Abstract
Multifunctional imaging nanoprobes have proven to be of great value in the research of pathological processes, as well as the assessment of the delivery, fate, and therapeutic potential of encapsulated drugs. Moreover, such probes may potentially support therapy schemes by the exploitation of their own physical properties, e.g., through thermal ablation. This review will present four classes of nanoparticulate imaging probes used in this area: multifunctional probes (1) that can be tracked with at least three different and complementary imaging techniques, (2) that carry a drug and have bimodal imaging properties, (3) that are employed for nucleic acid delivery and imaging, and (4) imaging probes with capabilities that can be used for thermal ablation. We will highlight several examples where the suitable combination of different (bio)materials like polymers, inorganic nanocrystals, fluorophores, proteins/peptides, and lipids can be tailored to manufacture multifunctional probes to accomplish nanomaterials of each of the aforementioned classes. Moreover, it will be demonstrated how multimodality imaging approaches improve our understanding of in vivo nanoparticle behavior and efficacy at different levels, ranging from the subcellular level to the whole body.
Intravenous administration of nanoparticles is increasingly being explored as a strategy to deliver therapeutic and diagnostic agents, for nucleic acid delivery, and thermal ablation.1 A range of advantages makes this delivery strategy exceptionally valuable and has resulted in explosive growth of the field that focuses on the development of novel nanoparticulate formulations.2–4 Nanoparticles are designed to have increased blood circulation half-lives, can carry high and diverse payloads, allow for easy derivatization and functionlization, and have the potential to reduce adverse systemic effects of their cargo.3 These qualities were recognized to be of great significance for targeted therapy and resulted in the development of liposomal formulations of cytostatic agents over 30 years ago.5 Because that time both the nature and the cargo of nanoparticles has been topic of investigation and has resulted in the use of a wide variety of materials and structures. Besides the aforementioned liposomes, these include other lipidic nanoparticles such as micelles,6 microemulsions,7,8 and disks,9 or naturally occurring lipidic nanoparticles such as lipoproteins.10 Polymeric nanoparticles have been investigated for the same purpose for an extended period of time,11 while the use of inorganic nanocrystals has increasingly been explored,12,13 mainly in the last decade. An overview of all the possible nanoparticles and nanomaterials for intravenous administration is beyond the scope of this review, but we refer the reader to some good reviews that have been published previously.1,3–5,14–16
Although therapy was the main initial application of nanoparticles, it was soon recognized that nanomaterials can also have exciting features for diagnostic purposes. For example, nanoparticles containing a high payload of paramagnetic ions or iodine can be employed for magnetic resonance imaging (MRI) or computed tomography (CT), respectively.17,18 Other nanoparticles, such as microemulsions and microbubbles may have echogenic properties, which makes them suitable as a contrast agent for ultrasound.19 Quantum dots (QDs), semiconductor nanocrystals, possess excellent fluorescence qualities and are being exploited for optical imaging purposes.12
One of the most exciting features of nanoparticles for their use in biomedicine is the possibility of including several payloads/features to enable their application of multiple purposes, e.g., diagnostics and therapy, or multimodality imaging.20 Although the field is young, the number of bifunctional nanoprobes available today is considerable. Nevertheless, most of these materials are employed for a maximum of two applications, so-called dual-modality nanoparticles. The number of reports about nanoparticles that exhibit features for more than two applications, termed multimodality nanoparticles, is relatively limited. In the current review we will focus on such state-of-the-art nanoparticle platforms that have been exploited for at least two imaging techniques and targeted therapy or a third imaging technique. We will refer to these nanoparticles as multifunctional imaging nanoprobes.
NANOPARTICLE DESIGN AND APPLICATIONS IN THERAPY AND DIAGNOSTICS
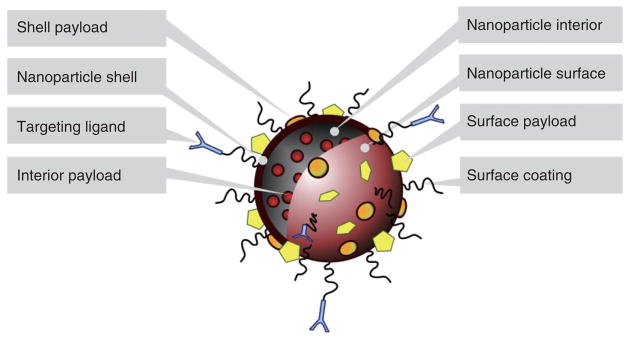
A generalized schematic of a multimodality nanoprobe is depicted in Figure 1. It has to be stressed that different variations are possible and numerous examples of nanoparticles that do not resemble this schematic have been developed. Nanoparticles may consist of a core material, i.e., nanocrystalline materials or hydrophobic oils, or may consist of a shell whose interior is hollow and can incorporate payloads. In the first case the core material itself may have distinct features suitable for diagnostic or therapeutic purposes, while in the latter case the interior may be used to carry diagnostic or therapeutic agents. The shell of a nanoparticle may have distinctively different properties from the core and, therefore, may be used to incorporate certain molecules that cannot be integrated in the nanoparticle lumen or core. Notably, the surface of most nanoparticles can be readily modified, which is a valuable feature that can be exploited to include diagnostic and therapeutic molecules, but also to alter the surface to increase biocompatibility and bioapplicability. Such coatings include hydrophilic polymers like PEG21 and polysaccharides like dextran.22 To increase target-specific uptake, ligands, such as antibodies, antibody fragments, sugars, peptides, peptidomimetics, and small molecules may be conjugated directly to the surface or to the surface coating of nanoparticles.5
FIGURE 1.
A generalized schematic of the ways in which a nanoparticle may be targeted, made biocompatible, and carry payloads such as drugs or contrast inducing materials.
