Abstract
Objectives:
To determine the incidence and magnitude of the rapid increase in the serum PSA (riPSA) level after high-intensity focused ultrasound (HIFU) therapy for prostate cancer, and its correlation with clinical factors.
Methods:
A total of 176 patients with localized prostate cancer underwent HIFU therapy. Serum riPSA was determined on the basis of the same criteria as those for “PSA bounce”, ie, an increase of ≥0.2 ng/ml with a spontaneous return to the prebounce level or lower. Patients were stratified according to neoadjuvant PSA level, T stage, risk group, age, Gleason score, pretreatment PSA level, post-treatment PSA nadir, and number of HIFU sessions.
Results:
riPSA was seen in 53% of patients during a median follow-up period of 43 months. A PSA nadir was achieved within 3 months for 85.1% of the treatments. In all cases, onset of riPSA was seen two days after HIFU therapy, and the median magnitude was 23.69 ng/ml. A magnitude of >2 ng/ml was seen in 89.4% of cases. Univariate analysis revealed that patients with riPSA were associated with usage of hormonal therapy and the post-treatment PSA nadir level. Multivariate Cox regression analysis revealed that riPSA and the number of HIFU sessions were predictors of biochemical recurrence. A significant statistical association was found between the presence of riPSA and the risk of biochemical failure only in the low- and intermediate-risk group.
Conclusion:
Patients treated with HIFU who experience post-treatment riPSA may have an increased risk of biochemical recurrence, especially in non-high-risk patients.
Keywords: HIFU, prostate cancer, PSA
Introduction
Prostate cancer is the most common cancer in men, and the second leading cause of cancer-related death in the western world. The mainstay of treatment remains radical surgery or radiation therapy, but several minimally invasive treatments are now being evaluated, and may prove to be of equivalent oncological value in the long term. Transrectal high-intensity focused ultrasound (HIFU) is a new minimally invasive therapeutic method that is able to achieve tissue coagulation transdermally and/or transmucosally, destroying tissue in various conditions. It has a number of clinical applications, including coagulation of pathology in the breast, uterus, kidney, spleen, liver, and bone. A rapid increase of the serum PSA level (riPSA) is usually recognized during the early phase after HIFU therapy. This riPSA can be explained by tissue necrosis and organ manipulation resulting from treatment. However, no study has analyzed the magnitude, timing, or clinical significance of the riPSA level after HIFU therapy for localized prostate cancer. Also, there are currently no definitive criteria for this phenomenon. In the present study, we employed the same definitive criteria as those for “PSA bounce”, ie, an increase in the serum PSA level of ≥0.2 ng/ml with spontaneous return to the prebounce level or lower.
The purpose of the present study was to investigate the timing and magnitude of riPSA, and its predictive value for biochemical recurrence in patients with prostate cancer receiving HIFU therapy.
Materials and Methods
Patient selection
Between June 2004 and November 2009, 176 men with clinically localized prostate cancer were treated using the Sonablate-500® (Focus Surgery, IN, USA), which consists of a power generator, a water-cooling system (‘Sonachill®’), a treatment probe and a probe-positioning system. In all patients prostate cancer had been diagnosed by at least a six-core prostate biopsy. All the patients provided written informed consent to the treatment following approval from the local institutional review board. Decision criteria for recommendation of HIFU therapy were: clinical stage T1/T2 (1997 TNM classification), normal bone scintigraphy results, normal abdominal CT results, and patient background, ie, either unwilling to undergo, or unfit for, radical prostatectomy. Any previous curative treatment for prostate cancer was considered as a formal exclusion criterion.
We stratified the patients into three risk categories according to the classification of D’Amico et al: low risk, clinical stage T1c or T2a, Gleason Score (GS) less than 6, and PSA < 10 ng/ml; intermediate risk, clinical stage T2b, or PSA 10–20 ng/ml, or GS 7; and high risk, clinical stage T2c, or PSA 20 ng/ml or more, or GS 8–10.
