated and analyzed as for panel A, except that apicidin was used.
Western blot analysis.
Cells were either left untreated or treated with chemical stimuli and were harvested at the times indicated in the legends to Fig. 3 to 6. Total-cell extracts were electrophoresed in sodium dodecyl sulfate (SDS)–8% polyacrylamide gels and were transferred to nitrocellulose membranes (Bio-Rad). Rabbit polyclonal antibodies were used to detect EBV ZEBRA (90) and acetylated histone H3 (Upstate). Mouse monoclonal antibodies were used to detect EA-D (R3.1) (71) and β-actin (catalog no. A5316; Sigma). Antibody-protein complexes were detected using 125I-labeled protein A.
Fig. 3.
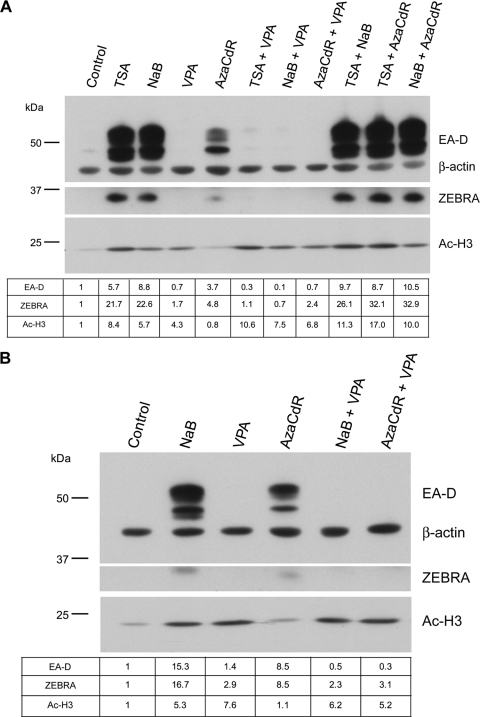
Valproic acid inhibits activation of the EBV lytic cycle in response to the HDAC inhibitor NaB or TSA or the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (AzaCdR). (A) HH514-16 cells were either left untreated or treated with TSA, NaB, VPA, AzaCdR, or the indicated combinations. Total-cell protein extracts harvested 24 h following treatment were resolved by 8% SDS-polyacrylamide gel electrophoresis. Immunoblots were probed with a mouse anti-EA-D or anti-β-actin monoclonal antibody followed by rabbit anti-mouse IgG or a rabbit anti-AcH3 or anti-ZEBRA polyclonal antibody. 125I-conjugated protein A was used for detection. EA-D, ZEBRA, and Ac-H3 protein levels were determined by densitometry, normalized to β-actin levels, and expressed relative to their levels in the control lane (untreated cells). (B) HH514-16 cells previously stored at −80°C were reconstituted and placed in culture. Cells were treated and analyzed as for panel A.
Real-time RT-PCR.
Total RNA was isolated using an RNeasy kit with on-column DNase digestion (Qiagen). The cells that were the source of RNA for the quantitative reverse transcription-PCR (qRT-PCR) experiments for which results are shown in Fig. 7 and Fig. 8A through D were the same cells that were the source of proteins for the immunoblot experiment for which results are shown in Fig. 3A. The relative transcript levels of selected cellular genes were determined using real-time RT-PCR with gene-specific primers using the iScript SYBR green RT-PCR kit (Bio-Rad). Relative expression levels were calculated using the ΔΔCT method and were normalized to 18S RNA. Individual samples were assayed in triplicate. Primers either were designed using Primer3 (77) or have been described previously (26, 101). The sequences of the primers are listed in Table 1.
Fig. 7.
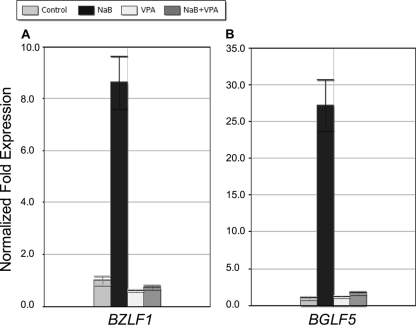
Valproic acid inhibits viral early lytic gene expression in response to NaB. HH514-16 cells were either left untreated or treated with either NaB, VPA, or both. Cells were harvested 24 h after treatment and total RNA extracted. Levels of RNA were measured by qRT-PCR relative to levels in untreated controls using the ΔΔCT method. Data are shown for BZLF1 (A) and BGLF5 (B).
Fig. 8.
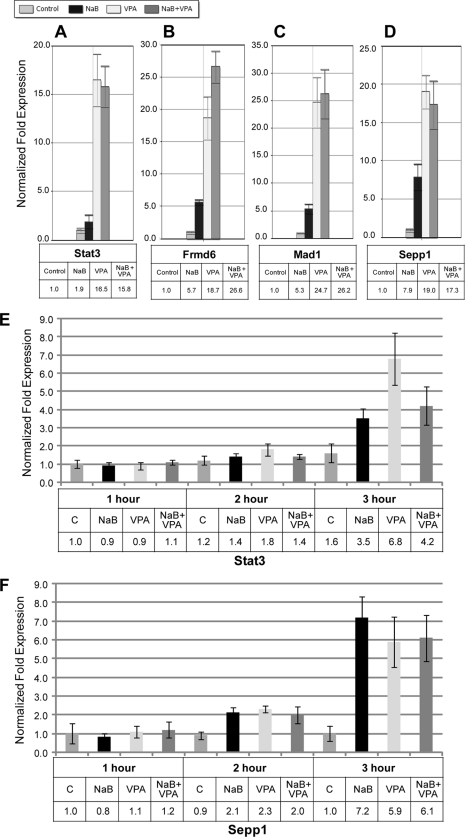
Valproic acid activates cellular gene expression. (A to D) Cells were treated as indicated, and total RNA was extracted after 24 h. Levels of RNA were measured by qRT-PCR relative to levels in untreated controls using the ΔΔCT method. Data are shown for Stat3 (A), Frmd6 (B), Mad1 (C), and Sepp1 (D). (E and F) HH514-16 cells were either left untreated or treated with NaB, VPA, or both. Cells were harvested 1, 2, or 3 h after treatment. Total RNA was extracted, and qRT-PCR was performed. Expression levels are shown relative to the levels in the 1-h untreated control for Stat3 (E) and Sepp1 (F).
Table 1.
Sequences of primers used to detect expression of viral and cellular mRNAs
| mRNA | Forward primer | Reverse primer |
|---|---|---|
| BZLF1 | TACAAGAATCGGGTGGCTTC | GCACATCTGCTTCAACAGGA |
| BGLF5 | GAGGACACGGTCAAGGACAT | CTCCGGTCGGTGAACAGTAT |
| STAT3 | GCCAGAGAGCCAGGAGCA | ACACAGATAAACTTGGTCTTCAGGTA |
| FRMD6 | TCCAGATGGAGCTAATATGCAA | CACTCCCCTGACTTGGTTTC |
| Mad1 | ACATGGTTATGCCTCCATGTTAC | AGATGAGCCCGTCTATTCTTCTC |
| SEPP1 | GATGGAGCAACTGAAAGGTG | CCCCTAGGTCATAGTTTACG |
| Jun | TCCCCTAACCTCTTTTCTGC | AACATCGCACTATCCTTTGG |
| Fos | ATGAGCCTTCCTCTGACTCG | ACGCACAGATAAGGTCCTCC |
| EGR1 | TACTGAGTAGGCGGCGATTT | TATCCCATGGGCAATAAAGC |
Microarray analysis.