This review paper will focus on a number of nanoparticle applications including diagnostics and therapies. A full and detailed summary is beyond the scope of this review, but we will briefly discuss the most prominent therapeutic applications and most relevant imaging modalities. The first and foremost application of nanoparticles is in the field of drug delivery. Numerous preclinical and clinical studies have shown significant benefits, including decreased adverse effects, lower dosages, increased specificity, controlled release, and the possibility to administer compounds that are not water-soluble.4 Another important application of nanoparticles lies in the field of nucleic acid delivery.23 This is because of the following difficulties in applying nucleic acid-based drugs: they are nuclease sensitive, it is difficult to direct them to the required site of action, and, once they reach the targeted site, an adjuvant is frequently required for translocation to the cytoplasm or nucleus. Lastly, nanoparticle facilitated thermal ablation using gold or iron oxide based agents is increasingly being explored, particularly in oncology.24,25
Nanoparticles can be radiolabeled to allow their visualization with nuclear imaging techniques, including scintigraphic imaging, positron emission tomography (PET) and single photon emission computed tomography (SPECT).26 These techniques exhibit high sensitivity but suffer from a poor anatomical definition and spatial resolution. MRI is one of the most versatile techniques in both clinical and research settings and gives excellent images of soft tissue with high spatial resolution.27 Nanoparticle visualization with MRI can be realized via a number of routes. First, nanoparticles can carry a payload of paramagnetic chelates to induce bright contrast in so-called T1-weighted images.27 Another MRI nanoparticle class consists of agents based primarily on superparamagnetic iron oxide that cause dark areas in T2/T2*-weighted images and, therefore, are termed negative contrast agents.27 More recent developments include the nanoparticulate formulations of chemical exchange saturation transfer (CEST) agents and fluorine-based agents.28,29 Both types of agents can be visualized in a ‘hot-spot’ fashion, without the necessity to perform a prescan, and also have the possibility for the simultaneous visualization of multiple species, i.e., multicolor imaging. Despite these advantages, the detection sensitivity of CEST and fluorine probes is relatively poor.
Contrast agents for CT imaging are typically based on iodine, colloidal gold, or bismuth nanoparticles.30 These materials can be integrated in or serve as the base of a nanoparticle platform. CT excels in the visualization of hard tissue with high temporal and spatial resolution, but high concentrations of exogenous administered agents are required for their detection.
Optical imaging techniques exhibit superb spatial and temporal resolution, are very sensitive and are capable of visualizing multiple fluorescent species simultaneously.31 A wide variety of fluorophores are available ranging from dyes, to proteins and semiconductor nanocrystals (QDs).12 In addition to confocal microscopy, multi-photon microscopy, intravital microscopy, near-infrared imaging techniques are increasingly being used on both cells in vitro and small laboratory animals in vivo. In contrast to the abovementioned techniques, most of the optical techniques are invasive, can usually only be applied to dissected tissues or are limited for application to tissues on or near the surface of small animals.
BEYOND DUAL MODALITY: MULTIFUNCTIONAL NANOPARTICLES
In this review we will discuss four classes of ‘imageable’ nanoparticle classes that have been employed for multifunctional purposes. The first class consists of nanoparticles that are employed for at least trimodal imaging studies. Nanoparticle platforms applied for both multimodal imaging and targeted drug delivery comprise the second class of nanoparticles, while the third class includes nanoparticles that are employed for multimodal imaging and nucleic acid delivery. The last and fourth class that will be discussed consists of nanoparticles that have both features for diagnostics and thermal therapy.
MULTIMODAL NANOPARTICLES EMPLOYED FOR AT LEAST THREE IMAGING MODALITIES
Multimodal probes have been demonstrated to be useful when combined with labels for different, preferably complementary imaging modalities. One of the first examples of a bimodal probe was reported by Huber, Meade et al.32 They described bifunctional contrast-enhancing agents for optical and MRI. The main advantage of this combination is that it integrates optical properties for high-resolution fluorescence microscopy and imaging and magnetic properties for in vivo MRI visualization of intact and opaque organisms. Various other fluorescently labeled MRI probes have also been explored, of which an overview can be found in two excellent review papers.33,34 In addition to fluorescently labeled MRI probes, many other types of dual imaging probes have also been developed. For example, Cai et al. developed a QD probe that was additionally labeled with 64Cu to enable its visualization by PET imaging.35
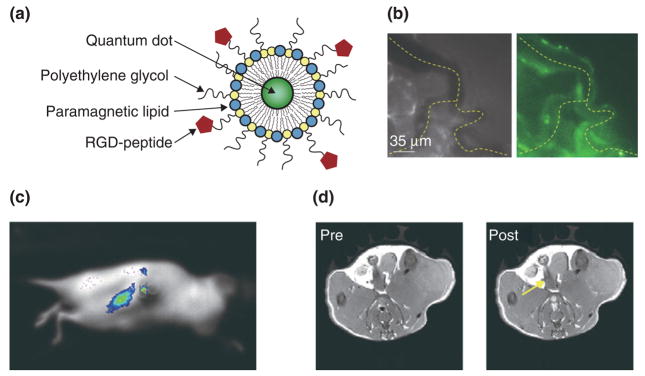
More recently, nanoprobes that exhibit features for their visualization with more than two imaging modalities have been investigated. Paramagnetic QD-micelles (Figure 2a) were introduced by Mulder et al. in 2006.36 In this study it was shown that QDs encapsulated in a monolayer of polyethylene glycol(PEG)ylated and paramagnetic lipids exhibit excellent features for both optical techniques and MRI. Moreover, the PEGylated lipids were functionalized with RGD-peptides to introduce specificity for the angiogenic marker αvβ3-integrin. In a follow-up study targeted multimodality imaging of tumor angiogenesis was performed on tumor-bearing mice that were intravenously injected with the αvβ3-specific QD-micelles and were studied with three complementary in vivo imaging modalities.37 Intravital fluorescence microscopy was used for the real-time monitoring of the fate of injected QD-micelles. In Figure 2b, a brightfield and a fluorescence image of tumor blood vessels are depicted 30 min after administration of the QD-micelles. Another optical technique, whole body fluorescence imaging, enabled the visualization of particle accumulation with high sensitivity and high temporal resolution in mice that had a tumor in their kidney (Figure 2c). As a third imaging modality, high-resolution T1-weighted MRI was performed before and 45 min following QD-micelle administration and revealed significant signal enhancement that was mainly found at the tumor periphery (Figure 2d), which corresponds with the regions of the tumor with highest angiogenic activity.
FIGURE 2.
