Abstract
Vesicular stomatitis virus (VSV)-based oncolytic virotherapy has the potential to significantly improve the prognosis of aggressive malignancies such as brain cancer. However, VSV's inherent neurotoxicity has hindered clinical development so far. Given that this neurotropism is attributed to the glycoprotein VSV-G, VSV was pseudotyped with the nonneurotropic envelope glycoprotein of the lymphocytic choriomeningitis virus (LCMV-GP→VSV-GP). Compared to VSV, VSV-GP showed enhanced infectivity for brain cancer cells in vitro while sparing primary human and rat neurons in vitro and in vivo, respectively. In conclusion, VSV-GP has a much wider therapeutic window than VSV and is thus more suitable for clinical applications, especially in the brain.
TEXT
Malignant gliomas are the most aggressive and lethal intracranial tumors, and despite advances in surgery, radiation, and chemotherapy, patient survival is still only 1 to 2 years (15, 40). Therefore, various innovative approaches are under investigation (25, 27). Several groups have developed oncolytic viruses (OV) for cancer therapy, but to date, most of these viruses are not sufficiently effective and safe for use in patient treatment (1, 2, 13, 46). The same holds true for the vesicular stomatitis virus (VSV), which is one of the most promising OV candidates, as it has a short, highly cytolytic, and productive replication cycle, a high susceptibility to interferon (IFN) responses, which mediates its cancer selectivity (38), and has virtually no preexisting immunity in humans (3, 32, 44). However, VSV is neurotoxic, which limits its application, especially in the brain (19, 22, 42).
Researchers have attempted to overcome this limitation by generating attenuated viruses (AV) (10, 23, 39). But the reduced toxicity comes at the expense of reduced replication competence and oncolytic activity, so that it is doubtful whether AVs would be effective in the patient (43). As the viral glycoprotein determines the tropism, the neurotropism is mainly attributed to the glycoprotein VSV-G (8, 41, 42). Hence, we aimed to detarget VSV from neurons at the entry level by pseudotyping. The glycoprotein GP of the viscerotropic lymphocytic choriomeningitis virus WE strain (LCMV-WE-HPI) was selected, based on previous findings with respect to the tropism of LCMV-GP (GP)-pseudotyped lentiviral vectors (4, 9). In contrast to VSV-G pseudotypes, GP-pseudotyped lentiviral vectors had little infectivity for neurons in vitro and in rat brains but were at least as infectious for malignant gliomas (28).
To reliably determine the cellular tropism of VSV-GP, we used two variants of a propagation-deficient, green fluorescent protein (GFP)-expressing VSV*ΔG vector (Indiana serotype) which lacks the G gene: (i) wild-type “cytopathic” VSV*McpΔG and (ii) VSV*MQΔG, which has reduced cytopathicity due to attenuating mutations in the M protein (16, 18). VSV*MQΔG was included to ensure longer survival of infected cells to facilitate monitoring of infection. To produce the single-cycle infectious VSV-G or VSV-GP virus, the respective viral glycoprotein was provided in trans by transient expression of VSV-G or stable expression of LCMV-GP. The stable cell line was generated by transduction of BHK-21 cells with the lentiviral self-inactivating (SIN) vector pHR′SIN-GPopt, encoding codon-optimized LCMV-GP, as previously described (4, 5, 14).
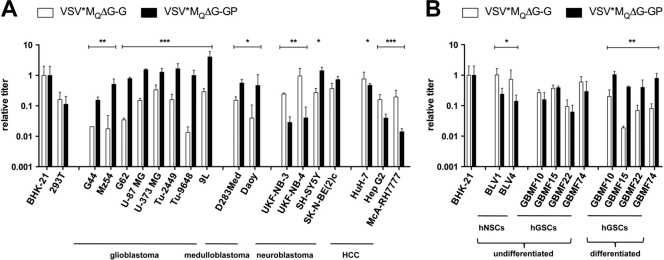
The GP pseudotype (VSV-GP) is more infectious for brain tumor cells than VSV-G.