Affymetrix U133 2.0 Plus arrays were used for all microarray experiments (26). HH514-16 cells were either left untreated or treated with NaB, TSA, or VPA and were harvested after 2, 3, or 6 h. Two separate gene expression microarray experiments were performed. In experiment a, the genes activated or repressed by TSA or NaB at 3 h and 6 h were compared. In experiment b, the genes activated or repressed by TSA or VPA at 2 h and 3 h were compared. RNA processing and initial data analysis were performed by the Keck Microarray Resource at Yale University. Gene expression data were processed using the Robust Multi-Array Average method (11), and quantile normalization was performed using the Affymetrix software packages from the R (version 2.10) Bioconductor open-source, open-development software project (37). Differences in gene expression between treatment groups were assessed using linear model methods from the limma package (82). Moderated t tests were used to compare the mean intensities of different samples. Statistical significance was determined by calculating P values based on the change in intensity of each probe across biological replicates. To control the false discovery rate (FDR), P values were adjusted for multiple testing using the method of Benjamini and Hochberg (3). Hierarchical clustering was performed on variable genes with standard deviation/mean ratios of >0.1 using Euclidean distance and complete linkage agglomeration. Venn diagrams were made with the Vennerable package using genes that had an FDR-adjusted P value of <0.05 for the comparison of cells treated with TSA or VPA for 3 h with untreated cells. The log2 gene expression values for all replicates of 20 genes thought to be related to EBV lytic activation were used to generate a heat map. The expression data for each gene were scaled to have a mean of zero and a standard deviation of 1 in order to allow better visualization of changes in gene expression, and the resulting Z scores are displayed in Fig. 9. Genes and samples with similar expression patterns were clustered together using the complete linkage agglomeration method with a Euclidean distance measure.
Fig. 9.
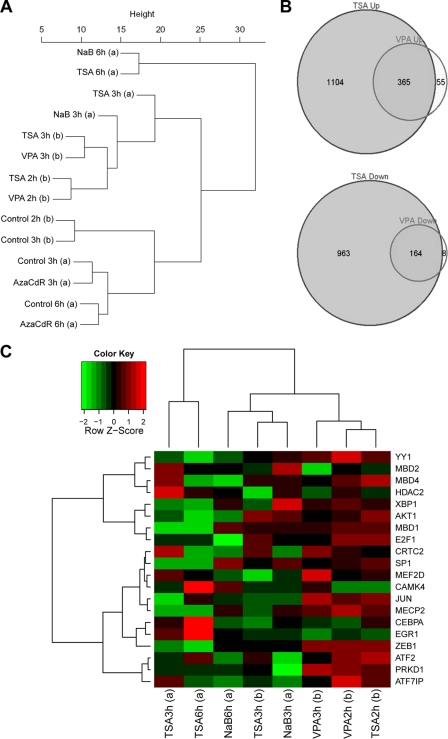
Comparison of cellular gene expression in HH514-16 cells after treatment with TSA, NaB, VPA, or AzaCdR. (A) Hierarchical clustering analysis of data from two gene expression microarray experiments. In experiment a, total RNA was extracted from HH514-16 cells harvested 3 or 6 h following no treatment or treatment with NaB, TSA, or AzaCdR. In experiment b, total RNA was extracted from HH514-16 cells harvested 2 or 3 h following no treatment or treatment with TSA or VPA. The data analysis was derived from 13,851 variable genes with standard deviation/mean ratios of >0.1. (B) Venn diagrams of microarray data comparing effects of TSA and VPA on cellular gene expression after 3 h of treatment relative to expression in untreated cells. (C) Heat map comparing the expression of 20 genes implicated in the EBV latent-to-lytic switch in the two gene expression microarray experiments. The heat map function shows Z scores, calculated by the equation Z = (x − μ)/σ where x is a raw score to be standardized, μ is the mean of the population, and σ is the standard deviation of the population.
RESULTS
Valproic acid activates the KSHV early lytic gene ORF50 but not its EBV homolog BRLF1.
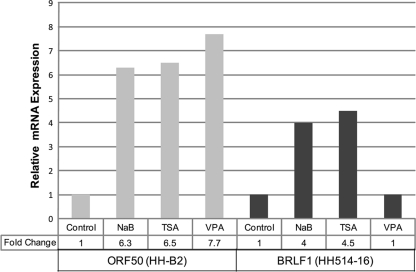
Representatives of two structural classes of HDAC inhibitors, NaB (an SCFA) and TSA (a hydroxamic acid) (Fig. 1), are known to activate viral lytic gene expression in certain cell lines harboring latent gammaherpesviruses (41, 61, 64, 102). However, we found previously that VPA, an SCFA HDAC inhibitor like NaB (Fig. 1), fails to activate EBV in the P3J-HR1 Burkitt lymphoma-derived HH514-16 cell line (24). VPA is known to activate the lytic cycle of another gammaherpesvirus, Kaposi's sarcoma-associated herpesvirus (KSHV) (80). We examined in parallel the abilities of NaB, TSA, and VPA to activate EBV or KSHV in two cell lines. The primary effusion lymphoma (PEL) cell line HH-B2 (40), which harbors latent KSHV, and EBV-infected HH514-16 cells were either left untreated or treated with NaB, TSA, or VPA and were harvested after 8 h. Expression of the KSHV early lytic gene ORF50 and the EBV homolog BRLF1 were analyzed using quantitative RT-PCR (qRT-PCR) (Fig. 2). In HH514-16 cells, expression of EBV BRLF1 was activated by NaB (4-fold) and TSA (4.5-fold) but not by VPA. In HH-B2 cells, NaB, TSA, and VPA all activated KSHV ORF50 (6.3, 6.5, and 7.7-fold, respectively). Therefore, the same VPA preparation activated lytic viral gene expression of KSHV but not of EBV.
Fig. 1.
Structures of HDAC inhibitors. Shown are representative examples of four classes of HDAC inhibitors: short-chain fatty acids (A), hydroxamic acids (B), benzamides (C), and cyclic tetrapeptides (D). SAHA, suberoylanilide hydroxamic acid.
Fig. 2.
Valproic acid activates the expression of KSHV ORF50 but not that of the EBV homolog BRLF1. HH-B2 cells (harboring latent KSHV) and HH514-16 cells (harboring latent EBV) were either left untreated or treated with NaB, TSA, or VPA. Cells were harvested 8 h after treatment and total RNA extracted. Relative levels of ORF50 and BRLF1 mRNAs were measured by qRT-PCR using the standard-curve method.
Valproic acid inhibits EBV lytic-cycle activation in HH514-16 cells in response to NaB, TSA, and AzaCdR.
Since EBV was not reactivated in HH514-16 cells treated with VPA, we asked whether VPA might possess a property that inhibits EBV reactivation. In addition to the HDAC inhibitors NaB and TSA, the DNA methyltransferase inhibitor AzaCdR also induces the EBV lytic cycle in HH514-16 cells. HH514-16 cells were treated with NaB, TSA, AzaCdR, VPA, or combinations of two of these agents (Fig. 3A). Expression of two EBV early proteins, the lytic switch transactivator (ZEBRA) and the DNA polymerase processivity factor (EA-D), was used as a marker for lytic-cycle induction. Lytic reactivation was observed in response to NaB, TSA, AzaCdR, or combinations of these agents. EBV lytic reactivation did not occur in response to VPA. Increased levels of histone H3 acetylation (AcH3) were observed in response to NaB, TSA, and VPA but not in response to AzaCdR. Therefore, the failure of VPA to induce EBV was not due to a lack of activity as an HDAC inhibitor. When combined with NaB, TSA, or AzaCdR, VPA drastically inhibited the induction of the ZEBRA and EA-D proteins but did not negatively affect histone hyperacetylation. One concern in investigating cell lines is that changes may occur during passage in culture over several months or years. To ensure that the inhibitory effect of VPA was not limited to a particular batch of cells, we reconstituted HH514-16 cells frozen at −80°C several years prior to these experiments. The reconstituted cells responded in the same manner as the population that had been cultured continuously (Fig. 3B). These results showed that VPA inhibits EBV lytic reactivation in response to different HDAC inhibitors (NaB and TSA) or the DNA methyltransferase inhibitor AzaCdR in HH514-16 cells.
