Abstract
B cell anergy represents an important mechanism of peripheral immunological tolerance for mature autoreactive B cells that escape central tolerance enforced by receptor editing and clonal deletion. While well documented in mice, the extent of its participation in human B cell tolerance remains to be fully established. In this study, we characterize the functional behavior of strictly defined human naïve B cells separated on the basis of their surface IgM (sIgM) expression levels. We demonstrate that cells with lower sIgM levels (IgMlo) are impaired in their ability to flux calcium in response to either anti-IgM or anti-IgD cross-linking, and contain a significantly increased frequency of autoreactive cells compared to naïve B cells with higher levels of sIgM. Phenotypically, in healthy subjects, IgMlo cells are characterized by the absence of activation markers, reduction of co-stimulatory molecules (CD19 and CD21) and increased levels of inhibitory CD22. Functionally, IgMlo cells display significantly weaker proliferation, impaired differentiation, and poor antibody production. In aggregate, the data indicates that hypo-responsiveness to BCR cross-linking associated with sIgM down-regulation is present in a much larger fraction of all human naïve B cells than previously reported, and is likely to reflect a state of anergy induced by chronic autoantigen stimulation. Finally, our results indicate that in SLE patients, naïve IgMlo cells display increased levels of CD95 and decreased levels of CD22, a phenotype consistent with enhanced activation of autoreactive naïve B cells in this autoimmune disease.
Keywords: Human B cells, anergy, tolerance
INTRODUCTION
The early B cell repertoire generated in mouse and human bone marrow is endowed with a high degree of autoreactivity that imposes the need for effective censoring mechanisms that enforce immunological tolerance for self-antigens. In transgenic models, central tolerance is largely imparted by receptor editing and clonal deletion of autoreactive B cells. Cells that escape these central checkpoints are subsequently censored by peripheral tolerance mechanisms including anergy (1, 2).
The density and binding affinity of surface B cell receptor (BCR) play critical roles in determining the fate of autoreactive B cells. Thus, in double transgenic mice, Hen-egg lysozyme (HEL)/anti-HEL antibody, strong BCR cross-linking induces deletion of autoreactive B cells while weaker signaling induces functional anergy (3). Anergic anti-HEL B cells are characterized by down-regulation of sIgM although expression of sIgD is maintained at normal levels. Similar down-regulation of sIgM has also been described in other mouse models including anti-dsDNA transgenic mice (4, 5). Less clear has been the applicability of these observations to non-transgenic cells. Of note however, early work in transgenic models also noted the expression of similarly low levels of sIgM in approximately 1/3 of all wild-type naïve B cells and suggested that anergy could also be operative in non-transgenic B cells. This suggestion has been recently confirmed by elegant studies in wild-type mice (6).
In humans, both clonal deletion and receptor editing play a major role in censoring the high frequency of autoreactive B cells that are constantly being generated in bone marrow (2, 3, 7). In contrast, the role of anergy in human B cell tolerance remains to be fully understood. The need for anergy or other mechanisms of peripheral tolerance is, nonetheless suggested by the high frequency of autoreactive cells (approximately 20% of all B cells) in the mature naïve compartment after avoiding editing or deletion at earlier checkpoints (7). Evidence for anergy is also provided by our earlier report of anergic responses in a significant fraction of human autoreactive naïve B cells identified by the 9G4 idiotype (8). It has also been recently shown that human naïve B cells lacking expression of IgM (BND) are highly autoreactive and display an anergic phenotype (9). Although this work demonstrated the participation of anergy in B cell tolerance, both the relatively low frequency of IgM-neg cells, which represents approximately 2.5% of total B cells, and the different phenotype displayed by anergic naïve 9G4 cells, which retain substantial expression of sIgM, suggested that anergic responses could contribute to the regulation of a significantly larger fraction of autoreactive naïve B cells. To address this important question we have systematically analyzed the phenotype, responsiveness, and autoreactivity of strictly defined naïve B cell fractions differentiated by their relative level of expression of surface IgM (sIgM). Our results indicate that naïve cells with low sIgM, representing up to 30% of all naïve cells, display an anergic phenotype and are substantially enriched in autoreactivity against HEp-2 cell antigens. The evidence presented indicates that anergic responses correlate with the level of down regulation of sIgM, and may therefore account for the high variability in sIgM expression observed in human naïve B cells. Finally, we present evidences indicating that this anergic phenotype is attenuated in patients with SLE (Systemic Lupus Erythematosus/Lupus), in whom down-regulation of sIgM appears to be secondary to naïve B cell activation.
MATERIALS AND METHODS
Human Samples
Peripheral blood (PBL) and tonsils were obtained from healthy donors according to protocols approved by the University of Rochester Medical Center Institutional Review Board. SLE patients were selected if they had a diagnosis of SLE with at least four of the American College of Rheumatology (ACR) criteria, and had an SLE disease activity index (SLEDAI) of 4 or more.
Cells isolation
Heparinized blood, and in some cases tonsils, were collected from healthy donors or SLE patients. Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation at 20°C using Ficoll-Paque (Amersham Biosciences).
Flow Cytometry
Single-cell suspensions (2×106/sample) were labeled at 4°C with predetermined optimal concentrations of fluorophore-conjugated mAbs and pair-matched Fluorescence Minus One (FMO) controls. The following antibodies were used: FcR blocking reagent (Miltenyi), anti-CD19–APC-Cy7 or PE-Cy5 (clone SJ25C1), anti-CD27–APC or Biotinylated (O323) protein, anti-IgD–PE (IA6-2), anti-IgM–PE-Cy5 (G20-127), anti-IgG PE-Cy5 or PE (G18-145), anti-CD22-APC (S-HCL-1), anti-CD24–FITC (ML5), anti-CD32b-Alexa Fluor647 (a generous gift from Dr. Robert Carter), anti-CD38–PE-Cy7 or APC-Cy5.5 (H1T2), anti-CD69-PE-Cy7 (FN50), anti-CD80-Biotinylated (2D10.4), anti-CD86-Biotinylated (IT2.2) or FITC (2331-FUN-1), anti-CD95-Biotinylated or FITC (DX2), and Streptavidin-PE-Cy7 (BD Biosciences-Pharmingen, and eBiosciences). In total, 5×105–106 events were collected for each sample an LSRII (10) flow cytometer. Data were analyzed using Flowjo software (Tree Star, San Carlos, CA).
