Abstract
Premature delivery is often complicated by neonatal growth restriction and neurodevelopmental impairment. Because global over-nutrition increases the risk of adult metabolic syndrome, we sought a targeted intervention. Premature delivery and perinatal growth restriction decrease circulating levels of the neurotrophic hormone leptin. We hypothesized leptin supplementation would normalize the outcomes of mice with incipient neonatal growth restriction. Pups were fostered into litters of 6 or 12 to elicit divergent growth patterns. Pups in each litter received injections of saline or leptin from day 4–14. At 4 months, mice underwent tail cuff blood pressure measurement, behavioral testing and MRI. Mice fostered in litters of 12 had decreased weanling weights and leptin levels. Neonatal leptin administration normalized plasma leptin levels without influencing neonatal growth. Leptin replacement also normalized the hypertension, stress-linked immobility, conditioned fear, and amygdala enlargement seen in neonatal growth restricted male mice. In control males, neonatal leptin administration led to hypothalamic enlargement, without overt neurocardiovascular alterations. Female mice were less susceptible to the effects of neonatal growth restriction or leptin supplementation. In conclusion, the effects of neonatal leptin administration are modulated by concurrent growth and gender. In growth restricted male mice, physiologic leptin replacement improves adult neurocardiovascular outcomes.
INTRODUCTION
With advances in obstetric and neonatal care, an increasing percentage of premature infants survive to hospital discharge (1). Unfortunately, premature infants do not maintain the intrauterine growth rates of the reference fetus. Once infants reach 36 weeks postmenstrual age, 89% weigh less than the 10th percentile (2). This postnatal growth restriction (GR) is associated with an increased incidence of neurodevelopmental impairment, anxiety disorders, and hypertension with male infants at disproportionate risk for such complications (1, 3–5). While nutritional interventions may improve neurodevelopmental outcomes, accelerated neonatal growth may increase the risk of type 2 diabetes mellitus and obesity (6–8). Targeted interventions to support brain growth without excessive somatic growth may thus optimize long-term neurologic, metabolic and cardiovascular outcomes.
Recent studies have demonstrated premature delivery and perinatal GR dramatically decrease circulating levels of the neurotrophic factor leptin (9–11). Leptin, the product of the obesity (ob) gene, is produced by adipocytes to signal satiety and suppress appetite. Leptin levels fall dramatically during fasting or undernutrition, a response that is accentuated as adiposity declines. Beginning in the third trimester of human fetal development, transplacental leptin exposure exerts critical neurotrophic effects (11–12).
Because rodents are born with neurodevelopmental immaturity, the developmental role of leptin has been modeled in neonatal mice and rats. In these studies, neonatal GR leads to circulating leptin deficiency, impaired hypothalamic development, and adult leptin resistance (13–16). Further studies have shown that mice genetically deficient for leptin (ob/ob mice) have impaired hypothalamic projections and decreased adult brain weights (17, 18). In leptin deficient mice, neonatal leptin replacement increases adult brain weight, myelination, and dendritic arborization (17, 18). We developed an isogenic GR model to test the hypothesis that neonatal leptin replacement will improve the neurodevelopmental and cardiovascular outcomes of neonatal GR mice.
METHODS
Animal Model
All animal procedures were approved by the University of Iowa Animal Care and Use Committee. C57BL/6J mice were bred from initial stock (Jackson Laboratories, Bar Harbor, ME). Utilizing an original breeding colony of 334 mice, we determined the natural distribution of birth weights and demonstrated a strong inverse relationship between litter size and pup growth (8). To isolate the effects of neonatal GR, only mice with birth weights above the 10th percentile for our colony (≥1.56g) were retained. These appropriate birth weight mice were cross-fostered into litters of 6 or 12 pups from day of life 1 to 21 to obtain control mice and neonatal GR mice, respectively. A total of 12 litters were utilized for these studies.
From postnatal days 4–14, half of the pups within each litter were randomized to daily intraperitoneal injections of leptin (80 ng/g) while the other half received vehicle (10 ml/kg of normal saline) (19). On day 10, during the critical phase of leptin-dependent hypothalamic development (14, 17), three litters of pups were isolated from their dam for 1 hour, immediately prior to the scheduled saline or leptin injection. Isoflurane anesthesia was induced, plasma was collected by cardiac puncture, and leptin levels were assessed by enzyme immunoassay, as previously described (20). The remaining pups were weaned on day 20. Adult phenotypes were evaluated beginning at 4 months.
Adult Phenotypes
Tail cuff systolic blood pressure (SBP) and heart rate were measured 30 times daily over 5 consecutive days, as previously described (20). At least one week after the hemodynamic measurements, mice were placed into a 40.6cm by 40.6cm brightly illuminated open field. Open field testing was utilized to assess overall locomotor activity. Measuring the relative avoidance of the 25.4cm by 25.4cm central region (thigmotaxis) further screened for the presence of unconditioned anxiety.