Inhibition of EBV lytic-cycle activation by valproic acid is dose dependent and not specific to HH514-16 cells.
We next asked two questions: (i) what is the concentration of VPA that inhibits EBV reactivation in HH514-16 cells? (ii) is the effect specific to HH514-16 cells? To answer the first question, HH514-16 cells were either left untreated or treated with TSA or VPA alone or with TSA plus varying concentrations of VPA from 100 nM to 10 mM. Cells were harvested 12 h posttreatment and were analyzed by Western blotting. The concentration of VPA used previously (10 mM) completely inhibited reactivation in response to TSA, as determined by the absence of the EBV early protein ZEBRA (Fig. 4A). This concentration of VPA was not cytotoxic as assessed by trypan blue staining of cells. We assessed whether VPA could block EBV reactivation in two other BL cell lines that are responsive to different inducing stimuli. The EBV-positive Burkitt lymphoma cell line Akata (87) either was left untreated or was treated with anti-IgG alone or with varying concentrations of VPA (100 nM to 10 mM). Cells were harvested at 16 h and were analyzed by Western blotting. VPA at concentrations of 10 mM and 1 mM significantly inhibited reactivation in response to anti-IgG (Fig. 4B). The protein kinase C (PKC) agonist TPA is the primary stimulus used to reactivate EBV in Raji cells. Therefore, we evaluated the ability of VPA to inhibit lytic-cycle activation following treatment of these cells with TPA. In Raji cells, TPA induced the EBV early-lytic-cycle proteins EA-D and ZEBRA (Fig. 4C). The HDAC inhibitors NaB and TSA promoted histone hyperacetylation but by themselves were unable to induce the expression of EA-D or ZEBRA. NaB or TSA synergized in activating early lytic genes when combined with TPA. Treatment with VPA in combination with TPA inhibited the appearance of EBV early lytic proteins by more than 90%. Thus, VPA inhibits lytic reactivation in response to at least four different categories of inducing stimuli in three independently derived Burkitt lymphoma cell lines.
Valproic acid inhibits EBV lytic reactivation in response to all classes of HDAC inhibitors.
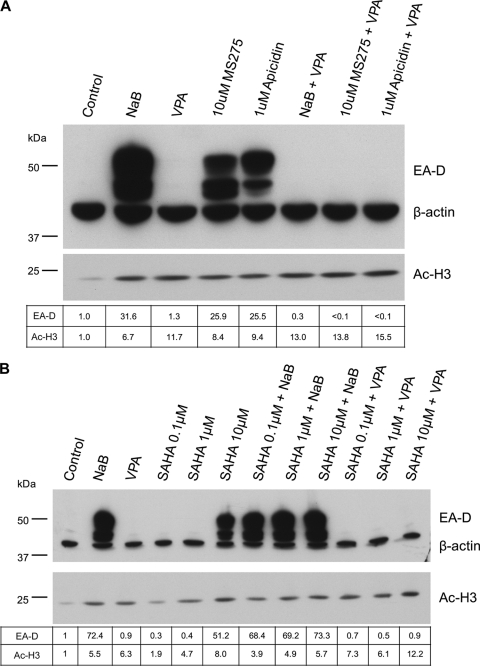
HDAC inhibitors are generally divided into four main classes: short-chain fatty acids, hydroxamic acids, benzamides, and cyclic tetrapeptides (103). NaB and TSA represent the short-chain fatty acid and hydroxamic acid classes, respectively (Fig. 1). The effects of inhibitors from the remaining two classes, benzamides and cyclic tetrapeptides, on EBV reactivation were not known. The benzamide MS-275 and the cyclic tetrapeptide apicidin were selected as representatives of these classes for further analysis (Fig. 1). We set out to answer two primary questions: (i) can all classes of HDAC inhibitors activate EBV in the HH514-16 cell line, or do other agents exhibit the inhibitory activity displayed by VPA? (ii) if other classes of HDAC inhibitors can reactivate latent EBV, does VPA also block the response to these agents? We treated HH514-16 cells with varying concentrations of MS-275 with or without NaB (Fig. 5A). Controls included cells that were either left untreated, treated with NaB, or treated with DMSO, the solvent for MS-275. Global increases in AcH3 levels indicated that MS-275 and NaB possessed comparable HDAC inhibitory activity. EBV lytic reactivation occurred in response to 10 μM MS-275 but not in response to 1.0 or 0.1 μM. The combination of 0.1, 1.0, or 10 μM MS-275 with NaB had no gross inhibitory or stimulatory effect on lytic induction. A parallel experiment using apicidin gave similar results, although apicidin induced lytic reactivation at a lower concentration of 1 μM (Fig. 5B). These results demonstrated that members of all known classes of HDAC inhibitors are competent to induce the EBV lytic cycle in HH514-16 cells. No combination of HDAC inhibitors capable of activating EBV in these cells inhibited lytic induction.
The effect of VPA on lytic induction by MS-275 or apicidin was tested using the minimum concentration of MS-275 (10 μM) or apicidin (1 μM) that was effective at EBV reactivation (Fig. 6A). Treatment with NaB, MS-275, or apicidin individually resulted in 25- to 30-fold increases in the levels of the EBV early lytic protein EA-D. No increase was observed following treatment with VPA. The combination of VPA with NaB, MS-275, or apicidin completely inhibited the accumulation of the EBV lytic protein EA-D. Global increases in AcH3 levels were observed for all agents relative to levels in untreated cells. Thus, in the HH514-16 cell line, VPA blocked EBV lytic induction by all classes of HDAC inhibitors.
Fig. 6.
Valproic acid blocks EBV lytic-cycle activation by representatives of the benzamide and cyclic tetrapeptide classes of HDAC inhibitors and by the hydroxamic acid SAHA. (A) HH514-16 cells were treated with the indicated compounds, harvested at 24 h, and analyzed by Western blotting as for Fig. 3. The concentrations used for apicidin (1 μM) and MS-275 (10 μM) were determined from Fig. 5 as the lowest concentration tested that exhibited robust activation of viral gene expression. (B) HH514-16 cells were treated with NaB, VPA, varying concentrations of suberoylanilide hydroxamic acid (SAHA), NaB plus SAHA, or VPA plus SAHA. Cells were collected and analyzed as for panel A.
Several HDAC inhibitors are currently in clinical trials for cancer therapy (10, 54, 65, 85, 88). Suberoylanilide hydroxamic acid (SAHA) (Fig. 1) was recently approved for use in treating cutaneous T-cell lymphoma (63). SAHA, a hydroxamic acid similar to TSA, has been shown to induce the EBV lytic cycle in cell culture, but this effect was observed primarily in epithelial, not lymphoid, cell lines (48). We tested the effect of SAHA, alone or in combination with NaB or VPA, on EBV reactivation in HH514-16 cells (Fig. 6B). Lytic-cycle induction was observed at 10 μM SAHA but not at 0.1 μM or 1.0 μM. Combination of SAHA with NaB did not inhibit or enhance lytic reactivation. However, combination of VPA with 10 μM SAHA completely blocked the appearance of the early lytic protein EA-D. Thus, the HDAC inhibitor SAHA induces the EBV lytic cycle in HH514-16 cells, and VPA potently inhibits this activity.
