Abstract
Adoptive immunotherapy is an appealing approach to cancer treatment, with the potential for more precise targeting and reduced toxicity. While early clinical trial data using adoptive T cells against post-transplant virus-associated hematologic malignancies, lymphoma and melanoma have been promising, treating other solid tumors has proven to be more challenging. Adoptive lymphocytes have been genetically modified in many ways to improve activity and circumvent tumor evasion, including transfer of transgenic T-cell receptors and chimeric antigen receptors to redirect T cell and natural killer cell antigen specificity. Gene transfer may also allow expression of homeostatic cytokines or their receptors to overcome the lack of stimulatory signals or expression of dominant-negative receptors for inhibitory cytokines to compensate for an immunosuppressive tumor milieu. In addition, suicide genes can install a ‘safety switch' on adoptively transferred cells to allow ablation if necessary. Although further refinement and validation are necessary, these genetic modification strategies offer hope for significant improvements in cancer immunotherapy.
INTRODUCTION
Over the past few decades, advances in cancer immunology have been increasingly translated into clinical testing of immune-based approaches to cancer treatment. By exploiting the exquisite specificity of the immune system, cancer immunotherapy offers the potential for more precise targeting and reduced toxicity compared with traditional chemotherapy. Numerous immune-based therapies have been evaluated, including monoclonal antibody treatment, cell- and DNA-based vaccines and adoptive transfer of natural killer (NK) and T lymphocytes (1).
Adoptive cell therapies have achieved promising results in clinical trials. Ex vivo-expanded autologous tumor-infiltrating lymphocytes (TILs) have induced regression in patients with metastatic melanoma (2), and virus-specific T cells have been shown to be effective in treatment of virus-associated hematologic malignancies (3). Unmanipulated donor lymphocyte infusions have been widely used to treat patients with relapsed hematologic malignancies after allogeneic hemopoietic stem cell transplantation (4). Allogeneic NK cell therapies have also shown activity in patients with acute myeloid leukemia with little or no toxicity (5). Although these results are promising, other studies, particularly in solid tumors, have shown less impressive responses, likely due to a combination of immune evasion by tumors and poor function of adoptively transferred cells. In this review, we discuss a variety of ex vivo genetic manipulations designed to overcome tumor evasion and improve function of adoptively transferred lymphocytes.
GENETIC AUGMENTATION OF ADOPTIVE T CELLS
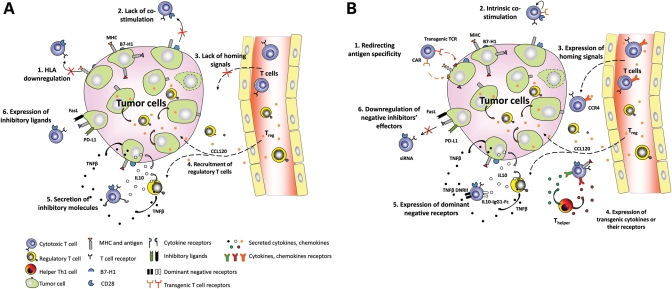
The development of T cell immunotherapy for cancer faces significant challenges. Most tumor-associated antigens that serve as targets for T cell therapies are self-proteins which are weakly antigenic, due to the development of tolerance. Therefore, endogenous antigen-specific T cells isolated from patients with cancer may be present in low frequency and will likely have low-affinity T-cell receptors (TCRs) due to negative selection during maturation, (6) limiting their cytotoxic activity against tumor cells. In addition, tumors have evolved to evade the immune system through both passive and active mechanisms (7). Tumors can passively avoid T cell targeting by downregulating major histocompatibility complex (MHC) or co-stimulatory molecules. Tumors actively antagonize the immune response by expressing inhibitory ligands and secreted factors. All of these evasion mechanisms can potentially be overcome by genetic modification of adoptively transferred T cells as summarized in Figure 1. Redirection of T cells to tumor antigens by expressing transgenic TCRs or chimeric antigen receptors (CARs) can bypass negative selection and yield much higher levels of tumor-specific cells, with reduced dependence on co-stimulation and target cell MHC expression. Lymphocyte activity can be restored by transgenic expression of activating cytokines such as interleukin-2 (IL-2) and IL-15, and T cells can also be made resistant to suppressive factors by overexpression of dominant-negative receptors (8,9). Homing of T cells to the tumor site can be improved by transgenic expression of receptors for tumor-secreted chemokines (10). T cells can also be provided with resistance to immunosuppressive drugs by genetic modification (11).
