Abstract
Muscular dystrophies are a heterogeneous group of genetic disorders characterized by muscle weakness and wasting. Duchenne muscular dystrophy (DMD) is the most common and severe form of muscular dystrophy, and although the molecular mechanisms of the disease have been extensively investigated since the discovery of the gene in 1986, there is currently no effective treatment. However, new gene-based therapies have recently emerged with particular noted advances in using conventional gene replacement strategies, RNA-based technology and pharmacological approaches. While the proof of principle has been demonstrated in animal models, several clinical trials have recently been undertaken to investigate the feasibility of these strategies in patients. In particular, antisense-mediated exon skipping has shown encouraging results and holds promise for the treatment of dystrophic muscle. Here, we summarize the recent progress in therapeutic approaches to muscular dystrophies, with an emphasis on gene therapy and exon skipping for DMD.
INTRODUCTION
Muscular dystrophies refer to a group of inherited disorders characterized by progressive muscle weakness, wasting and degeneration. Amongst these, Duchenne muscular dystrophy (DMD) is the most severe and common form, affecting ∼1 in 3500 newborn males. DMD is caused by the loss of dystrophin, which is located beneath the sarcolemna, assembles the dystrophin–glycoprotein complex at the sarcolemna, and links the internal cytoplasmic actin filament network and extracellular matrix, providing physical strength to muscle fibers (1). At present, there is no effective therapy to stop the lethal progression of the disease, although several promising experimental strategies are currently under investigation. These include gene therapy aiming at reintroducing a functional recombinant version of the dystrophin gene using adeno-associated, lentiviral or adenoviral vectors, as well as modification of the dystrophin pre-mRNA, commonly referred to as exon skipping. Both strategies hold great promises with several clinical trials ongoing, but cell-based therapies and pharmacological approaches such as the up-regulation of utrophin or read-through strategies for nonsense mutations have also been investigated. All these strategies face major challenges, imposed by the nature of muscular dystrophies such as the need to target different muscles in the body, including respiratory and cardiac muscles, the need for long-term effect, the potential immune response and the problem of fibrosis.
In this review, we focus on the main genetic and pharmacological approaches currently being designed which the aim to either replace or compensate for dystrophin. While the majority of them have focused on DMD, the basic principles and strategies summarized here are broadly applicable to many neuromuscular disorders. We do not review the many strategies to treat the secondary effects of the disease pathology.
VIRAL VECTOR-MEDIATED GENE DELIVERY
As DMD is a recessively inherited disorder, the prospect of using gene replacement is a conceptually simple approach to therapy. Harnessing the ability of viruses to enter a variety of cell types and deposit their genomes has led to the development of various shuttle vectors to deliver genes to muscle. With gene replacement, the aim is to provide an alternative copy of the functional dystrophin gene for patients rather than repair the locus within the patient's genome. An advantage of gene replacement for DMD is that it is not dependent on any particular mutation in a patient's gene. However, identifying safe and efficient methods to deliver a replacement dystrophin or dystrophin-like gene to muscles body-wide is a daunting challenge. Much of the work in the field is currently aimed at optimizing delivery methods for targeted and long-term expression of dystrophin in muscles throughout the body. Results from recent studies in larger animal models and in early human trials highlight immunological complications associated with viral vector-mediated gene transfer as the major barrier to clinical success.
TYPES OF VIRAL VECTORS
Three types of viral vectors have primarily been used by researchers studying the muscular dystrophies: adenoviral, adeno-associated viral and lentiviral vectors. All three vectors have shown some success in transduction and stable expression of striated muscles; however, only adeno-associated virus (AAV) vectors have progressed to being used in clinical trials.
Lentiviral vectors are a class of retroviral vectors that stably integrate transgenes into the genomes of quiescent and non-quiescent cells (2,3). Integration into the host genome, however, can cause insertional mutagenesis, leading to potential activation of proto-oncogenes (4,5), although this occurs at a low frequency. Lentiviral vectors have low inherent immunogenicity and a relatively large carrying capacity (∼9 kb), but they have not been shown to achieve widespread transduction of tissues in vivo (2,6). For example, intramuscular injection of lentiviral vectors expressing a micro- or mini-dystrophin gene resulted in successful transduction and stable retention in myogenic stem cells as well as myofibers in mdx mouse muscles (7,8). However, the efficiency at present is too low for direct therapeutic application. Consequently, the utility of this vector system for the muscular dystrophies appears primarily for stable transduction of myogenic stem cells or stem cell progenitors and mesoangioblasts with mini-dystrophin expression cassettes (3,9,10). These latter approaches could be developed for transduction of myogenic stem cells derived from a patient, expansion of those cells in vitro and subsequently using those cells for transplantation into dystrophic muscles to provide new dystrophin genes while contributing to muscle regeneration (i.e. ex vivo gene therapy). This is particularly advantageous for DMD treatments, as lentiviral reconstitution of dystrophin into myogenic stem cells would provide an ongoing source of dystrophin during repeated cycles of regeneration.
In contrast to lentiviruses, adenoviral vectors do not integrate into the host genome and exist as non-integrated episomes following infection. Adenoviruses are attractive for gene transfer because they have a large carrying capacity (in some formulations >30 kb) and can be produced in high titers. However, adenoviral gene transfer is more efficient in immature or regenerating muscle due to down-regulation of the primary coxsackie and adenovirus receptor during muscle maturation (11). The latest generation of high-capacity adenoviruses is devoid of viral genes and showed increased longevity of expression. In addition, the lack of viral coding sequences increased the capacity of these vectors, allowing gene transfer of full-length dystrophin (12–15). Earlier trials using mini-dystrophin showed good transduction of neonatal muscle, but immuno-suppression was required for long-term transduction in adult mdx mice and the dog model (16,17). Despite these promising results, the development of these vectors for dystrophin gene transfer was set back following reports of the acute cytotoxic T cell response and toxicity in non-human primates (18), and a severe innate immune response and death in one patient after receiving gene therapy to treat partial ornithine transcarbamylase deficiency (19).
AAVs are single-stranded DNA parvoviruses that require a helper virus for replication and assembly (20). However, the recombinant form (rAAV), despite carrying no viral genes, can be produced at high titers in the absence of a helper virus and infect both dividing and non-dividing cells (21). Stable gene expression following intramuscular rAAV injection has been reported for up to 2 years in mice and more than 7 years in dogs and rhesus monkeys (22–25). To date, more than nine primate serotypes (AAV1-9) have been described, with the different serotypes all displaying different tissue tropisms (26,27). In striated muscles, high transduction efficiencies have been achieved with rAAV1, 6, 7, 8 and 9 (28). Of all the serotypes tested, rAAV6 also showed the best capability of achieving high transduction in the heart of mice (29). Recombinant AAV vectors have attracted widespread interest for the development of muscular dystrophy therapies due to the ability of several serotypes to be efficiently extravasated from capillaries and subsequently transduce underlying muscle tissues. This ability has been exploited to develop systemic gene delivery techniques for body-wide delivery of dystrophin to striated muscles (12,30–34).
Unlike adenovirus, AAV vectors appeared to exhibit low immunogenicity. A phase I trial for hemophilia b using rAAV2 reported no adverse events in patients receiving intramuscular injections of up to 1014 vector genomes (23). However, further studies in dogs and humans now suggest that these vectors have the potential for elicitation of a cellular immune response that could greatly influence the nature of clinical application (35–38).