Valproic acid inhibits the accumulation of EBV early lytic gene transcripts.
The results presented in Fig. 3 to 6 indicated that VPA prevented increases in levels of viral early lytic proteins that are contingent on the exposure of HH514-16 cells to members of all known classes of HDAC inhibitors. However, these experiments did not ascertain the stage at which this inhibition occurred. Did VPA block the translation of viral mRNAs or the accumulation of early viral messages? To answer this question, total RNA was isolated from HH514-16 cells 24 h after treatment with NaB, VPA, or the combination of NaB and VPA. Expression of the EBV early lytic genes BZLF1 (ZEBRA) and BGLF5, which encodes an alkaline exonuclease and is involved in host cell shutoff (76), was analyzed by qRT-PCR using gene-specific primers (Fig. 7A and B). When cells were treated with NaB alone, expression of both viral early genes was upregulated; BZLF1 expression increased 8.6-fold, and BGLF5 expression increased 27.2-fold, relative to that in untreated cells. VPA treatment alone did not significantly alter BZLF1 (0.6-fold) or BGLF5 (1.2-fold) mRNA levels. The addition of VPA inhibited the stimulatory effect of NaB on the expression of BZLF1 by 92% and on that of BGLF5 by 94%. In related experiments, VPA blocked the expression of the BZLF1 and BRLF1 mRNAs as measured by Northern blotting 8 h after the addition of NaB (data not shown). Thus, in the HH514-16 cell line, VPA inhibits EBV reactivation in response to different chemical stimuli by preventing the accumulation of transcripts of early lytic viral genes.
Valproic acid activates the expression of cellular genes in a manner similar to that of sodium butyrate.
Identified as an inhibitor of HDAC activity (39, 73), VPA acts as an epigenetic modifier, leading to increased acetylation of histones and subsequent alterations in cellular gene expression. Our results, showing that VPA treatment failed to activate lytic viral gene expression and blocked the lytic viral gene expression promoted by other HDAC inhibitors, provoked the question of whether VPA shuts down cellular gene expression in HH514-16 cells. To address this question, we initially assessed the expression of four specific cellular genes that we have previously shown to be activated in HH514-16 cells in response to NaB or TSA (Fig. 8A to D) (26). Total RNA isolated from HH514-16 cells that were either left untreated or treated with NaB, VPA, or both for 24 h was analyzed by qRT-PCR using gene-specific primers. The cellular genes Stat3, Frmd6, Mad1, and Sepp1 were all upregulated in response to NaB. Treatment with VPA resulted in greater increases in the expression of these four genes than did treatment with NaB. The combination of VPA and NaB did not inhibit the expression of these four cellular genes; the extent of upregulation with VPA plus NaB was equivalent to that seen with VPA alone. Similar studies were carried out on the cellular immediate-early genes c-fos, c-jun, and egr1. VPA activated the expression of these 3 genes to an extent similar to that induced by NaB, and its effects on c-fos and egr1 were additive to those of NaB. In a separate experiment, Northern blotting showed that VPA completely blocked the expression of BZLF1 and BRLF1 mRNAs induced 8 h after the addition of NaB; the same Northern blot showed that VPA had no inhibitory effort on the 35-fold induction of c-fos mRNA by NaB (data not shown). Thus, VPA does not generally inhibit cellular gene expression, and in keeping with its HDAC-inhibitory function, it potently stimulates the expression of some cellular genes.
Early viral lytic gene expression in HH514-16 cells, in response to NaB or TSA, is detectable by 8 h (22). Since we initially analyzed changes in cellular gene expression after 24 h of treatment (Fig. 8A to D), it remained possible that VPA might delay changes in gene expression. We had previously shown that increases in the expression of some cellular genes, including Stat3 and Sepp1, occur rapidly and are detectable by 3 h after treatment of HH514-16 cells with NaB (26). To observe the time course of cellular gene expression after treatment with HDAC inhibitors of the SCFA class, we analyzed the kinetics of expression of two cellular genes, Stat3 and Sepp1, at early times (1, 2, and 3 h) after treatment with NaB, VPA, or both agents (Fig. 8E and F). Increased expression of Stat3 was first detectable 3 h after treatment with NaB (3.5-fold), VPA (6.8-fold), or NaB plus VPA (4.2-fold) (Fig. 8E). The expression of Sepp1 was slightly increased after 2 h of treatment with NaB (2.1-fold), VPA (2.3-fold), or NaB plus VPA (2.0-fold) and was further upregulated at 3 h with NaB (7.2-fold), VPA (5.9-fold), or NaB plus VPA (6.1-fold) (Fig. 8F). Thus, the kinetics and magnitude of changes in the expression of these two representative cellular genes were similar following exposure to NaB, VPA, or a combination of the two agents.
Valproic acid generally affects cellular gene expression in a manner similar to that of TSA and NaB.
The results in Fig. 8 showed similar responses of a small subset of cellular genes following treatment with VPA or NaB. One potential explanation for the paradoxical effects of VPA on EBV lytic activation is that globally VPA induces significantly different patterns of cellular gene expression than do other HDAC inhibitors. To begin to explore this possibility, data from Affymetrix U133 Plus 2.0 human genome arrays were examined for changes in the expression of cellular genes in HH514-16 cells following treatment with VPA, TSA, NaB, or AzaCdR from expression in untreated controls. No significant differences were seen in the overwhelming majority of genes following treatment with VPA compared to NaB or TSA. A group of 13,851 variable genes with a standard deviation/mean ratio of >0.1 was analyzed by hierarchical clustering (Fig. 9A). Overall, the time of exposure to the individual agent was more important in determining the patterns of gene expression in response to HDAC inhibitors than were differences among the individual agents. As the dendrogram illustrates, data for gene expression 2 h after treatment with VPA or TSA clustered together, while the data for the samples harvested 3 h after treatment with the same agents formed a separate cluster. The overall effects of AzaCdR, which also induces the lytic cycle, on cellular gene expression clustered with the data for the untreated control samples and were distinctly different from the effects of the HDAC inhibitors.
Through Venn diagrams, Fig. 9B takes a closer look at differentially expressed genes (FDR-adjusted P value, <0.05) from the comparisons of VPA- or TSA-treated cells with untreated cells at 3 h. The expression of a total of 1,469 genes was increased by TSA. VPA treatment led to an increase in the expression of 420 genes; of these, 365, or 87%, were also upregulated by TSA. The expression of 1,127 genes was reduced following TSA treatment, whereas that of 172 genes was reduced following VPA treatment. Only 8 of the 172 genes (4.6%) inhibited by VPA were unique to this agent; the expression of the remaining 164 genes was also reduced by TSA. The Venn diagram shows that although the expression of many more genes was upregulated or downregulated by TSA than by VPA, the genes affected by VPA are almost exclusively a subset of the genes altered by treatment with TSA. The conclusion from both types of analysis is that the global patterns of alterations in gene expression in response to VPA, NaB, and TSA are remarkably similar despite the different biological outcomes in terms of viral lytic activation. These results reinforce the conclusion that VPA at the concentrations used is not generally toxic to cellular gene expression.
Comparison of the effects of valproic acid and other HDAC inhibitors on the expression of cellular genes implicated in the EBV lytic-cycle switch.