Figure 1.
(A) Tumor evasion mechanisms. (1) Downregulation of HLA; (2) lack of co-stimulation; (3) lack of homing signals for effector T cells; (4) recruitment of Treg and Th2 subsets; (5) secretion of immunosupressive molecules; (6) expression of inhibitory ligands. (B) Genetic modifications of adoptive T cells to overcome tumor evasion. (1) Redirecting T cell antigen specificity with transgenic TCRs or bypassing the need for HLA with CARs; (2) intrinsic co-stimulatory signals; (3) expression of homing signals; (4) expression of transgenic cytokines or their receptors; (5) expression of dominant-negative receptors; (6) downregulation of negative effectors’ signaling cascade.
Redirecting T cell specificity with transgenic TCRs
Tumor-specific T cells are frequently negatively selected during development because tumor antigens are typically recognized as ‘self'. Those tumor-specific T cells that survive have low affinity TCRs, are often anergic and consequently have poor tumor-killing activity. However, tumor specificity can be conferred on non-tumor-specific T cells by expression of transgenic TCRs specific for antigens expressed on tumor cells. This approach permits large numbers of highly active, tumor-specific T cells to be generated in a short period of time. Transgenic TCRs are typically generated by cloning the α and β subunits of class I human leukocyte antigen (HLA)-restricted TCRs from tumor-reactive cytotoxic T cell clones. The cloned TCR is then transferred ex vivo into patient T cells using integrating retroviral or lentiviral vectors or, in some studies, plasmids.
In one example of this strategy, Morgan et al. (12) treated metastatic melanoma patients with autologous T cells genetically modified with a retroviral vector to express a transgenic TCR targeting melanoma antigen recognized by T cells (MART)-1. The infused cells persisted up to a year and objective regression of tumor was seen in 4 of 31 patients. None experienced clinical signs of graft-versus-host disease (GvHD) or melanocyte toxicity (13).
One of the advantages of transgenic TCR expression is the ability to optimize affinity between TCRs and their target antigens to improve activation. In follow-up studies, higher avidity TCRs recognizing MART-1 and the human melanocyte differentiation antigen gp100 were generated. Objective cancer regression was observed in 19–30% of patients who received these higher affinity TCRs (14). However, in the study with the higher affinity TCR targeting MART, some patients also experienced toxicity to normal melanocytes in the skin, eye and ear, requiring administration of steroids to treat hearing loss and uveitis (14). Another example of the effects of highly avid TCRs on normal tissue was observed in a study in which autologous T cells expressing a murine TCR against human carcinoembryonic antigen (CEA) were administered to three patients with metastatic colorectal cancer (15). Although one patient had an objective tumor regression, all three developed an inflammatory colitis, likely due to recognition of low levels of CEA in normal colonic mucosa.
Using a large pool of non-specific T cells as a source for adoptive transfer also has some potential drawbacks. Although these T cells gain new antigen specificity through the presence of a transgenic TCR, their original TCRs are still functional. Studies in murine models with TCR gene transfer have shown that mispairing of endogenous and introduced TCR chains can lead to off-target effects or GvHD (16). However, GvHD has not occurred in the >100 human patients who have received autologous T cells transduced with human or mouse TCRs, illustrating that preclinical models do not always predict clinical effects in humans (17).
While transgenic TCRs overcome the problems of low number and avidity of cancer-specific T cells, they are still HLA restricted, limiting the treatment scope of each transgenic TCR to MHC-matched tumors and tumors whose HLA antigens have not been downregulated. In addition, they can dimerize with native TCR, leading to loss of function. An alternative approach that avoids some of these issues is the use of transgenic CARs instead of TCRs.
Redirecting T cell specificity with CARs
CARs are synthetic constructs that can confer target antigen specificity without HLA restriction and avoid development of hybrid TCRs. A CAR contains an extracellular antigen-binding domain, a transmembrane region and a signaling endodomain. The extracellular domain is typically a single-chain variable fragment (scFv) derived from a tumor-specific monoclonal antibody. The hinge/spacer region between the binding and transmembrane domains permits flexibility and increases the CAR's access to antigens by allowing it to protrude higher than other molecules on the plasma membrane (18). The endodomain consists of one or more intracellular signaling components of the TCR complex.