Mice then underwent fear conditioning. On the training day, mice were placed in a fear conditioning chamber (MED Associates, St. Albans, VT). After 3 minutes, they were presented with a tone (80 dB, 20 sec) that co-terminated with an electric foot-shock (0.5 mA, 1 sec) a total of 5 times at 2 minute intervals. The time spent in a characteristic posture (“freezing”) was recorded by automated video tracking software whenever the only movement was respiration. The following day, cue conditioned fear was assessed by placing the mice in a novel context. The amount of time spent freezing was continuously recorded for 10 minutes, including 3 minutes of baseline, 3 minutes of representation of the learned auditory cue (tone), and 4 minutes of recovery. This fear conditioning protocol measures memory of an aversive experience (freezing during the tone) as well as the persistence of the fear-related response (post-tone freezing).
Adult Morphology
After the cardiovascular and behavioral phenotypes were assessed, MRI was completed with a 4.7 Tesla Varian Unity/INOVA system (Varian Inc., Palo Alto, CA). T1-weighted images were acquired in axial, sagittal, and coronal planes using a fast spin-echo pulse sequence with TR/TE = 600/12 ms, and echo train length of 2. Additionally, a more T2-weighted scan with TR/TE = 1600/48 ms and echo train length of 8 was acquired. Following acquisition, MR data were transferred to the Psychiatry Iowa Neuroimaging Consortium image processing laboratory for post-processing with the BRAINS2 software package (21). The skull stripped T2 images were registered to the MAP2006.t2avg.nii.gz image from the MBAT Atlas created by Mouse BIRN using a non-rigid affine transformation. MRI acquisition and processing were completed by investigators blinded to group assignment. At the completion of the studies, weight and length were recorded and isoflurane anesthesia was induced for organ measurement. Lengths were not obtained for the first 10 males (4 control-saline, 4 GR-saline, 1 control-leptin, and 1 GR-leptin).
Data Analysis
All values are presented as mean ± SE. Each group was comprised of mice from at least 5 separate litters. The effect of litter size or leptin administration on pup weight from day 4 to 14 was assessed by 2 way repeat measures ANOVA. Absolute MRI volumes were compared by two-tailed Student’s t-tests. All other data were compared by 2-way ANOVA, factoring for neonatal GR and leptin administration. Post hoc analysis (Holm-Sidak method) was performed if statistically significant differences were detected. When ANOVA identified a significant interaction between neonatal GR and leptin, data were compared by unpaired two-tailed Student’s t-tests. A value of P<0.05 was considered significant. Figure legends provide the ANOVA F-statistics and degrees of freedom, followed by the results of post-hoc testing. All analyses were performed using SigmaStat 3.0 (SPSS Inc., Chicago, IL).
RESULTS
Animal Model
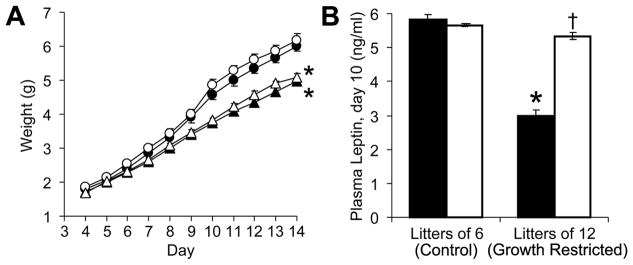
Mice fostered in litters of 12 pups developed neonatal GR and decreased plasma leptin levels (Figure 1). While leptin administration did not significantly alter neonatal growth (Figure 1A), the daily leptin injections increased circulating leptin levels in GR mice (Figure 1B, P<0.001). At 4 months, we began to assess the effects of neonatal leptin supplementation on adult neurocardiovascular outcomes.
Figure 1.
Male and female pups were cross-fostered into litters of 6 (circles) or 12 (triangles). From day 4 to 14, half the pups in each litter received leptin (80 ng/g/d, open symbols or bars), while littermates received an equal volume of normal saline (10 ml/kg/d, solid symbols or bars). While mice fostered in litters of 12 had decreased neonatal weights (F(1,740)=52, *P<0.001), leptin administration did not acutely influence pup weight (F(1,720)=1.4, P=0.24) (A). Plasma leptin levels were influenced by an interaction between litter size and leptin (B; F(1,7)=67, *P<0.001 versus litters of 6 and †P<0.001 versus saline).