The gene expression microarrays were specifically interrogated for 20 genes, encoding 12 DNA binding transcription factors, 4 methylated CpG binding proteins, 3 kinases, and 1 HDAC, that have been implicated in regulation of the expression of the EBV lytic-cycle activator genes BZLF1 and BRLF1 (Fig. 9C). At 3 h after treatment, the expression of 6 genes was upregulated by VPA compared with TSA. These were the YY1, MEF2D, c-Jun, MECP2, ZEB1, and protein kinase D (PRKD1) genes. At least 5 genes of this group have the potential to act as repressors of the EBV lytic cycle. YY1 binds to cis-active negative regulatory elements in Zp (66) and Rp (106). MEF2D binds Zp at ZI sites and is associated with class II HDACs (42). ZEB1 binds ZV sites in Zp and is a documented repressor (53). MECP2 binds CpG-methylated DNA, which can produce the epigenetic silencing of many viral lytic promoters (4). c-Jun binds the ZII sites in Zp and, in its unactivated state, may act as a repressor (34). The repressive function of many of these proteins might depend on their associated proteins or modification state (5). Activation of a cellular environment that is repressive of Zp or Rp is a plausible hypothesis to account for the ability of VPA to inhibit EBV lytic-cycle activation.
DISCUSSION
The HDAC inhibitors NaB, TSA, and VPA have been used to reactivate EBV in cell culture. The efficiency of lytic induction is variable, is cell line dependent, and often requires the presence of another inducing agent with which these HDAC inhibitors act in synergy (33, 40, 61, 101). These early studies of the switch from latency into the lytic phase of the EBV life cycle in cultured cells have evolved into attempts to manipulate EBV reactivation in a clinical setting. Strategies involving the use of epigenetic modifying agents, such as HDAC inhibitors, to induce the EBV lytic cycle in vivo have the potential to clear the latent reservoir or to destroy tumor cells containing viral genomes. Understanding how individual HDAC inhibitors affect the viral life cycle will be critical to these efforts.
Previously we reported that unlike NaB, TSA, or AzaCdR, VPA failed to induce EBV in the Burkitt lymphoma HH514-16 cell line (24). Here we show that VPA inhibits EBV reactivation when combined with the inducing stimulus NaB, TSA, or AzaCdR in HH514-16 cells, anti-IgG in Akata cells, or TPA in Raji cells. Representatives of all classes of HDAC inhibitors reactivated EBV in HH514-16 cells; lytic activation in response to each of these HDAC inhibitors was blocked by VPA. While VPA prevents the accumulation of EBV early lytic transcripts, it activates cellular gene expression alone and in combination with other HDAC inhibitors (Fig. 9). The changes in cellular gene expression observed after treatment with NaB, TSA, or VPA, in terms of kinetics, magnitude, and specific genes affected, are similar. This report thus describes a unique antagonistic relationship between VPA and other HDAC inhibitors that are used as medical therapy, a phenomenon that will provide clues to the mechanisms that regulate EBV lytic reactivation.
Previous studies of valproic acid and EBV reactivation.
It was reported previously that VPA enhanced the ability of chemotherapeutic agents, such as gemcitabine and doxorubicin, to activate EBV lytic protein expression in lymphoblastoid and epithelial cell lines harboring the latent virus (33). In this study, however, VPA treatment alone resulted in little or no EBV lytic protein expression in any cell background. Cells of the EBV-positive AGS line, a gastric carcinoma cell line in which there is a high level of spontaneous EBV lytic activation, exhibited the highest levels of EBV lytic gene expression following VPA treatment. A separate study recently confirmed that VPA can induce EBV lytic proteins in EBV-positive AGS/BX1 cells, although VPA failed to activate EBV lytic proteins in Burkitt lymphoma or lymphoblastoid cell lines (48). These two studies suggest that the ability of VPA to activate the EBV lytic cycle may be specific to certain cell backgrounds and may require synergy with other agents.
EBV reactivation in response to several different stimuli in three cell lines is blocked by valproic acid.
One advantage of using HH514-16 cells in studies of EBV reactivation is the ability to induce the viral lytic cycle using different classes of stimuli with distinct mechanisms of action. Here we show that representatives of all classes of HDAC inhibitors reactivated EBV in this cell line. Treatment of HH514-16 cells with HDAC inhibitors, such as TSA or NaB, leads to extensive changes in cellular gene expression, with thousands of transcripts increased or decreased in abundance (Fig. 9) (26). Treatment of this cell line with AzaCdR, a DNA methyltransferase inhibitor, also results in EBV reactivation, though cellular gene expression is only slightly changed at times preceding lytic induction (Fig. 9A). The potent inhibition of EBV reactivation when VPA was combined with any of the other HDAC inhibitors tested or with AzaCdR suggests that VPA may interfere with a common essential component or pathway in HH514-16 cells that is shared by these different inducing stimuli. Inhibition of induction of the EBV lytic cycle by VPA was not limited to the HH514-16 cell system. Cross-linking of the B cell receptor using anti-IgG initiates several signaling cascades that ultimately activate BZLF1 transcription (2, 84). VPA blocked lytic activation in response to anti-IgG treatment in Akata cells (Fig. 4B). The PKC agonist TPA activates the EBV lytic cycle in Raji cells; NaB augments this effect (24). VPA blocked lytic activation in response to TPA treatment in Raji cells (Fig. 4C). The ability of VPA to inhibit lytic activation by multiple chemical stimuli in different cell lines should provide an important tool for dissecting critical components of the reactivation pathway.
Valproic acid inhibits the accumulation of BZLF1 transcripts.
The first viral gene known to be activated upon induction of the lytic cycle is BZLF1, which encodes the viral transcriptional activator protein ZEBRA. In our reactivation experiments, VPA prevented the accumulation of BZLF1 transcripts (Fig. 7); therefore, the nature of the block induced by VPA may be to inhibit transcriptional activation at this viral promoter. Here we confirm (Fig. 3, 4, and 6) that VPA is a bona fide HDAC inhibitor that promotes global hyperacetylation of histone H3. Previously we have shown that VPA treatment leads to increased acetylation of histone H3 at the BZLF1 promoter (Zp) in the HH514-16 cell line, a result similar to that observed upon NaB or TSA treatment (24). This result suggests that VPA is not deficient in making Zp accessible to regulatory proteins such as transcription factors and supports the conclusion that opening of the chromatin on Zp alone is not sufficient to activate BZLF1 expression (24). Since VPA inhibits the transcription of BZLF1, it may prevent the interaction of positively acting cellular proteins with Zp, or it may enhance the interaction of cellular repressors, as suggested by the preliminary data of Fig. 9C.
VPA may not inhibit transcriptional initiation or elongation but may destabilize BZLF1 mRNA. It has been reported that TSA treatment destabilizes DNMT1 and DNMT3B mRNAs (50, 97). It has been suggested that expression of an RNase following TSA treatment could account for the decrease in DNMT3B mRNA levels (97). Destabilization of BZLF1 mRNA would need to be a specific trait of VPA treatment, since other HDAC inhibitors lead to substantial increases in the abundance of BZLF1 transcripts.
Effects of valproic acid and other HDAC inhibitors on cellular gene expression.
We considered the possibility that VPA may differentially affect cellular gene expression relative to other HDAC inhibitors. Our preliminary analysis of 20 genes related to EBV lytic activation suggests that VPA may upregulate genes that promote the refractory state. Activation of a direct or indirect repressor of lytic activation may surmount any activating signals initiated by other agents. Additionally, VPA may fail to activate genes that are needed to promote the lytic state.