There are several advantages to using an antibody-derived domain for antigen recognition. Antibodies are not dependent on MHC presentation, so CARs can recognize both protein-derived peptides and surface proteins with varying degrees of post-translational modification (19). In addition, antibodies bind antigens with much greater affinity than TCRs, permitting the formation of a more stable immunological synapse (20).
Current CARs can be grouped into three generations, with progressively increasing co-stimulatory activity. These differ primarily in the structure of the signaling endodomain. First-generation CARs contain a single signaling unit derived from the CD3ζ chain or FcεRIγ IgG receptor (21). Overall, modest clinical responses have been achieved in studies where first-generation CARs have been transferred to adoptively transferred lymphocytes for treatment of lymphoma, neuroblastoma, ovarian and renal cancer as summarized in Table 1. The results from these studies suggest that CAR signaling through the CD3ζ chain alone is not adequate to achieve full T cell activation. Pule et al. (22) attempted to overcome this limitation and enhance in vivo T cell persistence by expressing a tumor-specific CAR in Epstein–Barr virus (EBV)-specific T cells. Unlike non-specific T cells, EBV-specific T cells experience additional co-stimulation when encountering EBV antigens in vivo. These investigators expressed distinguishable CARs targeting the neuroblastoma antigen GD2 in EBV-specific T cells and T cells non-specifically activated with CD3 antibody. They found better persistence of the EBV-specific cytotoxic T lymphocytes (CTLs) and an encouraging response rate, with tumor regression or necrosis in four out of eight patients with active disease.
Table 1.
Clinical trials with T cells expressing CARs
| Reference | Type of T cell | CAR construct | Cell dose | Targeted cancer/number of patients | Serious adverse effects | Persistence | Responses |
|---|---|---|---|---|---|---|---|
| Kershaw et al. (57) | OKT3-activated T cells (8 patients) | α-Folate receptor CAR retroviral vector with neomycin resistance gene | 3 × 109 to 5 × 1010 (OKT3) | Ovarian cancer/14 patients | None (IL-2 effects in cohort receiving high dose IL-2) | Up to 3 weeks in 13 patients | None |
| Alloantigen activated T cells (6 patients) | 4.0 × 109 to 1.69 × 1011 (alloantigen) | 12 months in 1 patient | |||||
| Park et al. (58) | OKT3-activated T cells (clones) | CE7R-CAR plasmid with HyTK | 108 to 109 cells/m2 | Neuroblastoma/6 patients | None | 1–42 days | 1 of 6 with evaluable tumor had a PR |
| Lamers et al. (59) | OKT3-activated T cells | G250-CAR retroviral vector | 0.38 to 2.13 × 109 | Renal cancer | Grade 2–4 liver toxicity | Up to 53 days | None |
| Till et al. (60) | OKT3-activated T cells (clones in 3 and lines in 4) | CD20-CAR plasmid with neomycin resistance gene | 108 to 3.3 × 109 cells/m2 | CD20+ low grade B cell lymphoma/7 patients | None | 1–3 weeks (clones) | 4 of 5 with evaluable disease had stable disease and one a PR |
| 5–9 weeks (T cell lines and low dose IL-2) | |||||||
| Pule et al. (22) | OKT3-activated T cells and EBV-specific CTLs | GD2-CAR retroviral vector | 2 × 107 to 2 × 108 cells/m2 of each product | Neuroblastoma/11 patients | None | Up to 3 weeks for the activated T cells and up to 6 months for CTLs | 4 of 8 with evaluable tumor had necrosis or responses with 1 CR |
| Jensen et al. (61) | OKT3-activated T cells | CD19 or CD20 CAR plasmid with HyTK | Up to 2 × 109 cells/m2 | Diffuse large cell lymphoma | None | 24 h to 7 days | 2 of 4 patients with antitransgene immune rejection |
| Morgan et al. (31) | OKT3-activated T cells | Her-2/neu retroviral vector with CD28 and 41BB | 1010 cells | Colon cancer | Patient died after 5 days due to multiorgan failure | ||
| Brentjens et al. (32) | OKT3-activated T cells | CD19-CAR retroviral vector with CD28 | 1.2–3 × 108 cells/kg | CLL | 1/6 patient died after 2 days due to sepsis-like syndrome | ||
| Kochenderfer et al. (28) | OKT3-activated T cells | CD19-CAR retroviral vector with CD28 | 108 cells day 1, 3 × 108 cells day 2, IL-2 q8hr × 8 | Follicular lymphoma | None | 27 weeks after infusion | 1 out of 1 patient with partial remission at 39 weeks |
CR, complete remission; PR, partial remission; HyTK, hygromycin thymidine kinase.
