Abstract
Helper-dependent adenoviral (HDAd) vectors devoid of all viral-coding sequences are promising non-integrating vectors for liver-directed gene therapy because they have a large cloning capacity, can efficiently transduce a wide variety of cell types from various species independent of the cell cycle and can result in long-term transgene expression without chronic toxicity. The main obstacle preventing clinical applications of HDAd for liver-directed gene therapy is the host innate inflammatory response against the vector capsid proteins that occurs shortly after intravascular vector administration resulting in acute toxicity, the severity of which is dependent on vector dose. Intense efforts have been focused on elucidating the factors involved in this acute response and various strategies have been investigated to improve the therapeutic index of HDAd vectors. These strategies have yielded encouraging results with the potential for clinical translation.
INTRODUCTION
Helper-dependent adenoviral (HDAd) vectors are deleted of all viral-coding sequences, can efficiently transduce a wide variety of cell types from various species independent of the cell cycle and can result in long-term transgene expression. Several small and large animal models of genetic disorders can be corrected effectively and for long term by HDAd without chronic toxicity (1). An important advantage of HDAd vectors is their large cloning capacity of up to ∼37 kb, which allows for the delivery of large therapeutic genes and even whole genomic loci, multiple transgenes and large cis-acting elements to enhance, prolong and regulate transgene expression. The HDAd vector genome remains episomal in the nuclei of transduced cells, where it associates with cellular histones and, depending on the nature of the stuffer sequence, may undergo repression or can be maintained transcriptionally active (2). Because of its non-integrating nature, HDAd vectors are not associated with an increased risk of insertional carcinogenesis (3). Although clearly superior to early-generation Ad vectors in terms of safety and efficacy, clinical applications of HDAd have been hampered by an acute toxic response mediated by the vector capsid proteins in a dose-dependent manner. This review will focus on the current progress towards the clinical applications of HDAd vectors for liver-directed gene therapy, highlighting particularly interesting in vivo studies and discussing the important obstacles preventing clinical translation and strategies that have recently been developed to overcome them.
LIVER-DIRECTED GENE THERAPY
The liver is a very attractive target for gene therapy because it is a central organ in many metabolic processes and several inherited metabolic disorders have their origin in the liver (Table 1). Therefore, the hepatocyte is a key target for gene transfer directed at the correction of inborn errors of metabolism and hemophilias. The majority of the preclinical studies for in vivo liver-directed gene therapy, performed in various disease animal models, have demonstrated that HDAd can lead to long-term, sometimes lifelong, phenotypic correction in the absence of chronic toxicity, supporting the potential of HDAd for clinical applications (4,5). Importantly, long-term expression by HDAd has also been recapitulated in clinically relevant large animal models, including dogs and non-human primates (6–10). These results indicate that HDAd are superior to first-generation adenoviral (FGAd) vectors in terms of efficacy and chronic toxicity. With FGAd, the expression of viral genes from the vector backbone in fact is directly cytotoxic and also provokes an adaptive cellular immune response against the transduced cells resulting in transient transgene expression and chronic toxicity. This type of toxicity does not occur with HDAd vectors because they are devoid of all viral-coding sequences. However, the viral capsid, which is identical for both FGAd and HDAd, is responsible for triggering an acute inflammatory response in a dose-dependent manner (11,12). This capsid-mediated acute toxicity is lethal in non-human primates following systemic intravascular injection of either FGAd or HDAd at doses ≥1 × 1013 viral particles (VP)/kg (11,13,14). However, while FGAd vectors also result in a late phase of toxicity, occurring days to weeks after vector injection owing to low-grade expression of viral proteins from the vector backbone, the toxic response elicited by HDAd appears to be restricted exclusively to the first 24–48 h post-vector administration owing to the absence of viral gene expression. There has been a single case of intravascular administration of HDAd into a human patient. In this clinical trial, 4.3 × 1011 VP/kg of a HDAd-expressing factor VIII (FVIII) was intravenously injected into a hemophilia A patient (15). This subject developed grade 3 liver toxicity, marked increase in interleukin-6 (IL-6), thrombocytopenia and laboratory signs of disseminated intravascular coagulopathy, but all these values returned to baseline by day 19 post-infusion. Unfortunately, no evidence of FVIII expression was detected (15). Also unfortunate is that this study has yet to be published in a peer-reviewed format so that much of the details remain unknown.