Cell sorting
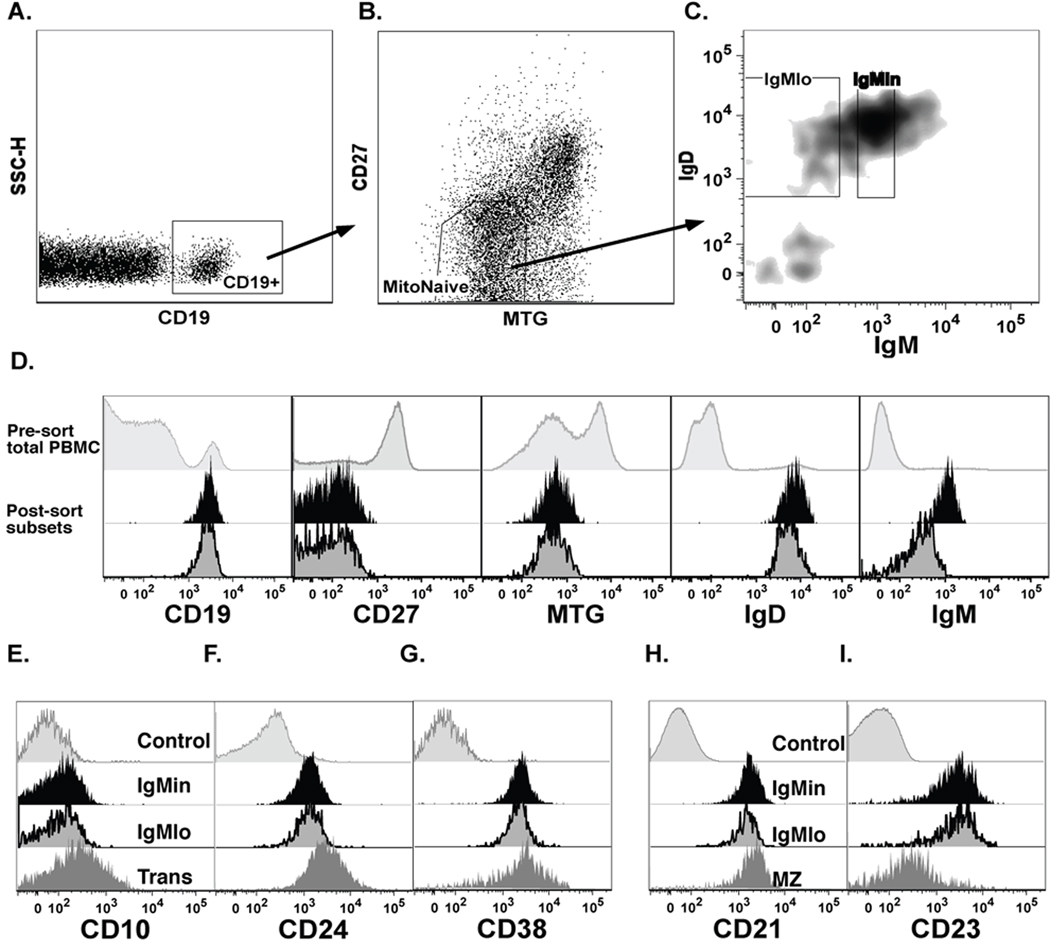
PBMC were stained with MitoTracker Green (Invitrogen), FcR blocking reagent (Miltenyi Biotec), anti-CD19 APC-Cy7, anti-IgD-PE, anti-IgM-PE-Cy5 (BD Bioscience) and anti-IgM-APC (Southern Biotech), anti-CD27-Biotin and SA-PE-Cy7 in cell sorting buffer (0.5% BSA in PBS), and then sorted using the gating scheme shown in Figure 2. Following gating on single lymphocytes, and CD19-pos, cells were gated on MitoTracker-neg, CD27-neg, and IgD-pos, and these cells were subdivided into three fractions (IgMhi, IgMin and IgMlo) based on sIgM expression levels.
Figure 2. Expression of naïve markers on IgMlo and IgMin cells.
A, Plots show the identification of IgMlo and IgMin cells. Upon gating on conventional naïve markers, CD19-pos, CD27-neg, MTG-pos, and IgD-pos, cells within the naïve compartment were divided into IgMlo and IgMin cells, as defined before. B, Overlay histograms showing representative post sort results of surface marker analysis of IgMlo and IgMin cells as compared to total PBMC (pre-sort). C and D, Total PBMC cells were stained with developmental markers, CD10, CD24, CD38, CD21, CD23 to identify the phenotypic differences of IgMlo as compared to IgMin cells and other B-cell subsets: Trans (Transitional cells: CD19-pos, CD27-neg, MTG-pos, IgD-pos) and MZ (Marginal Zone/Unswitched Memory cells: CD19-pos, CD27-pos, IgD-pos).
In vitro cultures
CFSE proliferation assay
After sort purification 3×104 cells of each fraction from the naïve compartment were loaded with 0.4 µM CFSE (Invitrogen) for 5 min at 37°C, and cultured untreated (media alone) or treated with CpG oligodeoxynucleotide 2006 (Oligos Etc; Wilsonville, OR) (2.5 µ/ml), anti-IgM F(ab’)2 (2.5 µg/ml) and IL-2 (10 ng/ml). Cultured cells were collected at various time points (from day 3 to 5) and analyzed for cell division using flow cytometry. The total cell numbers in each division (ni) was determined using a proliferation-fitting model from FlowJo (Treestar, San Carlos, CA). The precursor cohort in each peak was calculated by dividing the number of cells in each division ni by 2i, where i represents the division number of the CFSE peak (11, 12). To estimate the time required for a cell that has divided once to go through subsequent divisions, we calculated the average division index at each time point (i.e., Σ (i × ni/2i)/ Σ (ni/2i)), and plotted them against time. The inverse of the slope of the linear regression line fit through each data set gives an indication of time to subsequent division (12).
Flow analysis of activation markers
After sort purification, 3×104 cells of each fraction were placed in culture with anti-IgM alone, anti-IgM plus IL-2, anti-IgM plus CpG and IL-2, or left untreated for 18 h, and then stained with SytoxBlue, anti-CD69, and anti-CD86.