We used both microarray and qRT-PCR analyses to address the question of whether VPA differentially affected cellular gene expression. It is well recognized that HDAC inhibitors tend to repress as many genes as they induce, although the mechanisms accounting for activation or repression are not yet understood (26, 38, 52, 98). Treatment with different HDAC inhibitors has previously been found to result in similar patterns of altered gene expression (98). Direct comparison of TSA and VPA at various doses in Xenopus and zebrafish embryos demonstrated very similar effects on cellular transcripts (43). We found extensive overlap in the specific genes whose expression was increased or decreased following treatment of HH514-16 BL cells with VPA compared with TSA (Fig. 9B). However, TSA, which is a more potent HDAC inhibitor, elicited changes in a greater number of genes than VPA (Fig. 9B). Hierarchical clustering analysis (Fig. 9A) revealed three remarkable features. (i) The effects of HDAC inhibitors cluster distinctly from the effects of AzaCdR, which also induces the lytic cycle. (ii) The pattern of cellular gene expression in cells treated with AzaCdR is remarkably similar to the pattern in control cells left untreated. (iii) Time is a better indicator of changes in cellular gene expression in response to HDAC inhibitors than the specific agent used. In summary, gross differences in the patterns of gene expression induced by VPA do not seem to explain the distinct effect this agent has on lytic reactivation. The role of VPA in altering cellular gene expression may or may not be related to its inhibitory effect on lytic reactivation.
Effects of HDAC inhibition on nonhistone proteins.
The effects of VPA acting as a deacetylase inhibitor are not likely to be limited to effects on histones. The functional importance of HDACs on other protein substrates within the proteome are only starting to be identified (8, 21, 65, 94, 105). Acetylation plays an important role in regulating protein interactions, localization, and stability. But how might VPA affect nonhistone protein acetylation differently than other HDAC inhibitors? One possible explanation could be HDAC isoform specificity. VPA was originally identified as being selective for class I HDACs, although it is now thought to inhibit the activity of most class II HDACs as well (10, 39, 73). TSA and SAHA are indiscriminate, inhibiting HDACs belonging to classes I, II, and IV, while MS-275 is one of the few inhibitors known to exhibit some selectivity for class I enzymes (10, 47). Should VPA exhibit greater activity than other HDAC inhibitors against a specific HDAC isoform, VPA could lead to downstream events that diverge from those seen with other agents. Although this theory is plausible, one argument against it is that TSA and SAHA, two of the most potent and broadly acting HDAC inhibitors known, activate, and do not inhibit, the EBV lytic cycle.
Other cellular effects of valproic acid.
The role of VPA as an HDAC inhibitor was discovered almost a decade ago (39, 73). However, valproic acid has been used for more than 30 years as an anticonvulsant for the treatment of epilepsy, and more recently as a treatment for migraine and bipolar disorders (6, 59, 67). VPA is also known to cause developmental anomalies, such as neural tube defects (12). Other HDAC inhibitors, such as TSA and NaB, do not exhibit anticonvulsant effects (14, 46) but cause the same developmental abnormalities (28, 73). Further studies have demonstrated that the teratogenic effects of NaB and TSA are tightly correlated with their activities as HDAC inhibitors (29). However, the anticonvulsant properties of VPA are not linked to HDAC inhibition (9, 29). These studies demonstrate that VPA has properties distinct from those of other HDAC inhibitors. The capacity of VPA to inhibit lytic activation may be more closely linked to its anticonvulsant properties than to its role as an HDAC inhibitor.
Effects of valproic acid on signal transduction.
One proposed mechanism underlying VPA's anticonvulsant activity is that it stimulates gamma-aminobutyric acid (GABA) signaling. VPA increases GABA levels and augments signaling through this pathway by altering GABA metabolic pathways and influencing GABA receptor activity (25, 67, 69). VPA impinges on several other molecular and cellular processes, such as the activity and expression of ionic channels, Wnt signaling, and Akt regulation (6, 17, 19, 27, 67, 92, 99). Activation of the extracellular signal-regulated kinase (ERK) pathway is commonly observed following VPA treatment (67, 104). In many signaling pathways, such as Akt regulation, the effects of VPA overlap with those of other HDAC inhibitors (19, 27). However, VPA differs from other HDAC inhibitors in signal transduction. VPA treatment often leads to decreased PKC activity; the levels of the PKCα and PKCε isoforms were found to be selectively decreased following prolonged exposure to VPA (6, 18, 67, 100). In contrast, differentiation of colon cancer cell lines was induced by NaB in a PKC-dependent manner (70). In erythroid cells, upregulation of PKCε levels was observed within 20 min after exposure to NaB (75). Caution needs to be exercised in making these comparisons, in view of the cell type-dependent effects of HDAC inhibitors (10, 65).
Interest in the clinical use of HDAC inhibitors in cancer therapy has grown exponentially in recent years. Combination therapy using HDACi in conjunction with other agents, such as AzaCdR, has shown promise (36, 83). Understanding how these drugs work, and how they interact, will be essential for the rational design of treatment regimens (10, 54). The killing of EBV-positive tumors by inducing the lytic cycle is also being investigated as a therapy. Our studies suggest that VPA may not be the best choice for oncolytic therapy. Ultimately it will be important to understand the mechanism by which different HDAC inhibitors used in medical treatment activate or repress the EBV lytic cycle in patients.
ACKNOWLEDGMENTS
This study was supported by NIH grants CA16038 and CA12055 to G.M. and by NIH grant 5 T32 GM07223 and an Anna Fuller Fellowship for Cancer Research to D.D. R.W. was supported by NIH MSTP TG 2T32GM07205. The Yale Center of Excellence in Molecular Hematology, and NIH 1 U24 NS051869 Neuroscience Microarray Consortium supported gene expression microarray studies.
We thank the Keck lab at Yale University and Shrikant Mane and Sheila Westman for generous assistance in microarray analysis. We thank Jill Countryman and Ayman El-Guindy for many helpful discussions.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Ambinder R. F., Robertson K. D., Tao Q. 1999. DNA methylation and the Epstein-Barr virus. Semin. Cancer Biol. 9:369–375 [DOI] [PubMed] [Google Scholar]
- 2. Amon W., Farrell P. J. 2005. Reactivation of Epstein-Barr virus from latency. Rev. Med. Virol. 15:149–156 [DOI] [PubMed] [Google Scholar]
- 3. Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 57:289–300 [Google Scholar]
- 4. Bergbauer M., et al. 2010. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog. 6:e1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhende P. M., Dickerson S. J., Sun X., Feng W. H., Kenney S. C. 2007. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J. Virol. 81:7363–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaheta R. A., Cinatl J., Jr 2002. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med. Res. Rev. 22:492–511 [DOI] [PubMed] [Google Scholar]
- 7. Blazkova J., et al. 2009. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 5:e1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bode A. M., Dong Z. 2004. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4:793–805 [DOI] [PubMed] [Google Scholar]
- 9. Bojic U., Elmazar M. M., Hauck R. S., Nau H. 1996. Further branching of valproate-related carboxylic acids reduces the teratogenic activity, but not the anticonvulsant effect. Chem. Res. Toxicol. 9:866–870 [DOI] [PubMed] [Google Scholar]
- 10. Bolden J. E., Peart M. J., Johnstone R. W. 2006. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5:769–784 [DOI] [PubMed] [Google Scholar]
- 11. Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 [DOI] [PubMed] [Google Scholar]
- 12. Cabrera R. M., Hill D. S., Etheredge A. J., Finnell R. H. 2004. Investigations into the etiology of neural tube defects. Birth Defects Res. C Embryo Today 72:330–344 [DOI] [PubMed] [Google Scholar]