Paramagnetic QD-micelles for multimodality imaging. (a) Schematic depiction of αvβ3-specific and paramagnetic QD-micelles. (b) Intravital microscopy brightfield (left) and fluorescence (right) images of microvessels in tumor-bearing mice after intravenous injection of RGD-pQDs. (c) Fluorescence image of a tumor-bearing mouse following intravenous administration of the nanoparticle agent. (d) T1-weighted MR images before and 45 min after the injection of the αvβ3-specific and paramagnetic QD-micelles. Reproduced with permission from the publisher.36,37
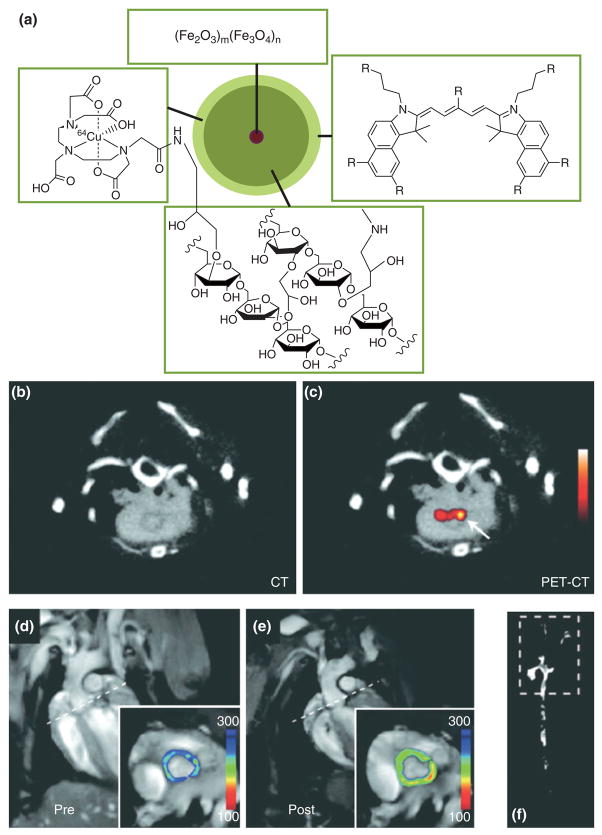
Iron oxide nanoparticles have shown great utility in the field of target-specific molecular MRI. Some pioneering studies were performed by Weissleder and colleagues who demonstrated the application of dextran coated iron oxide nanoparticles conjugated to human holo-transferrin to image transgene expression in tumor-bearing mice.38 In the field of cardiovascular disease a number of processes have been studied using iron oxide facilitated MR molecular imaging, including apoptosis after myocardial infarction39 and the upregulation of cell adhesion molecules in mouse models of atherosclerosis.40 In the aforementioned studies these iron oxide probes were additionally labeled with a fluorescent dye to allow colocalization with immunofluorescent techniques and perform near-infrared fluorescence (NIRF) imaging of live animals and excised organs, such as the heart and aorta. Most recently, Nahrendorf, Weissleder, and colleagues have shown two approaches to additionally label these magnetofluorescent probes with a radiolabel to allow their visualization with PET.41,42 In one approach the fluorescent and magnetic nanoprobes were conjugated with 18F-based radiotracer via so-called ‘click’ chemistry,41 while in another approach the chelator diethylenetriaminepentaacetic acid (DTPA) was conjugated to the nanoparticle dextran coating to allow complexation with the radiotracer 64Cu (Figure 3a).42 The latter nanoparticle platform was employed to quantitatively study macrophage inflammation in the aorta of atherosclerotic apolipoprotein E knockout (apoE-KO) mice. In Figure 3b and c a CT and combined PET/CT image reveal enhancement of the posterior aortic root, an area of the aorta that is known to have a high plaque burden in this mouse model. MRI scans of the aortic root prior to (Figure 3d) and postadministration (Figure 3e) of the triply labeled nanoprobe revealed a decrease of T2, which is indicative of iron oxide accumulation. Moreover, NIRF imaging of excised and intact aortas revealed the distribution of the nanoprobe in the atherosclerotic aorta and confirmed a prominent accumulation in the aortic root (Figure 3f).
FIGURE 3.
(a) Schematic of the trimodality reporter 64Cu-TNP. The chelator DTPA allows attachment of radiotracer 64Cu, the iron oxide core provides contrast in MRI and the fluorophore for fluorescence techniques. (b), (c) 64Cu-TNP accumulates in atherosclerotic lesions; PET-CT shows enhancement of the posterior aortic root (arrow). (d) Preinjection and (e) postinjection MRIs of the aortic root (inset). The dotted line in the long-axis views demonstrates slice orientation for shortaxis root imaging. Signal intensity (pseudocolored with identical scaling for preinjection and postinjection image) decreased significantly after injection of 64Cu-TNP. (f) Near-infrared fluorescence reflectance imaging (NIRF) of excised aortas shows accumulation of the probe in plaques residing in the root, thoracic aorta, and carotid bifurcation. Reproduced with permission from the publisher.42
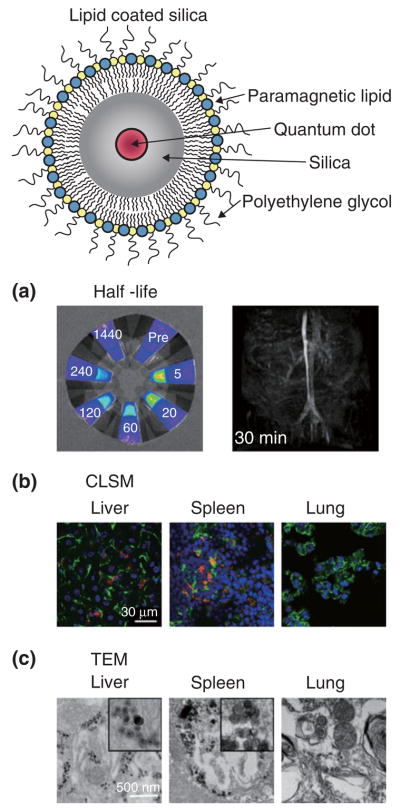
Silica nanoparticles can be synthesized in a wide range of desired sizes (50–1000 nm) and in the past decade, silica-based nanoparticles have increasingly been exploited for biomedical applications, including drug and gene delivery as well as a carrier vehicle for different contrast-generating materials. Despite the intrinsic utility of silica for biomedical applications, a serious drawback of these inorganic nanoparticles is their inherently low biocompatibility. To address this issue Koole et al. have recently developed a novel method to obtain hydrophobic silica nanoparticles coated with a physically adsorbed monolayer of PEGylated phospholipids (Figure 4, top).43 This highly flexible coating method allows, next to the inclusion of PEGylated lipids, the incorporation of many other lipid species, e.g., paramagnetic lipids for MRI and bio-functional lipids to achieve target specificity. In a recent study this nanoparticle platform was functionalized with αvβ3-specifc RGD-peptides and it was shown with fluorescence microscopy, MRI, and fluorescence imaging that these particles were preferentially taken up by proliferating endothelial cells (HUVEC, human umbilical vein endothelial cells) in vitro. The cytotoxicity and pharmacokinetics of paramagnetic and PEG-lipid coated QD containing silica nanoparticles was studied by van Schooneveld et al. using a variety of imaging techniques.44 Lipid-coated silica exhibited a prolonged circulation half-life as determined by quantitative fluorescence imaging of blood samples and in vivo MRI (Figure 4a). Confocal microscopy (Figure 4b) and transmission electron microscopy (TEM) (Figure 4c) of tissue sections of mice sacrificed 24 h after the administration of lipid-coated silica nanoparticles revealed the particles to accumulate in the liver and spleen. Interestingly, the bare silica particles were also found to accumulate in the lungs of the animals, indicating the value of the lipid coating.