Antibody and autoantibody analysis
After sort purification, 3×104 cells were cultured in medium alone (untreated) or treated with CpG (2.5 µg/ml) plus anti-IgM F(ab’)2 (2.5 µg/ml) and IL-2 (10 ng/ml), or with a CD40L-expressing murine cell line and IL-21 (50 ng/ml). Culture supernatants were collected on day 7 and tested for total IgM antibody production by ELISA.
Sorted cells were cultured with ODN2006 CpG (2.5 µg/ml) plus anti-IgM F(ab’)2 (2.5 µg/ml) and IL-2 (10 ng/ml) as described above. Cells were harvested on day 4 and placed in multiscreen ELISPOT plates for the detection of IgM production using goat anti-human IgM F(ab’)2, at 10 µg/ml (Biosource), or for the detection of autoantibodies binding to nuclear and cytoplasmic autoantigens. The latter was accomplished by coating ELISPOT plates with HEp-2 extracts conventionally used for the detection of autoreactivity in immunofluorescence and ELISA assays (7), using the equivalent of 107 cells/extract/plate. Similar methods have been used by others to measure antibody reactivity against malaria antigens and human brain extracts (13). After extensive washing, spot-forming cells producing either total IgM or autoreactive IgM were detected by incubation with Alkaline Phosphatase-conjugated goat anti-human IgM (μ chain specific; Jackson) for 2 hours, and developed with VECTOR Blue, Alkaline Phosphatase Substrate Kit III (Vector Laboratories, Burlingame, CA). Spots in each well were counted using a CTL ImmunoSpot plate reader and counting software (Cellular Technology Limited, Cleveland, OH).
Intracellular calcium measurements
B cells were purified by negative selection from heparinized PBL according to the manufacturer’s protocol (Human B Cell Enrichment Cocktail, RosetteSep, StemCell Technologies). To identify naive B cell populations of interest, IgMlo, IgMin, and IgMhi, the cells were stained with an exclusion cocktail of biotinylated non-B cell markers CD2, CD14, CD16, CD36, CD43 and CD235a antibodies (Miltenyi Biotec) as well as anti-CD27 and anti-IgG to gate out remaining non B cells and memory B cells at the time of the calcium measurements. Calcium responses were measured on a BD FACS Vantage SE with UV excitation. Data were collected and displayed as the relative ratio of intensities of Indo fluorescence (Ca++-bound Indo violet emission 405 nm/free Indo blue emission 485 nm) for each cell over time and analyzed with FlowJo software. Samples were analyzed for a 50 to 60-second baseline in the respective gated naive B cell populations (IgMlo, IgMin) at 37°C followed by the addition of 20 µ/ml F(ab′)2 goat anti-human IgM or anti-IgD (Jackson Laboratories).
Recombinant monoclonal antibodies
Single cell sorting
PBMC were stained with MitoTracker Green (Invitrogen), FcR blocking reagent (Miltenyi Biotec), anti-CD19 APC-Cy7, anti-IgD-PE, anti-IgM-PE-Cy5, anti-CD27-Biotin and SA-PE-Cy7 in cell sorting buffer (0.5% BSA in PBS), and then sorted into IgMlo and IgMin cells as described above. Subsequently, single cells from both subsets were re-sorted into 96-well PCR plates containing 4µl lysis buffer (0.1 M DTT, 40 U/ul rRNasin, in PBS and dH2O), immediately frozen on dry ice and stored at −70°C as previously reported (14).
Monoclonal antibody production and ANA ELISA
Single cell PCR, cloning strategy, expression vectors, and monoclonal antibody production were as described in Tiller et al. (14). Antibody concentrations in tissue culture supernatants were determined by anti-human IgG ELISA using whole human IgG as standard (Jackson Research). Self-reactivity screens were performed using QUANTA Lite™ ANA ELISA plates (INOVA Diagnostics, Inc.), and the result was calculated as suggested by the manufacturer. Purified antibodies were used at 25 µg/ml, and followed with three 1:4 dilutions in the diluent provided by the manufacturer.
Statistical analysis
All statistical analyses were done using Graphpad Prism 5.0 and verified with SAS statistical analysis software. Wilcoxon Matched Pairs/Signed Rank test was used to compare results of cells from IgMlo fraction vs. IgMin fraction.
RESULTS
Surface IgM (sIgM) expression level determines BCR responsiveness of naïve B cells
Human naïve B cells express a relatively homogenous level of expression of surface IgD (sIgD) but heterogeneous expression levels of sIgM, with mean fluorescence intensity values ranging from undetectable (background staining) to the 4th or –5th log decade. In some transgenic mice, low sIgM is a hallmark of anergic B cells, and as many as 50% of all transitional B cells in wild-type mice have low sIgM and represent anergic autoreactive B cells (6). In order to test the functional consequences of sIgM levels in human B cells, we determined the ability of naïve cells to respond to BCR cross-linking. We compared the response of naïve cells at the lower end of sIgM expression (hereafter termed IgMlo) with naïve cells expressing higher levels of sIgM. As shown in figure 1, IgMlo cells displayed both significantly lower global Ca++ oscillations and a significantly decreased frequency of responding cells. Comparable differences were consistently observed irrespective of whether cells were stimulated through sIgM (Fig. 1B) or sIgD (Fig. 1C) despite relatively similar levels of sIgD expression between the compared populations (Fig. 2B). Importantly, the data indicated a strong correlation between sIgM expression level and the degree of response to either type of stimulation (Figure 1E). Overall, these data suggest that the level of sIgM expression plays a significant role in determining the response of human naïve B cells, and that hypo-responsiveness to antigen ligation is not rescued in these cells by IgD signaling.
Figure 1. Correlation of sIgM expression level on peripheral human naïve B cells and their responsiveness.