Table 1.
Preclinical liver-directed gene therapy studies with HDAd vectors
| Disease |
Species | Outcome | Reference | |
|---|---|---|---|---|
| Hemophilias | Hemophilia A | Mouse | Long-term expression or anti-hFVIII antibody response; tolerance in newborn-injected mice | (62–65) |
| Dog | Long-term correction | (66,67) | ||
| Humana | No evidence of FVIII expression; toxic response | (15) | ||
| Hemophilia B | Mouse | Long-term correction | (68,69) | |
| Dog | Long-term correction | (10,70) | ||
| Inborn errors of metabolism | Ornithine transcarbamylase deficiency | Mouse | Long-term correction | (71,72) |
| Arginase deficiency | Mouse | Short-term correction in newborn mice | (73) | |
| Propionic acidemia | Mouse | Increased survival | (74) | |
| Glycogen storage disease 1a | Mouse | Long-term correction | (75) | |
| Crigler-Najjar syndrome | Rat | Long-term correction | (5,76) | |
| Pompe disease | Mouse | Long-term correction | (77) | |
| ApoE deficiency | Mouse | Long-term correction | (4,78) | |
| Familial Hypercholesterolemia | Mouse | Long-term correction | (79–81) | |
| ApoA1 deficiency hypoalphalipoproteinemia | Mouse | Long-term correction | (82) | |
| Diabetes | Type 1 diabetes | Mouse | Islet neogenesis and reverse of diabetes | (83) |
| Type 2 diabetes | Mouse | Improved glucose homeostasis | (84) | |
aThis study includes a single human patient.
INNATE INFLAMMATORY RESPONSE
Although the acute toxicity is clearly an obstacle for clinical applications of HDAd vectors, the severity of this response is dose-dependent and preferential, high efficiency hepatocyte transduction does not, at least alone, appear to necessarily cause a potent innate inflammatory response in mice (16). Therefore, major efforts have been focused in understanding the biology of the acute toxic response with the goal of developing strategies to block it or to improve the vector's ability at transducing hepatocytes so that lower, non-toxic doses can be administered.
Understanding the causes of the toxic response elicited by systemic injection of Ad-based vectors turned out to be a difficult task because multiple factors are involved. Within the bloodstream, the Ad comes into contact with the blood cells and several blood-borne proteins, all of which play a role in vector biodistribution and acute toxicity. In the blood, Ad binds to factor X (FX), as well as other vitamin K-dependent serine proteases, such as factors VII, IX and protein C (17–19), and efficiently target low-density lipoprotein receptor-related protein and heparan sulfate proteoglycans on hepatocytes that facilitate virus entry (19,20). The Ad particles activate the complement system through binding and activation of proteins in the classical and alternative complement pathways (21). Interactions of Ad with complement, including C3 and C4BP (22–24), result in the adhesion and migration of infiltrating leukocytes and platelet aggregation, and high levels of proinflammatory cytokines and chemokines (22).
Human plasma components (notably Ad type-specific antibodies), preventing the re-administration of the same vector serotype, play a role in the acute toxicity. Both neutralizing anti-Ad antibodies (22,25) and non-neutralizing or naturally occurring (non-specific cross-reacting) antibodies (22,26,27) may contribute to the acute toxicity by opsonizing the viral particles and rendering them more susceptible to Fc-mediated uptake by macrophages, which in turn become activated to secrete proinflammatory cytokines. This model is supported by studies showing that pre-existing immunity to Ad is associated with increased mortality shortly after the systemic administration of Ad in mice (28) and significantly higher IL-6 levels in non-human primates (25).
The clinical features of the acute toxic response observed in non-human primates resemble the shock septic reaction with hypotension, hemoconcentration, tissue edema and vasocongestion (11). In rodents, this reaction is dependent on reticuloendothelial system (RES)-derived platelet-activating factor (PAF), a lipid signaling molecule that is a known shock inducer. Interestingly, systemic intravenous injection of Ad stimulates the RES to upregulate PAF within minutes, consistent with the rapid onset of the toxic reactions observed in large animal models (29).