- 13. Chang L. K., Liu S. T. 2000. Activation of the BRLF1 promoter and lytic cycle of Epstein-Barr virus by histone acetylation. Nucleic Acids Res. 28:3918–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman A. G., Croucher M. J., Meldrum B. S. 1984. Anticonvulsant activity of intracerebroventricularly administered valproate and valproate analogues. A dose-dependent correlation with changes in brain aspartate and GABA levels in DBA/2 mice. Biochem. Pharmacol. 33:1459–1463 [DOI] [PubMed] [Google Scholar]
- 15. Chau C. M., Lieberman P. M. 2004. Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus. J. Virol. 78:12308–12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chau C. M., Zhang X. Y., McMahon S. B., Lieberman P. M. 2006. Regulation of Epstein-Barr virus latency type by the chromatin boundary factor CTCF. J. Virol. 80:5723–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen G., Huang L. D., Jiang Y. M., Manji H. K. 1999. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 72:1327–1330 [DOI] [PubMed] [Google Scholar]
- 18. Chen G., Manji H. K., Hawver D. B., Wright C. B., Potter W. Z. 1994. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J. Neurochem. 63:2361–2364 [DOI] [PubMed] [Google Scholar]
- 19. Chen J., Ghazawi F. M., Bakkar W., Li Q. 2006. Valproic acid and butyrate induce apoptosis in human cancer cells through inhibition of gene expression of Akt/protein kinase B. Mol. Cancer 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J., et al. 2001. Activation of latent Kaposi's sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc. Natl. Acad. Sci. U. S. A. 98:4119–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen H. Y., et al. 2004. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell 13:627–638 [DOI] [PubMed] [Google Scholar]
- 22. Countryman J., et al. 2009. Stimulus duration and response time independently influence the kinetics of lytic cycle reactivation of Epstein-Barr virus. J. Virol. 83:10694–10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Countryman J., Jenson H., Seibl R., Wolf H., Miller G. 1987. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J. Virol. 61:3672–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Countryman J. K., Gradoville L., Miller G. 2008. Histone hyperacetylation occurs on promoters of lytic cycle regulatory genes in Epstein-Barr virus-infected cell lines which are refractory to disruption of latency by histone deacetylase inhibitors. J. Virol. 82:4706–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunningham M. O., Woodhall G. L., Jones R. S. 2003. Valproate modifies spontaneous excitation and inhibition at cortical synapses in vitro. Neuropharmacology 45:907–917 [DOI] [PubMed] [Google Scholar]
- 26. Daigle D., et al. 2010. Upregulation of STAT3 marks Burkitt lymphoma cells refractory to Epstein-Barr virus lytic cycle induction by HDAC inhibitors. J. Virol. 84:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Sarno P., Li X., Jope R. S. 2002. Regulation of Akt and glycogen synthase kinase-3β phosphorylation by sodium valproate and lithium. Neuropharmacology 43:1158–1164 [DOI] [PubMed] [Google Scholar]
- 28. Di Renzo F., Broccia M. L., Giavini E., Menegola E. 2007. Relationship between embryonic histonic hyperacetylation and axial skeletal defects in mouse exposed to the three HDAC inhibitors apicidin, MS-275, and sodium butyrate. Toxicol. Sci. 98:582–588 [DOI] [PubMed] [Google Scholar]
- 29. Eikel D., Lampen A., Nau H. 2006. Teratogenic effects mediated by inhibition of histone deacetylases: evidence from quantitative structure activity relationships of 20 valproic acid derivatives. Chem. Res. Toxicol. 19:272–278 [DOI] [PubMed] [Google Scholar]
- 30. Epstein M. A., et al. 1966. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J. Natl. Cancer Inst. 37:547–559 [PubMed] [Google Scholar]
- 31. Feng W. H., Hong G., Delecluse H. J., Kenney S. C. 2004. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J. Virol. 78:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng W. H., Israel B., Raab-Traub N., Busson P., Kenney S. C. 2002. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 62:1920–1926 [PubMed] [Google Scholar]
- 33. Feng W. H., Kenney S. C. 2006. Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res. 66:8762–8769 [DOI] [PubMed] [Google Scholar]
- 34. Feng W. H., et al. 2007. ZEB1 and c-Jun levels contribute to the establishment of highly lytic Epstein-Barr virus infection in gastric AGS cells. J. Virol. 81:10113–10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fu D. X., et al. 2008. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat. Med. 14:1118–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Manero G., et al. 2006. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood 108:3271–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gentleman R. C., et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glaser K. B., et al. 2003. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol. Cancer Ther. 2:151–163 [PubMed] [Google Scholar]
- 39. Göttlicher M., et al. 2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20:6969–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gradoville L., et al. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gradoville L., Kwa D., El-Guindy A., Miller G. 2002. Protein kinase C-independent activation of the Epstein-Barr virus lytic cycle. J. Virol. 76:5612–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gruffat H., Manet E., Sergeant A. 2002. MEF2-mediated recruitment of class II HDAC at the EBV immediate early gene BZLF1 links latency and chromatin remodeling. EMBO Rep. 3:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gurvich N., et al. 2005. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 19:1166–1168 [DOI] [PubMed] [Google Scholar]
- 44. Hardwick J. M., Lieberman P. M., Hayward S. D. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heston L., Rabson M., Brown N., Miller G. 1982. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature 295:160–163 [DOI] [PubMed] [Google Scholar]
- 46. Hoffmann K., Czapp M., Loscher W. 2008. Increase in antiepileptic efficacy during prolonged treatment with valproic acid: role of inhibition of histone deacetylases? Epilepsy Res. 81:107–113 [DOI] [PubMed] [Google Scholar]
- 47. Hu E., et al. 2003. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J. Pharmacol. Exp. Ther. 307:720–728 [DOI] [PubMed] [Google Scholar]
- 48. Hui K. F., Chiang A. K. 2010. Suberoylanilide hydroxamic acid induces viral lytic cycle in Epstein-Barr virus-positive epithelial malignancies and mediates enhanced cell death. Int. J. Cancer 126:2479–2489 [DOI] [PubMed] [Google Scholar]
- 49. Israel B. F., Kenney S. C. 2003. Virally targeted therapies for EBV-associated malignancies. Oncogene 22:5122–5130 [DOI] [PubMed] [Google Scholar]
- 50. Januchowski R., Dabrowski M., Ofori H., Jagodzinski P. P. 2007. Trichostatin A down-regulate DNA methyltransferase 1 in Jurkat T cells. Cancer Lett. 246:313–317 [DOI] [PubMed] [Google Scholar]
- 51. Jenkins P. J., Binne U. K., Farrell P. J. 2000. Histone acetylation and reactivation of Epstein-Barr virus from latency. J. Virol. 74:710–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joseph J., et al. 2004. Expression profiling of sodium butyrate (NaB)-treated cells: identification of regulation of genes related to cytokine signaling and cancer metastasis by NaB. Oncogene 23:6304–6315 [DOI] [PubMed] [Google Scholar]
- 53. Kraus R. J., Perrigoue J. G., Mertz J. E. 2003. ZEB negatively regulates the lytic-switch BZLF1 gene promoter of Epstein-Barr virus. J. Virol. 77:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lane A. A., Chabner B. A. 2009. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 27:5459–5468 [DOI] [PubMed] [Google Scholar]
- 55. Lechowicz M., et al. 2009. Molecular and clinical assessment in the treatment of AIDS Kaposi sarcoma with valproic acid. Clin. Infect. Dis. 49:1946–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lehrman G., et al. 2005. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet 366:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lieberman P. M. 2008. Chromatin organization and virus gene expression. J. Cell. Physiol. 216:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lieberman P. M. 2006. Chromatin regulation of virus infection. Trends Microbiol. 14:132–140 [DOI] [PubMed] [Google Scholar]