A panel of human [G44, G62 (17, 28), Mz54, U-87 MG, U-373 MG, D283Med, Daoy, UKF-NB-3, UKF-NB-4, SK-N-BE(2)c, SH-SY5Y (6, 12), HuH7, and HepG2], murine (Tu-2449 and Tu-9648), and rat (9L and McA-RH7777) cancer cell lines from three different central nervous system (CNS) neoplasms (glioma, medulloblastoma, and neuroblastoma) as well as from hepatocellular carcinomas (HCC), as an unrelated control, were tested for their susceptibility to VSV*ΔG pseudotypes. Virus titers were determined by transduction of cells with serial 10-fold dilutions of the inoculum and flow cytometric analysis to determine GFP expression at 16 h postransduction. Titers are given relative to the titer determined for BHK-21 as the reference cell line.
The LCMV-GP pseudotype VSV*MQΔG-GP was significantly (5- to 80-fold) more infectious for all malignant glioma cell lines (P < 0.01 to P < 0.0001) and both medulloblastoma cell lines (P < 0.05) than VSV*MQΔG-G (Fig. 1A). The neuroblastoma cell lines UKF-NB-3 and UKF-NB-4 were more susceptible to VSV*MQΔG-G (P < 0.01), while SH-SY5Y cells were slightly more susceptible to VSV*MQΔG-GP (P < 0.05). Titers of VSV*MQΔG-G and VSV*MQΔG-GP on SK-N-BE(2)c cells were not significantly different. Cell lines derived from HCC were all more susceptible to VSV*MQΔG-G than to VSV*MQΔG-GP (P < 0.05 to P < 0.001). The tropism of the cytopathic VSV*McpΔG pseudotypes was consistent with that of the VSV*MQΔG pseudotypes (see Fig. S1 in the supplemental material).
Fig. 1.
Preferential tropism of VSV*ΔG-GP for malignant glioma cells. Different tumor cell lines (A) and “differentiated” as well as undifferentiated human neural stem cell (hNSC) and human glioma stem cell (hGSC) cultures (B) were infected with 10-fold serial dilutions of VSV*MQΔG-G and VSV*MQΔG-GP. Titers were analyzed 16 h postransduction by flow cytometry based on GFP expression. Relative titers were determined in relation to BHK-21 as the reference cell line. The data shown represent the means and standard deviations of the results of at least three infection experiments. VSV*MQΔG-G and VSV*MQΔG-GP infection titers were compared. HCC, hepatocellular carcinoma; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In addition, we tested infectivity by the use of primary human glioma-derived stem cell (hGSC) cultures, which have previously been shown to better reflect the genetic heterogeneity of the originating tumor (26). Human neural stem cells (hNSCs) were used as an internal control (Fig. 1B). Isolation and expansion of hNSCs (BLV1 and BLV4) and hGSCs (GBMF10, -15, -22, and -74) from primary tissues of different patients were essentially carried out as previously described for murine neural stem cells (21). While the hNSCs were more susceptible to VSV*MQΔG-G (P < 0.05), the levels of susceptibility of hGSCs for the two variants did not differ significantly. However, after induced “differentiation” of hGSCs by dissociation of neurospheres and replating in serum-supplemented Dulbecco's modified Eagle medium-F12 (DMEM-F12), the malignant cells became substantially (5- to 30-fold) more susceptible to VSV*MQΔG-GP than to VSV*MQΔG-G (P < 0.01 to P < 0.001), analogous to serum-grown glioma cell line results (Fig. 1A). Thus, these two types of brain cancer, malignant glioma and medulloblastoma, were especially susceptible to GP-pseudotyped VSV-complemented vectors relative to VSV-G-complemented vectors.