‘MINI’- AND ‘MICRO'-DYSTROPHINS
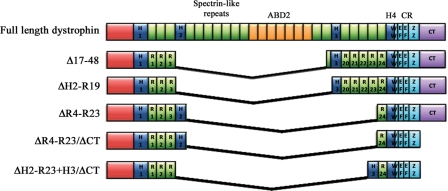
The limited carrying capacity of the rAAV vectors (∼4.7 kb), compared with the sheer size of the dystrophin gene, which encodes 14 kb mRNA and spans ∼2.2 Mb, has meant that truncated versions of dystrophin must be used. The construction of these ‘mini’- or ‘micro’-dystrophins follows the observation that some very mildly affected Becker muscular dystrophy (BMD) patients can carry large genomic deletions of dystrophin (39,40). Work from several labs has shown that two large regions of dystrophin—most of the rod domain (which normally contains 24 spectrin-like repeats) and the C-terminal domain—can be truncated with minimal functional impact (30,41–44) (Fig. 1). The best-studied 4 spectrin-like repeat (ΔR4–R23) micro-dystrophin containing only the first three and the last spectrin-like repeats has been shown to almost fully prevent dystrophy in transgenic dystrophin-deficient mdx mice (41). When combined with a C-terminal deletion, the ΔR4–R23/ΔCT micro-dystrophin is able to reverse most morphological abnormalities in young and old mdx mice and the more severe dystrophin:utrophin double-knockout mouse model following systemic delivery using rAAV vectors, although muscle strength is not fully restored (30,31,41,45). These studies raise the prospects for gene therapy of DMD using rAAV-mediated delivery of micro-dystrophin expression cassettes.
Figure 1.
Schematic representation of micro-dystrophin constructs. Full-length dystrophin consists of an actin binding domain (ABD1) at the N-terminus, four hinge domains (H1-4), 24 spectrin-like repeats (R1–R24) (within which lies a second ABD (ABD2)), a cysteine rich domain (CR) and the carboxy-terminal domain (CT). The CR itself consists of a WW domain, two EF-hand regions and a zinc finger domain (ZZ) that together form the dystroglycan binding site (122). Dystrophin also binds to α1-syntrophin and β1-syntrophin at the CT domain (123). Expression of the construct generated by deletion of exons 17–48 (Δ17–48), which was originally observed in a mildly affected Becker's muscular dystrophy patient, corrected 95% of the morphology in transgenic mdx mice and supports near normal force development (39,124). Later modification of this construct (ΔH2-R19) and one carrying deletions of R4–R23 (ΔR4–R23) have also been shown to reverse dystrophic pathology in transgenic mdx mice (41). Further truncation of dystrophin with deletion of the CT domain (ΔR4–R23/ΔCT) resulted in significant improvement in force in older dystrophic mice (45), confirming that the CT domain is not critical for dystrophin function. Recent work has now shown that replacing hinge 2 with hinge 3 in micro-dystrophin (ΔH2-R23 + H3/ΔCT) significantly improves the functional capacity of truncated dystrophins as demonstrated by prevention of muscle degeneration in mdx mice injected with rAAV6-ΔH2-R23 + H3/ΔCT (125). The size of a construct dictates which vectors can be used for delivery. The full-length cDNA only fits into gutted or high-capacity adenoviral vectors, the larger mini-dystrophins can be delivered with lentiviral vectors, while the smallest micro-dystrophins can be systemically delivered using rAAV.
IMMUNOLOGICAL COMPLICATIONS AND SOLUTIONS TO GENE TRANSFER USING RAAV: LESSONS FROM LARGER ANIMAL STUDIES AND EARLY HUMAN TRIALS
While AAV vectors do not elicit a cellular immune response in mice, multiple labs have now observed that AAVs 1, 2, 6 and 8 can elicit an immune response in the dog model for DMD (38,46–48), in monkeys (49) and in humans (35–37,50,51). However, studies by Wang et al. (52) in dystrophic dogs also showed that this T-cell response could be blocked with transient immuno-suppression. Transient immune suppression has also been applied with success in non-human primates (49,53).
There is also some concern that dystrophin itself may be immunogenic in dystrophin-deficient patients. In the only human trial of dystrophin gene transfer by Mendell et al. (54), intramuscular injections of rAAV2-minidystrophin into the biceps of six patients showed robust mini-dystrophin-specific T-cell activity from as early as day 15 post-vector treatment in one patient. Another patient showed a T-cell response before vector treatment and this response was intermittently positive over 2 years follow-up. No dystrophin-specific antibodies were detected in the serum of any study patients. However, none of the six patients displayed any detectable exogenous dystrophin (54), regardless of the presence or absence of an immune response against dystrophin, and all patients appeared to express ‘revertant' dystrophin-positive myofibers. The authors proposed that dystrophin epitopes from revertant dystrophin fibers expressing functional truncated dystrophin after spontaneous in-frame splicing in some patients could prime the T-cell response. However, other investigators postulate that the immune system tends to tolerize patients against revertant fibers—and this could explain the lack of immune response to the transfer of mouse dystrophin into mdx mice (55). Capsid-specific T-cell responses are also sometimes primed by the delivery of the rAAV vector to muscle (36,56), but this does not necessarily cause loss of transgene expression (56).
STRATEGIES TO ENHANCE DYSTROPHIN GENE TRANSFER AND REDUCE IMMUNE REJECTION
It has been shown that an inflammatory immune response is often observed following delivery of rAAV vectors that express immunogenic proteins such as bacterial proteins under the control of a ubiquitously active promoter, like cytomegalovirus (31,57–59). The use of a muscle-specific promoter such as creatine kinase has been shown to effectively block this response (58–60). Thorough purification of the viral vectors from contaminants that could potentially be immunogenic, such as products of residual viral genes in vectors or serum products, can also help reduce the immune response (61). Strategies to reduce the vector genome dosage would also help prevent elicitation of an immune response and improve expression levels following systemic administration. This could be achieved by codon optimization of the micro-dystrophin transgenes (62), or by the use of vascular ligatures or compression bandages to increase the dwell time of the vector in order to increase the chance of transduction (63–66). Other strategies that have been employed to ensure body-wide dissemination of the vector include the use of high-volume or high-pressure injections (66–68), or simultaneous perfusion with agents that promote endothelial permeabilization, such as histamine (69). Finally, delivery of micro-utrophin in place of micro-dystrophin could circumvent any innate immune response against dystrophin in DMD patients. Recent work has shown that micro-utrophin performed favorably relative to micro-dystrophin in dystrophin:utrophin double-knockout mice (70).
ANTISENSE-MEDIATED EXON SKIPPING
Principle and proof of concept in animal models
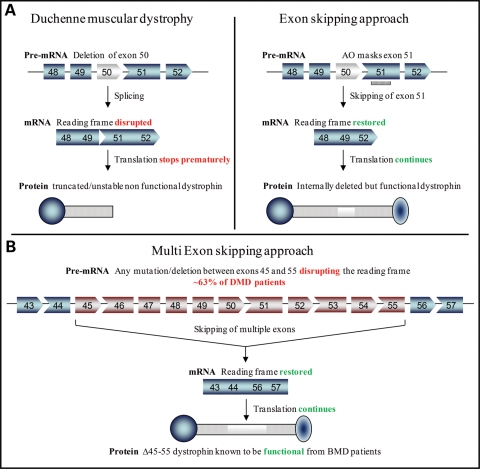
The majority of DMD mutations disrupt the open reading frame, resulting in the absence of a functional dystrophin protein at the sarcolemma of muscle fibers. The related allelic disorder BMD is caused by mutations that create shortened but in-frame transcripts with production of partially functional dystrophin, leading to variable symptoms that range from borderline DMD to virtually asymptomatic (71). Antisense-induced exon-skipping strategies, which aim to remove the mutated or additional exon(s) to restore the reading frame, have been shown to induce the expression of these ‘Becker MD-like' shortened forms of dystrophin protein, retaining crucial functions (Fig. 2A) (72–75). Antisense oligonucleotide (AO)-mediated exon skipping uses different oligonucleotide chemistries to target specific exon(s). The principle of the exon-skipping therapy for DMD has first been demonstrated by Pramono et al. (76) in 1996 in lymphoblastoid cells and by Dunckley et al. (73) in 1998 in cultured mouse cells in vitro. Since then, numerous in vivo studies have provided pre-clinical evidence for the therapeutic potential of an antisense strategy for DMD in several animal models. In particular, the mdx mouse model, which harbors a nonsense mutation in exon 23, has been used extensively to test efficacy of the AO approach using various oligonucleotide chemistries, such as 2′OMe (75), phosphorodiamidate morpholino oligomers (PMO) (77,78), locked nucleic acid or peptide nucleic acid (79,80). Intramuscular and systemic injections in canine models of the disease have also demonstrated restoration of dystrophin expression associated with functional benefits (81).
