Abstract
Alpha-1 antitrypsin (AAT) deficiency is a common single-gene disorder among Northern Europeans and North Americans. The carrier frequency for the common missense mutation (Z-AAT) ranges from 4% in the US to nearly 25% in the Republic of Ireland. Severe AAT deficiency (plasma levels below 11 μm) is most commonly associated with an adult-onset lung disease, with pan-acinar emphysema and airway inflammation, which is thought to be primarily owing to the loss of function of AAT in neutralizing neutrophil elastase and other pro-inflammatory enzymes. In 5–10% of patients, severe liver disease may develop. This may occur at any time from infancy to adulthood, and is thought to be owing to toxicity from the Z-AAT mutant protein that folds poorly and forms insoluble polymers within the hepatocyte, which is the primary site for AAT production. Thus, gene therapy for AAT lung disease is conceived of as augmentation of serum levels (a prolonged form of protein replacement, which is currently in use), while gene therapy for liver disease presents the problem of also having to downregulate the production of Z-AAT protein. Over the years, numerous strategies have been employed for the gene therapy of both AAT-deficient lung disease and liver disease. These will be reviewed with an emphasis on modalities that have reached clinical trials recently.
NORMAL FUNCTIONS OF AAT
AAT is the most abundant circulating serum antiprotease, with a steady-state concentration in normal individuals of 15–30μm, and a threshold level for susceptibility to lung disease at 11 μm (570 μg/ml). AAT is structurally homologous to other serine proteinase inhibitors (serpins), although it has a broad spectrum of inhibitory activity for pro-inflammatory enzymes and mediators. These include neutrophil elastase (NE), proteinase-3, a number of cathespins, caspase 3 and alpha defensins. The capacity for the inhibition of trypsin, for which it was classically named, is probably not of physiological importance.
Under normal circumstances, AAT is produced primarily within the hepatocytes and secreted directly into the serum. Other sites of production include monocytes, macrophages and neutrophils. AAT secreted from the liver circulates at high levels (as mentioned above) throughout the serum and interstitial fluid. In the lungs, AAT protects the alveolar interstitial elastin from degradation by the NE. Neutrophils are abundant in pulmonary capillaries (the main location of the so-called ‘marginating pool') and as their lifespan outside the bone marrow is less than 2 days, the alveolar capillaries are a site of ongoing neutrophil death and release of NE. Thus, a steady production of AAT is necessary in order to maintain an appropriate protease–antiprotease balance.
Intermittently, lung infections or other noxious insults may recruit additional neutrophils to the alveolar spaces and airways in the lungs, and the ability of the AAT to localize the effects of NE and related neutrophil products to the site of maximal host response. Of note, AAT's reactive loop is inactivated by the oxidation of a key methionine residue, so that dense accumulations of neutrophils at the site of serious infection can, via their oxidative burst, inactivate AAT and allow for the unabated action of NE against the attacking pathogens. As the acute infection resolves, however, AAT protects the surrounding interstitial elastin from destruction and restores the protease–antiprotease balance, with the antiprotease activity always remaining in excess.
AAT DEFICIENCY
Mutation of the AAT gene is very common in Northern Europeans and North Americans, with mutation rates nearing 25% in the Republic of Ireland. In the USA, estimates of mutation rates range from 3 to 4%. The vast majority of mutant alleles are comprised of one specific missense mutation (Glu342Lys, known as PI*Z) accounting for over 90% of disease-causing alleles, and the second most common (Glu264Val, known as PI*S) accounting for much of the remaining disease, particularly when in the compound heterozygous state with PI*Z. A number of other missense and null alleles have been identified, but these are collectively rare.
The pathobiology of the Z-AAT mutation has been carefully characterized. The Glu to Lys amino acid substitution disrupts an important salt bridge in a beta-pleated sheet region of the protein, allowing the reactive loop from one AAT molecule to insert itself into the beta sheet of an adjacent AAT molecule (1,2). The ‘loop-sheet' polymer form of AAT has been crystallized and its structure fully defined (3).