Because full activation and proliferation of T cells require signaling through the CD28 receptor, in second-generation CARs the CD28 intracellular domain is inserted proximal to the CD3ζ endodomain to enhance the stimulatory effects of the CAR (23). The synergistic effect of combining the two signaling domains results in increased proliferation, decreased activation-induced apoptosis and increased cytokine secretion in response to antigen (19).
The improvement in T cell function with addition of the CD28 endodomain to the CAR encouraged further addition of other signaling sequences such as CD137 (4-1BB) and CD134 (OX40) to third-generation CARs (24,25). In preclinical studies, these second- and third-generation CARs showed superior ability to eliminate tumor xenograft models (26) and are currently under evaluation in the clinic (27). In one recent report, a complete response was seen in a patient with high-grade progressive follicular lymphoma who received T cells transduced with an anti-CD19 CAR (FMC63 antibody-CD28-CD3ζ) (28). A summary of other active clinical trials involving all three generations of CARs as of 2010 is listed by Cooper and colleagues (29) in a recent review.
While second- and third-generation CARs showed superior ability to target tumor xenografts (26), their potentially supraphysiological signal is also a source of concern. By lowering the activation threshold, later generation CARs have a higher risk for low-avidity off-target binding (30) and may also produce an overly vigorous activation signal, with on-target binding resulting in adverse effects from cytokine release. Morgan et al. (31) recently reported an adverse event when a patient with metastatic colon cancer receiving autologous T cells transduced with an ERBB2-specific CAR (herceptin-CD28-CD137-CD3ζ) rapidly developed acute respiratory distress syndrome within 15 min after infusion, and died 5 days after treatment despite intensive medical intervention. This event was associated with very high cytokine levels attributed to cross-reactivity with normal tissues expressing HER2. In a second report, a patient with bulky chronic lymphocytic leukemia developed fever and hypotension with elevated cytokine levels within 24 h of receiving autologous T cells transduced with a CD19-28ζ CAR (32). In this case, low-grade sepsis was considered the most likely cause, but elevated cytokine levels may also have enhanced the in vivo activation of modified T cells. It is worth noting that similar ‘on-target, off-organ' adverse events have been observed in other studies using native T cells (33), suggesting that this is not solely due to genetic modifications. Modification to reduce T cell dose, as well as splitting infusions across multiple days, has been suggested to reduce the risk of such serious adverse events (31,32).
Supplying homeostatic cytokines
Administration of cytokines such as IL-2 and IL-15 can overcome the lack of stimulatory signals within the tumor microenvironment and enhance antitumor effects of adoptively transferred T cells (9). However, systemic toxicity and expansion of regulatory T cells limit the use of these cytokines when administered systemically. An alternative approach is to modify T cells to express cytokine or cytokine receptor genes that recapitulate the milieu found during lymphoid regeneration and restoration of homeostasis. Transgenic expression of IL-2 and IL-15 has been shown to increase antigen-specific T cell expansion in vivo and enhance antitumor activity without systemic toxicity in preclinical models (34). For cytokines like IL-7, whose receptors are downregulated upon exposure, T cells can be modified to stably express IL-7Rα (35). Antigen-specific T cells transduced with transgenic cytokines or cytokine receptors showed improved antitumor effects in animal models (36,37). However, in a clinical trial of adoptive transfer of TILs to treat metastatic melanoma, overexpression of IL-2 did not increase in vivo persistence or overall clinical effectiveness compared with unmodified T cells (38).
Enhancing homing signals
It is well established that tumors secrete chemokines that selectively recruit only Th2 and regulatory subsets of T cells. In murine studies, transgenic expression of CCR4 on CD8(+) T cells that are specific for the Hodgkin's lymphoma marker CD30 helped to direct them to the tumor site while retaining their cytotoxic function and cytokine secretion in vivo (10). Similar enhancements in antitumor effects were obtained when T cells were co-transduced with anti-GD2 CAR and the receptor for CCL2, a chemokine which is highly secreted by neuroblastoma cells (39). At present, strategies involving genetic modification of T cells to enhance homing signals are still in the preclinical phase and need to consider chemokines produced by tumor stromal cells as well as tumor cells.