Figure 2.
Antisense-mediated exon skipping rationale for DMD. (A) Patients with DMD have mutations which disrupt the open reading frame of the dystrophin pre-mRNA. In this example, exon 50 is deleted, creating an out-of-frame mRNA and leading to the synthesis of a truncated non-functional or unstable dystrophin (left panel). An antisense oligonucleotide directed against exon 51 can induce effective skipping of exon 51 and restore the open reading frame, therefore generating an internally deleted but partly functional dystrophin (right panel). (B) Multi exon-skipping rationale for DMD. The optimal skipping of exons 45–55 leading to the del45–55 artificial dystrophin could transform the DMD phenotype into the asymptomatic or mild BMD phenotype. This multiple exon skipping could theoretically rescue up to 63% of DMD patients with a deletion (126).
Ongoing therapeutic trials using antisense oligonucleotides
Following the very encouraging results obtained on these animal models, groups both in the Netherlands (Dutch consortium from the Leiden University medical School) and in the UK (MDEX consortium) have worked towards clinical evaluation of the antisense-mediated exon skipping in DMD patients. The Dutch consortium together with the RNA therapy company Prosensa selected a 20-mer antisense oligonucleotide of 2′OMe phosphorothioate RNA chemistry targeting exon 51 (PRO051). Four DMD patients were injected locally in the tibialis anterior muscle with a single dose of 0.8 mg PRO051. This treatment was well tolerated and accompanied by specific exon 51 skipping as well as detection of dystrophin in the vast majority of muscle fibers at levels varying between 17 and 35% (82). The MDEX consortium in collaboration with AVI Biopharma selected a PMO antisense oligonucleotide, based on pre-clinical studies with this backbone chemistry (77,78,83). The 30-mer PMO optimized to skip exon 51 (AVI-4658) (84) was injected unilaterally into the extensor digitorum brevis muscles of seven patients, in a placebo-controlled dose–escalation protocol. Results from this trial demonstrated that PMO oligonucleotides were well tolerated by all patients and that the dystrophin protein was expressed at up to 42% of normal levels in dystrophin-positive fibers of patients treated with the higher dose of 0.9 mg (85).
Both studies have been followed by repeated systemic administration studies. The Dutch consortium recently completed a phase I/IIa trial involving four groups of DMD boys receiving escalating doses of 0.5, 2.0, 4.0 and 6.0 mg/kg of 2′OMe weekly for 5 weeks. While the results of this study have not yet been published, Dr Goemans reported at the World Muscle Society (WMS) meeting in 2009 that PRO051 was well tolerated in each DMD patient and that novel dystrophin expression was observed (86). All boys who participated in this study entered an open label extension study, receiving weekly subcutaneous injections of 6 mg/kg regarless of earlier dose. Twenty-four week follow-up data presented at the WMS meeting in 2010 reported that this treatment was generally well tolerated over the 24-week period and that encouraging gains in the 6min walk test were observed in some boys (87). Encouraging results have also been announced by the UK consortium and AVI Biopharma who have completed their systemic study enrolling 19 patients receiving weekly IV administrations of AVI-4658 (six cohorts receiving 0.5, 1.0, 2.0, 4.0, 10.0 or 20.0 mg/kg). Shrewsbury et al. (88) reported at the WMS meeting in 2010 that the study drug was well tolerated and that exon 51 skipping was detected in patients at the 2 mg/kg dose and above, giving rise to expression of dystrophin.
As attested by the above accounts, the potential of exon skipping as a therapeutic strategy for DMD has developed from a plausible notion in the mid-nineties (73,89) to the point where early clinical trials show it holds realistic prospects of providing genuine therapeutic benefit. However, substantial barriers remain, some scientific and some regulatory.
Delivery and regulatory challenges
Hurdles limiting the systemic delivery of AO include the poor cellular uptake and relative rapid clearance from circulation, but also the high variability in exon-skipping efficiency among muscle types. Experiments in animal models demonstrated that large doses ranging from 100 mg/kg/week in the mouse (90) to 200 mg/kg/week in dogs (81) over several weeks were required for functional improvement but not enough to achieve uniform expression of dystrophin, and more importantly, expression in cardiac muscle (91). The reasons for the low efficiency of cardiac dystrophin restoration are unclear, but are probably related to the poor ability of unmodified oligonucleotides to penetrate the heart. Recent developments using cell penetrating peptide-conjugated PMO (PPMO) have addressed most of these delivery issues and could therefore represent an effective strategy to reduce the dose level and dose frequency, as well as delivering the AO to non-leaky fibers and the heart (92–96). However, the toxicity of current PPMO chemistry poses a challenge for determination of an effective and safe regimen in man. One PPMO targeting human exon 50 (AVI-5038), currently in pre-clinical development for DMD, was found to cause mild tubular degeneration in the kidneys of cynomolgus monkeys injected weekly with 9 mg/kg for 4 weeks (96), although the same peptide conjugated to PMOE23 did not exhibit any toxic effect in the kidneys of mdx mice treated with higher doses (30 mg/kg biweekly for 3 months) (94). In addition, use of peptides to enhance delivery raises the possibility of an immune response that may prevent repeated administration or cause rejection of targeted tissues, especially because DMD patients would require regular life-long treatment. Although no immune response was observed in mice models (78,94), immunogenicity varies considerably between species, arguing for longer term studies in a range of animal models.
Despite the very promising results of the initial trials targeting exon 51, the clinical applicability of the AO-mediated exon-skipping approach for DMD still faces a major hurdle regarding regulatory approval. The sequence-specific nature of the strategy has implications for future personalized medicine. The different deletions that cause DMD would require skipping of different exons, and therefore design and optimization of many specific AOs. If each AO is considered a new drug—which is the current food and drug administration (FDA) regulation, then each of them will have to go through the expensive and lengthy clinical trial stages. On the top of being an insuperable barrier in terms of money and time, it might be very problematic to find enough patients to even perform clinical trials. Some AO may be applicable to a very restricted number of patients such as those targeting exons 71, 72, 75, 77 or 78 (representing 0.02% of all mutations) (97). A practical resolution of this problem would be to consider AOs (PMO or 2′OMe) as one drug, even if their sequences are different. Therefore, the realization of the clinical applicability of AO-based exon-skipping as a treatment for DMD might lie in the approval of antisense sequences as a class of drugs. This type of approval would be a first for the FDA, but the prospect of personalized molecular medicine might justify such a change in approach.