It is clear that the Z-AAT protein polymers are not secreted efficiently from the hepatocytes, resulting in the intracellular accumulation of AAT in hepatocytes and low circulating serum levels (<11 μm). Patients with AAT deficiency frequently develop lung disease, which is primary thought to be owing to the deficient serum levels, leading to an inadequate protease protection for the lung. This has been confirmed by bronchoalveolar lavage studies on deficient patients, which show zero levels of anti-NE capacity and some baseline active NE in many cases. The lack of AAT in the lung thus exposes the alveolar interstitial elastin to gradual degradation by NE, and leads to emphysema, which is histologically termed as ‘pan-acinar' or ‘pan-lobular', uniformly affecting a pulmonary lobule, in contrast with emphysema due solely to exposure to cigarette smoke, which is classically ‘centri-lobular'. Because AAT also is normally inactivating a number of other pro-inflammatory molecules in the airways and alveoli, AAT-deficient patients also demonstrate airway inflammation, wheezing and an asthma-like clinical picture. The average age of diagnosis is 52 years, but most patients retrospectively report up to 10 years of symptoms preceding their diagnosis.
Tobacco smoking is an important co-factor in the development of AAT lung disease. This is most likely owing to the fact that cigarette smoke can lead to oxidation of the reactive site methionine, as described above. The fact that cigarette smoking is often a coincident historical finding has probably contributed to the rather remarkable ‘under-diagnosis' of this problem. Of the 30 000 estimated PI*ZZ homozygous individuals in this country, only 6000 have been diagnosed. It seems likely that chronic lung disease in a patient with a history of cigarette smoking is often assumed to be purely environmental even when AAT deficiency is present.
There is also some evidence that AAT lung disease could be due, in part, to the presence of Z-AAT polymers within alveolar macrophages and in the extracellular space. Such polymers are themselves pro-inflammatory and could lead to propagation of the inflammatory cascade and further skewing the protease–antiprotease imbalance. Finally, AAT has been shown to be directly anti-apoptotic in the pulmonary endothelial cells owing to the inhibition of caspase-3 in those cells. Furthermore, loss of endothelial maintenance by inhibition of the VEGF-receptor leads to a form of experimental emphysema that can be cured by AAT augmentation. Taken together, the combined effects of AAT deficiency and Z-AAT polymerization lead to loss of both alveolar interstitium and alveolar capillary endothelial cells, as well as an active cycle of airway inflammation that causes progressive loss of lung function in most AAT-deficient patients over time.
AAT LIVER DISEASE
The spectrum of liver disease in AAT deficiency is quite broad. In an early report by Sharp et al. (4), infants and children with AAT deficiency were recognized to be at risk for cirrhosis. Subsequently, Sveger undertook screening of 200 000 newborns with AAT deficiency and determined that a much higher proportion of AAT-deficient children suffered mild or subclinical cases of either jaundice or elevated liver enzymes (5). While the underlying mechanisms of liver disease are not fully understood, it is generally accepted that it is owing to the downstream consequences of the accumulation of Z-AAT within the hepatocytes. Both the unfolded protein response and endoplasmic reticulum overload caused by the distension of accumulating Z-AAT have been described within the hepatocytes of these patients, which ultimately have been associated with pro-inflammatory signaling (6,7). In addition, the usually present Z-AAT aggresomes of insoluble Z-AAT polymers have also been linked to an increase in hepatocyte autophagy (8). Although many of these phenomena may be present in most of the Z-homozygote patients, only about a 10% go on to develop a full-blown liver disease. This would suggest that the progression of the liver disease could be dependent on or accelerated by environmental factors such as alcohol exposure, viral infections or the presence of modifier gene. However, current association studies have not linked a modifier gene to the liver disease, but there is some evidence that viral infection precipitates the progression of established hepatitis.