In every case, prostate volume was evaluated using ultrasound. In some cases, the HIFU session was combined with neoadjuvant hormonal therapy, either when the prostate volume was above 30 ml or in cases of lower urinary tract voiding symptoms. For postoperative follow-up, PSA monitoring was performed routinely at 2 days after treatment, at 1 and 3 months, and then every 3 months. Biochemical recurrence of the disease was defined as a rise in the serum PSA level of 2 ng/ml or more above the PSA nadir, in accordance with the latest Phoenix criteria. The investigated PSA nadir value was used as an indicator for prediction of biochemical failure. In addition, we performed sextant prostate biopsies in cases of biochemical failure. Also, a follow-up control biopsy 3–6 months after treatment was recommended for all patients. Patients with a rising PSA level but negative control biopsy underwent bone scintigraphy and a computed tomography scan to exclude distant metastatic disease. Biochemical recurrence-free survival rate was evaluated from the date of the first HIFU session to the date of the last follow-up visit, or death, in all patients as mentioned above.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism® version 5.0 statistical package for Windows (GraphPad Software, San Diego, California USA) and PASW software version 17 for Windows (SPSS Inc., Chicago, IL, USA). The Fisher’s exact test and chi-squared test were used to evaluate categorical comparisons. Biological relapse-free survival was defined as the period from random assignment to the date of biological relapse, or censored at last follow-up. Survival functions were calculated using the Kaplan-Meier method. Survival distributions were compared between the two or three arms using a log-rank test. All tests and reported P values were 2-sided, and significance was defined as P < 0.05.
Results
The clinical disease characteristics and dosimetric parameters of the 176 patients are shown in Table 1. The median follow-up period for the entire group was 43 (range, 2–70) months. At the time of analysis, 40 men (22.7%) had biochemical failure, of whom 28 underwent biopsy and 13 (46.4%) had positive biopsy findings. The median PSA level after HIFU was 9.91 (range, 0–268.9) ng/mL, and the median PSA nadir was 0.03 (range, 0.03–3.31) ng/mL. Of the 176 patients, 106 (60.2%) had a PSA follow-up of >2 years.
Table 1.
Patients background characteristics.
| riPSA+ | riPSA– | P value | |
|---|---|---|---|
| Neoadjuvant hormonal therapy* | 0.000 | ||
| Yes | 6.3 | 38.3 | |
| No | 46.3 | 9.1 | |
| Stage* | 0.343 | ||
| T1c | 16.6 | 10.7 | |
| T2a | 13 | 17.2 | |
| T2b | 13 | 10.7 | |
| T2c | 10.7 | 8.3 | |
| D’Amico risk group* | 0.181 | ||
| Low | 18.5 | 11.3 | |
| Intermediate | 17.3 | 13.1 | |
| High | 17.9 | 22 | |
| Age; median (min-max) | 62 (52–86) | 62 (54–82) | 0.595 |
| GS; median (min-max) | 5 (5–10) | 7 (4–10) | 0.485 |
| iPSA; median (min-max) | 10.87 (2.20–26.91) | 9.70 (3.8–45.69) | 0.558 |
| PSA nadir (ng/ml); median (min-max) | 0.51 (0.01–3.31) | 0.01 (0.00–2.77) | 0.018 |
| Number of HIFU sessions* | 0.993 | ||
| Once | 47.7 | 42.6 | |
| Twice | 5.1 | 4.5 |
Note:
Values are expressed as the percent of entities.
Abbreviations: riPSA, rapid increase in the serum PSA; GS, Gleason score; iPSA, initial PSA.
riPSA was detected in 93 men (52.8%). In all patients, riPSA was seen at 2 days after HIFU therapy. The median amplitude of the increase was 23.69 (range, 0.21–258.73) ng/mL. Twenty-four (25.8%) of these 93 patients were found to have biochemical failure. The biochemical recurrence-free (BCRF) survival rate was 29% and 21% for those with and without a riPSA, respectively.
Univariate analysis (Table 1) showed that neoadjuvant hormonal therapy and the PSA nadir were associated with riPSA. A riPSA magnitude of >2 ng/mL was detected in 89.4% of the patients.