Viral vector-mediated exon skipping
Among the challenges faced by the exon-skipping approach is the long-lasting effect of the therapy. Since the duration of the effects of AOs is limited in vivo, patients will have to be re-injected weekly or monthly to maintain therapeutic levels of dystrophin. An alternative to this limitation is to deliver the AO using viral vectors and, in particular, AAV vectors which are extremely efficient for muscle gene transfer. Studies using U7 or U1 small-nuclear RNA (snRNA) to carry the AOs have demonstrated efficient and sustained rescue of dystrophin in mdx mice (98,99). Linking the AO to a snRNA enhances its activity by allowing its proper subcellular localization and thereby facilitates the AO inclusion into the spliceosome (100,101). This type of viral vector-mediated exon-skipping approach can also be combined with cell-based therapies using lentiviral vectors to correct the reading frame of muscle stem cell before re-injection (102). In addition, this vectorized approach offers the possibility to efficiently deliver multiple snRNA cassettes to target multiple exons which could be extremely useful to overcome the personalized medicine issue for DMD treatment (Fig. 2B).
PHARMACOLOGICAL APPROACHES
Utrophin upregulation
A promising pharmacological treatment for DMD aims to increase levels of utrophin, an orthologue of dystrophin, in muscle fibers of affected patients to compensate for the absence of dystrophin. Proof of principle studies in mdx mice have established that elevation of utrophin levels in dystrophic muscle fibers can restore sarcolemnal expression of the dystrophin associated protein complex (DAPC) members and alleviate the dystrophic pathology (103,104). The mechanisms of transcriptional control of utrophin provide multiple targets for pharmacological interventions (105). In particular, knowledge of the utrophin-A promoter has initiated the search for small molecules that can stimulate the transcription of utrophin. Such utrophin-based drug therapy for DMD presents many advantages as it should be effective for all DMD patients, regardless of the specific gene defect and could be administered to patients systemically because utrophin overexpression in tissues other than muscles does not seem to cause detrimental effect (106). High-throughput screenings, which enable the rapid assessment of thousands of potentially beneficial compounds from chemical libraries, have allowed the identification of a small molecule (BMN195) that has recently been tested in phase I clinical trial by BioMarin Pharmaceuticals. While BMN195 showed promising utrophin upregulation potential in pre-clinical models (Tinsley, Davies et al., submitted for publication), it could not reach efficient plasma level concentration due to poor bioavailability during the clinical study (http://phx.corporateir.net/phoenix.zhtml?c=106657&p=irolnewsArticle&ID=1455247&highlight=). Since there were no safety issues with BMN195, Summit plc is investigating the possibility of better formulations of the drug. Additional candidates overcoming the limitation of BMN195 are being developed.
Several other groups have reported approaches for increasing the levels of utrophin such as RhoA (107), heregulin and l-arginine (reviewed in 105,108) and calpain inhibition (109), although none increases the levels substantially. Increased expression of integrins (110,111) and ADAM12 (112) have been shown to improve pathology in addition to increasing the levels of utrophin. Agents such as biglycan that stablize the DAPC also hold therapeutic promise (113). Glucocorticoids have been shown to enhance activity of an internal ribosome entry site located within the utrophin A 5′UTR (114) which may be used to stabilize utrophin in muscle. Custom-designed proteins with zinc finger motifs have been shown to act as strong transcriptional activators of the utrophin A promoter (115). Ervasti and colleagues (116) are also attempting to deliver utrophin protein directly.
Read-through strategies for suppression of nonsense mutations
Novel strategies designed to induce ribosomal read-through of premature termination mutations can produce full-length functional protein. In the case of DMD, the small molecule drugs gentamicin and ataluren (also known as PTC124) have achieved convincing proof of concept in vitro and in vivo, with the production of a full-length dystrophin protein that localizes correctly to the sarcolemna (117,118). Recent clinical trials of gentamicin and ataluren have not demonstrated clinical efficacy, making the path toward regulatory approval for DMD a difficult one (119,120). Despite eliciting a favorable pharmacodynamic response to the drug, demonstrating clinical benefit remains problematic. Lessons learned from these trials will hopefully prove useful in the design of future clinical trials for DMD, especially regarding long-term placebo-controlled studies and determination of the age and state of the disease when treatment will have clinically meaningful benefit.
CONCLUSION
Since the cloning of the dystrophin gene almost 25 years ago (121), DMD has gone from a disorder viewed often as incurable or hopeless to one with numerous potential treatment options. From a genetic standpoint, methods to increase dystrophin or utrophin production are showing great promise in animal models and are beginning to be tested in the clinic. Despite this potential, it is clear that considerable optimization of all of the candidate approaches to a treatment will be needed before success is in hand. Such optimization must now be performed with a delicate balancing act between animal studies and human clinical trials. None of the available animal models fully mimics the human disorder, and the larger animal models either display significant variation from one individual to another (dogs) or do not have muscular dystrophy (non-human primates). Consequently, it will be important to pursue phase I human trials on all promising therapeutics that show safety in the existing animal models such that approaches which do not show promising results in humans can be deprioritized in favor of other emerging strategies. Those strategies that show encouraging results in human trials can then be further optimized in cycles of animal/human studies. With continued success, we will hopefully see a gradual implementation of new therapies over the coming years that will increasingly extend lifespan and improve the quality of life for patients with all forms of muscular dystrophy.
Conflict of Interest statement: K.E.D. is share holder of Summit plc.
FUNDING
This work was supported by the UK Medical Research Center, the Muscular Dystrophy Campaign, the Muscular Dystrophy Association USA, the Association Monegasque contre les myopathies and the Duchenne Parent project France (A.G. and K.E.D.). J.C. is supported by NIH grant R37AR40864. J.T.S. is supported by the Sir Keith Murdoch Fellowship from the American Australian Association.