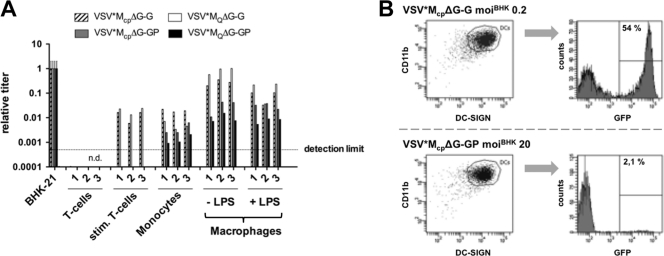
Low susceptibility of immune cells to VSV-GP.
Peripheral blood mononuclear cells (PBMCs), as off-target cells, could mediate viral spread, thereby promoting systemic toxicity but possibly also enhancing therapeutic efficacy (20). Furthermore, infection of monocyte-derived macrophages and dendritic cells (DCs) could boost the antiviral immune response, restraining OV replication. Hence, the infectivity of VSV*ΔG pseudotypes was analyzed using human T lymphocytes, monocytes, macrophages, and DCs of three healthy donors (Fig. 2).
Fig. 2.
VSV*ΔG-GP shows negligible transduction of PBMCs, macrophages, and dendritic cells. PBMCs were isolated from blood and T cells were stimulated as previously described (30). CD14+ monocytes were purified by magnetic sorting using anti-CD14 microbeads (Miltenyi Biotec). CD14+ monocyte-derived macrophages were differentiated in vitro by adding 50 ng/ml recombinant human macrophage colony stimulation factor (rhM-CSF) (Invitrogen) for 6 days. Monocyte-derived DCs were generated after culturing CD14+ cells with IL-4 and GM-CSF in vitro (35). Macrophages were activated overnight by LPS stimulation (100 ng/ml). Freshly isolated T cells and activated T cells as well as monocytes and macrophages (A) and DCs (B) were infected with 10-fold serial dilutions of VSV*ΔG-G and VSV*ΔG-GP. Titers were analyzed by flow cytometry based on the expression of GFP and normalized to the reference BHK-21 hamster cell line. GFP expression by DCs (gated on CD11b+/DC-SIGN+) was quantified 16 h postinfection by flow cytometry. n.d., not detectable.
No differences in infectivity between the reduced cytopathic and cytopathic VSV variants in T lymphocytes were observed (Fig. 2A). Freshly isolated T cells were more than 104-fold less susceptible for both VSV pseudotypes than BHK-21 cells; even after infection with 20 transducing units (TU)/cell, infection levels remained below the detection limit. After stimulation (30), detection of T lymphocyte infection required 100-fold-higher multiplicities of infection (MOIs) than BHK-21 infection for VSV*ΔG-G and no infection of stimulated T cells was detected for VSV*ΔG-GP. In monocytes, the cytopathic variant tended to be more infectious, whereas unstimulated and lipopolysaccharide (LPS)-stimulated macrophages were slightly more susceptible to VSV*MQΔG-G than to VSV*MQΔG-GP. Monocyte titers were around 100-fold and 1,000-fold lower than BHK-21 titers for VSV*ΔG-G and VSV*ΔG-GP, respectively. After differentiation, the resulting macrophages became more susceptible to both pseudotypes but again slightly lost susceptibility after LPS-induced activation (Fig. 2A). In general, VSV*ΔG-G infected monocytes and macrophages more efficiently than VSV*ΔG-GP. Monocyte-derived DCs were not at all susceptible to either VSV*MQΔG variant (see Fig. S2 in the supplemental material), presumably due to an induced interferon response. However, as shown in Fig. 2B, DCs are susceptible to infection with VSV*McpΔG-G but could not be efficiently infected with the GP pseudotype. Infection with VSV*McpΔG-G (MOIBHK of 0.2) resulted in 54% GFP+/CD11b+/DC-SIGN+ DCs at 16 h postexposure, whereas 20 TU/cell of VSV*McpΔG-GP resulted in 2.1% infected DCs. In conclusion, all immune cells tested were considerably less susceptible to the GP pseudotype than to VSV-G-complemented vectors. Thus, VSV-GP may be less susceptible to viral clearance via the host immune system.