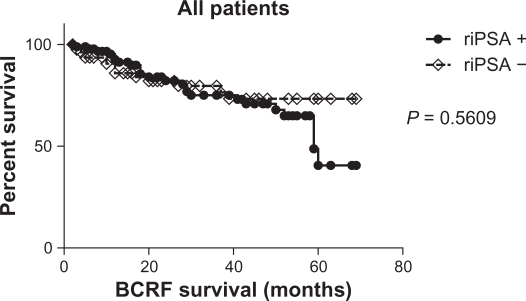
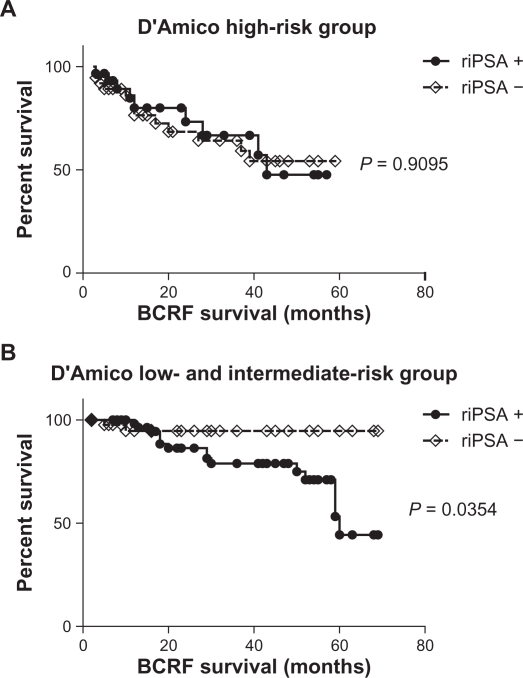
Biochemical recurrence-free survival showed no significant difference between patients with and without riPSA (Fig. 1). We analyzed the impact of riPSA in the risk group using Kaplan–Meier curves (Fig. 2). Log-rank test revealed no significant association (P = 0.9095) between the presence of riPSA and the risk of biochemical failure in the high-risk group (Fig. 2A), but demonstrated a significant association (P = 0.0354) between the presence of riPSA and the risk of biochemical failure in the low- and intermediate-risk group (Fig. 2B).
Figure 1.
Biochemical recurrence-free survival curve for all patients who underwent HIFU treatment. riPSA = rapid increase of the PSA level.
Figure 2.
Biochemical recurrence-free survival curve for the patients who underwent HIFU treatment. riPSA = rapid increase of the PSA level. A) Biochemical recurrence-free survival curve for the D’Amico high-risk group. B) Biochemical recurrence-free survival curve for the D’Amico low- and intermediate-risk group.
On univariate analysis (Table 2), among all of the clinical and dosimetric parameters analyzed, only the number of HIFU sessions was significant (hazard ratio, 18.834; 95% confidence interval, 3.736–94.947, P = 0.000). riPSA remained of borderline relevance without statistical significance, exhibiting a tendency to be associated with a higher biochemical failure rate (hazard ratio, 4.239; 95% confidence interval, 0.967–18.576, P = 0.055). Multivariate analysis among paremeters, including Gleason score, riPSA, PSA nadir, HIFU session numbers, showed that riPSA and the number of HIFU sessions were significant (hazard ratio, 4.955; 95% confidence interval, 1.023–23.997, P = 0.047; hazard ratio 22.460; 95% confidence interval, 3.729–135.266; P = 0.001 for riPSA and number of HIFU sessions, respectively) (Table 2).
Table 2.
Multivariate Cox regression analysis of BCRF survival.
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Neoadjuvant hormonal therapy | ||||||||
| No | 1 | – | ||||||
| Yes | 0.430 | 0.123 | 1.502 | 0.186 | ||||
| Stage | ||||||||
| T1c and T2a | 1 | – | ||||||
| T2b and T2c | 0.462 | 0.171 | 1.252 | 0.129 | ||||
| D’Amico | ||||||||
| Low-risk | 1 | – | ||||||
| Intermediate-risk | 1.738 | 0.659 | 4.582 | 0.264 | ||||
| Age (continuous variable) | 0.985 | 0.920 | 1.055 | 0.675 | – | |||
| Gleason score | ||||||||
| Lesser than 7 | 1 | 1 | ||||||
| 7 or greater | 0.726 | 0.208 | 2.531 | 0.615 | 0.435 | 0.112 | 1.689 | 0.229 |
| riPSA | ||||||||
| No | 1 | 1 | ||||||
| Yes | 4.239 | 0.967 | 18.576 | 0.055 | 4.955 | 1.023 | 23.997 | 0.047 |
| iPSA (continuous variable) | 1.007 | 0.935 | 1.085 | 0.844 | – | |||
| PSA nadir (ng/ml) (continuous variable) | 1.675 | 0.931 | 3.012 | 0.085 | 2.024 | 0.991 | 4.135 | 0.053 |
| Number of HIFU sessions | ||||||||
| Once | 1 | 1 | ||||||
| Twice | 18.834 | 3.736 | 94.947 | 0.000 | 22.460 | 3.729 | 135.266 | 0.001 |
Abbreviations: riPSA, rapid increase in the serum PSA; BCRF survival, biochemichal recurrence free survival; iPSA, initial PSA.