Resisting hostile tumor environment
Even after migration to tumor tissues, T cells continue to encounter challenges such as suppressive cytokines and inhibitory ligands produced by tumors and their stroma. One of the most potent inhibitory cytokines is transforming growth factor (TGF)β, and expression of a dominant-negative TGFβ type II receptor in T cells rendered them resistant to TGFβ-secreting EBV-positive lymphoma in in vitro studies and murine models (40). This approach is currently being evaluated in a phase I clinical trial. Similar strategies to confer resistance to other negative signals such as IL-10 are also under evaluation (41). T cells can also be protected from apoptosis by knockdown of inhibitory ligands such as Fas using retrovirally encoded small interfering RNAs (siRNAs) (42).
Resisting concomitant immunosuppressive therapy
Adoptive transfer of EBV-specific T cells has been shown to be safe and efficacious in preventing post-transplantation lymphoproliferative diseases (43,44). However, most solid organ transplant recipients require continuous administration of immunosuppressive drugs to prevent graft rejection, which can limit the efficacy and long-term persistence of adoptively transferred T cells (45). By knocking down FK506-binding protein (FKBP12) with a stably expressed siRNA, De Angelis et al. (11) enabled EBV-specific T cells to continue to expand and maintain their cytotoxicity in the presence of FK506 in a xenogenic mouse model.
Installing a safety switch on adoptively transferred T cells
Along with enhancing potency, sustainability and resistance to suppressive signals, genetic modification can also install a ‘safety switch' so that genetically modified T cells can be ablated if adverse effects occur. One of the most well-studied suicide strategies is the herpes simplex viral thymidine kinase (TK) gene. In the presence of ganciclovir or acyclovir prodrugs, TK will phosphorylate the substrate to produce a toxic product that can interfere with the infused T cell's DNA synthesis. Thus, prodrugs can be administered when transferred cells have deleterious effects. T cells genetically modified with TK have been infused in clinical trials to >120 patients after allogeneic stem cell transplants (13) and have controlled GvHD in every occurrence, confirming the ability of this approach to control alloreactivity. The strategy is now being evaluated in phase III clinical trials in preventing GvHD when donor lymphocytes are infused following stem cell transplantation (46).
There are, however, several shortcomings to using TK as a suicide gene. One is its immunogenicity, which might lead to premature clearance of infused cells. Second is the removal of a therapeutically valuable drug as an option in treating viral infection post-transplant. Another concern is the time required to ablate infused cells, usually days to weeks. This is acceptable in treatment of GvHD, but would be inadequate in cases where infused cells cause acute on- or off-target toxicity. An attractive alternative suicide strategy is the inducible Caspase9 transgene (iCaspase9), which is non-immunogenic and rapidly produces apoptosis even in non-dividing cells (47). iCaspase9 is trigged upon administration of a small molecule dimerizer, AP20187, yields >90% apoptosis and is currently being tested in a phase I study.
GENETIC MODIFICATIONS OF NK CELLS
In recent years, interest in using NK cells for cancer immunotherapy has been increasing. In a study in which acute myeloid leukemia patients were infused with freshly isolated and expanded haploidentical, related-donor NK cells, complete hematologic remissions were seen in 5 out of 19 patients (5). Unlike T cells, NK cells are not antigen-specific, and their cytotoxicity is directed at a number of targets on cells expressing low levels of MHC class I (48). Genetic modification with CARs can retarget NK cells specifically to tumor antigens, as demonstrated by reports of improved killing of a Her-2/neu breast cancer cell line and CD20-positive lymphoma (49,50). A recent comparison between the classical CAR endodomain of CD28-CD3ζ versus 2B4 (CD244), an important regulator of NK cell activation, showed that 2B4 signaling on its own can only trigger a degranulation response but not full NK cell activation (51). However, addition of the 2B4 endodomain proximally to CD3ζ significantly enhances NK cell activation as well as cytokine secretion in a tumor-specific manner (51). As with T cells, genetic modifications to produce cytokines (IL-2, IL-12 and IL-15) can increase NK cell in vivo survival and antitumor activities (52–54). NK cells may be an attractive platform for redirecting antigen specificity modification after allogeneic transplant due to their lower potential to induce GvHD compared with T cells. However, NK cell trials are at an earlier stage compared with T cells, mostly due to technical difficulties in NK cell in vitro expansion (29). This limitation may be overcome by development of an efficient ex vivo culturing system using gas-permeable cultureware (55) and artificial antigen-presenting cells (56).