A, Gating strategy for the analysis of Ca++ flux in IgMlo cells. Total peripheral blood B cells were purified using human B cell enrichment RosetteSep Kit (86–94% purity), loaded with Indo-AM, and then stained with exclusion markers (anti-CD2, CD14, CD16, CD36, CD43 and CD235a), anti-CD27, IgM and IgG antibodies. Live naïve B cells were gated on those that were indo-pos, CD27-neg and IgG-neg, exclusion-neg. Subsequently, cells with low surface IgM as compared to control naïve cells expressing higher levels were analyzed according to the gates shown in the figure. B and C, Indo ratio reflecting mean fluorescence of fluxing calcium (upper panels), and the frequency of responding cells (lower panels), upon stimulation with anti-IgM (B) or anti-IgD (C) were analyzed vs. time to compare the calcium flux ability of IgM “negative” (IgMlo) cells with IgM “positive” (IgMin-hi) cells. D, Live CD27-neg, Exclusion-neg cells were gated and IgM density was displayed. Events in this gate were subdivided into 50 fractions (2% each). E, IgM median fluorescence intensity (MFI) value from each fraction from D was obtained using Flowjo and plotted against the frequency of responding cells above the pre-stimulation baseline. Representative plots, from 14 independent experiments (donors, n=12), show the sIgM MFI vs. the frequency of responding cells upon stimulation with either anti-IgM (left) or anti-IgD (right). The correlation of sIgM MFI and frequency of responding cells was calculated by the Graphpad Prism and was then rechecked with SAS statistical analysis software. SAS were used to calculate the ED90, the sIgM MFI value at which the response achieved 90% of the maximal response (dash line, nonlinear fit curve; solid line, smooth curve). F and G, IgMlo cells, from B and C, were reanalyzed in the absence of BND (the 25% fraction of IgMlo cells that are at the lowest end of sIgM expression spectrum) to evaluate their ability to flux Ca++ in response to the stimulants, and compared to those of IgMin-hi
A previous report by Duty et al. showed that a subset of anergic, autoreactive naive cells lacking expression of sIgM (BND), which represent on average 2.5% of all peripheral blood B cells (9). Accordingly, to assess the contribution of this subset to the overall behavior of IgMlo cells, experiments were repeated excluding BND cells from the analysis. Figure 1F and G show that after exclusion of BND cells, IgMlo cells retained a consistently hypo-responsive in response to anti-IgM (Fig. 1F) and anti-IgD (Fig. 1G) as compared to control IgMin-hi cells.
In order to determine the fraction of naïve cells that were hypo-responsive, we calculated the ED90 Ca++ flux values (the sIgM MFI at which the frequency of responding cells achieved 90% of the maximum responses). Based on this ED90 cut off value, we determined that BCR hypo-responsive cells were contained in the 15–30% fraction of naïve cells at the lower end of sIgM expression (n=12–14) (Fig. 1E). Accordingly, for further phenotypic and functional analyses we consistently defined, and purified when needed, IgMlo cells as the dimmest 20% of naïve B cells, and compared them to naïve cells expressing higher sIgM (IgMin).
Phenotypic characterization of human IgMlo naïve B cells
We have recently reported that late transitional cells express sIgM levels that are similar to naïve cells, and that the two populations cannot be clearly separated on the basis of conventional markers including CD10 (15). However, early transitional cells (T1 and T2), which are hypo-responsive to BCR stimulation (15), are unlikely to contribute significantly to the signaling results observed for IgMlo cells because, in healthy peripheral blood, transitional cells express significantly higher levels of sIgM. To ensure a rigorous phenotypic and functional analysis of naïve IgMlo cells, we took advantage of the observation that, in contrast to transitional and memory B cells, naïve B cells express a functional ATP-binding cassette transporter (ABCB1) and, as a result, fail to retain rhodamine and similar dyes, such as Mito-tracker green (MTG) (16). Accordingly, we used a multicolor flow cytometric approach previously described in our laboratory, which incorporates MTG to provide a more conclusive differentiation between naïve cells (CD19+IgD+CD24+CD38+/−CD27−CD10−MTG−), and late (T3) transitional cells (CD19+IgD+CD24+CD38+CD27−CD10+/−MTG+) than can be obtained with more commonly markers such as IgD, CD24, CD38, and CD10 (15). Our data indicate that IgMlo cells do not represent late transitional cells, but have a mature naïve phenotype with expression patterns for other developmental antigens, CD21 and CD23, similar to other naïve cells irrespective of their sIgM level. Using this phenotype and based on the signaling results, we applied a stringent definition of naïve cells to sort purify the 20% of naïve cells with the lowest sIgM levels (IgMlo) and analyzed these cells for their surface phenotype and cellular properties. As shown in figure 2A and B, this approach allowed for a consistently clear separation of the subset of interest from the majority of naïve B cells, which express approximately 3-fold higher levels of sIgM with relatively little difference in IgD expression (IgM intermediate or IgMin).
Functional properties of naïve IgMlo B cells
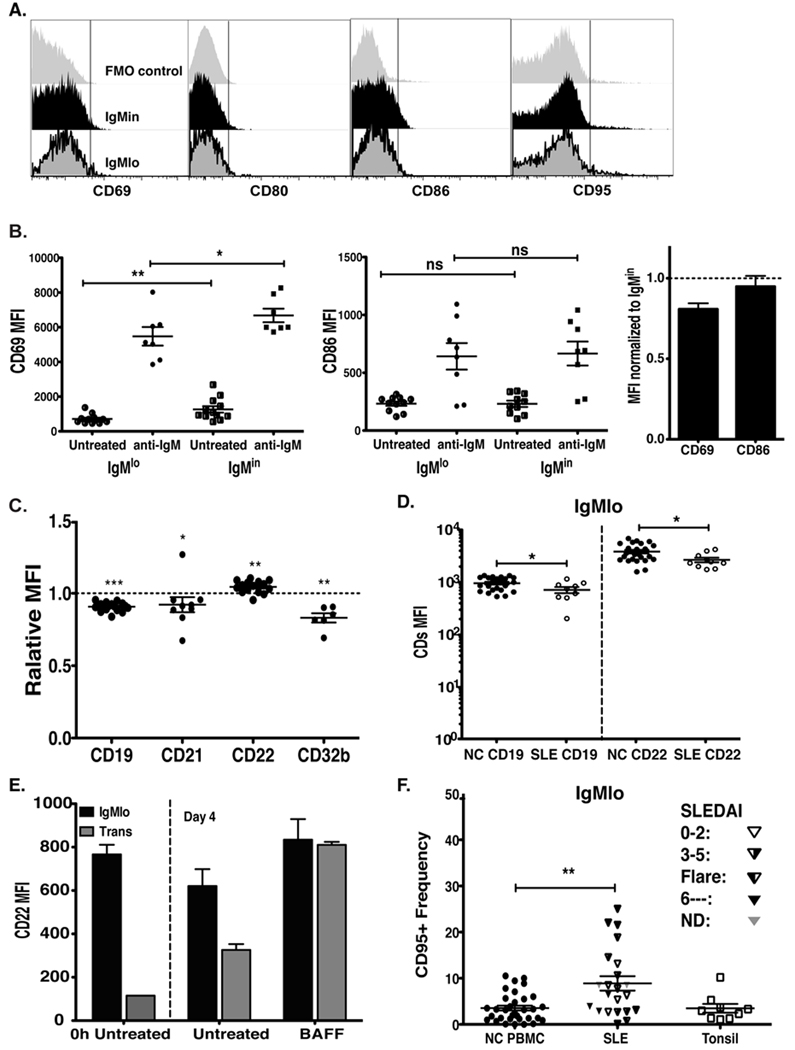
Given that BCR down regulation could either be secondary to acute antigenic stimulation or chronic engagement by exposure to self-antigens, we examined the expression of conventional activation markers on freshly isolated PBMC. As shown in Figure 3A, IgMlo cells lacked expression of CD69, CD80, CD86, and CD95 suggesting that sIgM down-regulation in these cells is not the consequence of acute antigenic stimulation. In addition, the IgMlo population had significantly lower up-regulation of the early activation marker, CD69, after in vitro BCR stimulation for 18 hours. In contrast, as has been reported for anergic anti-insulin transgenic B cells (17), BCR-stimulated IgMlo cells were able to up-regulate CD80/CD86 to similar levels as the control IgMin naïve B cells (Fig. 3B).