FIGURE 4.
Top: Schematic depiction of quantum dot containing silica particle with a lipid coating. (a) Determination of blood circulation half-life values by fluorescence imaging (left) and magnetic resonance angiography (right). (b) Confocal microscopy of sections from different organs collected from mice at 24 h after injection and stained for endothelial cells to visualize blood vessels (green) and nuclei (blue), shows particle accumulation in red. (c) TEM was used to show particle uptake by cells in the liver, spleen, and lung. Reproduced with permission from the publisher.44
A number of studies have focused on nanoparticulate MRI probes that were also labeled for optical detection. Interestingly, the nature of the majority of such particles allows their visualization with TEM, rendering them trimodal. Excellent examples based on silica nanoparticles were independently published by Kim et al.,45 Rieter et al.,46 and Zhang et al.47
The combination of using different imaging techniques, as demonstrated in the abovementioned studies, has shown to be very valuable for a variety of reasons. PET/CT imaging allows quantification of nanoparticle accumulation, be it with a relatively poor spatial resolution, while MRI methods can generate images of opaque tissue with a very good spatial resolution, superb tissue contrast, albeit with limited sensitivity to detect contrast-generating materials. Optical techniques have the advantage of a high sensitivity and the capability to detect multiple species simultaneously, but the penetration depth of light is limited which restricts the application of these techniques to the surface or small species. Microscopic techniques allow the investigation of the (sub)cellular distribution of nanoparticulate materials, while electron microscopy techniques can visualize cellular organelles and can also identify individual particles within these organelles. Therefore, these combinatory studies allow investigators to study particle distribution, kinetics, and fate at different levels, ranging from the entire organism down to cellular and particle level.
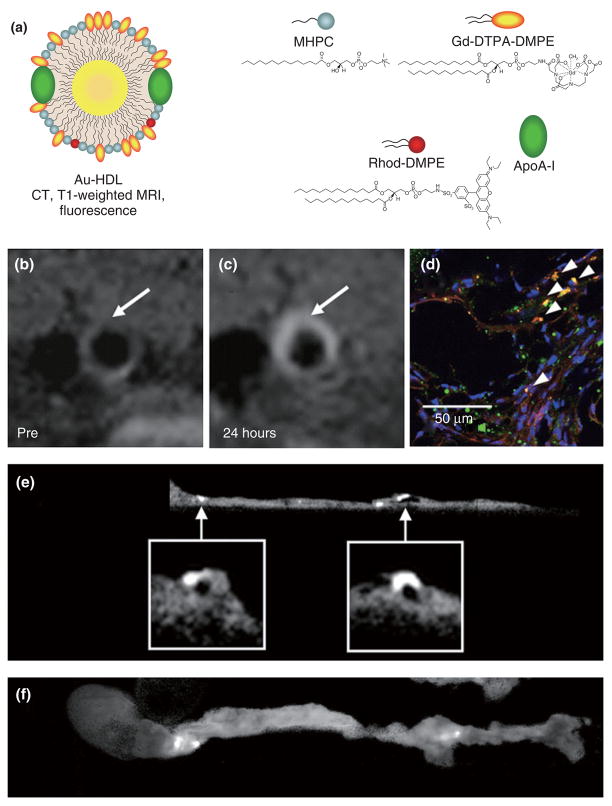
Recently, Cormode et al. modified high density lipoprotein (HDL) particles to create endogenous nanoparticle-inorganic material composites.48 A method was developed to modify both the hydrophobic core and the phospholipid coating to provide contrast for medical imaging. A variety of nanocrystals, i.e., gold nanoparticles, iron oxide nanoparticles, and QDs, were included in the HDL core to produce a broad range of novel contrast agents for multimodality imaging. The gold core HDL was additionally labeled with fluorescent and paramagnetic lipids to create a trimodal particle that has properties for detection with CT, fluorescence, and MRI (Figure 5a). Characterization of the particles revealed these nanoparticles to be very similar to native HDL.
FIGURE 5.
(a) Schematic depiction of gold core high density lipoprotein (Au-HDL). The particle corona consists of ordinary phospolipids, paramagnetic lipids, fluorescent lipids and apolipoprotein A-I. T1-weighted MR images of the aorta of an apoE-KO mouse (b) before and (c) 24 h postinjection with Au-HDL. (d) Confocal microscopy image of an aortic section revealed colocalization of nanocrystal HDL (red) with macrophages (green) as indicated by arrowheads. The nuclei are depicted in blue. (e) Ex vivo sagittal CT image of an aorta of a mouse injected with Au-HDL. (f) Corresponding fluorescence image of the aorta depicted in (e). Reproduced with permission from the publisher.48
In vitro experiments revealed that these particles were preferentially taken up by macrophages, as evidenced by confocal laser scanning microscopy, cell pellet MR imaging, CT imaging, and TEM. In vivo experiments using the gold core HDL nanoparticles were performed with the apoE-KO mouse model of atherosclerosis. The abdominal aorta of mice injected with Au-HDL appeared brighter on T1-weighted MR images at 24 h (Figure 5b and c, respectively), while ex vivo confocal microscopy of aortic sections that were stained for macrophages (green) and for nuclei (blue) revealed the Au-HDL particles (red) to be associated with macrophages (Figure 5d). CT images of intact aorta specimen from Au-HDL-injected and control animals revealed specific uptake of Au-HDL (Figure 5e). Importantly, these CT data were corroborated with fluorescence imaging of the same specimen (Figure 5f).