Adult Phenotypes
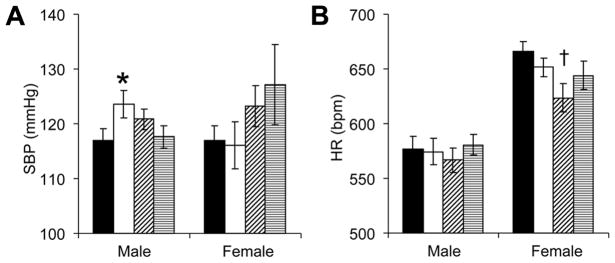
Among male mice, tail cuff SBP was influenced by an interaction between neonatal growth and neonatal leptin administration (Figure 2A). Post-hoc testing showed neonatal GR increased the SBP of mice that had not received concurrent leptin supplementation. Although neonatal growth or leptin administration did not significantly alter female SBP (P=0.88 and P=0.08, respectively), neonatal leptin administration significantly decreased adult female heart rates (Figure 2B). Because tail cuff measurements are obtained within an unfamiliar stress-evoking environment, we proceeded to investigate anxiety-related behavioral phenotypes.
Figure 2.
Tail cuff systolic blood pressures (SBP, A) and heart rates (HR, B) were measured in adult mice that received neonatal injections of saline or leptin (■: control-saline, n=8 male, 10 female; □: GR-saline, n=15 male, 8 female; ▨: control-leptin, n=14 male, 9 female; ▤: GR-leptin, n=14 male, 6 female). Male SBP was influenced by a significant interaction between neonatal GR and leptin administration (A; F(1,47)=4.2, *P<0.05 versus control-saline and GR-leptin mice). In female controls, neonatal leptin administration tended to increase adult SBP (A; F(1,29)=3.2, P=0.08) and significantly decreased adult HR (B; F(1,29)=4.4, †P<0.05 versus control-saline).
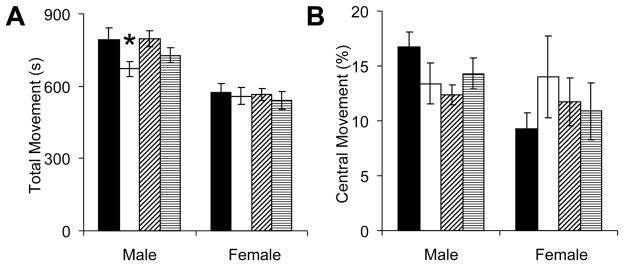
Mice were first placed within a brightly illuminated open field. Among male mice, neonatal GR led to an overall reduction in open field activity (P<0.01), and post-hoc testing identified a significant reduction in saline-treated but not leptin-treated mice (Figure 3A). Although the same mice tended to avoid movement into the center of the open field (Figure 3B), this did not approach statistical significance (P=0.17). No significant alterations in open field movement were observed in female mice. Since the overall reduction in open field activity may reflect either sedentary behavior or anxiety-related immobility, we further assessed murine activity with a fear conditioning protocol that assesses baseline activity and fear-related freezing.
Figure 3.
At least one week after the tail cuff measurements, adult mice that received neonatal saline or leptin were placed within an open field (■: control-saline, n=8 male, 10 female;□s: GR-saline, n=14 male, 8 female; ▨: control-leptin, n=12 male, 9 female; ▤: GR-leptin, n=15 male, 6 female). Video tracking software recorded movement throughout the 1648 cm2 of open space (A), including movements exploring the central 645 cm2 (B). Neonatal growth restriction decreased the activity of adult males (A; F(1,45)=7.0, *P<0.05 versus control-saline).
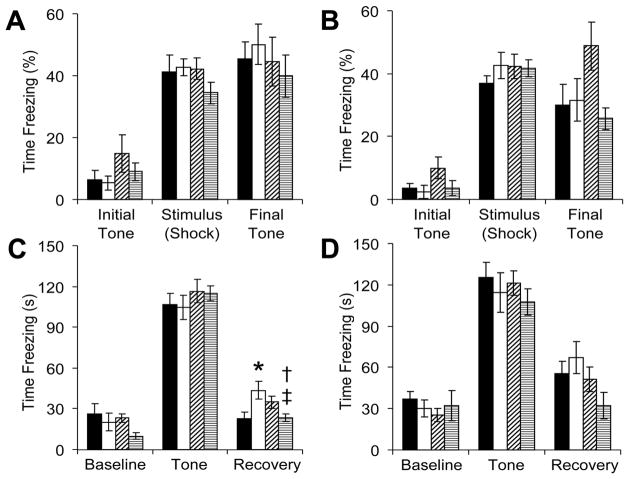
During fear conditioning, neonatal GR or leptin administration did not significantly alter baseline activity, the acute response to electrical stimulation or the conditioned response to the paired tone (Figure 4). A significant interaction between neonatal GR and neonatal leptin administration influenced freezing time in the immediate post-tone recovery phase. For male mice (Figure 4C), neonatal GR significantly increased post-tone freezing and neonatal leptin administration decreased this response. Although leptin tended to increase the post-tone freezing of control mice, this did not reach statistical significance (P=0.09). For female mice (Figure 4D), neonatal leptin administration tended to decrease post-tone freezing behavior, but this was not statistically significant (ANOVA P=0.06). Although ANOVA did not identify an overall effect of GR or leptin among females, it is noteworthy that neonatal leptin decreased the freezing of GR female mice, both during training (Figure 4B, final tone, P<0.05 versus control-leptin) and during the post-tone recovery phase (Figure 4C, P<0.05 versus control-leptin). Because cue-based fear conditioning involves the same brain regions (amygdala and hippocampus) implicated in human post-traumatic stress disorder (PTSD) (22), we next investigated the effects of neonatal GR and leptin administration on regional brain volumes in male mice.