REFERENCES
- 1.Campbell K.P. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. doi:10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 2.Kafri T., Blomer U., Peterson D.A., Gage F.H., Verma I.M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. doi:10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 3.Li S., Kimura E., Fall B.M., Reyes M., Angello J.C., Welikson R., Hauschka S.D., Chamberlain J.S. Stable transduction of myogenic cells with lentiviral vectors expressing a minidystrophin. Gene Ther. 2005;12:1099–1108. doi: 10.1038/sj.gt.3302505. doi:10.1038/sj.gt.3302505. [DOI] [PubMed] [Google Scholar]
- 4.Beard B.C., Keyser K.A., Trobridge G.D., Peterson L.J., Miller D.G., Jacobs M., Kaul R., Kiem H.P. Unique integration profiles in a canine model of long-term repopulating cells transduced with gammaretrovirus, lentivirus, or foamy virus. Hum. Gene Ther. 2007;18:423–434. doi: 10.1089/hum.2007.011. doi:10.1089/hum.2007.011. [DOI] [PubMed] [Google Scholar]
- 5.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. doi:10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie T.C., Kobinger G.P., Louboutin J.P., Radu A., Javazon E.H., Sena-Esteves M., Wilson J.M., Flake A.W. Transduction of satellite cells after prenatal intramuscular administration of lentiviral vectors. J. Gene Med. 2005;7:50–58. doi: 10.1002/jgm.649. doi:10.1002/jgm.649. [DOI] [PubMed] [Google Scholar]
- 7.Kimura E., Li S., Gregorevic P., Fall B.M., Chamberlain J.S. Dystrophin delivery to muscles of mdx mice using lentiviral vectors leads to myogenic progenitor targeting and stable gene expression. Mol. Ther. 2010;18:206–213. doi: 10.1038/mt.2009.253. doi:10.1038/mt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobinger G.P., Louboutin J.P., Barton E.R., Sweeney H.L., Wilson J.M. Correction of the dystrophic phenotype by in vivo targeting of muscle progenitor cells. Hum. Gene Ther. 2003;14:1441–1449. doi: 10.1089/104303403769211655. doi:10.1089/104303403769211655. [DOI] [PubMed] [Google Scholar]
- 9.Bachrach E., Li S., Perez A.L., Schienda J., Liadaki K., Volinski J., Flint A., Chamberlain J., Kunkel L.M. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc. Natl Acad. Sci. USA. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. doi:10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampaolesi M., Blot S., D'Antona G., Granger N., Tonlorenzi R., Innocenzi A., Mognol P., Thibaud J.L., Galvez B.G., Barthelemy I., et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. doi:10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 11.Larochelle N., Teng Q., Gilbert R., Deol J.R., Karpati G., Holland P.C., Nalbantoglu J. Modulation of coxsackie and adenovirus receptor expression for gene transfer to normal and dystrophic skeletal muscle. J. Gene Med. 2010;12:266–275. doi: 10.1002/jgm.1433. [DOI] [PubMed] [Google Scholar]
- 12.Koppanati B.M., Li J., Xiao X., Clemens P.R. Systemic delivery of AAV8 in utero results in gene expression in diaphragm and limb muscle: treatment implications for muscle disorders. Gene Ther. 2009;16:1130–1137. doi: 10.1038/gt.2009.71. doi:10.1038/gt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eghtesad S., Zheng H., Nakai H., Epperly M.W., Clemens P.R. Effects of irradiating adult mdx mice before full-length dystrophin cDNA transfer on host anti-dystrophin immunity. Gene Ther. 2010;17:1181–1190. doi: 10.1038/gt.2010.108. doi:10.1038/gt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Z., Schiedner G., van Rooijen N., Liu C.C., Kochanek S., Clemens P.R. Sustained muscle expression of dystrophin from a high-capacity adenoviral vector with systemic gene transfer of T cell costimulatory blockade. Mol. Ther. 2004;10:688–696. doi: 10.1016/j.ymthe.2004.07.020. doi:10.1016/j.ymthe.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z., Schiedner G., Gilchrist S.C., Kochanek S., Clemens P.R. CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Ther. 2004;11:1453–1461. doi: 10.1038/sj.gt.3302315. doi:10.1038/sj.gt.3302315. [DOI] [PubMed] [Google Scholar]
- 16.Guibinga G.H., Lochmuller H., Massie B., Nalbantoglu J., Karpati G., Petrof B.J. Combinatorial blockade of calcineurin and CD28 signaling facilitates primary and secondary therapeutic gene transfer by adenovirus vectors in dystrophic (mdx) mouse muscles. J. Virol. 1998;72:4601–4609. doi: 10.1128/jvi.72.6.4601-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell J.M., Lochmuller H., O'Hara A., Fletcher S., Kakulas B.A., Massie B., Nalbantoglu J., Karpati G. High-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscle of dystrophic dogs: prolongation of expression with immunosuppression. Hum. Gene Ther. 1998;9:629–634. doi: 10.1089/hum.1998.9.5-629. doi:10.1089/hum.1998.9.5-629. [DOI] [PubMed] [Google Scholar]
- 18.Brunetti-Pierri N., Palmer D.J., Beaudet A.L., Carey K.D., Finegold M., Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. doi:10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 19.Raper S.E., Chirmule N., Lee F.S., Wivel N.A., Bagg A., Gao G.P., Wilson J.M., Batshaw M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. doi:10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 21.Podsakoff G., Wong K.K., Jr, Chatterjee S. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. J. Virol. 1994;68:5656–5666. doi: 10.1128/jvi.68.9.5656-5666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzog R.W., Yang E.Y., Couto L.B., Hagstrom J.N., Elwell D., Fields P.A., Burton M., Bellinger D.A., Read M.S., Brinkhous K.M., et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med. 1999;5:56–63. doi: 10.1038/4743. doi:10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 23.Manno C.S., Chew A.J., Hutchison S., Larson P.J., Herzog R.W., Arruda V.R., Tai S.J., Ragni M.V., Thompson A., Ozelo M., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. doi:10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 24.Monahan P.E., Samulski R.J., Tazelaar J., Xiao X., Nichols T.C., Bellinger D.A., Read M.S., Walsh C.E. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. doi:10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 25.Rabinowitz J.E., Samulski J. Adeno-associated virus expression systems for gene transfer. Curr. Opin. Biotechnol. 1998;9:470–475. doi: 10.1016/s0958-1669(98)80031-1. doi:10.1016/S0958-1669(98)80031-1. [DOI] [PubMed] [Google Scholar]
- 26.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. doi:10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. doi:10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 28.Schultz B.R., Chamberlain J.S. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. doi:10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zincarelli C., Soltys S., Rengo G., Koch W.J., Rabinowitz J.E. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin. Transl. Sci. 2010;3:81–89. doi: 10.1111/j.1752-8062.2010.00190.x. doi:10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregorevic P., Allen J.M., Minami E., Blankinship M.J., Haraguchi M., Meuse L., Finn E., Adams M.E., Froehner S.C., Murry C.E., et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 2006;12:787–789. doi: 10.1038/nm1439. doi:10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Meuse L., Miller D.G., Russell D.W., Chamberlain J.S. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 2004;10:828–834. doi: 10.1038/nm1085. doi:10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koppanati B.M., Li J., Reay D.P., Wang B., Daood M., Zheng H., Xiao X., Watchko J.F., Clemens P.R. Improvement of the mdx mouse dystrophic phenotype by systemic in utero AAV8 delivery of a minidystrophin gene. Gene Ther. 2010;17:1355–1362. doi: 10.1038/gt.2010.84. doi:10.1038/gt.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Zhu T., Qiao C., Zhou L., Wang B., Zhang J., Chen C., Li J., Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. doi:10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 34.Pacak C.A., Conlon T., Mah C.S., Byrne B.J. Relative persistence of AAV serotype 1 vector genomes in dystrophic muscle. Genet. Vaccines Ther. 2008;6:14. doi: 10.1186/1479-0556-6-14. doi:10.1186/1479-0556-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendell J.R., Rodino-Klapac L.R., Rosales X.Q., Coley B.D., Galloway G., Lewis S., Malik V., Shilling C., Byrne B.J., Conlon T., et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann. Neurol. 