NANOPARTICLES EMPLOYED FOR DUAL-MODALITY IMAGING AND DRUG DELIVERY
Nanoparticle platforms for simultaneous drug delivery and diagnostic purposes offer a number of unique advantages that add to our understanding of the efficacy, mode of action and specificity of the therapy, especially in a preclinical setting. This field of diagnostic therapy is popularly referred to as ‘theranostics’ and has the ultimate goal to improve and personalize nanotherapeutic approaches. Lanza, Wickline, and colleagues have published a number of theranostic studies in which perfluorocarbon microemulsions were used for simultaneous drug delivery and imaging.49 For example, Winter et al. have shown the application of fumagillin loaded nanoemulsions to inhibit angiogenesis in rabbit models of atherosclerosis and cancer, while using MRI as a readout for therapeutic efficacy.50
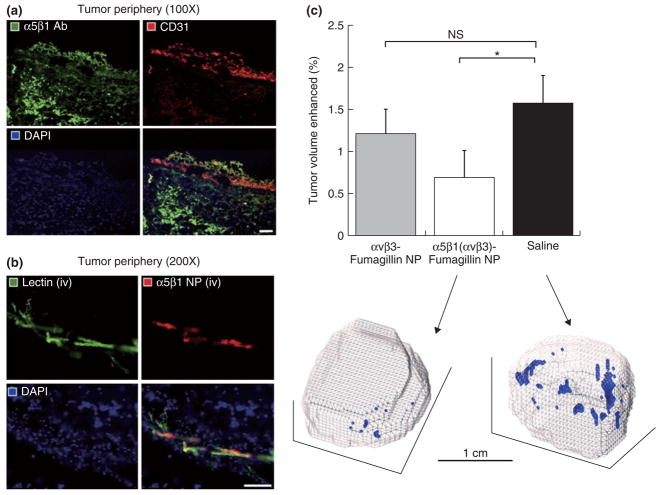
A study where the abovementioned perfluorocarbon microemulsion platform was labeled for both MRI and fluorescence, and also carried a payload of fumagillin, has been applied to tumor model recently.51 In this study, the nanoparticles were functionalized with different angiogenesis specific targeting ligands and their neovascularization-reducing efficacy was extensively studied. It was shown that in a xenograft mouse model with sparse angiogenesis dual targeted nanoparticles to α5β1-and αvβ3-intergin specifically bound to tumor blood vessels in the tumor periphery (Figure 6a and b) as determined with immunofluorescent techniques. Three dimensional MR mapping of the angiogenic response revealed a significant reduction of the percentage of the enhanced tumor volume for the dual α5β1(αvβ3)-integrin targeted particles, while αvβ3-integrin targeted did not significantly reduce tumor angiogenesis.
FIGURE 6.
Histological examination of α5β1-integrin expression and nanoparticle localization. (a) Fluorescent microscopy images (×200) of conventional immunohistochemistry of 8 μm sections of tumor border stained for α5β1-integrin (green) and endothelium (red), with nuclei counterstained blue. There is prevalent expression of α5β1-integrin throughout the tissue section, both within and outside of the vasculature. (b) The merged images confirm that the nanoparticles colocalized with the angiogenic vasculature, and did not reach the α5β1-integrin expressed by tumor and other cells in the extravascular matrix. Reproduced with permission from the publisher.51
A polymeric platform based on poly(D, L-lactic-co glycolic acid) and the surfactant F127, with iron oxide and QD nanocrystals as well as doxorubicin incorporated was developed by Kim and colleagues.52 Using an oil-in-water and subsequent evaporation method spherical nanoparticles in a size range of 100–200 nm were produced and tested on KB cancer cells. It was shown that the particles were taken up by the cells as evidenced by microscopic techniques and MRI. Via a cell viability assay it was demonstrated that the nanoparticles without doxorubicin exhibited a high degree of biocompatibility, while the doxorubicin loaded particles reduced the cell viability significantly.
Prior to the aforementioned study a polymeric micellar particle containing iron oxide nanocrystals, an amphiphilic polymer, doxorubicin and a fluorophore was reported by Nasongkla et al.53 Conjugation of αvβ3-specific RGD-peptides resulted in their preferential uptake by tumor SLK endothelial cells and the subsequent inhibition of their growth.
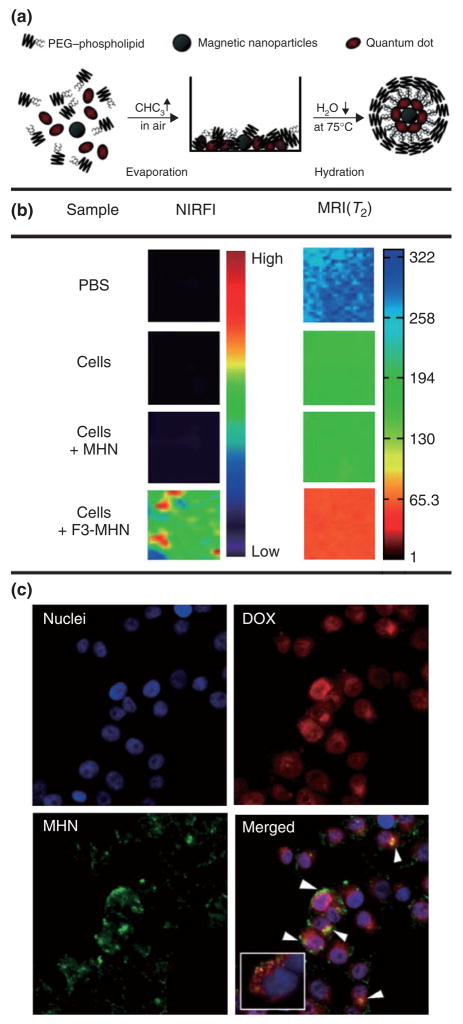
Another polymeric micellar nanoparticle platform with a core composed of iron oxide, QDs and doxorubicin is shown in Figure 7.54 A simple evaporation and hydration method (Figure 7a) was used to create the particles that have a hydrodynamic size of 60–70 nm. MDA-MB-435 human cancer cells were incubated with peptide functionalized nanoparticles and were shown to be preferentially taken up as compared to nonfunctionalized nanoparticles as was shown by near-infrared imaging and MRI (Figure 7b). Moreover, because doxorubicin has fluorescent properties it was demonstrated that this nanoparticle vehicle caused intracellular delivery of the drug, within endosomes (Figure 7c).