Figure 4.
Adult mice that received neonatal saline or leptin underwent fear conditioning (■: control-saline, n=9 male, 10 female; □: GR-saline, n=15 male, 8 female; ▨: control-leptin, n=14 male, 9 female; ▤: GR-leptin, n=15 male, 6 female). On the initial training day, male (A) and female mice (B) were presented with a repetitive tone that co-terminated with a mild electrical stimulus, and fearful behavior (freezing) was digitally captured. The following day, male (C) and female mice (D) were presented with an unpaired cue (tone) in a novel context. The post-cue freezing of male mice was influenced by a significant interaction between neonatal growth and leptin administration (C; F(1,49)=11, *P<0.05 versus control-saline, †P<0.01 versus GR-saline, ‡P<0.05 versus control-leptin). In female mice, neonatal leptin administration tended to decrease overall post-tone freezing (D; F(1,29)=3.8, P=0.06).
Adult Morphology
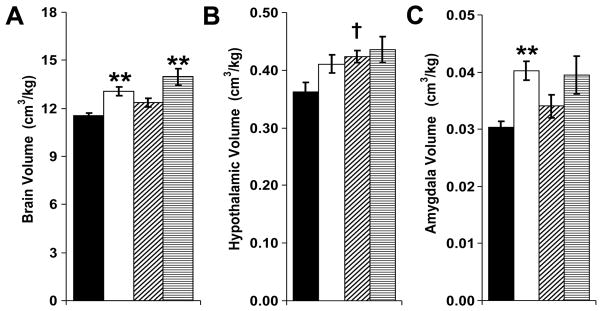
When normalized to body weight, relative brain volume was significantly increased by neonatal GR (“brain sparing”) (Figure 5A). Neonatal leptin increased relative hypothalamic volumes, specifically for control mice (Figures 5B). Adult male amygdala volumes were dramatically increased in GR mice that did not receive neonatal leptin supplementation, whether amygdala volumes were normalized to body weight or compared in absolute terms (Figures 5C and Table 1, respectively). Compared to leptin-treated controls (that had relative hypothalamic enlargement), leptin-treated GR mice had increased basal ganglia volumes and decreased cerebral cortex and hypothalamic volumes (Table 1). In female mice, neonatal GR led to an absolute increase in brain stem volumes (Table 1). Since female GR-saline mice did not have significant alterations in fear conditioning or brain volumes, MRIs were not obtained on leptin-exposed females. These data are consistent with a “brain-sparing” effect of neonatal GR, a trophic effect of neonatal leptin on hypothalamic development (23), and a potential relationship between programmed susceptibility to fear and adult amygdala volumes. To better define the tissue-specific effects of neonatal GR and neonatal leptin, organ weights were obtained.
Figure 5.
Magnetic resonance imaging was performed to assess male mice for neonatal GR or leptin-induced alterations in regional brain volumes (■: control-saline, n=15; □: GR-saline, n=12; ▨: control-leptin, n=8; ▤: GR-leptin, n=6). Compared to control mice, GR mice had increased brain volume to body weight ratios (A; F(1,37)=26, **P<0.01 versus corresponding control). Although neonatal leptin administration also increased relative brain volumes (A; F(1,37)=7.3, P=0.01), no significant pair-wise differences were present (P=0.06 for control-leptin versus control-saline and P=0.07 for GR-leptin versus GR-saline). Relative hypothalamic volumes were significantly increased by neonatal leptin supplementation (B; F(1,37)=5.1, †P=0.02 for control-leptin versus control-saline). Neonatal GR significantly increased relative amygdala volumes (C; F(1,37)=14, **P<0.001 GR-saline versus control-saline, P=0.11 GR-leptin versus control-leptin).
TABLE 1.
Adult brain and brain stem volumes were measured by MRI for neonatal growth restricted (GR) and control mice with or without neonatal leptin administration. MRI was not performed on leptin-exposed females.