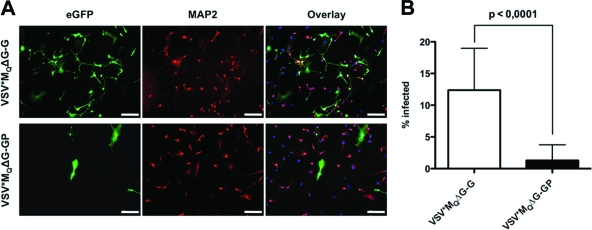
Reduced neurotropism of GP pseudotypes in vitro and in vivo.
Neurotoxicity is dose limiting and has so far precluded VSV treatment of brain cancer. As we had determined that GP pseudotypes efficiently target brain tumor cells (Fig. 1), we next analyzed the tropism of VSV*ΔG-GP relative to VSV*ΔG-G for primary human neurons. Cells were infected with 4 TU/cell and stained with anti-microtubule-associated protein 2 (anti-MAP-2; for human neurons) and DAPI (4′,6-diamidino-2-phenylindole) at 16 h postinfection. VSV*MQΔG-G infection was much more efficient than VSV*MQΔG-GP infection (Fig. 3A). For quantification, cultures were infected with 1 TU/cell and GFP+/MAP2+ cells were counted. The percentage of infected neurons was significantly (P < 0.001) higher in VSV*MQΔG-G (12.37 ± 1.21%) infected cultures than in VSV*MQΔG-GP infected cultures (1.29 ± 0.46%) (Fig. 3B).
Fig. 3.
Reduced neurotropism of LCMV-GP pseudotypes in vitro. Primary human neurons (ScienCell Research Laboratories) were grown on 2 μg/cm2 poly-l-lysine-coated culture vessels and infected with VSV*MQΔG-G and VSV*MQΔG-GP at an MOIBHK of 4 (A) or 1 (B). At 16 h postransduction, cultures were fixed in 4% paraformaldehyde. Neurons were stained with mouse anti-MAP2 (Sigma) primary antibody and goat anti-mouse Alexa Fluor 568-conjugated (Invitrogen) secondary antibody. Nuclei were counterstained with DAPI (Roche). (A) A representative microscopic field is shown. eGFP, enhanced GFP. Bars, 200 μm. (B) The percentages of GFP+/MAP2+ cells were determined by fluorescence microscopy, counting 30 microscopic fields per pseudotype. Values are expressed as means ± standard deviations (SD) and were tested for significance by Student's t test (P < 0.0001).