Figure 3. Surface expression of co-stimulatory/inhibitory molecules on IgMlo cells.
PBMC were stained with CD19, MTG, CD27, IgD, IgM, and CD69, plus CD80, CD86, CD95, CD21, CD22, or CD32b for flow analysis. A, Representative histograms showing CD69, CD80, CD86, and CD95 surface expression on IgMlo (bottom) and IgMin (middle) cells compared to FMO control (fluorescence minus one, top row). B, B cells from naïve compartments (IgMlo and IgMin) were sorted and placed in culture with or without anti-IgM for 18 hours, and stained for CD69 and CD86 expression (each data point represents MFI results from an independent experiment). The bar graph shows the relative CD69 and CD86 MFI of IgMlo cells as compared to IgMin cells after stimulating with anti-IgM for 18 hours. C, Relative MFI ratios of tested BCR co-regulators, CD19, CD21, and inhibitors, CD22, CD32b expression levels of IgMlo / IgMin (*p < 0.05, **p < 0.005, ***p < 0.0001). D, MFI values of surface CD19 and CD22 of IgMlo cells from healthy control (NC) and Lupus donors (SLE). E, CD22 expression by IgMlo and transitional B cells (CD19-pos, CD27-neg, MTG-pos, IgD-pos) cultured with or without BAFF for 4 days. F, CD95-pos frequency of IgMlo cells from SLE PBMC, NC PBMC and NC tonsil. **p < 0.005 (Mann-Whitney test).
Ultimately, B cell responses are determined by the balance between BCR and co-stimulatory signals and the engagement of inhibitory receptors capable of dampening B cell activation (18–20). Thus, we used flow cytometry to examine the expression levels of accessory molecules known to modulate BCR signaling including CD19, CD21, CD22, and CD32b. As shown in figure 3C, although IgMlo cells express CD19 and CD21 (Fig. 2B and 2D), they express significantly lower levels of the BCR co-stimulatory complex CD19–CD21 as compared to naïve IgMin cells. Interestingly, IgMlo cells also have elevated surface expression of CD22 but reduced surface expression of CD32b, both members of the immunoglobulin-like superfamily receptors carrying immunotyrosine-based inhibitory motifs (ITIM) in their cytoplasmic tails that powerfully dampens positive BCR-induced signaling (21). Expression of CD22 has been shown to increase as immature/transitional cells enter the mature naïve compartment and its expression declines with B cell activation (22). Consistently, we observed that IgMlo naïve cells expressed significantly higher levels of CD22 than do transitional cells (Fig. 3E and data not shown). Interestingly, CD22 expression increased significantly in transitional cells but not in IgMlo cells when cultured with BAFF (Fig. 3E), the main B cell maturation factor for transitional B cells (15, 23).
To better understand the significance of these findings, we also examined the expression of these markers in patients with Systemic Lupus Erythematosus (SLE), an autoimmune condition in which one would expect to detect decreased anergy and increased activation of autoreactive B cells. As shown in figure 3D and F, SLE IgMlo B cells have significantly lower expression of CD22 but significantly enhanced expression of the activation marker CD95, both findings consistent with an activated phenotype and/or lower activation threshold in SLE IgMlo naïve cells.