| Male Control | Male GR | Male Control Leptin | Male GR Leptin | Female Control | Female GR | ||
|---|---|---|---|---|---|---|---|
| N | 15 | 12 | 8 | 6 | 7 | 6 | |
| Total Volume | (mm3) | 445 ± 5 | 445 ± 4 | 454 ± 4 | 440 ± 5 | 449 ± 8 | 457 ± 5 |
| Cerebral Cortex | (mm3) | 148 ± 4 | 148 ± 3 | 154 ± 2 | 144 ± 3* | 147 ± 4 | 152 ± 4 |
| (% total) | 33.2 ± 0.4 | 33.2 ± 0.4 | 33.7 ± 0.3 | 32.7 ± 0.3* | 32.7 ± 0.3 | 33.3 ± 0.5 | |
| Brain Stem | (mm3) | 80.8 ± 1.5 | 80.7 ± 1.0 | 83.0 ± 1.1 | 81.0 ± 1.4 | 80.6 ± 1.1 | 84.4 ± 1.1* |
| (% total) | 18.1 ± 0.2 | 18.1 ± 0.1 | 18.2 ± 0.1 | 18.4 ± 0.1 | 17.9 ± 0.1 | 18.4 ± 0.1* | |
| Fiber Tracts | (mm3) | 79.7 ± 1.6 | 78.8 ± 1.3 | 79.8 ± 1.6 | 77.6 ± 1.2 | 83.0 ± 2.3 | 83.7 ± 1.0 |
| (% total) | 17.9 ± 0.2 | 17.6 ± 0.2 | 17.5 ± 0.3 | 17.6 ± 0.2 | 18.5 ± 0.3 | 18.3 ± 0.2 | |
| Cerebellum | (mm3) | 43.2 ± 0.7 | 43.3 ± 1.0 | 44.5 ± 0.5 | 43.5 ± 0.9 | 44.9 ± 0.8 | 43.5 ± 0.8 |
| (% total) | 9.69 ± 0.15 | 9.69 ± 0.20 | 9.77 ± 0.10 | 9.87 ± 0.10 | 10.00 ± 0.09 | 9.51 ± 0.27 | |
| Basal Ganglia | (mm3) | 34.0 ± 0.5 | 34.3 ± 0.4 | 33.0 ± 0.4 | 34.4 ± 0.2* | 33.4 ± 0.4 | 33.2 ± 0.3 |
| (% total) | 7.64 ± 0.21 | 7.69 ± 0.14 | 7.26 ± 0.11 | 7.82±0.10** | 7.45 ± 0.18 | 7.26 ± 0.10 | |
| Hippocampus | (mm3) | 22.7 ± 0.4 | 23.4 ± 0.4 | 22.8 ± 0.6 | 22.8 ± 0.3 | 23.0 ± 0.6 | 21.7 ± 0.3 |
| (% total) | 5.10 ± 0.15 | 5.24 ± 0.13 | 5.01 ± 0.17 | 5.18 ± 0.11 | 5.13 ± 0.19 | 4.74 ± 0.11 | |
| Thalamus | (mm3) | 18.9 ± 0.3 | 18.7 ± 0.2 | 18.6 ± 0.2 | 18.9 ± 0.1 | 18.8 ± 0.2 | 18.9 ± 0.2 |
| (% total) | 4.25 ± 0.12 | 4.19 ± 0.06 | 4.08 ± 0.05 | 4.30 ± 0.07 | 4.18 ± 0.06 | 4.14 ± 0.06 | |
| Hypothalamus | (mm3) | 11.3 ± 0.7 | 11.4 ± 0.6 | 12.7 ± 0.4 | 11.1 ± 0.4* | 11.4 ± 0.8 | 12.5 ± 1.0 |
| (% total) | 2.53 ± 0.14 | 2.54 ± 0.12 | 2.79 ± 0.08 | 2.50 ± 0.06* | 2.53 ± 0.14 | 2.72 ± 0.21 | |
| Ventricles | (mm3) | 3.4 ± 0.2 | 3.2 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.1 |
| (% total) | 0.76 ± 0.03 | 0.72 ± 0.03 | 0.71 ± 0.01 | 0.75 ± 0.03 | 0.70 ± 0.02 | 0.75 ± 0.03 | |
| Pituitary | (mm3) | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.2 | 2.2 ± 0.2 |
| (% total) | 0.47 ± 0.03 | 0.50 ± 0.04 | 0.48 ± 0.02 | 0.49 ± 0.03 | 0.45 ± 0.04 | 0.49 ± 0.03 | |
| Amygdala | (mm3) | 0.95 ± 0.04 | 1.12±0.07** | 1.01 ± 0.04 | 0.99 ± 0.07 | 0.96 ± 0.05 | 1.08 ± 0.05 |
| (% total) | 0.21 ± 0.01 | 0.25±0.02** | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.24 ± 0.01 |
P<0.05 or
P<0.01 versus control.