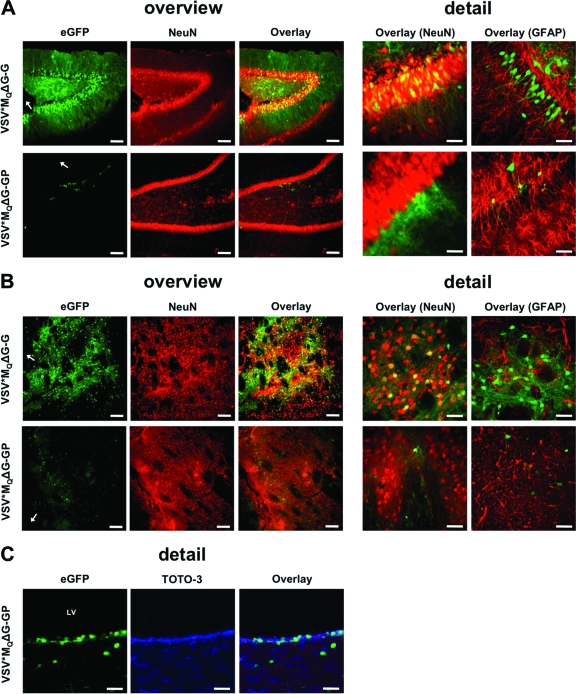
Next, we injected 7 × 105 TU of each pseudotype (reduced cytopathic variant) into the striatum and the hippocampus region of rat brains (28). On the following day, animals were sacrificed and microscopic slides were prepared. Neurons were stained with anti-NeuN and astrocytes with anti-glial fibrillary acidic protein (anti-GFAP), and expression of GFP was analyzed by confocal laser scanning microscopy. Massive infection, almost exclusively of neurons, was observed for VSV*MQΔG-G, as shown by GFP+/NeuN+ cells and neurite-localized GFP expression (Fig. 4). The virus was able to efficiently penetrate the examined brain region and infected cells as far as >2 mm from the injection site (Fig. 4A and B). These observations are in agreement with the previously described tropism of VSV and VSV-G-pseudotyped lentiviral vectors (29, 42). In contrast, VSV*MQΔG-GP infection rates were considerably lower, with only a few predominantly glial cells infected (Fig. 4A, detail). This is in accordance with our in vitro data on GP-pseudotyped VSV (Fig. 1B, Fig. 3) as well as with the tropism of lentiviral GP pseudotypes (28, 37). In particular, Stein et al. found a transduction preference of LCMV-WE-HPI-pseudotyped feline immunodeficiency virus for GFAP-positive astrocytes in the striatum and subventricular zone (SVZ) as well as for SVZ neural progenitor cells in vivo (37).
Fig. 4.
Reduced neurotropism of LCMV-GP pseudotypes in vivo. Adult Fischer F344/NCrHsd rats were inoculated intracranially (hippocampus or striatum region) with 7 × 105 TU/ml of VSV-G-complemented or GP-pseudotyped VSV*MQΔG vectors. At 24 h postinfection, brains were removed and suspended in phosphate-buffered saline (PBS)–4% paraformaldehyde. Coronal brain sections (40 μm thick) were prepared. Neurons were counterstained with mouse anti-NeuN (clone A60) and astrocytes with rabbit anti-GFAP (Dako) primary antibody. The respective Alexa Fluor 568-conjugated antibodies were used as secondary antibodies, and nuclear counterstaining was performed with DAPI and TOTO-3 iodide (Invitrogen). (A) Transduction of hippocampal neurons with VSV-G- and GP-pseudotyped vectors. (B) Transduction of striatal neurons by VSV-G- and GP-pseudotyped vectors. (C) Transduction of ependymal cells by VSV*MQΔG-GP. Bars for overview, 400 μm; bars for detail, 50 μm; arrow, injection site; LV, lateral ventricle.
As the LCMV-WE-HPI envelope glycoprotein displays high-affinity binding to its α-dystroglycan cellular receptor (24), which is likewise expressed on neurons and astrocytes (36, 45), the observed scarce infection of neurons may be due to (i) different glycosylation patterns of α-dystroglycan in distinct cell types of the CNS (33), (ii) alternative, yet-unidentified receptor and/or coreceptor usage in the brain (24), or (iii) the use of propagation-deficient vectors, which, in contrast to wild-type LCMV vectors, cannot spread via direct cell-cell contact from glial cells to proximal neurons (7). Further studies to analyze whether replication-competent VSV-GP can spread from glial cells to neurons are ongoing. In accordance with previous studies on LCMV-WE tropism, VSV*MQΔG-GP was able to efficiently infect the ependymal cell layer of the lateral ventricle (Fig. 4C) (11, 34).