Discussion
Reduction of the PSA level after curative treatment is a hallmark by which treatment success for prostate cancer is defined. In the setting of radical prostatectomy, stably undetectable PSA levels are usually achieved within a few weeks after surgery. This situation is different in the setting of prostate cancer that is treated using non-surgical methods including radiation therapy and HIFU, because measurable PSA levels are almost universally present for an extended period, showing great variability among patients and at different time points. Hence, for definition of successful therapy, the expected course of the PSA level following treatment is a decline to a nadir.
It is generally thought that the PSA level decreases continuously after potentially successful radiation treatment for prostate cancer, and that an increase in the PSA level might reflect disease recurrence. However, prostate cancer patients often show a temporary rise in their PSA levels after radiation therapy. This is known as the “PSA bounce”, which does not reflect disease recurrence.
In the present study, riPSA was defined as an increase ≥0.2 ng/ml with spontaneous return to the prebounce level or lower—the same definition as that used for the PSA bounce. The PSA bounce phenomenon was first recognized in patients receiving combined external beam radiation therapy and permanent prostate implants, and its exact cause is unclear, although suggestions have included radiation therapy-induced prostatitis resulting from compromised membrane integrity. Previous studies have also implicated recent instrumentation such as biopsy, bicycle riding, or recent ejaculation as causes. There are various definitions of the PSA bounce.1–3 Many have defined it as an increase in the PSA level by 0.1%,4 0.2%,5,6 or 15%7 above the nadir. T stage, prostate volume, irradiation dose, hormonal therapy, and age have been reported as predictive factors for the PSA bounce.8 In a recent review, younger age was the only consistent predictive factor.8 Most studies that have examined the PSA bounce have been based on patients who underwent radiation therapy. The onset of PSA bounce after radiation therapy usually occurs during mid-term through long-term follow-up. In patients undergoing permanent implant brachytherapy, the time of onset of the PSA bounce varies from 12 months–24 months depending on the definition.5,9 Although it is believed that the PSA bounce never occurs with HIFU,10 no researchers have yet focused on the PSA increase in the acute phase after HIFU therapy. In our present series, after each treatment, PSA showed a marked increase at 2 days, and accordingly we coined the term “rapid increase of the PSA level” to describe it. To our knowledge, this is the first published study to hav analyzed patients showing a rapid increase of the PSA level after HIFU therapy. Because HIFU is non-ionizing, the cause of this PSA increse may differ from radiation therapy. It can be speculated that ultrasound-induced prostate tissue necrosis may be the histological basis of this phenomenon. At the point where the waves emitted from the HIFU unit are focused, the sudden and intense absorption of the ultrasound beam creates a sudden elevation of temperature (to greater than 85 °C), which destroys the prostate cancer cells located in the target zone. The extremely high intensity of the ultrasound in prostate tissue may itself account for discrete tissue necrosis, a phenomenon that is likely absent after external beam radiation therapy, which in turn can also result in a steep increase of the PSA level. If this riPSA can be regarded as a form of bounce phenomenon, then the time until the bounce is much shorter than that resulting from radiation therapy. PSA kinetics after HIFU may follow a course different from that with other modalities, especially radiation therapy. Unlike radiation, HIFU is an ablation technology, which rapidly increases the temperature, resulting in coagulative necrosis; this means that PSA levels decline quickly after rapid increase of PSA in response to tissue necrosis and take only 3 through 6 months to reach a nadir. For radiation, the behaviour of PSA is more complex. The radiation does not of itself kill cells. Radiation therapy works by damaging the DNA of cells. Generally, this is repairable but sometimes it produces fatal damage so that the DNA damage is inherited through cell division, accumulating damage to the cancer cells, causing them to die or reproduce slowly. The cells with fatally damaged DNA still continue to produce PSA and this only ceases when they die. As a result, PSA declines quite slowly after radiotherapy, showing the marked contrast to HIFU.