Naïve IgMlo B cells are hyporesponsive to TLR co-stimulation
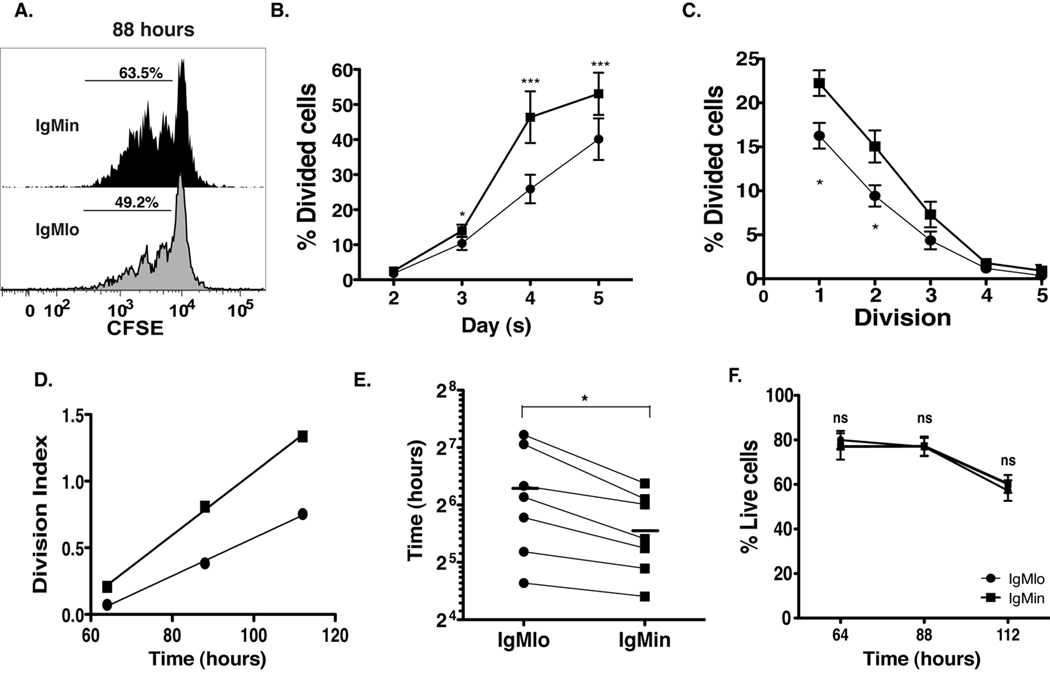
In HEL/anti-HEL double transgenic mice, anergic B cells are refractory to either TLR9 or TLR4 stimulation although their hypo-responsiveness can be at least partly overcome by the high concentration of the corresponding ligands (CpG DNA and LPS, respectively) (24). We have shown that even in the absence of BCR engagement, TLR9 stimulation, with appropriate cytokines, can also drive proliferation of human naïve B cells, and similar results have been published for transitional cells (25, 26). Accordingly, we analyzed cell divisions using measurements of CFSE dilution after in vitro stimulation with anti-IgM, IL-2, and CpG (note that expression of TLR9, the receptor for CpG, on IgMlo cells is similar to that of IgMin cells, supplemental fig. 1A–B). As shown in figure 4, after 4 days in culture, the frequency of IgMlo cells that underwent at least one division was diminished by 40% as compared to IgMin cells (Fig. 4A and B). Moreover, IgMlo cells had a lower frequency of dividing cells within each division cycle (Fig. 4C), a feature reflected in the lower division index of IgMlo cells and accounted for by the longer time to subsequent division experienced by these cells (Fig. 4D–E). Of note, the diminution of cell proliferation among IgMlo cells was not due to rapid cell death in culture as the frequency of live cells obtained from IgMlo culture was similar to those of IgMin cells, specially on day 3 when cells were first observed to undergo proliferation (Fig. 4F).
Figure 4. IgMlo cells have reduced proliferative capacity in response to in vitro stimulation.
Naïve cells from peripheral blood were sorted as described in Figure 1, loaded with CFSE, and placed in culture with CpG (2.5 µg/ml), F(ab)’2 anti-IgM (2.5 µg/ml), IL-2 (10 ng/ml). Cultured cells were collected on day 3, 4, and 5 for proliferation and cell survival analysis. A, CFSE histogram shows the 4-day proliferation of IgMin and IgMlo cells. B, Frequency of cells having undergone at least one division (* p<0.05 & ***p<0.005). C, Frequency of dividing cells within each cell division. D, Division index (includes only cells that made at least one division) vs. time. Reciprocal slope of regression line gives time to subsequent divisions. E, Time to subsequent divisions of IgMlo and IgMin cells. F, Graph shows the percentage of live cells within total culture cells (ns, not significant). All data were collected from 7 independent experiments, and the analyses were performed as described in the Materials and Methods.
IgMlo cells display decreased antibody secreting ability and are enriched for autoreactivity
A critical downstream consequence of B cell stimulation is the ability to secrete antibody. Hence, we stimulated IgMlo cells under different conditions known to induce antibody secretion by human naïve B cells (27). Figure 5A and B show that IgMlo cells produce significantly less IgM antibody than IgMin cells. Importantly, decreased antibody production can also be demonstrated under powerful IgM-independent stimulation conditions, CD40L plus IL-21 (Fig. 5A). This result, since CD40 expression is similar between IgMlo and IgMin cells (supplemental fig. 1C–D), is consistent with the anti-IgD Ca++ flux results, and indicates a global hypo-responsiveness of IgMlo cells that cannot be attributed simply to lower levels of IgM, but rather is a consequence of an intrinsic refractoriness to stimulation. Diminished antibody production was confirmed by ELISPOT experiments using equal numbers of input cells from 4-day cultures stimulated with CpG DNA, F(ab)’2 anti-IgM, and IL-2. As shown in figure 5A, in keeping with their lower division rate (28), IgMlo cells were significantly impaired in terms of differentiation to antibody secreting cells.
Figure 5. IgMlo cells have reduced total IgM production but increased frequency of autoreactive IgM antibody producing cells.
A, Plot displays amount of total IgM antibody production of IgMlo and IgMin cells upon stimulation with CpG (2.5 µg/ml), F(ab)’2 anti-IgM (2.5 µg/ml), IL-2 (10 ng/ml), or CD40L and IL-21 (50 ng/ml). B, graph displays frequency of IgM-producing cells (left Y-axis) and frequency of autoantibody-producing cell (right Y-axis) among IgMlo* (IgMlo cells that were excluded of BND cells during cell sorting), IgMin, IgMhi cells (49) following stimulation with CpG, F(ab)’2 anti-IgM, and IL-2. C and D, Images show developed spots from ELISPOT assays for total IgM (C) and for autoreactive IgM antibodies (D). Equal numbers of viable cells from each population were assayed. Anti-IgM autoantibody producing cells (D) upon stimulation with CpG (2.5 µg/ml), F(ab)’2 anti-IgM (2.5 µg/ml), IL-2 (10ng/ml) (* p<0.05 & **p<0.005)
Importantly, although IgMlo cells were inefficient in generating total anti-IgM antibody producing cells, they contained a high frequency of autoreactive producing cells as indicated by a newly developed HEp-2 ELISPOT, a high-throughput method to elucidate at the single cell level the global autoreactivity of large numbers of cultured cells (Fig. 5D). To confirm that autoreactive cells detected with this method were not derived from the expansion of BND cells, HEp-2 ELISPOT experiments were repeated after the exclusion of BND cells, as described in the figure legend, from the initial culture, and we obtained the similar results (Fig. 5B). The autoreactivity was detected up to 20% of antibody-producing IgMlo cells, but in only 3% of IgMin cells. Of note, the frequency of HEp-2 autoreactive cells observed in our experiments was consistent with the frequency of HEp-2 reactivity observed by others for unfractionated naïve B cells in single cell analysis using recombinant monoclonal antibodies (7). Also of interest, cells with the highest sIgM levels (IgMhi) appeared to contain the lowest frequency of autoreactive cells even though they yielded the highest frequency of IgM-secreting cells. The overall magnitude of autoreactivity detected in the current study is consistent with the level detected by Merrel et al. in their analysis of wild-type mouse anergic naïve B cells showing decreased expression of sIgM reactive with purified antigens contained in the HEp-2 extracts (6).