In summary, relative to VSV, LCMV-GP-pseudotyped VSV exhibits an increased infectivity for brain cancer cells, whereas its neurotropism is highly attenuated. Immune cells (as potential off-target cells) are also considerably less susceptible to VSV-GP than to VSV-G. Taken together, these consistent data indicate that GP-pseudotyped VSV has a beneficial toxicity as well as efficacy profile and may render VSV eligible for use in the brain for potential treatment of brain cancer. However, use of a propagation-incompetent VSV-GP would presumably result in minor antitumoral effectiveness, since preclinical and clinical trials have shown that incomplete viral transduction or distribution within the tumor is a major obstacle that eventually contributes to therapy failure (31). Thus, the use of carrier cell-assisted viral delivery as well as a replication-competent recombinant rVSV-GP is a promising strategy currently under evaluation.
Supplementary Material
Acknowledgments
The work was supported by grants from the Wilhelm Sander Foundation and the Deutsche Forschungsgemeinschaft (Graduate College 1172).
We thank Patricia Schult-Dietrich for expert technical assistance.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Aghi M., Martuza R. L. 2005. Oncolytic viral therapies—the clinical experience. Oncogene 24:7802–7816 [DOI] [PubMed] [Google Scholar]
- 2. Aghi M. K., Chiocca E. A. 2009. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol. Ther. 17:8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barber G. N. 2004. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 17:516–527 [DOI] [PubMed] [Google Scholar]
- 4. Beyer W. R., Miletic H., Ostertag W., von Laer D. 2001. Recombinant expression of lymphocytic choriomeningitis virus strain WE glycoproteins: a single amino acid makes the difference. J. Virol. 75:1061–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beyer W. R., Westphal M., Ostertag W., von Laer D. 2002. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J. Virol. 76:1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biedler J. L., Spengler B. A. 1976. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science 191:185–187 [DOI] [PubMed] [Google Scholar]
- 7. Bonthius D. J., Mahoney J., Buchmeier M. J., Karacay B., Taggard D. 2002. Critical role for glial cells in the propagation and spread of lymphocytic choriomeningitis virus in the developing rat brain. J. Virol. 76:6618–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boritz E., Gerlach J., Johnson J. E., Rose J. K. 1999. Replication-competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway. J. Virol. 73:6937–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cannon J. R., Sew T., Montero L., Burton E. A., Greenamyre J. T. 2011. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp. Neurol. 228:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarke D. K., et al. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cole G. A., Gilden D. H., Monjan A. A., Nathanson N. 1971. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed. Proc. 30:1831–1841 [PubMed] [Google Scholar]
- 12. Ebener U., Wehner S., Cinatl J., Gussetis E. S., Kornhuber B. 1990. Expression of markers shared between human haematopoietic cells and neuroblastoma cells. Anticancer Res. 10:887–890 [PubMed] [Google Scholar]
- 13. European Medicines Agency 2009. Questions and answers on the recommendation for the refusal of the marketing authorisation for Cerepro (sitimagene ceradenovec). EMA/828428/2009 European Medicines Agency, London, United Kingdom [Google Scholar]
- 14. Fischer Y. H., et al. 2007. A retroviral packaging cell line for pseudotype vectors based on glioma-infiltrating progenitor cells. J. Gene Med. 9:335–344 [DOI] [PubMed] [Google Scholar]
- 15. Furnari F. B., et al. 2007. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21:2683–2710 [DOI] [PubMed] [Google Scholar]
- 16. Hanika A., et al. 2005. Use of influenza C virus glycoprotein HEF for generation of vesicular stomatitis virus pseudotypes. J. Gen. Virol. 86:1455–1465 [DOI] [PubMed] [Google Scholar]
- 17. Heese O., Disko A., Zirkel D., Westphal M., Lamszus K. 2005. Neural stem cell migration toward gliomas in vitro. Neuro. Oncol. 7:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann M., et al. 2010. Fusion-active glycoprotein G mediates the cytotoxicity of vesicular stomatitis virus M mutants lacking host shut-off activity. J. Gen. Virol. 91:2782–2793 [DOI] [PubMed] [Google Scholar]