Two precipitating factors—lack of hormonal therapy and a higher PSA nadir—were found to increase the risk of riPSA (Table 2). Hormonal therapies have a repressive effect on follow-up PSA levels, as might be expected. In our series, 83.5% of patients without hormonal therapy showed the riPSA, compared with 14.1% of patients who received hormonal therapy. Although, neither hormonal therapy nor riPSA showed a significant tendency to be related to biochemical recurrence on univariate analysis (Table 2), multivariate Cox regression analysis showed that riPSA and the number of HIFU sessions were predictors of biochemical recurrence (Table 2). This provides evidence that riPSA may not automatically dictate hormonal therapy. In our series, log-rank test demonstrated a significant association (P = 0.0354) between the presence of riPSA and the risk of biochemical failure only in the low- and intermediate-risk group (Fig. 2B). riPSA may thus be potentially predictive of biochemical recurrence in selected patient populations, especially those with non-high-risk cancer. In our cohort, prognostic value of riPSA was lost in high-risk cancer. This is explainable by the fact that the definition for high-risk group does not account for some adverse variables. High-risk prostate cancer is a quite heterogeneous group that includes patients with clinically locally advanced stage disease at diagnosis, in which some have micrometastatic disese, some have local extension, and some have neither. Hence, high-risk patients with a riPSA after irradiation may be a heterogeneous group, including patients with truly localized failure as well as those with metastatic disease. Further risk stratification within this heterogeneous high-risk group would be useful to assess which patients are most likely to fail HIFU therapy.
Conclusions
We have found that patients with low- and intermediate-risk prostate cancer who demonstrate a post-HIFU riPSA have an increased risk of biochemical failure.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Rosser CJ, Kamat AM, Wang X, et al. Is patient age a factor in the occurrence of prostate-specific antigen bounce phenomenon after external beam radiotherapy for prostate cancer? Urology. 2005;66:327–31. doi: 10.1016/j.urology.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Pickles T. Prostate-specific antigen (PSA) bounce and other fluctuations: which biochemical relapse definition is least prone to PSA false calls? An analysis of 2030 men treated for prostate cancer with external beam or brachytherapy with or without adjuvant androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2006;64:1355–9. doi: 10.1016/j.ijrobp.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell DM, Swindell R, Elliott T, et al. Analysis of prostate-specific antigen bounce after I(125) permanent seed implant for localised prostate cancer. Radiother Oncol. 2008;88:102–7. doi: 10.1016/j.radonc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Stock RG, Stone NN, Cesaretti JA. Prostate-specific antigen bounce after prostate seed implantation for localized prostate cancer: descriptions and implications. Int J Radiat Oncol Biol Phys. 2003;56:448–53. doi: 10.1016/s0360-3016(02)04470-x. [DOI] [PubMed] [Google Scholar]
- 5.Crook J, Gillan C, Yeung I, et al. PSA kinetics and PSA bounce following permanent seed prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2007;69:426–33. doi: 10.1016/j.ijrobp.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Bostancic C, Merrick GS, Butler WM, et al. Isotope and patient age predict for PSA spikes after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2007;68:1431–7. doi: 10.1016/j.ijrobp.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 7.Das P, Chen MH, Valentine K, et al. Using the magnitude of PSA bounce after MRI-guided prostate brachytherapy to distinguish recurrence, benign precipitating factors, and idiopathic bounce. Int J Radiat Oncol Biol Phys. 2002;54:698–702. doi: 10.1016/s0360-3016(02)03036-5. [DOI] [PubMed] [Google Scholar]
- 8.Caloglu M, Ciezki J. Prostate-specific antigen bounce after prostate brachytherapy: review of a confusing phenomenon. Urology. 2009;74:1183–90. doi: 10.1016/j.urology.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–9. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- 10.Crouzet S, Rebillard X, Chevallier D, et al. Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients. Eur Urol. 2010;58:559–66. doi: 10.1016/j.eururo.2010.06.037. [DOI] [PubMed] [Google Scholar]