2010;68:629–638. doi: 10.1002/ana.22251. doi:10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E., Ragni M.V., Manno C.S., Sommer J., Jiang H., et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. doi:10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 37.Mingozzi F., Meulenberg J.J., Hui D.J., Basner-Tschakarjan E., Hasbrouck N.C., Edmonson S.A., Hutnick N.A., Betts M.R., Kastelein J.J., Stroes E.S., et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. doi:10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Storb R., Lee D., Kushmerick M.J., Chu B., Berger C., Arnett A., Allen J., Chamberlain J.S., Riddell S.R., et al. Immune responses to AAV in canine muscle monitored by cellular assays and noninvasive imaging. Mol. Ther. 2010;18:617–624. doi: 10.1038/mt.2009.294. doi:10.1038/mt.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.England S.B., Nicholson L.V., Johnson M.A., Forrest S.M., Love D.R., Zubrzycka-Gaarn E.E., Bulman D.E., Harris J.B., Davies K.E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. doi:10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 40.Matsumura K., Burghes A.H., Mora M., Tome F.M., Morandi L., Cornello F., Leturcq F., Jeanpierre M., Kaplan J.C., Reinert P., et al. Immunohistochemical analysis of dystrophin-associated proteins in Becker/Duchenne muscular dystrophy with huge in-frame deletions in the NH2-terminal and rod domains of dystrophin. J. Clin. Invest. 1994;93:99–105. doi: 10.1172/JCI116989. doi:10.1172/JCI116989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harper S.Q., Hauser M.A., DelloRusso C., Duan D., Crawford R.W., Phelps S.F., Harper H.A., Robinson A.S., Engelhardt J.F., Brooks S.V., et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. doi:10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 42.Wang B., Li J., Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl Acad. Sci. USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. doi:10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakamoto M., Yuasa K., Yoshimura M., Yokota T., Ikemoto T., Suzuki M., Dickson G., Miyagoe-Suzuki Y., Takeda S. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem. Biophys. Res. Commun. 2002;293:1265–1272. doi: 10.1016/S0006-291X(02)00362-5. doi:10.1016/S0006-291X(02)00362-5. [DOI] [PubMed] [Google Scholar]
- 44.Rafael J.A., Cox G.A., Corrado K., Jung D., Campbell K.P., Chamberlain J.S. Forced expression of dystrophin deletion constructs reveals structure-function correlations. J. Cell Biol. 1996;134:93–102. doi: 10.1083/jcb.134.1.93. doi:10.1083/jcb.134.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregorevic P., Blankinship M.J., Allen J.M., Chamberlain J.S. Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol. Ther. 2008;16:657–664. doi: 10.1038/mt.2008.28. doi:10.1038/mt.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z., Allen J.M., Riddell S.R., Gregorevic P., Storb R., Tapscott S.J., Chamberlain J.S., Kuhr C.S. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum. Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. doi:10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- 47.Yuasa K., Yoshimura M., Urasawa N., Ohshima S., Howell J.M., Nakamura A., Hijikata T., Miyagoe-Suzuki Y., Takeda S. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. doi:10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- 48.Yue Y., Ghosh A., Long C., Bostick B., Smith B.F., Kornegay J.N., Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. doi:10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mingozzi F., Hasbrouck N.C., Basner-Tschakarjan E., Edmonson S.A., Hui D.J., Sabatino D.E., Zhou S., Wright J.F., Jiang H., Pierce G.F., et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. doi:10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingozzi F., High K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. doi:10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 51.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. doi:10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z., Kuhr C.S., Allen J.M., Blankinship M., Gregorevic P., Chamberlain J.S., Tapscott S.J., Storb R. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol. Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 53.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. doi:10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S., Bowles D., Gray S., Li C., Galloway G., et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. doi:10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrer A., Wells K.E., Wells D.J. Immune responses to dystropin: implications for gene therapy of Duchenne muscular dystrophy. Gene Ther. 2000;7:1439–1446. doi: 10.1038/sj.gt.3301259. doi:10.1038/sj.gt.3301259. [DOI] [PubMed] [Google Scholar]
- 56.Brantly M.L., Chulay J.D., Wang L., Mueller C., Humphries M., Spencer L.T., Rouhani F., Conlon T.J., Calcedo R., Betts M.R., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl Acad. Sci. USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. doi:10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuasa K., Sakamoto M., Miyagoe-Suzuki Y., Tanouchi A., Yamamoto H., Li J., Chamberlain J.S., Xiao X., Takeda S. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. doi:10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- 58.Cordier L., Gao G.P., Hack A.A., McNally E.M., Wilson J.M., Chirmule N., Sweeney H.L. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum. Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. doi:10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 59.Hartigan-O'Connor D., Kirk C.J., Crawford R., Mule J.J., Chamberlain J.S. Immune evasion by muscle-specific gene expression in dystrophic muscle. Mol. Ther. 2001;4:525–533. doi: 10.1006/mthe.2001.0496. doi:10.1006/mthe.2001.0496. [DOI] [PubMed] [Google Scholar]
- 60.Salva M.Z., Himeda C.L., Tai P.W., Nishiuchi E., Gregorevic P., Allen J.M., Finn E.E., Nguyen Q.G., Blankinship M.J., Meuse L., et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. doi:10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 61.Wright J.F., Wellman J., High K.A. Manufacturing and regulatory strategies for clinical AAV2-hRPE65. Curr. Gene Ther. 2010;10:341–349. doi: 10.2174/156652310793180715. [DOI] [PubMed] [Google Scholar]
- 62.Foster H., Sharp P.S., Athanasopoulos T., Trollet C., Graham I.R., Foster K., Wells D.J., Dickson G. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol. Ther. 2008;16:1825–1832. doi: 10.1038/mt.2008.186. doi:10.1038/mt.2008.186. [DOI] [PubMed] [Google Scholar]
- 63.Gregorevic P., Schultz B.R., Allen J.M., Halldorson J.B., Blankinship M.J., Meznarich N.A., Kuhr C.S., Doremus C., Finn E., Liggitt D., et al. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol. Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. doi:10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodino-Klapac L.R., Montgomery C.L., Bremer W.G., Shontz K.M., Malik V., Davis N., Sprinkle S., Campbell K.J., Sahenk Z., Clark K.R., et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol. Ther. 2010;18:109–117. doi: 10.1038/mt.2009.254. doi:10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bridges C.R., Gopal K., Holt D.E., Yarnall C., Cole S., Anderson R.B., Yin X., Nelson A., Kozyak B.W., Wang Z., et al. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J. Thorac. Cardiovasc. Surg. 2005;130:1364. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 66.Su L.T., Gopal K., Wang Z., Yin X., Nelson A., Kozyak B.W., Burkman J.M., Mitchell M.A., Low D.W., Bridges C.R., et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. doi:10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- 67.Cho W.K., Ebihara S., Nalbantoglu J., Gilbert R., Massie B., Holland P., Karpati G., Petrof B.J. Modulation of Starling forces and muscle fiber maturity permits adenovirus-mediated gene transfer to adult dystrophic (mdx) mice by the intravascular route. Hum. Gene Ther. 2000;11:701–714. doi: 10.1089/10430340050015608. doi:10.1089/10430340050015608. [DOI] [PubMed] [Google Scholar]