GENE THERAPY FOR AAT DEFICIENCY
Currently, the only Food and Drug Administration (FDA)-approved therapy for AAT deficiency is protein replacement by weekly intravenous infusions. Several products exist in the market for this purpose and they have been shown to be very safe and effective in restoring AAT serum levels to the therapeutic threshold of 11 μm as set by the FDA. While there have been few properly powered prospective studies to demonstrate the therapeutic benefits on lung disease resulting from such therapies, there is evidence from the retrospective analysis of the AAT foundation's registry that patients on protein replacement trended towards a decrease in mortality. While this approach may be effective, the nuisance of weekly infusions for life, along with the high cost of this therapy permits a reasonable opportunity for a gene augmentation approach with a gene therapy vector. The FDA-set therapeutic threshold of 11 μm is a convenient endpoint for a gene therapy intervention because if plasma levels meet or cross this level, the gene therapy product would be considered therapeutic. Another attractive need for gene therapy in AAT deficiency is the idea that the use of gene augmentation could offer the advantage of a single administration; this would considerably decrease the burden of the costly weekly protein infusions and would be of great benefit to the patients.
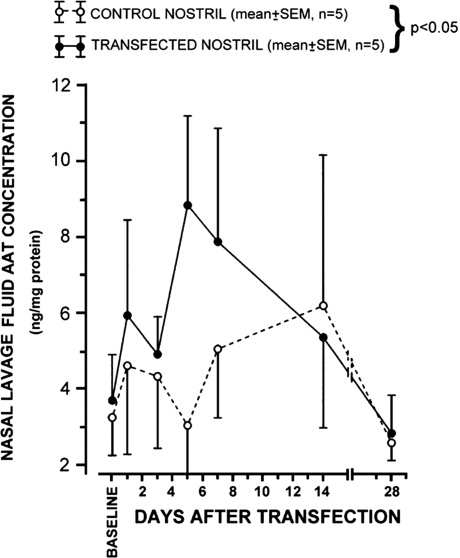
Over the last decade, there have been three completed and one ongoing gene therapy clinical trials in which the normal (PiM) AAT (M-AAT) gene has been delivered to AAT-deficient patients. Three of these trials used recombinant adeno-associated viral vectors with the aim of achieving therapeutic systemic AAT correction after intramuscular delivery. However, the first gene therapy clinical trial to be performed on this patient population used non-viral vectors to deliver AAT locally. This trail consisted of instilling an unmodified cationic liposome (DOTMA:DOPE) complexed with a plasmid (lipoplex) encoding the normal AAT gene driven by a cytomegalovirus (CMV) promoter into one of the nostrils of the human subject (9). Five patients homozygous for the PiZ mutation were enrolled and each was instilled with the lipoplex in one nostril thus leaving the contralateral nostril to serve as a control. Lavage samples for each nostril demonstrated a rise in AAT levels on the treated nostril, peaking at day 5 and returning to basal levels by day 14 (Fig. 1). During the peak of expression, AAT levels where one-third of normal (9). This trial served as a proof-of-concept for the use of local non-viral gene delivery of AAT; however, there is a wide gap in the translation of this concept as delivery of the whole lung would necessitate frequent re-administrations owing to the transient nature of the expression and would be hampered by the toxicity of cationic liposomes.
Figure 1.
Concentrations of transgene-derived normal AAT protein in nasal lavage fluid from subjects with PiZZ AAT deficiency. Time-course of responses to intranasal lipoplex delivery of a normal AAT gene with the untreated contralateral nostril serving as control. Data are normalized to total protein concentration. Reprinted with permission from Brigham et al. (9).
As mentioned above, all the remaining clinical trials have focused on the intramuscular delivery of recombinant adeno associated virus (rAAV). The idea is simply to restore plasma levels at or above 11 μm, and since the site or cell type that is producing the AAT is not relevant, the muscle was chosen for its easy accessibility and the long-lived non-dividing nature of its cells. In theory, this would allow for a minimally invasive delivery of vector with a sustained expression of AAT. This idea is based on numerous animal studies that showed robust long-term AAT detection in serum in some cases for up to a year after a single rAAV intramuscular injection (10).