Intravascular delivery of Ad vectors induces an innate immune response very rapidly, which results in the activation of NF-κB pathway and transcription of host chemokine and cytokine genes (30). This innate immune response is activated by the interaction of Ad particles with Toll-like receptors (TLRs), the crucial components in pathogen recognition processes (31–37). TLRs have been implicated in Ad recognition at the level of the plasma membrane where TLR2 interacts with the Ad capsid proteins (38) and at the endosome level where TLR9 interacts with the vector genome (32,36,37). Internalized vector double-stranded DNA recognition occurs also via a TLR-independent nucleic acid-sensing mechanism (39), which is dependent on NALP3 and ASC, components of the innate cytosolic molecular complex known as the inflammasome (40).
The innate inflammatory response to Ad is clearly complex and multifactorial. However, a simple transient pre-treatment with the synthetic anti-inflammatory glucocorticoid Dexamethasone (DEX) prior to intravascular administration of Ad greatly reduced the associated innate toxicity in mice (41). Transient DEX pre-treatment also decreased the induction of neutralizing anti-Ad antibodies. Importantly, DEX pre-treatment did not reduce the efficacy of hepatocyte transduction by Ad.
BARRIERS TO HEPATOCYTE TRANSDUCTION
Within the liver, there are two major cell types that play an important role in transduction efficiency and acute toxicity: endothelial cells and Kupffer cells. Endothelial cells of the liver have a unique characteristic, i.e. the presence of fenestrations that allow small blood-borne particles to cross the vascular space to get in close contact with the hepatocytes. The liver fenestrations also form a structural barrier preventing large circulating macromolecules from accessing the hepatocytes. Several studies suggest that the size (∼100 nm) and the number of the endothelial fenestrations of the liver play a key role in the efficiency of Ad-mediated hepatocyte transduction (Ad virion ≥ 100 nm) (16,42,43). Specifically, it was demonstrated that there is a positive correlation between the size of the fenestrations and the efficiency of hepatocyte transduction following systemic administration of Ad vectors (42,43). Fenestration size varies significantly among species in number and size (44) and these differences may also contribute to differences in hepatocyte transduction efficiency among species and possibly among subjects within the same species. Liver fibrosis and cirrhosis can lead to a decreased number of fenestrations and capillarization of normal sinusoids, resulting in changes of Ad vector biodistribution (45,46).
Kupffer cells of the liver avidly remove intravenously injected Ad vectors from the circulation predominantly through binding with scavenger receptors (47). In contrast to hepatocytes, uptake of Ad vectors by Kupffer cells is independent from the binding with FX (47,48). Ad uptake by the Kupffer cells reduces the efficiency of hepatic transduction, and is responsible for a nonlinear dose response (26,49,50). The relative contribution of Kupffer cells to Ad vector clearance may be dependent on the specific genetic context. For example, viral vector uptake in non-parenchymal liver cells in BALB/c mice is nearly 6-fold higher than in C57BL/6 mice. This difference in vector scavenging between mouse strains results in the approximately 3-fold higher transgene expression levels in C57BL/6 mice when compared with BALB/c mice (51). Similar variations may explain the well-known differences in hepatocyte transduction efficiency among different species.
Kupffer cells have also been implicated in the pathogenesis of the acute innate inflammatory response at least in mice. They are rapidly killed following the uptake of Ad but this does not result in elevated serum IL-6 (52,53). It is also interesting to note that systemic injection of low vector doses into mice and non-human primates resulting in low-efficiency hepatocyte transduction almost certainly results in substantial Ad uptake by Kupffer cells as they are the barrier responsible for the threshold effect to efficient hepatocyte transduction (26,27). Yet, these animals exhibit little, if any, manifestations of acute toxicity. Indeed, it has been suggested that Kupffer cells may, in fact, play a protective role (54).
In summary, Kupffer cells of the liver remove a significant proportion of injected vector, thus reducing the amount of vector available for hepatocyte transduction. Possible strategies currently under investigation to overcome the Kupffer cell barrier are based on modified Ad vector particles capable of evading Kupffer cell uptake (55). These vectors can transduce hepatocytes with higher efficiency and have potential for improving the therapeutic index of the vector (55). Alternative strategies of ‘masking’ the viral capsid have also been reported to attenuate the severity of the innate inflammatory response. Systemic administration of PEGylated Ad into mice resulted in a reduction in serum IL-6 compared with unPEGylated vector, but it also reduced hepatic transduction efficiency in non-human primates (56–58). Therefore, there is uncertainty regarding the real clinical potential of this approach.