- 68.Budker V., Zhang G., Danko I., Williams P., Wolff J. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther. 1998;5:272–276. doi: 10.1038/sj.gt.3300572. doi:10.1038/sj.gt.3300572. [DOI] [PubMed] [Google Scholar]
- 69.Greelish J.P., Su L.T., Lankford E.B., Burkman J.M., Chen H., Konig S.K., Mercier I.M., Desjardins P.R., Mitchell M.A., Zheng X.G., et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat. Med. 1999;5:439–443. doi: 10.1038/7439. doi:10.1038/7439. [DOI] [PubMed] [Google Scholar]
- 70.Odom G.L., Gregorevic P., Allen J.M., Finn E., Chamberlain J.S. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol. Ther. 2008;16:1539–1545. doi: 10.1038/mt.2008.149. doi:10.1038/mt.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monaco A.P., Bertelson C.J., Liechti-Gallati S., Moser H., Kunkel L.M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. doi:10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 72.Sherratt T.G., Vulliamy T., Dubowitz V., Sewry C.A., Strong P.N. Exon skipping and translation in patients with frameshift deletions in the dystrophin gene. Am. J. Hum. Genet. 1993;53:1007–1015. [PMC free article] [PubMed] [Google Scholar]
- 73.Dunckley M.G., Manoharan M., Villiet P., Eperon I.C., Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum. Mol. Genet. 1998;7:1083–1090. doi: 10.1093/hmg/7.7.1083. doi:10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- 74.Mann C.J., Honeyman K., Cheng A.J., Ly T., Lloyd F., Fletcher S., Morgan J.E., Partridge T.A., Wilton S.D. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc. Natl Acad. Sci. USA. 2001;98:42–47. doi: 10.1073/pnas.011408598. doi:10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Q.L., Mann C.J., Lou F., Bou-Gharios G., Morris G.E., Xue S.A., Fletcher S., Partridge T.A., Wilton S.D. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat. Med. 2003;9:1009–1014. doi: 10.1038/nm897. doi:10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- 76.Pramono Z.A., Takeshima Y., Alimsardjono H., Ishii A., Takeda S., Matsuo M. Induction of exon skipping of the dystrophin transcript in lymphoblastoid cells by transfecting an antisense oligodeoxynucleotide complementary to an exon recognition sequence. Biochem. Biophys. Res. Commun. 1996;226:445–449. doi: 10.1006/bbrc.1996.1375. doi:10.1006/bbrc.1996.1375. [DOI] [PubMed] [Google Scholar]
- 77.Fletcher S., Honeyman K., Fall A.M., Harding P.L., Johnsen R.D., Wilton S.D. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J. Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. doi:10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- 78.Fletcher S., Honeyman K., Fall A.M., Harding P.L., Johnsen R.D., Steinhaus J.P., Moulton H.M., Iversen P.L., Wilton S.D. Morpholino oligomer-mediated exon skipping averts the onset of dystrophic pathology in the mdx mouse. Mol. Ther. 2007;15:1587–1592. doi: 10.1038/sj.mt.6300245. doi:10.1038/sj.mt.6300245. [DOI] [PubMed] [Google Scholar]
- 79.Ivanova G.D., Arzumanov A., Abes R., Yin H., Wood M.J., Lebleu B., Gait M.J. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008;36:6418–6428. doi: 10.1093/nar/gkn671. doi:10.1093/nar/gkn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin H., Lu Q., Wood M. Effective exon skipping and restoration of dystrophin expression by peptide nucleic acid antisense oligonucleotides in mdx mice. Mol. Ther. 2008;16:38–45. doi: 10.1038/sj.mt.6300329. doi:10.1038/sj.mt.6300329. [DOI] [PubMed] [Google Scholar]
- 81.Yokota T., Lu Q.L., Partridge T., Kobayashi M., Nakamura A., Takeda S., Hoffman E. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann. Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. doi:10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Deutekom J.C., Janson A.A., Ginjaar I.B., Frankhuizen W.S., Aartsma-Rus A., Bremmer-Bout M., den Dunnen J.T., Koop K., van der Kooi A.J., Goemans N.M., et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. doi:10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 83.Gebski B.L., Mann C.J., Fletcher S., Wilton S.D. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum. Mol. Genet. 2003;12:1801–1811. doi: 10.1093/hmg/ddg196. doi:10.1093/hmg/ddg196. [DOI] [PubMed] [Google Scholar]
- 84.Arechavala-Gomeza V., Graham I.R., Popplewell L.J., Adams A.M., Aartsma-Rus A., Kinali M., Morgan J.E., van Deutekom J.C., Wilton S.D., Dickson G., et al. Comparative analysis of antisense oligonucleotide sequences for targeted skipping of exon 51 during dystrophin pre-mRNA splicing in human muscle. Hum. Gene Ther. 2007;18:798–810. doi: 10.1089/hum.2006.061. doi:10.1089/hum.2006.061. [DOI] [PubMed] [Google Scholar]
- 85.Kinali M., Arechavala-Gomeza V., Feng L., Cirak S., Hunt D., Adkin C., Guglieri M., Ashton E., Abbs S., Nihoyannopoulos P., et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. doi:10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goemans N.M., Buyse G., Tulinius M., Verschuuren J.J.G., de Kimpe S.J., van Deutekom J.C.T. T.O.4 A phase I/IIa study on antisense compound PRO051 in patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 2009;19:659–660. doi:10.1016/j.nmd.2009.06.359. [Google Scholar]
- 87.Goemans N., Tulinius M., Buyse G., Wilson R., de Kimpe S., van Deutekom J., Campion G. O.15 24 week follow-up data from a phase I/IIa extension study of PRO051/GSK2402968 in subjects with Duchenne muscular dystrophy. Neuromuscul. Disord. 2010;20:639. doi:10.1016/j.nmd.2010.07.139. [Google Scholar]
- 88.Shrewsbury S.B., Cirak S., Guglieri M., Bushby K., Muntoni F. O.16 Current progress and preliminary results with the systemic administration trial of AVI-4658, a novel phosphorodiamidate morpholino oligomer (PMO) skipping dystrophin exon 51 in Duchenne muscular dystrophy (DMD) Neuromuscul. Disord. 2010;20:639–640. doi:10.1016/j.nmd.2010.07.140. [Google Scholar]
- 89.Takeshima Y., Nishio H., Sakamoto H., Nakamura H., Matsuo M. Modulation of in vitro splicing of the upstream intron by modifying an intra-exon sequence which is deleted from the dystrophin gene in dystrophin Kobe. J. Clin. Invest. 1995;95:515–520. doi: 10.1172/JCI117693. doi:10.1172/JCI117693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alter J., Lou F., Rabinowitz A., Yin H., Rosenfeld J., Wilton S.D., Partridge T.A., Lu Q.L. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat. Med. 2006;12:175–177. doi: 10.1038/nm1345. doi:10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 91.Lu Q.L., Rabinowitz A., Chen Y.C., Yokota T., Yin H., Alter J., Jadoon A., Bou-Gharios G., Partridge T. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc. Natl Acad. Sci. USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. doi:10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin H., Moulton H.M., Seow Y., Boyd C., Boutilier J., Iverson P., Wood M.J. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum. Mol. Genet. 2008;17:3909–3918. doi: 10.1093/hmg/ddn293. doi:10.1093/hmg/ddn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jearawiriyapaisarn N., Moulton H.M., Sazani P., Kole R., Willis M.S. Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc. Res. 2010;85:444–453. doi: 10.1093/cvr/cvp335. doi:10.1093/cvr/cvp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu B., Moulton H.M., Iversen P.L., Jiang J., Li J., Li J., Spurney C.F., Sali A., Guerron A.D., Nagaraju K., et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc. Natl Acad. Sci. USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. doi:10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu B., Lu P., Benrashid E., Malik S., Ashar J., Doran T.J., Lu Q.L. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 2010;17:132–140. doi: 10.1038/gt.2009.120. doi:10.1038/gt.2009.120. [DOI] [PubMed] [Google Scholar]