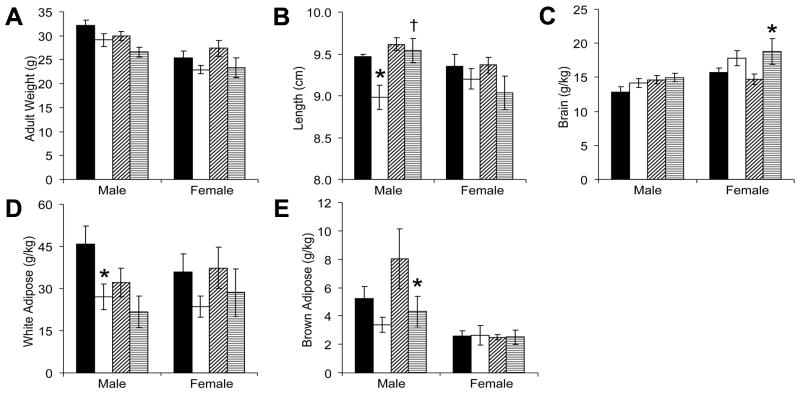
Overall, neonatal GR decreased the adult weights of male and female mice (Figure 6A), but no significant pair-wise differences were identified on post hoc testing. For male mice, neonatal leptin supplementation increased (normalized) the length of GR mice (Figure 6B). For female mice, neonatal GR led to increased brain weight to body weight ratios (Figure 6C). As expected from our prior studies (20), neonatal GR decreased adult white adipose (Figure 6D) and brown adipose (Figure 6E).
Figure 6.
At study completion, male and female GR mice weighed significantly less than their control counterparts (A; males: F(1,28)=7.7, P=0.01; females: F(1,27)=4.9, P<0.05), but post-hoc testing did not isolate significant differences within subgroups of saline-treated or leptin-treated mice (■: control-saline, n=7 male, 8 female; □: GR-saline, n=9 male, 8 female; ▨: control-leptin, n=7 male, 9 female; ▤: GR-leptin, n=9 male, 6 female ). Neonatal GR deceased adult male length (B; F(1,18)=4.3, *P<0.05 versus control-saline). Furthermore, neonatal leptin administration increased the length of adult male mice (B, F(1,18)=7.9, †P<0.01 versus GR-saline). In female mice, neonatal GR increased relative brain weights (C, F(1,27)=8.1, *P<0.05 versus control-leptin). In male mice, neonatal GR decreased white adipose tissue (D, F(1,28)=7.6, *P<0.05 GR-saline versus control-saline and P=0.18 GR-leptin versus control-leptin) and brown adipose tissue (E, F(1,28)=6.0, P=0.28 GR-saline versus control-saline and *P<0.05 GR-leptin versus control-leptin).
DISCUSSION
The sequelae of prematurity include both neurocognitive impairment and a higher incidence of psychiatric illness (1, 24, 25). Compared to females, premature males have both a survival and a developmental disadvantage. The etiologies of these sexually dimorphic outcomes are unknown, but may relate to sex-specific developmental trajectories (1, 4, 26, 27). Our previous studies showed that catch-up growth normalized markers of anxiety and hypertension in males but not in females, suggesting gender-specific windows of developmental susceptibility or reversibility exist, with males have a relative delay in neuromaturation (8).
Our current studies are the first to show that neonatal leptin replacement improves the behavioral outcomes and blood pressures of neonatal GR male mice. Phenotypic and morphologic correction after leptin supplementation demonstrates the potential protective effects of targeted neonatal interventions. Our data further support a series of studies in which both the dosage and timing of leptin administration are key determinants of the regimen’s acute and long-term effects (19, 28, 29). The administration of exogenous leptin (80 to 250 ng/g/d) to leptin-replete animals induces adult leptin resistance, inactivity and obesity, while leptin administration to GR offspring elicits protective effects (28, 29, 30). Consistent with those studies, we noted altered regional brain volumes and trends towards higher blood pressures and heightened anxiety in well-nourished mice that received neonatal leptin. Interestingly, the dose of leptin we utilized (80 ng/g/d) did not increase the pre-injection leptin levels of control mice. This may have been due to a down regulation of endogenous leptin production.
In addition to the dose of leptin, the timing of administration influences short and long-term responses. Leptin administration beyond the critical window of neurodevelopmental plasticity does not elicit persistent neurotrophic effects, while normalization of circulating leptin levels during the establishment of the leptin-dependent anorexigenic pathway permanently influences adult brain structure and function (17). Furthermore, when the same dose of leptin (80 ng/g/d) is administered to the lactating dam rather than the pup, diametrical results are noted, with decreased rather than heightened anxiety-like behavior in the exogenous leptin-exposed adult mice (19, 31). Further studies are necessary to clarify the optimal dose, route and timing of leptin supplementation for at-risk populations.
We utilized two tests that assess both baseline activity and anxiety or fear-related behavior. Intriguingly, neonatal GR led to decreased open field activity without altering baseline testing during fear conditioning. It is important to note our open field testing protocol measured horizontal beam breaks over 30 minutes while fear conditioning measured all movement beyond respiration over 3 minutes. Overall, mice were moving horizontally 37% of the time they were in the open field, and had movements beyond respiration 87% of the time they were in the fear conditioning chamber. We speculate the open field measurements are a better marker of general activity, while baseline movement during fear conditioning assesses exploratory behavior.