Another barrier to hepatocyte transduction by Ad vectors appears to be erythrocytes, at least in humans. Over 90% of a typical dose of Ad vectors binds to and is neutralized by human erythrocytes ex vivo (59). Detail studies showed that erythrocytes from humans, but not from mice or rhesus macaques, bear the Ad receptor Coxsackie and Adenovirus Receptor (CAR) responsible for the sequestration of the vector (60). Furthermore, human erythrocytes, but not mice, bear the complement receptor 1 (CR1) that binds Ad in the presence of antibodies and complements. These results highlight the important potential limitations of animal models. A strategy to overcome sequestration by human erythrocytes is to coat the Ad vector with polymers containing quaternary amines (61).
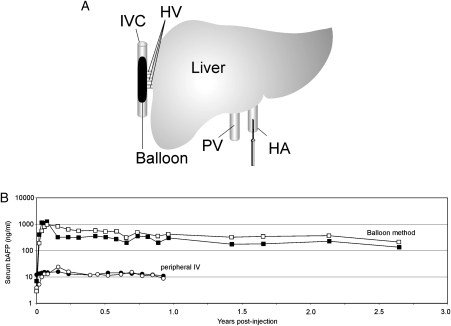
Other strategies to improve the therapeutic index of the vector are based on physical approaches aimed at achieving preferential hepatocyte transduction. One such strategy was to deliver the HDAd into the surgically isolated liver, thus limiting systemic exposure (7). This approach resulted in preferential hepatocyte transduction compared with systemic injection, no chronic toxicity and long-term, stable transgene expression for several years, post-vector administration in non-human primates. More recently, a minimally invasive, percutaneous balloon occlusion catheter-based method was reported to achieve preferential hepatocyte transduction, resulting in high level, stable transgene expression in non-human primates using clinically relevant low doses of HDAd (6,9). In this method, a sausage-shaped balloon is inflated in the inferior vena cava to occlude the hepatic venous outflow and the HDAd is injected directly into the liver via the hepatic artery (Fig. 1A). This novel method of vector delivery resulted in up to 80-fold higher level of transgene expression compared with the peripheral intravenous injection (Fig. 1B). Furthermore, this high-level transgene expression persisted for several years in the absence of chronic toxicity (Fig. 1B).
Figure 1.
(A) A sausage-shaped balloon catheter is percutaneously positioned in the inferior vena cava under fluoroscopic guidance. Inflation of the balloon results in hepatic venous outflow occlusion from the hepatic veins. The HDAd is administered by injection through a percutaneously positioned hepatic artery catheter. (B). Serum levels of the reporter baboon α-fetoprotein (bAFP) following the administration of 3 × 1010VP/kg of a HDAd-expressing bAFP into baboons using the balloon method described above (squares) or by simple peripheral intravenous injection (circles). The balloon method of vector delivery yielded up to 80-fold higher level of transgene expression compared with peripheral intravenous injection of vector, and transgene expression persisted at high levels for at least 2.5 years. Adapted from Brunetti-Pierri et al. (9). IVC, inferior vena cava; HV, hepatic veins; HA, hepatic artery; PV, portal vein; bAFP, baboon α-fetoprotein; IV, intravenous.
In conclusion, strategies designed to overcome the barriers to efficient hepatocyte transduction with low vector doses represent a reasonable and likely successful approach to address the serious, but potentially surmountable, problem of dose-dependent acute toxicity.
CONCLUSIONS
The main obstacle preventing clinical applications of HDAd for liver-directed gene therapy is the host innate inflammatory response against the vector capsid proteins that occurs shortly after intravascular vector administration. This response is multifactorial and its mechanism(s) remains only partially understood. Clearly, a better understanding of this acute response is necessary for the development of novel strategies to overcome it. However, as the severity of this innate inflammatory response is dependent on the vector dose, strategies to overcome the barrier to efficient hepatocyte transduction so that sub-toxic amount of vector could be administered would also probably be beneficial in minimizing acute toxicity.
Conflict of Interest statement. None declared.
FUNDING
N.B.P. is supported by Fondazione Telethon (Rome, Italy) (TCBP37TELC). P.N. is supported by the National Institutes of Health (R01 DK069369 and R01 HL083047).