- 96.Moulton H.M., Moulton J.D. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta. 2010;12:2296–2303. doi: 10.1016/j.bbamem.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Aartsma-Rus A., Fokkema I., Verschuuren J., Ginjaar I., van Deutekom J., van Ommen G.J., den Dunnen J.T. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. doi:10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 98.Goyenvalle A., Vulin A., Fougerousse F., Leturcq F., Kaplan J.C., Garcia L., Danos O. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. doi:10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- 99.Denti M.A., Rosa A., D'Antona G., Sthandier O., De Angelis F.G., Nicoletti C., Allocca M., Pansarasa O., Parente V., Musaro A., et al. Body-wide gene therapy of Duchenne muscular dystrophy in the mdx mouse model. Proc. Natl Acad. Sci. USA. 2006;103:3758–3763. doi: 10.1073/pnas.0508917103. doi:10.1073/pnas.0508917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Angelis F.G., Sthandier O., Berarducci B., Toso S., Galluzzi G., Ricci E., Cossu G., Bozzoni I. Chimeric snRNA molecules carrying antisense sequences against the splice junctions of exon 51 of the dystrophin pre-mRNA induce exon skipping and restoration of a dystrophin synthesis in Delta 48–50 DMD cells. Proc. Natl Acad. Sci. USA. 2002;99:9456–9461. doi: 10.1073/pnas.142302299. doi:10.1073/pnas.142302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grimm C., Stefanovic B., Schumperli D. The low abundance of U7 snRNA is partly determined by its Sm binding site. EMBO J. 1993;12:1229–1238. doi: 10.1002/j.1460-2075.1993.tb05764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benchaouir R., Meregalli M., Farini A., D'Antona G., Belicchi M., Goyenvalle A., Battistelli M., Bresolin N., Bottinelli R., Garcia L., et al. Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell. 2007;1:646–657. doi: 10.1016/j.stem.2007.09.016. doi:10.1016/j.stem.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 103.Tinsley J.M., Potter A.C., Phelps S.R., Fisher R., Trickett J.I., Davies K.E. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. doi:10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- 104.Gilbert R., Nalbantoglu J., Petrof B.J., Ebihara S., Guibinga G.H., Tinsley J.M., Kamen A., Massie B., Davies K.E., Karpati G. Adenovirus-mediated utrophin gene transfer mitigates the dystrophic phenotype of mdx mouse muscles. Hum. Gene Ther. 1999;10:1299–1310. doi: 10.1089/10430349950017987. doi:10.1089/10430349950017987. [DOI] [PubMed] [Google Scholar]
- 105.Miura P., Jasmin B.J. Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: how close are we? Trends Mol. Med. 2006;12:122–129. doi: 10.1016/j.molmed.2006.01.002. doi:10.1016/j.molmed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Fisher R., Tinsley J.M., Phelps S.R., Squire S.E., Townsend E.R., Martin J.E., Davies K.E. Non-toxic ubiquitous over-expression of utrophin in the mdx mouse. Neuromuscul. Disord. 2001;11:713–721. doi: 10.1016/s0960-8966(01)00220-6. doi:10.1016/S0960-8966(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 107.Gauthier-Rouviere C., Bonet-Kerrache A. RhoA leads to up-regulation and relocalization of utrophin in muscle fibers. Biochem. Biophys. Res. Commun. 2009;384:322–328. doi: 10.1016/j.bbrc.2009.04.127. doi:10.1016/j.bbrc.2009.04.127. [DOI] [PubMed] [Google Scholar]
- 108.Khurana T.S., Davies K.E. Pharmacological strategies for muscular dystrophy. Nat. Rev. Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. doi:10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- 109.Courdier-Fruh I., Briguet A. Utrophin is a calpain substrate in muscle cells. Muscle Nerve. 2006;33:753–759. doi: 10.1002/mus.20549. doi:10.1002/mus.20549. [DOI] [PubMed] [Google Scholar]
- 110.Burkin D.J., Wallace G.Q., Nicol K.J., Kaufman D.J., Kaufman S.J. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J. Cell Biol. 2001;152:1207–1218. doi: 10.1083/jcb.152.6.1207. doi:10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rooney J.E., Gurpur P.B., Burkin D.J. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA. 2009;106:7991–7996. doi: 10.1073/pnas.0811599106. doi:10.1073/pnas.0811599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kronqvist P., Kawaguchi N., Albrechtsen R., Xu X., Schroder H.D., Moghadaszadeh B., Nielsen F.C., Frohlich C., Engvall E., Wewer U.M. ADAM12 alleviates the skeletal muscle pathology in mdx dystrophic mice. Am. J. Pathol. 2002;161:1535–1540. doi: 10.1016/S0002-9440(10)64431-8. doi:10.1016/S0002-9440(10)64431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amenta A.R., Yilmaz A., Bogdanovich S., McKechnie B.A., Abedi M., Khurana T.S., Fallon J.R. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc. Natl Acad. Sci. USA. 2011;108:762–767. doi: 10.1073/pnas.1013067108. doi:10.1073/pnas.1013067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miura P., Andrews M., Holcik M., Jasmin B.J. IRES-mediated translation of utrophin A is enhanced by glucocorticoid treatment in skeletal muscle cells. PLoS ONE. 2008;3:e2309. doi: 10.1371/journal.pone.0002309. doi:10.1371/journal.pone.0002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu Y., Tian C., Danialou G., Gilbert R., Petrof B.J., Karpati G., Nalbantoglu J. Targeting artificial transcription factors to the utrophin A promoter: effects on dystrophic pathology and muscle function. J. Biol. Chem. 2008;283:34720–34727. doi: 10.1074/jbc.M804518200. doi:10.1074/jbc.M804518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sonnemann K.J., Heun-Johnson H., Turner A.J., Baltgalvis K.A., Lowe D.A., Ervasti J.M. Functional substitution by TAT-utrophin in dystrophin-deficient mice. PLoS Med. 2009;6:e1000083. doi: 10.1371/journal.pmed.1000083. doi:10.1371/journal.pmed.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Welch E.M., Barton E.R., Zhuo J., Tomizawa Y., Friesen W.J., Trifillis P., Paushkin S., Patel M., Trotta C.R., Hwang S., et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. doi:10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 118.Barton-Davis E.R., Cordier L., Shoturma D.I., Leland S.E., Sweeney H.L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest. 1999;104:375–381. doi: 10.1172/JCI7866. doi:10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malik V., Rodino-Klapac L.R., Viollet L., Wall C., King W., Al-Dahhak R., Lewis S., Shilling C.J., Kota J., Serrano-Munuera C., et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 2010;67:771–780. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- 120.Finkel R.S. Read-through strategies for suppression of nonsense mutations in Duchenne/Becker muscular dystrophy: aminoglycosides and ataluren (PTC124) J. Child Neurol. 2010;25:1158–1164. doi: 10.1177/0883073810371129. doi:10.1177/0883073810371129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monaco A.P., Neve R.L., Colletti-Feener C., Bertelson C.J., Kurnit D.M., Kunkel L.M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. doi:10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 122.Chung W., Campanelli J.T. WW and EF hand domains of dystrophin-family proteins mediate dystroglycan binding. Mol. Cell Biol. Res. Commun. 1999;2:162–171. doi: 10.1006/mcbr.1999.0168. doi:10.1006/mcbr.1999.0168. [DOI] [PubMed] [Google Scholar]
- 123.Yang B., Jung D., Rafael J.A., Chamberlain J.S., Campbell K.P. Identification of alpha-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J. Biol. Chem. 1995;270:4975–4978. doi: 10.1074/jbc.270.10.4975. doi:10.1074/jbc.270.10.4975. [DOI] [PubMed] [Google Scholar]
- 124.Phelps S.F., Hauser M.A., Cole N.M., Rafael J.A., Hinkle R.T., Faulkner J.A., Chamberlain J.S. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum. Mol. Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. doi:10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- 125.Banks G.B., Judge L.M., Allen J.M., Chamberlain J.S. The polyproline site in hinge 2 influences the functional capacity of truncated dystrophins. PLoS Genet. 2010;6:e1000958. doi: 10.1371/journal.pgen.1000958. doi:10.1371/journal.pgen.1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beroud C., Tuffery-Giraud S., Matsuo M., Hamroun D., Humbertclaude V., Monnier N., Moizard M.P., Voelckel M.A., Calemard L.M., Boisseau P., et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum. Mutat. 2007;28:196–202. doi: 10.1002/humu.20428. doi:10.1002/humu.20428. [DOI] [PubMed] [Google Scholar]