Finally, in order to validate the frequency and type of autoreactivity observed with our newly developed HEp-2 ELISPOT assay, we generated monoclonal antibodies from single cells sorted from both the IgMlo and IgMin populations using recombinant technology (as described in the Material and Methods). This approach provides important complementary information as it reflects the frequency of autoreactive cells without the bias of preferential proliferation of particular subsets that may affect the result outcomes of the ELISPOT assays. Reassuringly, commercial ANA ELISA assay testing of recombinant monoclonal antibodies generated from single IgMlo and IgMin cells showed that 40% of cells obtained from the IgMlo population were autoreactive compared to 7% obtained from the IgMin population (supplemental fig.2 A–B).
DISCUSSION
The data presented herein are consistent with the presence of anergy in a significant fraction of human naïve B cells as documented by low Ca++ flux, attenuated proliferation, and substantially decreased ability to secrete antibody in response to both BCR crosslinking and BCR-independent stimulation (CD40L + IL-21). These cells are characterized by low surface expression of IgM, absence of activation markers, increased autoreactivity, and abnormal expression of inhibitory receptors as compared to control naïve B cells, which express higher levels of sIgM and respond more vigorously to BCR cross-linking. Of note the hypo-response observed to BCR stimulation was not due solely to the decreased levels of sIgM since similar attenuated responses were obtained in response to anti-IgD stimulation. Rather, our results indicate that, as was observed in mouse anergic B cells (29, 30), the level of sIgM determines the threshold of response to stimulation.
Interestingly, intracellular staining detects similar levels of cytoplasmic IgM in IgMlo and IgMin cells (supplemental fig. 3A–C) indicating that decreased sIgM levels are not due to decreased transcription (as also supported by the sustained level of sIgD). Instead, these observations are consistent with increased IgM internalization and/or defective transport to the cell surface reported by Bell et al. (31) These mechanisms have also been invoked to explain low sIgM levels in anergic transgenic B cells, a phenotype that can be reversed when the responsible self-antigen is removed from the system. Our results indicate that similarly, human IgMlo cells are able to up-regulate sIgM after resting for 48 hours in culture medium in the absence of stimulation (supplementary figure 3C). These conditions would also preclude IgMlo cells from engaging self-antigens that might have been present in vivo, other than those present in the surface of naïve B cells themselves or those released from dead or apoptotic cells. Given that we observed negligible levels of cell death/apoptosis in the 48-hours culture (not shown), our results suggest that down-regulation of sIgM may be secondary to chronic engagement of self-antigen and may be reversed by interrupting this interaction.
As we and others have established (7, 15), transitional cells share some of the properties assigned here to anergic naïve B cells (including attenuated BCR responses, hypo-proliferation to TLR9 stimulation, and increased autoreactivity), and the differentiation between these populations may be difficult because the surface phenotypic markers typically used (IgM, IgD, CD24, CD38 and CD10) are expressed as a continuum in transitional and naïve populations. The mature nature of IgMlo cells, however, is supported by their competency to extrude rhodamine or mitrotracker, a property characteristic of mature naïve B cells owing to their expression of the ABCB1 transporter (15) (16). IgMlo maturity is also supported by the higher expression of CD22 and by the differential up-regulation of this marker in response to BAFF stimulation. Collectively, our results are consistent with the recent description of An1 anergic B cells in wild-type mice rather with a T3 transitional phenotype (6).
CD22 is a critical inhibitory receptor that becomes operational in mature follicular B cells and is reduced upon B cell activation (22). In this compartment, CD22 determined the threshold for BCR-stimulated activation and is critical for the enforcement of B cell tolerance. In keeping with this model, CD22 deficiency may result in autoimmunity (32, 33). Thus, our finding of increased CD22 levels in IgMlo cells strongly indicates that, also in keeping with signaling, proliferation and antibody secretion studies, these cells have a higher activation threshold as compared with other naïve cells with lesser degree of autoreactivity. IgMlo cells expressed lower levels of CD19 and CD21, lacked expression of activation markers, and displayed attenuated up-regulation of some early activation markers in response to in vitro stimulation. Collectively, this phenotype suggests that in healthy subjects IgMlo cells represent cells chronically stimulated in vivo, presumably by self antigens, leading to decreased levels of IgM and co-stimulatory BCR molecules. Also of interest, IgMlo cells express lower level of CD32b suggesting that in these cells low levels of BCR signaling fail to recruit this important inhibitory receptor for the feedback inhibition of B cells engaged in productive antigen-specific responses (34). These features also argue against the alternative possibility that sIgM down-regulation could reflect the recent BCR internalization in response to acute B cell activation. Instead, our data suggest that IgMlo cells from SLE patients may represent acutely activated cells as indicated by increased CD95 and decreased CD22 expression.
While the relative decrease in CD19 and increase in CD22 should impose an enhanced threshold for activation of IgMlo cells, thereby contributing to enforcing tolerance in autoreactive B cells, the up-regulation of CD80/86 observed in vitro indicates that the state of unresponsiveness in IgMlo cells is reversible; therefore, these cells represent a dangerous reservoir of potentially pathogenic autoreactive B cells. Although some transgenic models suggest that this danger can be minimized by the shortened lifespan of anergic B cells (35, 36), our data highlight the risk created by the persistence of these cells. Consistent with their lack of CD95 expression, IgMlo cells did not appear to be pro-apoptotic, and in the absence of stimulation, on average 90% of the 18 hours cultured cells survived, which is similar to the survival of control cells (data not show).
Similar scenarios indicating the ability of anergic B-cell to be activated by both T-dependent and independent stimulation are well described in different animal models (17, 24, 37–39). Antigen-activated, but not resting, naïve B cells can up-regulate co-stimulatory molecules and effectively serve as antigen-presenting cells (APC) that mediate cognate activation of T cells (40–42). B cell APC activity may critically contribute to the initiation of T cell-mediated autoimmunity (43), and therefore, failure to up-regulate critical T cell co-stimulatory molecules such as CD80 and CD86 has tolerogenic effects beyond the censoring of the B cells themselves. However, the ability of anergic B cells to express these co-stimulatory molecules has been inconsistent in different autoreactive transgenic models (17, 44, 45). The unaltered up-regulation of CD86 in vitro culture suggests that the APC activity of IgMlo cells could be intact, as shown in the anti-insulin T125tg B cells (17), and thus the pathway leading to anergy of IgMlo cells may be distinct from the pathway leading to becoming an APC.