FIGURE 7.
(a) Synthetic procedure used to prepare micellar hybrid nanoparticles that encapsulate magnetic nanoparticles and quantum dots within a single PEG-modified phopholipid micelle. (b) Multimodal images (NIR fluorescence in the Cy5.5 channel and MRI) of PBS and MDA-MB-435 human carcinoma cells that were left untreated, were treated with untargeted nanoparticles and with targeted nanoparticles. (c) Targeted drug delivery of nanoparticles containing DOX into MDAMB-435 human carcinoma cells. The DOX-loaded nanoparticles were incubated with the cells for 2 h. Arrowheads indicate colocalization of DOX and nanoparticles. The inset shows the colocalization of some DOX (red) and the endosome marker (green) 30 min after incubation with DOX-loaded nanoparticles. The nuclei were stained with 4–6-diamidino-2-phenylindole. Reproduced with permission from the publisher.54
In addition to lipidic and polymeric nanoparticles, solid inorganic nanoparticles have also been explored as a multimodal imaging drug delivery vehicle. For example, Liong et al. have developed magnetic and fluorescent silica particles for the delivery of paclitaxel,55 while Lai et al. developed iron oxide core silica nanoparticles carrying iridium complexes for simultaneous MRI, luminescent imaging and photodynamic therapy.56
NANOPARTICLES EMPLOYED FOR DUAL-MODALITY IMAGING AND NUCLEIC ACID DELIVERY
A variety of difficulties have limited the application of gene-specific interventions as a therapeutic strategy. Among those are degradation of the nucleic acids, poor bioavailability, and biodistribution upon intravenous administration, as well as problems that are associated with diminished acitivity in the cells of interest because of compartmentalization.23 Nanoparticulate formulations may have significant advantages to deal with the aforementioned limitations and, therefore, have been applied extensively in this field of research. More recently studies have appeared where the nucleic acid delivery vehicle was additionally labeled for visualization with diagnostic imaging techniques. For example, nucleic acid delivery platforms based on diagnostically active nanocrystals such as iron oxide,57 QD58 or gold59 nanoparticles have been topic of investigation, which allows their visualization with MRI, optical techniques, and CT. In this section we will highlight some topnotch studies where nanoparticles with bimodal imaging properties and nucleic acid delivery potential were studied.
Medarova et al. reported a study where they developed and employed a dextran coated iron oxide nanoparticle that had a near-infrared fluorescent dye, a translocation peptide and small interfering RNA, specific for green fluorescent protein (GFP) mRNA, covalently conjugated (Figure 8a).57 This design allows the nanoparticle to be visualized with MRI and near-infrared fluorescent imaging, to be efficiently taken up by cells and to subsequently silence GFP expression. The authors showed, with MRI (Figure 8b) and NIRF imaging, that upon intravenous administration this nanoparticle accumulated in the tumors of mice that had two tumor types inoculated on the flank, of which one tumor expressed red fluorescent protein (RFP) and one tumor expressed GFP. Using in vivo optical imaging it was shown that GFP expression was selectively silenced 48 h after probe administration, while RFP expression remained unaffected (Figure 8c). Moreover, the authors demonstrate silencing of the Birc5 gene using this nanoparticle platform. A siRNA was chosen that reduces Survivin expression in the tumor and thereby induces apoptosis in this tissue (Figure 8d). This study convincingly demonstrated how the combination of nanotechnology, imaging, and genetics allows investigators to visualize nanoparticle delivery and subsequent silencing in live animals.
FIGURE 8.
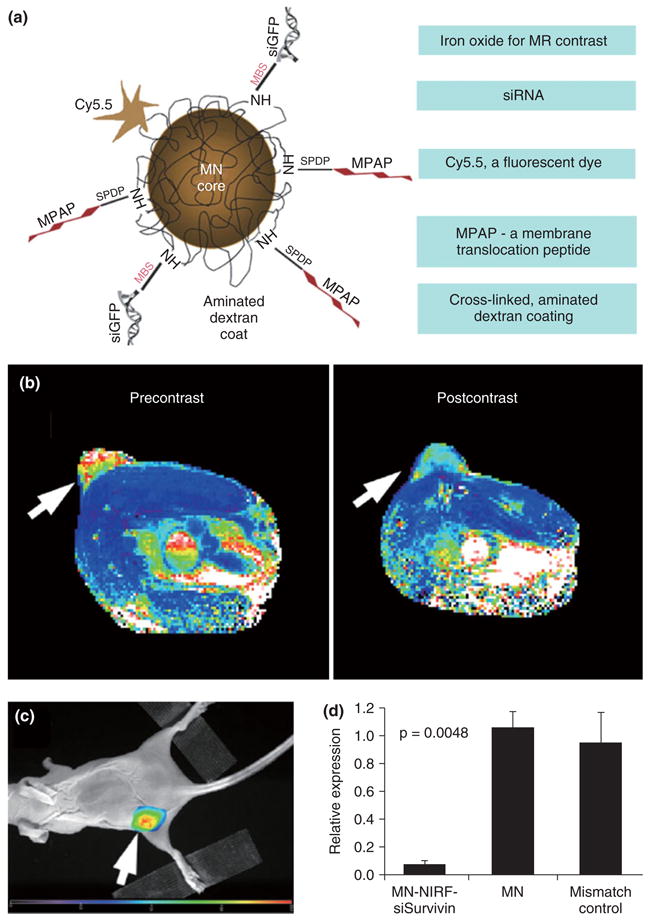
(a) Schematic depiction of an iron oxide based MR contrast agent that can deliver siRNA. (b) MR image of a mouse pre- and 24 h postadministration of the agent. Change in pixel color in the tumor (arrow) from reddish yellow to blue indicates accumulation of the agent. (c) Fluorescence image of the mouse with emission seen from the tumor, also indicating agent accumulation. (d) Silencing of the Birc5 gene by the siRNA reduces Survivin expression in the tumor and leads to apoptosis in this tissue. Reproduced with permission from the publisher.57
Subsequent to the aforementioned study Bartlett et al. reported a study where PET and bioluminscence imaging was applied to study the biodistribution and efficacy of siRNA complexed and transferring targeted nanoparticles.60 Most recently, Lee et al. reported a PEGylated iron oxide based particle that was functionalized with αvβ3-integrin specifc RGD-peptides, a fluorescent dye and siRNA to enable the visualization of target-specific delivery with MRI and microscopic techniques.61
NANOPARTICLES EMPLOYED FOR DUAL-MODALITY IMAGING AND THERMAL ABLATION
The last category that will be discussed are nanoparticles that can be employed for dual-modality imaging and thermal ablation. Thermal ablation of nanoparticles has been explored for some time and thus far two strategies have shown effective. In the first approach iron oxide nanoparticles are heated by applying a fluctuating magnetic field and because iron oxide also functions as an MRI contrast agent the combination of magnetothermal ablation and MRI is trivial. Second, gold nanoparticles may be heated through photothermal ablation using laser illumination, preferably in the near-infrared region to allow deep tissue photothermal ablation.