The first of these Phase I trials was completed in 2006 using the original rAAV2 vectors expressing the AAT gene driven by a hybrid chicken beta-actin promoter with a CMV enhancer (CB) (11). Twelve patients were enrolled into four dose cohorts with a range of 2.1 × 1012 to 6.9 × 1013 vector genomes (vg) per patient. Injections were performed in a sequential dose-escalating manner after a 28-day wash-out period for those patients on protein replacement (11). While vgs were detected in the blood of patients 1–3 days post-administration in most patients, and there was an eventual rise in the anti-AAV2 antibodies, further serum analysis failed to show any sustained expression of PiM AAT (M-AAT). An important lesson from this initial Phase I trial was that the study design which relied on a 28-day wash-out period was not optimal as residual M-AAT levels from protein replacement were still present and could possibly be masking any rAAV-derived M-AAT expression.
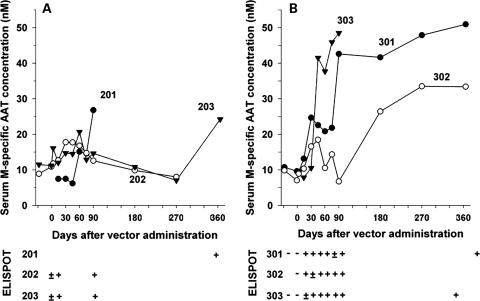
With the advent of new serotypes and the feasibility of cross-packaging AAV2 genomes into different AAV capsids to create pseudotypes, rAAV vectors with expanded tropisms and greatly increased efficiency of gene transfer to certain tissues were discovered. At the time of the first AAV2-AAT trial, numerous studies had already demonstrated the superior expression achieved with rAAV1-pseudotyped vectors after intramuscular injection in mice (12–14). It was on the basis of these findings that the next clinical trial focused on rAAV1 muscle-directed gene transfer. This second Phase I trial was sponsored by Applied Genetic Technologies Corporation and enrolled nine AAT-deficient subjects into cohorts of three patients each with a dose escalation of 6.9 × 1012, 2.2 × 1013 and 6.0 × 1013vgs per patient (15). The deltoid muscle of either arm was instilled with a constant 9.9 ml volume regardless of the vector dose containing the same expression cassette used in the first trial but pseudotyped into an AAV1 capsid. Aside from the change in vector capsid and the increase in dose range, this second trial also amended the clinical protocol to include a 56-day wash-out period for patients on protein replacement for cohorts 2 and 3. The injections were well-tolerated with only minor bruising and swelling in some instances and there was only one serious adverse event reported (bacterial epididymitis), which was deemed unrelated to vector administration. In contrast to the first trial, M-AAT levels for cohorts 2 and 3 were all detected above the baseline (Fig. 2). The increase was dose-dependent, and in cohort 3 it was sustained for at least 1 year in those patients who were followed for that time period. The three patients in the high-dose cohort all achieved M-AAT levels between 30 and 50 nm, which is 200-fold lower than the 11 μm target. As seen in the first trial, all patients in this study also developed neutralizing antibodies against AAV capsid, however this trial also included a more in-depth analysis of T-cell response, which demonstrated a concomitant interferon gamma-positive ELISPOTs against a peptide library for the AAV1 capsid (Fig. 1). Further evaluation of capsid-specific response by flow cytometry with peripheral blood mononuclear cells from two of the patients in the high-dose cohort revealed both functional CD4+ and CD8+ T-cell specific for AAV1 capsid epitopes. Interestingly, in this trial, despite the evidence for positive functional CD8+ T-cells against AAV1 capsid, in the two high-dose patients who were followed for a year, M-AAT levels were sustained.
Figure 2.