CONCLUSION
Potentially immunogenic tumors have evolved many different immune evasion strategies, including reduced antigen presentation and inhibition of effector lymphocyte function. Genetic modifications of adoptively transferred cells may be able to overcome these challenges and improve clinical outcomes. Early clinical reports have been promising; however, there have also been increased rates of both on- and off-target toxicities, some of which may be amenable to dose optimization. Inclusion of a safety switch with other genetic modifications can further ensure long-term safety of adoptively transferred lymphocytes. With these novel approaches, the results of on-going and future clinical trials with genetically modified NK and T cells promise to be exciting.
FUNDING
This work was supported in part by CA094237 and P50CA126752 from the NCI, U54HL081007 from NHLBI and a Leukemia and Lymphoma Society Specialized Center of Research award. J.M.H. was supported by T32HL092332.
ACKNOWLEDGEMENT
We would like to thank Juan F Vera for his assistance in designing the figure.
REFERENCES
- 1.Perez-Gracia J.L., Berraondo P., Martinez-Forero I., Alfaro C., Suarez N., Gurpide A., Sangro B., Hervas-Stubbs S., Ochoa C., Melero J.A., Melero I. Clinical development of combination strategies in immunotherapy: are we ready for more than one investigational product in an early clinical trial? Immunotherapy. 2009;1:845–853. doi: 10.2217/imt.09.51. doi:10.2217/imt.09.51. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S.A., Restifo N.P., Yang J.C., Morgan R.A., Dudley M.E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. doi:10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leen A.M., Rooney C.M., Foster A.E. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. doi:10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 4.Moosmann A., Bigalke I., Tischer J., Schirrmann L., Kasten J., Tippmer S., Leeping M., Prevalsek D., Jaeger G., Ledderose G., et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115:2960–2970. doi: 10.1182/blood-2009-08-236356. doi:10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 5.Miller J.S., Soignier Y., Panoskaltsis-Mortari A., McNearney S.A., Yun G.H., Fautsch S.K., McKenna D., Le C., Defor T.E., Burns L.J., et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. doi:10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 6.Brenner M.K., Heslop H.E. Adoptive T cell therapy of cancer. Curr. Opin. Immunol. 2010;22:251–257. doi: 10.1016/j.coi.2010.01.020. doi:10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart T.J., Abrams S.I. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. doi:10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- 8.Bollard C.M., Rössig C., Calonge M.J., Huls M.H., Wagner H., Massague J., Brenner M.K., Heslop H.E., Rooney C.M. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. doi:10.1182/blood.V99.9.3179. [DOI] [PubMed] [Google Scholar]
- 9.Westwood J.A., Kershaw M.H. Genetic redirection of T cells for cancer therapy. J. Leukoc. Biol. 2010;87:791–803. doi: 10.1189/jlb.1209824. doi:10.1189/jlb.1209824. [DOI] [PubMed] [Google Scholar]
- 10.Di Stasi A., De Angelis B., Rooney C.M., Zhang L., Mahendravada A., Foster A.E., Heslop H.E., Brenner M.K., Dotti G., Savoldo B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. doi:10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Angelis B., Dotti G., Quintarelli C., Huye L.E., Zhang L., Zhang M., Pane F., Heslop H.E., Brenner M.K., Rooney C.M., Savoldo B. Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506) Blood. 2009;114:4784–4791. doi: 10.1182/blood-2009-07-230482. doi:10.1182/blood-2009-07-230482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. doi:10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonini C., Brenner M.K., Heslop H.E., Morgan R.A. Genetic modification of T cells. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011;17:S15–S20. doi: 10.1016/j.bbmt.2010.09.019. doi:10.1016/j.bbmt.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson L.A., Morgan R.A., Dudley M.E., Cassard L., Yang J.C., Hughes M.S., Kammula U.S., Royal R.E., Sherry R.M., Wunderlich J.R., et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. doi:10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkhurst M.R., Yang J.C., Langan R.C., Dudley M.E., Nathan D.N., Feldman S.A., Davis J.L., Morgan R.A., Merino M.J., Sherry R.M., et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2010 doi: 10.1038/mt.2010.272. doi:10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendle G.M., Linnemann C., Hooijkaas A.I., Bies L., de Witte M.A., Jorritsma A., Kaiser A.D.M., Pouw N., Debets R., Kieback E., et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16:565–570. doi: 10.1038/nm.2128. (1p following 570) doi:10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg S.A. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol. Ther. 2010;18:1744–1745. doi: 10.1038/mt.2010.195. doi:10.1038/mt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moritz D., Groner B. A spacer region between the single chain antibody- and the CD3 zeta-chain domain of chimeric T cell receptor components is required for efficient ligand binding and signaling activity. Gene Ther. 1995;2:539–546. [PubMed] [Google Scholar]