Composite materials that consist of both gold and iron oxide were developed by Lim et al.62 Larson et al.,63 and, most recently, by Wang et al.64 In the first two studies the materials consisted of an iron oxide core that was subsequently covered by a gold shell, while in the study by Wang et al. the composite material consisted of iron oxide nanoparticles that were adsorbed on the surface of gold nanorods. It was shown in these studies that the materials can be functionalized with targeting moieties, can be employed for both MRI and optical imaging, as well as photothermal ablation. An alternative approach, where the MRI properties were introduced by the conjugation of Gd-DTPA to the surface of gold nanoparticles via an Anti-HER2 antibody, was published by Kim and colleagues.65 As for the aforementioned composite materials this material can be employed for target-specific photothermal ablation, MRI, and optical imaging.
CONCLUSION
In this review paper we have shown topnotch examples of the development and application of nanomaterials that can be employed for at least dual-modality imaging and a third application, i.e., a third or fourth imaging modality, drug delivery, nucleic acid delivery, or thermal ablation. Multimodality imaging has been demonstrated to be especially useful in a pre-clinical setting where it may serve to validate the findings obtained by a clinical imaging modality such as MRI, CT, PET, or SPECT with a preclinical imaging modality such as near-infrared fluorescence imaging, intravital microscopy, or ex vivo techniques such as confocal microscopy and electron microscopy. Moreover, this multimodality approach allows investigators to better understand the accumulation and targeting kinetics of the new materials, as well as the mechanism of particle interaction with tissues and cells and particle secretion pathways. Composite materials that allow both multimodality imaging and a therapeutic intervention are of particular interest, because these approaches may better clarify the mode of action of a given therapy and, therefore, improve the decision making and timing of therapy. Although this type of personalized medicine will benefit patients, the extra costs and technical complications that are involved will likely limit a widespread implementation. On the other hand, in a preclinical setting, multimodality imaging of nanotherapies is highly valuable and will enhance the developmental process and allows more rapid and precise screening in vivo. Advances in the field of nanochemistry will contribute to expand the field of multifunctional imaging nanoprobes and will allow more exotic nanocomposites to be developed. A major issue will remain the biocompatibility of the material and the benefits the eventual application will have. Those aspects will drive the translation of the technologies from a preclinical setting to the clinic. Irrespective if the ultimate application in humans will eventually occur the establishment of the field has already had a major impact in the areas of nanotechnology and nanochemistry and has shown great benefits in preclinical biomedical research.
References
- 1.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29:992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 3.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 5.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 7.Ansari MJ, Kohli K, Dixit N. Microemulsions as potential drug delivery systems: a review. PDA J Pharm Sci Technol. 2008;62:66–79. [PubMed] [Google Scholar]
- 8.Bagwe RP, Kanicky JR, Palla BJ, Patanjali PK, Shah DO. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst. 2001;18:77–140. [PubMed] [Google Scholar]
- 9.Vucic E, Sanders HM, Arena F, Terreno E, Aime S, et al. Well-defined, multifunctional nanostructures of a paramagnetic lipid and a lipopeptide for macrophage imaging. J Am Chem Soc. 2009;131:406–407. doi: 10.1021/ja808310u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormode DP, Mulder WJM, Fisher EA, Fayad ZA. Modified lipoproteins as contrast agents for molecular imaging. Future Lipidol. 2007;2:587–590. [Google Scholar]
- 11.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 14.Mulder WJ, Strijkers GJ, van Tilborg GA, Cormode DP, Fayad ZA, et al. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc Chem Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva GA. Nanomedicine: seeing the benefits of ceria. Nat Nanotechnol. 2006;4:92–94. doi: 10.1038/nnano.2006.111. [DOI] [PubMed] [Google Scholar]
- 16.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 17.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 18.Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 19.Morawski AM, Lanza GA, Wickline SA. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr Opin Biotechnol. 2005;16:89–92. doi: 10.1016/j.copbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Mulder WJ, Cormode DP, Hak S, Lobatto ME, Silvera S, et al. Multimodality nanotracers for cardiovascular applications. Nat Clin Pract Cardiovasc Med. 2008;(suppl 2):S103–S111. doi: 10.1038/ncpcardio1242. [DOI] [PubMed] [Google Scholar]
- 21.Bailon P, Won CY. PEG-modified biopharmaceuticals. Expert Opin Drug Deliv. 2009;6:1–16. doi: 10.1517/17425240802650568. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy JR, Kelly KA, Sun EY, Weissleder R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomedicine. 2007;2:153–167. doi: 10.2217/17435889.2.2.153. [DOI] [PubMed] [Google Scholar]
- 23.Schiffelers RM, de Wolf HK, van RI, Storm G. Synthetic delivery systems for intravenous administration of nucleic acids. Nanomed. 2007;2:169–181. doi: 10.2217/17435889.2.2.169. [DOI] [PubMed] [Google Scholar]
- 24.Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, et al. Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Invest Radiol. 2002;37:580–586. doi: 10.1097/00004424-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 25.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Mitra A, Nan A, Line BR, Ghandehari H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Curr Pharm Des. 2006;12:4729–4749. doi: 10.2174/138161206779026317. [DOI] [PubMed] [Google Scholar]