Time-course of vector-mediated AAT expression and enzyme-linked immunosorbent spot (ELISPOT) responses to AAV1 capsid peptides in patients receiving (A) 2.2 × 1013 or (B) 6.0 × 1013 vgs intramuscularly of a recombinant AAV1 vector expressing the M-AAT gene. Serum M-specific AAT levels are plotted at the top of each panel and interferon-gamma ELISPOT responses to an AAV1 capsid peptide library are plotted at the bottom of each panel. M-AAT levels past day 90 for subjects 201 and 303 are not shown as they resumed protein replacement at this time-point. The day 365 sample for subject 202 could not be tested because of severe hemolysis. ELISPOT responses are characterized as – (negative in both ex vivo and cultured assays),±(positive in cultured assay but negative in ex vivo assay) or + (positive in both ex vivo and cultured assays). Samples for ELISPOT analysis were not available beyond day 90 for subjects 202, 203 and 302. Reprinted with permission from Brantly et al. (15).
Based on this, a new Phase I/II trial with rAAV1-AAT vector was initiated, which include a top dose 7-fold higher over the high dose in the first rAAV1-AAT trial. This trial also differed in the packaging method used for vector production as it relied on HSV1-helper system as opposed to the traditionally used triple transfection (16). This change in production had two effects—first, it facilitated the production of large amounts of recombinant vector needed for the study and second, HSV-produced vector had increased potency as determined by transduction when compared with equally titered transfection-made vector (16). The trial design was similar to the previous trial with three dose cohorts receiving intramuscular injections at doses ranging form 6 × 1011, 6.0 × 1012 and 4.2 × 1014vgs per a 70 kg subject. Unlike the previous trial and due to the need for higher doses, the number of intramuscular injections administered in each dose group was different. All injections consisted of a suspension of 1.35 ml, in the first dose cohort this was delivered by 10 intramuscular injections, increasing to 32 and 100 individual intramuscular injections administered in a single day for cohorts 2 and 3, respectively. In addition to monitoring the immune response through ELISPOT and flow cytometry, this trial also included the addition of muscle biopsies at day 90 to evaluate inflammation and M-AAT secretion at the site of injection. To date, all patients have been dosed and no adverse events have been reported; the trial is ongoing and the results for serum M-AAT levels should be forthcoming.
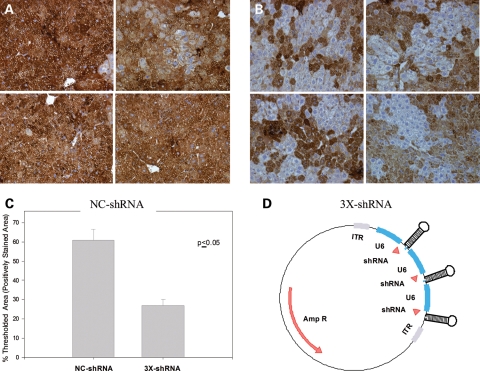
Currently, all the clinical gene therapy trials for AAT deficiency have focused on restoring M-AAT protein to ultimately address lung disease. However, as mentioned above, the PiZ homozygote patients will accumulate misfolded AAT polymers in the hepatocytes. These aggregates have been linked to liver disease ranging from mild jaundice in infants to hepatocellular carcinoma and fulminate liver failure. Several gene-therapy strategies have been employed throughout the years to reduce the accumulations of misfolded PIZ. Some of the earlier attempts to target PiZ mRNA were performed using ribozymes specific for PiZ mRNA (17–19). Since then and with the transformational discovery of RNAi, strategies involving the use of short hairpin RNA (shRNA) for the knockdown of Z-AAT have been used (20–22). Preclinical studies using shRNA for the knockdown of PiZ protein show a reduction in the serum Z-AAT levels in transgenic mice and significant clearance of Z-AAT protein from the hepatocytes (Fig. 3) (20). Despite the fact that no liver toxicity was observed in these studies, the use of shRNA has been shown to have toxicity both in the liver and in the brain in two other studies (21,23). Other more promising approaches rely on the use of polymerase II-driven miRNAs instead of polymerase III-driven shRNAs to decrease the toxicity associated with the interference RNA (23,24). In fact, the use of pol II-driven miRNAs against the Z-AAT mRNA will allow the simultaneous expression of M-AAT cDNA, which should address both liver and lung disease. While these recent breakthroughs are promising and have been shown to work in mice, preclinical toxicology studies are needed to understand the effects of long-term miRNA expression.