- 19.Sadelain M., Brentjens R., Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. doi:10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckman R.A., Weiner L.M., Davis H.M. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–179. doi: 10.1002/cncr.22402. doi:10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 21.Weijtens M.E., Willemsen R.A., Valerio D., Stam K., Bolhuis R.L. Single chain Ig/gamma gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity. J. Immunol. 1996;157:836–843. [PubMed] [Google Scholar]
- 22.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z., et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. doi:10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiger T.L., Nguyen P., Leitenberg D., Flavell R.A. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood. 2001;98:2364–2371. doi: 10.1182/blood.v98.8.2364. doi:10.1182/blood.V98.8.2364. [DOI] [PubMed] [Google Scholar]
- 24.Pulè M.A., Straathof K.C., Dotti G., Heslop H.E., Rooney C.M., Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. doi:10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Brentjens R.J., Santos E., Nikhamin Y., Yeh R., Matsushita M., La Perle K., Quintás-Cardama A., Larson S.M., Sadelain M. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. doi:10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed N., Salsman V.S., Yvon E., Louis C.U., Perlaky L., Wels W.S., Dishop M.K., Kleinerman E.E., Pule M., Rooney C.M., Heslop H.E., Gottschalk S. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. doi:10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohn D.B., Dotti G., Brentjens R., Savoldo B., Jensen M., Cooper L.J., June C.H., Rosenberg S., Sadelain M., Heslop H.E. CARs on track in the clinic. Mol. Ther. J. Am. Soc. Gene Ther. 2011;19:432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.N., Lanier B.J., Morgan R.A., Rosenberg S.A. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. doi:10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jena B., Dotti G., Cooper L.J.N. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. doi:10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heslop H.E. Safer CARS. Mol. Ther. 2010;18:661–662. doi: 10.1038/mt.2010.42. doi:10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. doi:10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brentjens R., Yeh R., Bernal Y., Riviere I., Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. doi:10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren E.H., Fujii N., Akatsuka Y., Chaney C.N., Mito J.K., Loeb K.R., Gooley T.A., Brown M.L., Koo K.K.W., Rosinski K.V., et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. doi:10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintarelli C., Vera J.F., Savoldo B., Giordano Attianese G.M.P., Pule M., Foster A.E., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. doi:10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vera J.F., Hoyos V., Savoldo B., Quintarelli C., Giordano Attianese G.M.P., Leen A.M., Liu H., Foster A.E., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol. Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. doi:10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. doi:10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markley J.C., Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–3519. doi: 10.1182/blood-2009-09-241398. doi:10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heemskerk B., Liu K., Dudley M.E., Johnson L.A., Kaiser A., Downey S., Zheng Z., Shelton T.E., Matsuda K., Robbins P.F., Morgan R.A., Rosenberg S.A. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum. Gene Ther. 2008;19:496–510. doi: 10.1089/hum.2007.0171. doi:10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craddock J.A., Lu A., Bear A., Pule M., Brenner M.K., Rooney C.M., Foster A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. doi:10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster A.E., Dotti G., Lu A., Khalil M., Brenner M.K., Heslop H.E., Rooney C.M., Bollard C.M. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J. Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. doi:10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terai M., Tamura Y., Alexeev V., Ohtsuka E., Berd D., Mastrangelo M.J., Sato T. Human interleukin 10 receptor 1/IgG1-Fc fusion proteins: immunoadhesins for human IL-10 with therapeutic potential. Cancer Immunol. Immunother. 