REFERENCES
- 1.Brunetti-Pierri N., Ng P. Progress towards liver and lung-directed gene therapy with helper-dependent adenoviral vectors. Curr. Gene Ther. 2009;9:329–340. doi: 10.2174/156652309789753310. [DOI] [PubMed] [Google Scholar]
- 2.Ross P.J., Kennedy M.A., Parks R.J. Host cell detection of noncoding stuffer DNA contained in helper-dependent adenovirus vectors leads to epigenetic repression of transgene expression. J. Virol. 2009;83:8409–8417. doi: 10.1128/JVI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen S.L., Montini E., Sivanandam V.G., Al-Dhalimy M., Kestler H.A., Finegold M., Grompe M., Kochanek S. Chromosomal integration of adenoviral vector DNA in vivo. J. Virol. 2010;84:9987–9994. doi: 10.1128/JVI.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim I.H., Jozkowicz A., Piedra P.A., Oka K., Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl Acad. Sci. USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toietta G., Mane V.P., Norona W.S., Finegold M.J., Ng P., McDonagh A.F., Beaudet A.L., Lee B. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc. Natl Acad. Sci. USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetti-Pierri N., Stapleton G.E., Palmer D.J., Zuo Y., Mane V.P., Finegold M.J., Beaudet A.L., Leland M.M., Mullins C.E., Ng P. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol. Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti-Pierri N., Ng T., Iannitti D.A., Palmer D.J., Beaudet A.L., Finegold M.J., Carey K.D., Cioffi W.G., Ng P. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum. Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- 8.Morral N., O'Neal W., Rice K., Leland M., Kaplan J., Piedra P.A., Zhou H., Parks R.J., Velji R., Aguilar-Cordova E., et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl Acad. Sci. USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunetti-Pierri N., Stapleton G.E., Law M., Breinholt J., Palmer D.J., Zuo Y., Grove N.C., Finegold M.J., Rice K., Beaudet A.L., Mullins C.E., Ng P. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunetti-Pierri N., Nichols T.C., McCorquodale S., Merricks E., Palmer D.J., Beaudet A.L., Ng P. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- 11.Brunetti-Pierri N., Palmer D.J., Beaudet A.L., Carey K.D., Finegold M., Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 12.Muruve D.A., Cotter M.J., Zaiss A.K., White L.R., Liu Q., Chan T., Clark S.A., Ross P.J., Meulenbroek R.A., Maelandsmo G.M., Parks R.J. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morral N., O'Neal W.K., Rice K., Leland M.M., Piedra P.A., Aguilar-Cordova E., Carey K.D., Beaudet A.L., Langston C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- 14.Nunes F.A., Furth E.E., Wilson J.M., Raper S.E. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum. Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- 15.White G.I., Monahan P.E. Gene therapy for hemophilia A. In: Lee C., Berntrop E., Hoots K., editors. Textbook of Hemophilia. Oxford, UK: Blackwell Publishing; 2005. pp. 226–228. [Google Scholar]
- 16.Brunetti-Pierri N., Palmer D.J., Mane V., Finegold M., Beaudet A.L., Ng P. Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol. Ther. 2005;12:99–106. doi: 10.1016/j.ymthe.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Shayakhmetov D.M., Gaggar A., Ni S., Li Z.Y., Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker A.L., Waddington S.N., Nicol C.G., Shayakhmetov D.M., Buckley S.M., Denby L., Kemball-Cook G., Ni S., Lieber A., McVey J.H., Nicklin S.A., Baker A.H. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 19.Kalyuzhniy O., Di Paolo N.C., Silvestry M., Hofherr S.E., Barry M.A., Stewart P.L., Shayakhmetov D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl Acad. Sci. USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waddington S.N., McVey J.H., Bhella D., Parker A.L., Barker K., Atoda H., Pink R., Buckley S.M., Greig J.A., Denby L., et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Tian J., Xu Z., Smith J.S., Hofherr S.E., Barry M.A., Byrnes A.P. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J. Virol. 2009;83:5648–5658. doi: 10.1128/JVI.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cichon G., Boeckh-Herwig S., Schmidt H.H., Wehnes E., Muller T., Pring-Akerblom P., Burger R. Complement activation by recombinant adenoviruses. Gene Ther. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., Wang Z., Serra D., Frank M.M., Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol. Ther. 2004;10:1140–1142. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Kiang A., Hartman Z.C., Everett R.S., Serra D., Jiang H., Frank M.M., Amalfitano A. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 2006;14:588–598. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Varnavski A.N., Zhang Y., Schnell M., Tazelaar J., Louboutin J.P., Yu Q.C., Bagg A., Gao G.P., Wilson J.M. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J. Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao N., Gao G.P., Parr M., Johnston J., Baradet T., Wilson J.M., Barsoum J., Fawell S.E. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 27.Schiedner G., Hertel S., Johnston M., Dries V., van Rooijen N., Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol. Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 28.Varnavski A.N., Calcedo R., Bove M., Gao G., Wilson J.M. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z., Smith J.S., Tian J., Byrnes A.P. Induction of shock after intravenous injection of adenovirus vectors: a critical role for platelet-activating factor. Mol. Ther. 2010;18:609–616. doi: 10.1038/mt.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girardin S.E., Sansonetti P.J., Philpott D.J. Intracellular vs. extracellular recognition of pathogens: common concepts in mammals and flies. Trends Microbiol. 2002;10:193–199. doi: 10.1016/s0966-842x(02)02334-x. [DOI] [PubMed] [Google Scholar]
- 31.Basner-Tschakarjan E., Gaffal E., O'Keeffe M., Tormo D., Limmer A., Wagner H., Hochrein H., Tuting T. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- 32.Cerullo V., Seiler M.P., Mane V., Brunetti-Pierri N., Clarke C., Bertin T.K., Rodgers J.R., Lee B. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 2007;15:378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- 33.Hartman Z.C., Black E.P., Amalfitano A. Adenoviral infection induces a multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology. 2007;358:357–372. doi: 10.1016/j.virol.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 34.Hartman Z.C., Kiang A., Everett R.S., Serra D., Yang X.Y., Clay T.M., Amalfitano A. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensley S.E., Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol. Ther. 2007;15:1417–1422. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- 36.Iacobelli-Martinez M., Nemerow G.R. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J., Huang X., Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appledorn D.M., Patial S., McBride A., Godbehere S., Van Rooijen N., Parameswaran N., Amalfitano A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 39.Nociari M., Ocheretina O., Schoggins J.W., Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muruve D.A., Petrilli V., Zaiss A.K., White L.R., Clark S.A., Ross P.J., Parks R.J., Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 41.Seregin S.S., Appledorn D.M., McBride A.J., Schuldt N.J., Aldhamen Y.A., Voss T., Wei J., Bujold M., Nance W., Godbehere S., Amalfitano A. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lievens J., Snoeys J., Vekemans K., Van Linthout S., de Zanger R., Collen D., Wisse E., De Geest B. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004;11:1523–1531. doi: 10.1038/sj.gt.3302326. [DOI] [PubMed] [Google Scholar]
- 43.Snoeys J., Lievens J., Wisse E., Jacobs F., Duimel H., Collen D., Frederik P., De Geest B. Species differences in transgene DNA uptake in hepatocytes after adenoviral transfer correlate with the size of endothelial fenestrae. Gene Ther. 2007;14:604–612. doi: 10.1038/sj.gt.3302899. [DOI] [PubMed] [Google Scholar]
- 44.Wisse E., De Zanger R.B., Charels K., Van Der Smissen P., McCuskey R.S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 45.Horn T., Christoffersen P., Henriksen J.H. Alcoholic liver injury: defenestration in noncirrhotic livers—a scanning electron microscopic study. Hepatology. 1987;7:77–82. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 46.Smith J.S., Tian J., Muller J., Byrnes A.P. Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther. 2004;11:431–438. doi: 10.1038/sj.gt.3302149. [DOI] [PubMed] [Google Scholar]