- 19. Huneycutt B. S., et al. 1994. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain Res. 635:81–95 [DOI] [PubMed] [Google Scholar]
- 20. Ilett E. J., et al. 2009. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 16:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johansson C. B., et al. 1999. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96:25–34 [DOI] [PubMed] [Google Scholar]
- 22. Johnson J. E., et al. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly E. J., Nace R., Barber G. N., Russell S. J. 2010. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J. Virol. 84:1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kunz S., Sevilla N., Rojek J. M., Oldstone M. B. 2004. Use of alternative receptors different than alpha-dystroglycan by selected isolates of lymphocytic choriomeningitis virus. Virology 325:432–445 [DOI] [PubMed] [Google Scholar]
- 25. Kyritsis A. P., Sioka C., Rao J. S. 2009. Viruses, gene therapy and stem cells for the treatment of human glioma. Cancer Gene Ther. 16:741–752 [DOI] [PubMed] [Google Scholar]
- 26. Lee J., et al. 2006. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9:391–403 [DOI] [PubMed] [Google Scholar]
- 27. Liu T. C., Galanis E., Kirn D. 2007. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 4:101–117 [DOI] [PubMed] [Google Scholar]
- 28. Miletic H., et al. 2004. Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum. Gene Ther. 15:1091–1100 [DOI] [PubMed] [Google Scholar]
- 29. Naldini L., Blomer U., Gage F. H., Trono D., Verma I. M. 1996. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 93:11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newrzela S., Gunda B., von Laer D. 2009. T cell culture for gammaretroviral transfer. Methods Mol. Biol. 506:71–82 [DOI] [PubMed] [Google Scholar]
- 31. Pulkkanen K. J., Yla-Herttuala S. 2005. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 12:585–598 [DOI] [PubMed] [Google Scholar]
- 32. Rose J. K., Whitt M. A. 2001. Rhabdoviridae: the viruses and their replication, p. 1221–1244 Fields virology, 4th ed Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 33. Satz J. S., et al. 2010. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J. Neurosci. 30:14560–14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwendemann G., Lohler J., Lehmann-Grube F. 1983. Evidence for cytotoxic T-lymphocyte-target cell interaction in brains of mice infected intracerebrally with lymphocytic choriomeningitis virus. Acta Neuropathol. 61:183–195 [DOI] [PubMed] [Google Scholar]
- 35. Shortman K., Naik S. H. 2007. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7:19–30 [DOI] [PubMed] [Google Scholar]
- 36. Smelt S. C., et al. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stein C. S., Martins I., Davidson B. L. 2005. The lymphocytic choriomeningitis virus envelope glycoprotein targets lentiviral gene transfer vector to neural progenitors in the murine brain. Mol. Ther. 11:382–389 [DOI] [PubMed] [Google Scholar]
- 38. Stojdl D. F., et al. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821–825 [DOI] [PubMed] [Google Scholar]
- 39. Stojdl D. F., et al. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275 [DOI] [PubMed] [Google Scholar]
- 40. Stupp R., et al. 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352:987–996 [DOI] [PubMed] [Google Scholar]
- 41. Tani H., et al. 2007. Replication-competent recombinant vesicular stomatitis virus encoding hepatitis C virus envelope proteins. J. Virol. 81:8601–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Pol A. N., Dalton K. P., Rose J. K. 2002. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 76:1309–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wollmann G., Rogulin V., Simon I., Rose J. K., van den Pol A. N. 2010. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J. Virol. 84:1563–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wollmann G., Tattersall P., van den Pol A. N. 2005. Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J. Virol. 79:6005–6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zaccaria M. L., Di Tommaso F., Brancaccio A., Paggi P., Petrucci T. C. 2001. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience 104:311–324 [DOI] [PubMed] [Google Scholar]
- 46. Zemp F. J., Corredor J. C., Lun X., Muruve D. A., Forsyth P. A. 2010. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 21:103–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.