2009;58:1307–1317. doi: 10.1007/s00262-008-0644-9. doi:10.1007/s00262-008-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dotti G., Savoldo B., Pule M., Straathof K.C., Biagi E., Yvon E., Vigouroux S., Brenner M.K., Rooney C.M. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. doi:10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heslop H.E., Ng C.Y., Li C., Smith C.A., Loftin S.K., Krance R.A., Brenner M.K., Rooney C.M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 1996;2:551–555. doi: 10.1038/nm0596-551. doi:10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 44.Heslop H.E., Slobod K.S., Pule M.A., Hale G.A., Rousseau A., Smith C.A., Bollard C.M., Liu H., Wu M., Rochester R.J., et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. doi:10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plosker G.L., Foster R.H. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59:323–389. doi: 10.2165/00003495-200059020-00021. doi:10.2165/00003495-200059020-00021. [DOI] [PubMed] [Google Scholar]
- 46.Ciceri F., Bonini C., Stanghellini M.T.L., Bondanza A., Traversari C., Salomoni M., Turchetto L., Colombi S., Bernardi M., Peccatori J., et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. doi:10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 47.Tey S., Dotti G., Rooney C.M., Heslop H.E., Brenner M.K. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol. Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. doi:10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smyth M.J., Cretney E., Kelly J.M., Westwood J.A., Street S.E.A., Yagita H., Takeda K., van Dommelen S.L.H., Degli-Esposti M.A., Hayakawa Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005;42:501–510. doi: 10.1016/j.molimm.2004.07.034. doi:10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 49.Uherek C., Tonn T., Uherek B., Becker S., Schnierle B., Klingemann H., Wels W. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 50.Müller T., Uherek C., Maki G., Chow K.U., Schimpf A., Klingemann H., Tonn T., Wels W.S. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol. Immunother. 2008;57:411–423. doi: 10.1007/s00262-007-0383-3. doi:10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altvater B., Landmeier S., Pscherer S., Temme J., Schweer K., Kailayangiri S., Campana D., Juergens H., Pule M., Rossig C. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin. Cancer Res. 2009;15:4857–4866. doi: 10.1158/1078-0432.CCR-08-2810. doi:10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., Sun R., Wei H., Zhang J., Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 2004;89:338–347. [PubMed] [Google Scholar]
- 53.Jiang W., Zhang J., Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10:265–274. doi: 10.1080/14653240801965156. doi:10.1080/14653240801965156. [DOI] [PubMed] [Google Scholar]
- 54.Goding S.R., Yang Q., Knudsen K.B., Potter D.M., Basse P.H. Cytokine gene therapy using adenovirally transduced, tumor-seeking activated natural killer cells. Hum. Gene Ther. 2007;18:701–711. doi: 10.1089/hum.2007.052. doi:10.1089/hum.2007.052. [DOI] [PubMed] [Google Scholar]
- 55.Vera J.F., Brenner L.J., Gerdemann U., Ngo M.C., Sili U., Liu H., Wilson J., Dotti G., Heslop H.E., Leen A.M., Rooney C.M. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J. Immunother. 2010;33:305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. doi:10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee D.A., Verneris M.R., Campana D. Acquisition, preparation, and functional assessment of human NK cells for adoptive immunotherapy. Methods Mol. Biol. 2010;651:61–77. doi: 10.1007/978-1-60761-786-0_4. doi:10.1007/978-1-60761-786-0_4. [DOI] [PubMed] [Google Scholar]
- 57.Kershaw M.H., Westwood J.A., Parker L.L., Wang G., Eshhar Z., Mavroukakis S.A., White D.E., Wunderlich J.R., Canevari S., Rogers-Freezer L., et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. doi:10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J.R., Digiusto D.L., Slovak M., Wright C., Naranjo A., Wagner J., Meechoovet H.B., Bautista C., Chang W., Ostberg J.R., Jensen M.C. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 59.Lamers C.H.J., Sleijfer S., Vulto A.G., Kruit W.H.J., Kliffen M., Debets R., Gratama J.W., Stoter G., Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. doi:10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 60.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J., et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. doi:10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen M.C., Popplewell L., Cooper L.J., DiGiusto D., Kalos M., Ostberg J.R., Forman S.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. doi:10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]