- 47.Xu Z., Tian J., Smith J.S., Byrnes A.P. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies and complement. J. Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Paolo N.C., van Rooijen N., Shayakhmetov D.M. Redundant and synergistic mechanisms control the sequestration of blood-borne adenovirus in the liver. Mol. Ther. 2009;17:675–684. doi: 10.1038/mt.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiedner G., Bloch W., Hertel S., Johnston M., Molojavyi A., Dries V., Varga G., Van Rooijen N., Kochanek S. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum. Gene Ther. 2003;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- 50.Wolff G., Worgall S., van Rooijen N., Song W.R., Harvey B.G., Crystal R.G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J. Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snoeys J., Mertens G., Lievens J., van Berkel T., Collen D., Biessen E.A., De Geest B. Lipid emulsions potently increase transgene expression in hepatocytes after adenoviral transfer. Mol. Ther. 2006;13:98–107. doi: 10.1016/j.ymthe.2005.06.477. [DOI] [PubMed] [Google Scholar]
- 52.Manickan E., Smith J.S., Tian J., Eggerman T.L., Lozier J.N., Muller J., Byrnes A.P. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Smith J.S., Xu Z., Tian J., Stevenson S.C., Byrnes A.P. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum. Gene Ther. 2008;19:547–554. doi: 10.1089/hum.2008.004. [DOI] [PubMed] [Google Scholar]
- 54.Jooss K., Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 55.Prill J.M., Espenlaub S., Samen U., Engler T., Schmidt E., Vetrini F., Rosewell A., Grove N., Palmer D., Ng P., Kochanek S., Kreppel F. Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells. Mol. Ther. 2010;19:83–92. doi: 10.1038/mt.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok H., Palmer D.J., Ng P., Barry M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Croyle M.A., Le H.T., Linse K.D., Cerullo V., Toietta G., Beaudet A., Pastore L. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 58.Wonganan P., Clemens C.C., Brasky K., Pastore L., Croyle M.A. Species differences in the pharmacology and toxicology of PEGylated helper-dependent adenovirus. Mol. Pharm. 2010;8:78–92. doi: 10.1021/mp100216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyons M., Onion D., Green N.K., Aslan K., Rajaratnam R., Bazan-Peregrino M., Phipps S., Hale S., Mautner V., Seymour L.W., Fisher K.D. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Carlisle R.C., Di Y., Cerny A.M., Sonnen A.F., Sim R.B., Green N.K., Subr V., Ulbrich K., Gilbert R.J., Fisher K.D., Finberg R.W., Seymour L.W. Human erythrocytes bind and inactivate type 5 adenovirus by presenting coxsackievirus-adenovirus receptor and complement receptor 1. Blood. 2009;113:1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subr V., Kostka L., Selby-Milic T., Fisher K., Ulbrich K., Seymour L.W., Carlisle R.C. Coating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood components. J. Control. Release. 2009;135:152–158. doi: 10.1016/j.jconrel.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Cerullo V., Seiler M.P., Mane V., Cela R., Clarke C., Kaufman R.J., Pipe S.W., Lee B. Correction of murine hemophilia A and immunological differences of factor VIII variants delivered by helper-dependent adenoviral vectors. Mol. Ther. 2007;15:2080–2087. doi: 10.1038/sj.mt.6300308. [DOI] [PubMed] [Google Scholar]
- 63.Brown B.D., Shi C.X., Rawle F.E., Tinlin S., McKinven A., Hough C., Graham F.L., Lillicrap D. Factors influencing therapeutic efficacy and the host immune response to helper-dependent adenoviral gene therapy in hemophilia A mice. J. Thromb. Haemost. 2004;2:111–118. doi: 10.1111/j.1538-7836.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 64.Hu C., Cela R.G., Suzuki M., Lee B., Lipshutz G.S. Neonatal helper-dependent adenoviral vector gene therapy mediates correction of hemophilia A and tolerance to human factor VIII. Proc. Natl Acad. Sci. USA. 2011;108:2082–2087. doi: 10.1073/pnas.1015571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy P.S., Sakhuja K., Ganesh S., Yang L., Kayda D., Brann T., Pattison S., Golightly D., Idamakanti N., Pinkstaff A., et al. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol. Ther. 2002;5:63–73. doi: 10.1006/mthe.2001.0510. [DOI] [PubMed] [Google Scholar]
- 66.McCormack W.M., Jr., Seiler M.P., Bertin T.K., Ubhayakar K., Palmer D.J., Ng P., Nichols T.C., Lee B. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J. Thromb. Haemost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown B.D., Shi C.X., Powell S., Hurlbut D., Graham F.L., Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–810. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- 68.Ehrhardt A., Kay M.A. A new adenoviral helper-dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo. Blood. 2002;99:3923–3930. doi: 10.1182/blood.v99.11.3923. [DOI] [PubMed] [Google Scholar]