Our prior studies have shown neonatal catch up growth normalizes neurobehavioral and cardiovascular phenotypes in IUGR male, but not female mice (8). Those studies emphasized the importance of the perinatal environment in the resetting of adult disease susceptibility and suggested a delayed window of vulnerability among male mice (26, 32). The present study supports and extends our earlier observations in demonstrating the protective effects of neonatal leptin and detrimental effects of neonatal GR on the cardiovascular, behavioral and growth phenotypes of male mice. Our hemodynamic data highlight the sexually dimorphic and interdependent effects of neonatal GR and leptin administration. In leptin treated females, the finding of relative bradycardia suggests the presence of alterations in central hemodynamic regulation that require further investigation, including assessment of baroreceptor sensitivity by radiotelemetry.
Beyond improved cardiovascular regulation, leptin treatment rescued the behavioral alterations seen in our novel model. Neonatal GR led to a constellation of phenotypes in male mice that are reminiscent of human PTSD, including anxiety, fearfulness, and hypertension. Consistent with our MRI and fear conditioning results, elegant studies have demonstrated a correlation between amygdala volumes and freezing to conditioned stimuli (33). Similarly, clinical studies have shown increased amygdala volumes in patients with autism, a disorder with marked male-dominated sexual dimorphism (32, 34, 35). The etiology of this amygdala overgrowth requires further investigation, with alterations in glucocorticoid signaling among the factors thought to influence amygdala development (36).
Leptin treatment decreased post-tone freezing in male and female mice, showing that a targeted intervention can reverse the programming effects of GR. The unique finding of increased post-tone freezing in our baseline studies is not the expected outcome in classic fear conditioning models (22). Fear conditioning studies generally assess the acute response to the conditioned stimulus (freezing during the tone). We speculate the failure of the GR male mice to resolve their fearful response immediately after the tone terminated may predict the risk for chronic stress-associated disorders. The mechanism for this has not been elucidated, but may be due to hypothalamic pituitary adrenal (HPA) axis dysregulation. Because fear conditioning and PTSD involve acquisition and retention of fearful memories, respond to the same therapies, and depend on the same anatomic substrates (amygdala and hippocampus), fear conditioning is widely used to model PTSD (33). As in PTSD, fear conditioning in our model was also associated with adverse cardiovascular endpoints (37).
Leptin’s role in fetal and infant neuronal development makes it an attractive candidate to reverse the deleterious neurocognitive and behavioral phenotypes associated with prematurity and IUGR. A recent post-hoc evaluation of data from a nutritional intervention trial showed strong correlations between breast milk intake, brain volumes and intelligence that was isolated to males (38). Breast milk contains a number of trophic factors, including leptin, which are not present in infant formulas. In our studies, neonatal leptin administration had robust effects on adult brain volumes.
Importantly, leptin supplementation offered neurodevelopmental protection without metabolic compromise as evidenced by a sustained decrease in adiposity for GR mice. The observed decrease in length of GR male mice is consistent with short stature in premature or IUGR infants. Our studies show that leptin supplementation can preemptively normalize linear growth. This effect on linear growth may reflect the hormone’s effects on hypothalamic development (12). The lack of an acute effect on pup growth is consistent with studies showing that during the critical neonatal window, leptin triggers the development of leptin-sensitive circuits. It is not until day 14 that these circuits have the capacity to alter orexic behavior (17).
Our studies demonstrate that leptin supplementation can block the programming of behavioral phenotypes in mice. Exogenous leptin administration has been shown to elicit neurotrophic effects for IUGR piglets and genetically leptin-deficient humans (39, 40). Our results translate most directly to the premature population who must undergo critical phases of neurodevelopment without the benefit of transplacental leptin. Future studies are necessary to determine if neonatal leptin can improve the behavioral and cardiovascular outcomes of premature or IUGR infants.
Acknowledgments
This study was funded by grants from the Children’s Miracle Network and the National Institues of Health, (HL102659.)