- 27.Strijkers GJ, Mulder WJ, van Tilborg GA, Nicolay K. MRI contrast agents: current status and future perspectives. Anticancer Agents Med Chem. 2007;7:291–305. doi: 10.2174/187152007780618135. [DOI] [PubMed] [Google Scholar]
- 28.Woods M, Woessner DE, Sherry AD. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem Soc Rev. 2006;35:500–511. doi: 10.1039/b509907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulte JW. Hot spot MRI emerges from the background. Nat Biotechnol. 2005;23:945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 30.Yu SB, Watson AD. Metal-based X-ray contrast media. Chem Rev. 1999;99:2353–2378. doi: 10.1021/cr980441p. [DOI] [PubMed] [Google Scholar]
- 31.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 32.Huber MM, Staubli AB, Kustedjo K, Gray MH, Shih J, et al. Fluorescently detectable magnetic resonance imaging agents. Bioconjug Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 33.Mulder WJ, Griffioen AW, Strijkers GJ, Cormode DP, Nicolay K, et al. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomed. 2007;2:307–324. doi: 10.2217/17435889.2.3.307. [DOI] [PubMed] [Google Scholar]
- 34.Frullano L, Meade TJ. Multimodal MRI contrast agents. J Biol Inorg Chem. 2007;12:939–949. doi: 10.1007/s00775-007-0265-3. [DOI] [PubMed] [Google Scholar]
- 35.Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, et al. microPET-based biodistribution of quantum dots in living mice. J Nucl Med. 2007;48:1511–1518. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulder WJ, Koole R, Brandwijk RJ, Storm G, Chin PT, et al. Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 37.Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, et al. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis. 2009;12:17–24. doi: 10.1007/s10456-008-9124-2. [DOI] [PubMed] [Google Scholar]
- 38.Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6:351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 39.Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54:718–724. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 40.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 41.Devaraj NK, Keliher EJ, Thurber GM, Nahrendorf M, Weissleder R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug Chem. 2009;20:397–401. doi: 10.1021/bc8004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koole R, van Schooneveld MM, Hilhorst J, Castermans K, Cormode DP, et al. Paramagnetic lipid-coated silica nanoparticles with a fluorescent quantum dot core: a new contrast agent platform for multimodality imaging. Bioconjug Chem. 2008;19:2471–2479. doi: 10.1021/bc800368x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, et al. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: a multimodality investigation. Nano Lett. 2008;8:2517–2525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Kim HS, Lee N, Kim T, Kim H, et al. Multi-functional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 46.Rieter WJ, Kim JS, Taylor KM, An H, Lin W, et al. Hybrid silica nanoparticles for multimodal imaging. Angew Chem Int Ed Engl. 2007;46:3680–3682. doi: 10.1002/anie.200604738. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Wang SN, Ma S, Guan JJ, Li D, et al. Self-assembly multifunctional nanocomposites with Fe3O4 magnetic core and CdSe/ZnS quantum dots shell. J Biomed Mater Res A. 2008;85:840–846. doi: 10.1002/jbm.a.31609. [DOI] [PubMed] [Google Scholar]
- 48.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanza GM, Winter P, Caruthers S, Schmeider A, Crowder K, et al. Novel paramagnetic contrast agents for molecular imaging and targeted drug delivery. Curr Pharm Biotechnol. 2004;5:495–507. doi: 10.2174/1389201043376544. [DOI] [PubMed] [Google Scholar]
- 50.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 51.Schmieder AH, Caruthers SD, Zhang H, Williams TA, Robertson JD, et al. Three-dimensional MR mapping of angiogenesis with alpha5beta1(alpha nu beta3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008;22:4179–4189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Lee JE, Lee SH, Yu JH, Lee JH, et al. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Adv Mater. 2008;20:478. [Google Scholar]
- 53.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 54.Park JH, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Micellar hybrid nanoparticles for simultaneous magnetofluorescent imaging and drug delivery. Angew Chem Int Ed Engl. 2008;47:7284–7288. doi: 10.1002/anie.200801810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai CW, Wang YH, Lai CH, Yang MJ, Chen CY, et al. Iridium-complex-functionalized Fe3O4/SiO2 core/shell nanoparticles: a facile three-in-one system in magnetic resonance imaging, luminescence imaging, and photodynamic therapy. Small. 2008;4:218–224. doi: 10.1002/smll.200700283. [DOI] [PubMed] [Google Scholar]
- 57.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 58.Qi L, Gao X. Quantum dot-amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano. 2008;2:1403–1410. doi: 10.1021/nn800280r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JS, Green JJ, Love KT, Sunshine J, Langer R, et al. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME, et al. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JH, Lee K, Moon SH, Lee Y, Park TG, et al. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl. 2009;48:4174–4179. doi: 10.1002/anie.200805998. [DOI] [PubMed] [Google Scholar]
- 62.Lim YT, Cho MY, Kim JK, Hwangbo S, Chung BH. Plasmonic magnetic nanostructure for bimodal imaging and photonic-based therapy of cancer cells. Chem-biochem. 2007;8:2204–2209. doi: 10.1002/cbic.200700416. [DOI] [PubMed] [Google Scholar]
- 63.Larson TA, Bankson J, Aaron J, Sokolov K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology. 2007;18:325101/1–325101/8. [Google Scholar]
- 64.Wang C, Chen J, Talavage T, Irudayaraj J. Gold nanorod/Fe3O4 nanoparticle “nano-pearl-necklaces” for simultaneous targeting, dual-mode imaging, and photothermal ablation of cancer cells. Angew Chem Int Ed Engl. 2009;48:2759–2763. doi: 10.1002/anie.200805282. [DOI] [PubMed] [Google Scholar]
- 65.Lim YT, Cho MY, Choi BS, Lee JM, Chung BH. Paramagnetic gold nanostructures for dual modal bioimaging and phototherapy of cancer cells. Chem Commun (Camb) 2008;28:4930–4932. doi: 10.1039/b810240f. [DOI] [PubMed] [Google Scholar]