- 59. Löscher W. 2002. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 16:669–694 [DOI] [PubMed] [Google Scholar]
- 60. Lu F., et al. 2003. Chromatin remodeling of the Kaposi's sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J. Virol. 77:11425–11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luka J., Kallin B., Klein G. 1979. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 94:228–231 [DOI] [PubMed] [Google Scholar]
- 62. Lukac D. M., Renne R., Kirshner J. R., Ganem D. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304–312 [DOI] [PubMed] [Google Scholar]
- 63. Marks P. A. 2007. Discovery and development of SAHA as an anticancer agent. Oncogene 26:1351–1356 [DOI] [PubMed] [Google Scholar]
- 64. Miller G., et al. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Minucci S., Pelicci P. G. 2006. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer 6:38–51 [DOI] [PubMed] [Google Scholar]
- 66. Montalvo E. A., Cottam M., Hill S., Wang Y. J. 1995. YY1 binds to and regulates cis-acting negative elements in the Epstein-Barr virus BZLF1 promoter. J. Virol. 69:4158–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Monti B., Polazzi E., Contestabile A. 2009. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. 2:95–109 [DOI] [PubMed] [Google Scholar]
- 68. Moore S. M., Cannon J. S., Tanhehco Y. C., Hamzeh F. M., Ambinder R. F. 2001. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob. Agents Chemother. 45:2082–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Motohashi N. 1992. GABA receptor alterations after chronic lithium administration. Comparison with carbamazepine and sodium valproate. Prog. Neuropsychopharmacol. Biol. Psychiatr. 16:571–579 [DOI] [PubMed] [Google Scholar]
- 70. Orchel A., Dzierzewicz Z., Parfiniewicz B., Weglarz L., Wilczok T. 2005. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig. Dis. Sci. 50:490–498 [DOI] [PubMed] [Google Scholar]
- 71. Pearson G. R., et al. 1983. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J. Virol. 47:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perrine S. P., et al. 2007. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 109:2571–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Phiel C. J., et al. 2001. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276:36734–36741 [DOI] [PubMed] [Google Scholar]
- 74. Ragoczy T., Heston L., Miller G. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978–7984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rivero J. A., Adunyah S. E. 1998. Sodium butyrate stimulates PKC activation and induces differential expression of certain PKC isoforms during erythroid differentiation. Biochem. Biophys. Res. Commun. 248:664–668 [DOI] [PubMed] [Google Scholar]
- 76. Rowe M., et al. 2007. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc. Natl. Acad. Sci. U. S. A. 104:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rozen S., Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365–386 [DOI] [PubMed] [Google Scholar]
- 78. Sagot-Lerolle N., et al. 2008. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS 22:1125–1129 [DOI] [PubMed] [Google Scholar]
- 79. Shaw J. E., Levinger L. F., Carter C. W., Jr 1979. Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J. Virol. 29:657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shaw R. N., Arbiser J. L., Offermann M. K. 2000. Valproic acid induces human herpesvirus 8 lytic gene expression in BCBL-1 cells. AIDS 14:899–902 [DOI] [PubMed] [Google Scholar]
- 81. Siliciano J. D., et al. 2007. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J. Infect. Dis. 195:833–836 [DOI] [PubMed] [Google Scholar]
- 82. Smyth G. K. 12 February 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article3 [DOI] [PubMed] [Google Scholar]
- 83. Soriano A. O., et al. 2007. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood 110:2302–2308 [DOI] [PubMed] [Google Scholar]
- 84. Speck S. H., Chatila T., Flemington E. 1997. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 5:399–405 [DOI] [PubMed] [Google Scholar]
- 85. Stimson L., Wood V., Khan O., Fotheringham S., La Thangue N. B. 2009. HDAC inhibitor-based therapies and haematological malignancy. Ann. Oncol. 20:1293–1302 [DOI] [PubMed] [Google Scholar]
- 86. Sun R., et al. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. U. S. A. 95:10866–10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Takada K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27–32 [DOI] [PubMed] [Google Scholar]
- 88. Tan J., Cang S., Ma Y., Petrillo R. L., Liu D. 2010. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J. Hematol. Oncol. 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tao Q., Robertson K. D. 2003. Stealth technology: how Epstein-Barr virus utilizes DNA methylation to cloak itself from immune detection. Clin. Immunol. 109:53–63 [DOI] [PubMed] [Google Scholar]
- 90. Taylor N., Countryman J., Rooney C., Katz D., Miller G. 1989. Expression of the BZLF1 latency-disrupting gene differs in standard and defective Epstein-Barr viruses. J. Virol. 63:1721–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tierney R. J., et al. 2000. Methylation of transcription factor binding sites in the Epstein-Barr virus latent cycle promoter Wp coincides with promoter down-regulation during virus-induced B-cell transformation. J. Virol. 74:10468–10479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. VanDongen A. M., VanErp M. G., Voskuyl R. A. 1986. Valproate reduces excitability by blockage of sodium and potassium conductance. Epilepsia 27:177–182 [DOI] [PubMed] [Google Scholar]
- 93. Van Lint C., Emiliani S., Ott M., Verdin E. 1996. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 15:1112–1120 [PMC free article] [PubMed] [Google Scholar]
- 94. Wang R., Cherukuri P., Luo J. 2005. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J. Biol. Chem. 280:11528–11534 [DOI] [PubMed] [Google Scholar]
- 95. Westphal E. M., Blackstock W., Feng W., Israel B., Kenney S. C. 2000. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60:5781–5788 [PubMed] [Google Scholar]
- 96. Williams S. A., Greene W. C. 2005. Host factors regulating post-integration latency of HIV. Trends Microbiol. 13:137–139 [DOI] [PubMed] [Google Scholar]
- 97. Xiong Y., et al. 2005. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 65:2684–2689 [DOI] [PubMed] [Google Scholar]
- 98. Xu W. S., Parmigiani R. B., Marks P. A. 2007. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26:5541–5552 [DOI] [PubMed] [Google Scholar]
- 99. Yamamoto R., Yanagita T., Kobayashi H., Yokoo H., Wada A. 1997. Up-regulation of sodium channel subunit mRNAs and their cell surface expression by antiepileptic valproic acid: activation of calcium channel and catecholamine secretion in adrenal chromaffin cells. J. Neurochem. 68:1655–1662 [DOI] [PubMed] [Google Scholar]
- 100. Yao C. P., Mather G. G., Stephens J. R., Levy R. H. 1999. Cytotoxicity induced by the combination of valproic acid and tumor necrosis factor-alpha: implication for valproic acid-associated hepatotoxicity syndrome. Biochem. Pharmacol. 58:455–459 [DOI] [PubMed] [Google Scholar]
- 101. Ye J., Gradoville L., Daigle D., Miller G. 2007. De novo protein synthesis is required for lytic cycle reactivation of Epstein-Barr virus, but not Kaposi's sarcoma-associated herpesvirus, in response to histone deacetylase inhibitors and protein kinase C agonists. J. Virol. 81:9279–9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ye J., Shedd D., Miller G. 2005. An Sp1 response element in the Kaposi's sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 79:1397–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yoo C. B., Jones P. A. 2006. Epigenetic therapy of cancer: past, present and future. Nat. Rev. Drug Discov. 5:37–50 [DOI] [PubMed] [Google Scholar]
- 104. Yuan P. X., et al. 2001. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 276:31674–31683 [DOI] [PubMed] [Google Scholar]
- 105. Yuan Z. L., Guan Y. J., Chatterjee D., Chin Y. E. 2005. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307:269–273 [DOI] [PubMed] [Google Scholar]
- 106. Zalani S., Coppage A., Holley-Guthrie E., Kenney S. 1997. The cellular YY1 transcription factor binds a cis-acting, negatively regulating element in the Epstein-Barr virus BRLF1 promoter. J. Virol. 71:3268–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zalani S., Holley-Guthrie E., Kenney S. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. U. S. A. 93:9194–9199 [DOI] [PMC free article] [PubMed] [Google Scholar]