Figure 3.
Liver human alpha-1 antitrypsin (hAAT) histology results for PiZ-transgenic mice transduced with AAV8-NC-shRNA or AAV8-3X-shRNA, 14 days post-rAAV8 delivery. Mice transgenic for the human Z-AAT gene were dosed via the portal vein with rAAV8 vectors expressing either three shRNAs directed against the AAT gene (3X-shRNA) or a scrambled negative control (NC-shRNA). (A) Representative hAAT-stained liver sections for mice injected with 1 × 1011 vector genomes of rAAV8-NC-shRNA. (B) Representative hAAT-stained liver sections for mice injected with 1 × 1011 vector genomes of rAAV8-3X-shRNA. (C) Quantification of hAAT-stained areas were performed using MetaMorph Software, sections for each rAAV8-shRNA-transduced livers were analyzed. Thresholded area represents the area on the section that is within the parameters considered to be positively stained. (D) Schematic of the proviral plasmid construct containing three U6 promoter-driven shRNAs, used to make the rAAV8 vectors. Results are expressed as the mean thresholded area for four different livers for each group ± S.E.M. Reprinted with permission from Cruz et al. (20).
Conflict of Interest statement. None declared.
FUNDING
This work was supported by grants from NHLBI (HL69877) the Cystic Fibrosis Foundation, the Alpha-1 Antitrypsin Foundation, a fellowship from the Parker B. Francis Foundation and the Diabetes and Endocrinology Research Center of the University of Massachusetts Medical School (supported by Grant P30 DK32520).
REFERENCES
- 1.Song H.K., Lee K.N., Kwon K.S., Yu M.H., Suh S.W. Crystal structure of an uncleaved alpha 1-antitrypsin reveals the conformation of its inhibitory reactive loop. FEBS Lett. 1995;377:150–154. doi: 10.1016/0014-5793(95)01331-8. [DOI] [PubMed] [Google Scholar]
- 2.Lomas D.A. Loop-sheet polymerization: the structural basis of Z alpha 1- antitrypsin accumulation in the liver. Clin. Sci. (Colch) 1994;86:489–495. doi: 10.1042/cs0860489. [DOI] [PubMed] [Google Scholar]
- 3.Huntington J.A., Pannu N.S., Hazes B., Read R.J., Lomas D.A., Carrell R.W. A 2.6 Ǻ structure of a serpin polymer and implications for conformational disease. J. Mol. Biol. 1999;293:449–455. doi: 10.1006/jmbi.1999.3184. [DOI] [PubMed] [Google Scholar]
- 4.Sharp H.L., Bridges R.A., Krivit W., Freier E.F. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J. Lab. Clin. Med. 1969;73:934–939. [PubMed] [Google Scholar]
- 5.Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N. Engl. J. Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- 6.Lawless M.W., Greene C.M., Mulgrew A., Taggart C.C., O'Neill S.J., McElvaney N.G. Activation of endoplasmic reticulum-specific stress responses associated with the conformational disease Z alpha 1-antitrypsin deficiency. J. Immunol. 2004;172:5722–5726. doi: 10.4049/jimmunol.172.9.5722. [DOI] [PubMed] [Google Scholar]
- 7.Hidvegi T., Schmidt B.Z., Hale P., Perlmutter D.H. Accumulation of mutant alpha1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and BAP31 but not the unfolded protein response. J. Biol. Chem. 2005;280:39002–39015. doi: 10.1074/jbc.M508652200. [DOI] [PubMed] [Google Scholar]
- 8.Teckman J.H., Perlmutter D.H. Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G961–G974. doi: 10.1152/ajpgi.2000.279.5.G961. [DOI] [PubMed] [Google Scholar]
- 9.Brigham K.L., Lane K.B., Meyrick B., Stecenko A.A., Strack S., Cannon D.R., Caudill M., Canonico A.E. Transfection of nasal mucosa with a normal alpha1-antitrypsin gene in alpha1-antitrypsin-deficient subjects: comparison with protein therapy. Hum. Gene Ther. 2000;11:1023–1032. doi: 10.1089/10430340050015338. [DOI] [PubMed] [Google Scholar]