Abbreviations
- GR
growth restriction
- PTSD
post-traumatic stress disorder
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 2.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–310. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson JG, Forsén TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- 4.Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, Maller-Kesselman J, Silbereis J, Constable RT, Makuch RW, Ment LR. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schothorst PF, Swaab-Barneveld H, van Engeland H. Psychiatric disorders and MND in non-handicapped preterm children. Prevalence and stability from school age into adolescence. Eur Child Adolesc Psychiatry. 2007;16:439–448. doi: 10.1007/s00787-007-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotteveel J, van Weissenbruch MM, Twisk JW, Delemarre-Van de Waal HA. Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely born young adults. Pediatrics. 2008;122:313–321. doi: 10.1542/peds.2007-2012. [DOI] [PubMed] [Google Scholar]
- 7.Beardsall K, Ong KK, Murphy N, Ahmed ML, Zhao JH, Peeters MW, Dunger DB. Heritability of childhood weight gain from birth and risk markers for adult metabolic disease in prepubertal twins. J Clin Endocrinol Metab. 2009;94:3708–3713. doi: 10.1210/jc.2009-0757. [DOI] [PubMed] [Google Scholar]
- 8.Hermann GM, Miller RL, Erkonen GE, Dallas LM, Hsu E, Zhu V, Roghair RD. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatr Res. 2009;66:53–58. doi: 10.1203/PDR.0b013e3181a7c5fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaskar SU, Ollesch C, Rajakumar RA, Rajakumar PA. Developmental changes in ob gene expression and circulating leptin peptide concentrations. Biochem Biophys Res Commun. 1997;238:44–47. doi: 10.1006/bbrc.1997.7237. [DOI] [PubMed] [Google Scholar]
- 10.Pighetti M, Tommaselli GA, D’Elia A, Di Carlo C, Mariano A, Di Carlo A, Nappi C. Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet Gynecol. 2003;102:535–543. doi: 10.1016/s0029-7844(03)00668-9. [DOI] [PubMed] [Google Scholar]
- 11.Val niene M, Verkauskiene R, Boguszewski M, Dahlgren J, Lasiene D, Lasas L, Wikland KA. Leptin levels at birth and in early postnatal life in small- and appropriate-for-gestational-age infants. Medicina (Kaunas) 2007;43:784–791. [PubMed] [Google Scholar]
- 12.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remmers F, Fodor M, Delemarre-van de Waal HA. Neonatal food restriction permanently alters rat body dimensions and energy intake. Physiol Behav. 2008;95:208–215. doi: 10.1016/j.physbeh.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 15.Passos MC, Vicente LL, Lisboa PC, de Moura EG. Absence of anorectic effect to acute peripheral leptin treatment in adult rats whose mothers were malnourished during lactation. Horm Metab Res. 2004;36:625–629. doi: 10.1055/s-2004-825927. [DOI] [PubMed] [Google Scholar]
- 16.Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- 17.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 18.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256:600–602. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- 19.Fraga-Marques MC, Moura EG, Claudio-Neto S, Trevenzoli IH, Toste FP, Passos M, Lisboa PC, Manhaes AC. Neonatal hyperleptinaemia programmes anxiety –like and novelty seeking behaviours but not memory/learning in adult rats. Horm Behav. 2009;55:272–279. doi: 10.1016/j.yhbeh.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Roghair RD, Aldape G. Naturally occurring perinatal growth restriction in mice programs cardiovascular and endocrine function in a sex-and strain-dependent manner. Pediatr Res. 2007;62:399–404. doi: 10.1203/PDR.0b013e31813cbf16. [DOI] [PubMed] [Google Scholar]
- 21.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 22.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–1115. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 24.Conrad AL, Richman L, Lindgren S, Nopoulos P. Biological and environmental predictors of behavioral sequelae in children born preterm. Pediatrics. 2010;125:e83–e89. doi: 10.1542/peds.2009-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hack M, Youngstrom ER, Cartar L, Schluchter M, Gerry Taylor H, Flannery D, Klein N, Borawski E. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 2004;114:932–940. doi: 10.1542/peds.2003-1017-L. [DOI] [PubMed] [Google Scholar]
- 26.Spring S, Lerch JP, Henkelman RM. Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. Neuroimage. 2007;35:1424–1433. doi: 10.1016/j.neuroimage.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Vasileiadis GT, Thompson RT, Han VK, Gelman N. Females follow a more “compact” early human brain development model than males. A case-control study of preterm neonates. Pediatr Res. 2009;66:551–555. doi: 10.1203/PDR.0b013e3181ba1ae7. [DOI] [PubMed] [Google Scholar]
- 28.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 29.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 30.Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr. 2006;95:830–837. doi: 10.1079/bjn20061726. [DOI] [PubMed] [Google Scholar]
- 31.Fraga-Marques MC, Moura EG, Silva JO, Claudio-Neto S, Pereira-Toste F, Passos MC, Lisboa PC, Manhães AC. Effects of maternal hyperleptinaemia during lactation on short-term memory/learning, anxiety-like and novelty-seeking behavioral traits of adult male rats. Behav Brain Res. 2010;206:147–150. doi: 10.1016/j.bbr.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008;121:1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- 33.Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33:2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- 34.Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry. 2010;49:552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med. 2009;34:125–132. doi: 10.3200/BMED.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- 38.Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67:357–362. doi: 10.1203/PDR.0b013e3181d026da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attig L, Djiane J, Gertler A, Rampin O, Larcher T, Boukthir S, Anton PM, Madec JY, Gourdou I, Abdennebi-Najar L. Study of hypothalamic leptin receptor expression in low-birth-weight piglets and effects of leptin supplementation on neonatal growth and development. Am J Physiol Endocrinol Metab. 2008;295:E1117–E1125. doi: 10.1152/ajpendo.90542.2008. [DOI] [PubMed] [Google Scholar]
- 40.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]