- 69.Brunetti-Pierri N., Grove N.C., Zuo Y., Edwards R., Palmer D., Cerullo V., Teruya J., Ng P. Bioengineered factor IX molecules with increased catalytic activity improve the therapeutic index of gene therapy vectors for hemophilia B. Hum. Gene Ther. 2009;20:479–485. doi: 10.1089/hum.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrhardt A., Xu H., Dillow A.M., Bellinger D.A., Nichols T.C., Kay M.A. A gene-deleted adenoviral vector results in phenotypic correction of canine hemophilia B without liver toxicity or thrombocytopenia. Blood. 2003;102:2403–2411. doi: 10.1182/blood-2003-01-0314. [DOI] [PubMed] [Google Scholar]
- 71.Mian A., McCormack W.M., Jr., Mane V., Kleppe S., Ng P., Finegold M., O'Brien W.E., Rodgers J.R., Beaudet A.L., Lee B. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol. Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 72.Brunetti-Pierri N., Clarke C., Mane V., Palmer D.J., Lanpher B., Sun Q., O'Brien W., Lee B. Phenotypic correction of ornithine transcarbamylase deficiency using low dose helper-dependent adenoviral vectors. J. Gene Med. 2008;10:890–896. doi: 10.1002/jgm.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gau C.L., Rosenblatt R.A., Cerullo V., Lay F.D., Dow A.C., Livesay J., Brunetti-Pierri N., Lee B., Cederbaum S.D., Grody W.W., Lipshutz G.S. Short-term correction of arginase deficiency in a neonatal murine model with a helper-dependent adenoviral vector. Mol. Ther. 2009;17:1155–1163. doi: 10.1038/mt.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofherr S.E., Senac J.S., Chen C.Y., Palmer D.J., Ng P., Barry M.A. Short-term rescue of neonatal lethality in a mouse model of propionic acidemia by gene therapy. Hum. Gene Ther. 2009;20:169–180. doi: 10.1089/hum.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koeberl D.D., Sun B., Bird A., Chen Y., Oka K., Chan L. Efficacy of helper-dependent adenovirus vector-mediated gene therapy in murine glycogen storage disease Type Ia. Mol. Ther. 2007;15:1253–1258. doi: 10.1038/sj.mt.6300188. [DOI] [PubMed] [Google Scholar]
- 76.Dimmock D., Brunetti-Pierri N., Palmer D., Beaudet A., Ng P. Correction of hyperbilirubinemia in Gunn rats using clinically relevant low doses of helper-dependent adenoviral vectors. Hum. Gene Ther. 2011;22:483–488. doi: 10.1089/hum.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiang A., Hartman Z.C., Liao S., Xu F., Serra D., Palmer D.J., Ng P., Amalfitano A. Fully deleted adenovirus persistently expressing GAA accomplishes long-term skeletal muscle glycogen correction in tolerant and nontolerant GSD-II mice. Mol. Ther. 2006;13:127–134. doi: 10.1016/j.ymthe.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Pastore L., Belalcazar L.M., Oka K., Cela R., Lee B., Chan L., Beaudet A.L. Helper-dependent adenoviral vector-mediated long-term expression of human apolipoprotein A-I reduces atherosclerosis in apo E-deficient mice. Gene. 2004;327:153–160. doi: 10.1016/j.gene.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 79.Oka K., Pastore L., Kim I.H., Merched A., Nomura S., Lee H.J., Merched-Sauvage M., Arden-Riley C., Lee B., Finegold M., Beaudet A., Chan L. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- 80.Belalcazar L.M., Merched A., Carr B., Oka K., Chen K.H., Pastore L., Beaudet A., Chan L. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 81.Nomura S., Merched A., Nour E., Dieker C., Oka K., Chan L. Low-density lipoprotein receptor gene therapy using helper-dependent adenovirus produces long-term protection against atherosclerosis in a mouse model of familial hypercholesterolemia. Gene Ther. 2004;11:1540–1548. doi: 10.1038/sj.gt.3302310. [DOI] [PubMed] [Google Scholar]
- 82.Oka K., Belalcazar L.M., Dieker C., Nour E.A., Nuno-Gonzalez P., Paul A., Cormier S., Shin J.K., Finegold M., Chan L. Sustained phenotypic correction in a mouse model of hypoalphalipoproteinemia with a helper-dependent adenovirus vector. Gene Ther. 2007;14:191–202. doi: 10.1038/sj.gt.3302819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kojima H., Fujimiya M., Matsumura K., Younan P., Imaeda H., Maeda M., Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 84.Samson S.L., Gonzalez E.V., Yechoor V., Bajaj M., Oka K., Chan L. Gene therapy for diabetes: metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model. Mol. Ther. 2008;16:1805–1812. doi: 10.1038/mt.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]