- 10.Song S., Morgan M., Ellis T., Poirier A., Chesnut K., Wang J., Brantly M., Muzyczka N., Byrne B.J., Atkinson M., et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl Acad. Sci. USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brantly M.L., Spencer L.T., Humphries M., Conlon T.J., Spencer C.T., Poirier A., Garlington W., Baker D., Song S., Berns K.I., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- 12.Louboutin J.P., Wang L., Wilson J.M. Gene transfer into skeletal muscle using novel AAV serotypes. J. Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 13.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller C., Braag S.A., Martino A.T., Tang Q., Campbell-Thompson M., Flotte T.R. The pros and cons of immunomodulatory IL-10 gene therapy with recombinant AAV in a Cftr(−/−)-dependent allergy mouse model. Gene Ther. 2009;16:172–183. doi: 10.1038/gt.2008.156. [DOI] [PubMed] [Google Scholar]
- 15.Brantly M.L., Chulay J.D., Wang L., Mueller C., Humphries M., Spencer L.T., Rouhani F., Conlon T.J., Calcedo R., Betts M.R., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl Acad. Sci. USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chulay J.D., Ye G.J., Thomas D.L., Knop D.R., Benson J.M., Hutt J.A., Wang G., Humphries M., Flotte T.R. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum. Gene Ther. 2011;22:155–165. doi: 10.1089/hum.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zern M.A., Ozaki I., Duan L., Pomerantz R., Liu S.L., Strayer D.S. A novel SV40-based vector successfully transduces and expresses an alpha 1-antitrypsin ribozyme in a human hepatoma-derived cell line. Gene Ther. 1999;6:114–120. doi: 10.1038/sj.gt.3300793. [DOI] [PubMed] [Google Scholar]
- 18.Duan Y.Y., Wu J., Zhu J.L., Liu S.L., Ozaki I., Strayer D.S., Zern M.A. Gene therapy for human alpha1-antitrypsin deficiency in an animal model using SV40-derived vectors. Gastroenterology. 2004;127:1222–1232. doi: 10.1053/j.gastro.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki I., Zern M.A., Liu S., Wei D.L., Pomerantz R.J., Duan L. Ribozyme-mediated specific gene replacement of the alpha1-antitrypsin gene in human hepatoma cells. J. Hepatol. 1999;31:53–60. doi: 10.1016/s0168-8278(99)80163-9. [DOI] [PubMed] [Google Scholar]
- 20.Cruz P.E., Mueller C., Cossette T.L., Golant A., Tang Q., Beattie S.G., Brantly M., Campbell-Thompson M., Blomenkamp K.S., Teckman J.H., et al. In vivo post-transcriptional gene silencing of alpha-1 antitrypsin by adeno-associated virus vectors expressing siRNA. Lab. Invest. 2007;87:893–902. doi: 10.1038/labinvest.3700629. [DOI] [PubMed] [Google Scholar]
- 21.Grimm D., Streetz K.L., Jopling C.L., Storm T.A., Pandey K., Davis C.R., Marion P., Salazar F., Kay M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 22.Grimm D., Pandey K., Kay M.A. Adeno-associated virus vectors for short hairpin RNA expression. Methods Enzymol. 2005;392:381–405. doi: 10.1016/S0076-6879(04)92023-X. [DOI] [PubMed] [Google Scholar]
- 23.McBride J.L., Boudreau R.L., Harper S.Q., Staber P.D., Monteys A.M., Martins I., Gilmore B.L., Burstein H., Peluso R.W., Polisky B., et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc. Natl Acad. Sci. USA. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giering J.C., Grimm D., Storm T.A., Kay M.A. Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol. Ther. 2008;16:1630–1636. doi: 10.1038/mt.2008.144. [DOI] [PubMed] [Google Scholar]