This manuscript expands the spectrum of anergic naïve B cells identified in humans. Indeed, our initial report on this tolerance mechanism identified anergic behavior in autoreactive 9G4-pos cells that represent 5–10% of all human naïve B cells in healthy subjects, and that display markedly depressed Ca++ responses upon BCR stimulation despite expressing intermediate levels of sIgM (46). While other autoreactivities may also be involved in the censoring of 9G4 B cells (Jenks et al., in preparation), the main antigenic target of 9G4-pos B cells is represented by the blood group i antigen, which is also expressed by a CD45/B220 glycoform preferentially up-regulated in naïve B cells (47). More recently, another population of human anergic naïve B cells has been reported. These cells, termed BND, lack expression of surface IgM, account for about 2.5% of all B cells and are likely to represent the IgM-negative tail of the population studied in our work. Given that naïve cells represent 50–80% of all peripheral blood B cells, BND cells would account for approximately 3–5% of all naïve cells. Of interest, BND cells demonstrate significant autoreactivity against nuclear and cytoplasmic antigens in anti-nuclear antibody (ANA) testing as well as polyreactivity against two or more antigens (ssDNA, dsDNA, insulin, and LPS) when tested by ELISA (9). A third type of human naïve anergic B cell has also been reported during the preparation of this manuscript. These cells, characterized by poor BCR responsiveness and autoreactivity similar to the one described above for BND cells, are distinguished by the down-regulation of CD21 (CD21lo cells). Of note, these anergic, autoreactive CD21lo cells were identified in patients with CVID and in a subset of Rheumatoid Arthritis patients who may be deficient in receptor editing mechanisms. However, CD21lo anergic cells did not seem to represent a substantial fraction of naïve B cells in healthy subjects (48). Thus, previously reported anergic populations represent relatively small fractions of all naïve B cells, and therefore, could not account for the large fraction of autoreactive cells consistently identified in the healthy naïve B cell compartment (9). Our data help fill this gap by identifying a larger subset of autoreactive naïve B cells with an anergic phenotype that are consistently identified in healthy subjects. Accordingly, this work contributes to the growing body of evidence identifying anergy as mechanism of tolerance enforcement in mature naïve cells that is present universally in healthy subjects. While our preliminary results suggest that this mechanism may be defective in SLE patients, larger and more detailed studies will be required to assess the participation of defective anergy in this and other autoimmune diseases.
Supplementary Material
ACKNOWLEGEMENTS
We thank Drs. J. Anolik, S. Jenks and members of the Sanz lab for helpful discussions and technical support. Special thanks to Dr. S. Jenks and E. Palmer for proofreading this manuscript; Drs. T. Bushnell, P. Keng and N. Laniewski and M. Strong for expert and technical help with flow cytometry. We are grateful to Dr. H. Miao and H. Motulsky for statistical analysis and technical support. Thanks to the staff and patients at the URMC for providing and procuring samples, specially, to D. Maffett and S. Kemshetti.
Footnotes
Supported in part by training grants to TDQ from NIH-T32-DE007202 and to IS from NIH-U19 Autoimmunity Center of Excellence AI49660 and NIH MERIT AWARD R37 AI049660; NMO was supported in part by CONACyT-Mexico
REFERENCES
- 1.Goodnow CC, Adelstein S, Basten A. The need for central and peripheral tolerance in the B cell repertoire. Science. 1990;248:1373–1379. doi: 10.1126/science.2356469. [DOI] [PubMed] [Google Scholar]
- 2.Carsett R, Kohler G, Lamers MC. Transitional B Cells Are the Target of Negative Selection in the B Cell Compartment. J. Exp. Med. 1995;181 doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 4.Roark JH, Bui A, Nguyen KA, Mandik L, Erikson J. Persistence of functionally compromised anti-double-stranded DNA B cells in the periphery of non-autoimmune mice. Int. Immunol. 1997;9:1615–1626. doi: 10.1093/intimm/9.11.1615. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Shen S, Manser T. Influence of B Cell Antigen Receptor Expression Level on Pathways of B Cell Tolerance Induction. J Immunol. 2009;182:398–407. doi: 10.4049/jimmunol.182.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of Anergic B Cells within a Wild-Type Repertoire. Immunity. 2006;26:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant Autoantibody Production by Early Human B Cell Precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 8.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duty JA, Szodoray P, Zheng N-Y, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdul-Majid KB, Abedi-Valurgerdi M. T cell dependent B cell activation occurs during the induction of T cell anergy by staphylococcal enterotoxin B in mice. Immunol Invest. 1998;27:73–88. doi: 10.3109/08820139809070891. [DOI] [PubMed] [Google Scholar]
- 11.Hodgkin AVGPD. A cellular calculus for signal integration by T cells. Nature Immunology. 2000;1:239–244. doi: 10.1038/79782. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Guajardo V, Borghans JAM, Marquez MA, Garcia S, Freitas AA. Different Competitive Capacities of Stat4- and Stat6- Deficient CD4+ T Cells during Lymphophenia-Driven Proliferation. J Immunol. 2005;174:1178–1187. doi: 10.4049/jimmunol.174.3.1178. [DOI] [PubMed] [Google Scholar]
- 13.Piriou E, Kimmel R, Chelimo K, Middeldorp JM, Odada PS, Ploutz-Snyder R, Moormann AM, Rochford R. Serological evidence for long-term epstein-barr virus reactivation in children living in a holoendemic malaria region of Kenya. Journal of Medical Virology. 2009;81:1088–1093. doi: 10.1002/jmv.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH. Novel Human Transitional B Cell Populations Revealed by B Cell Depletion Therapy. J Immunol. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. European Journal of Immunology. 2005;35:3433–3441. doi: 10.1002/eji.200535364. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo-Suarez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of Anergy from Developmental Arrest in Anti-Insulin B Cells Supports the Development of Autoimmune Diabetes. J Immunol. 2005;174:827–833. doi: 10.4049/jimmunol.174.2.827. [DOI] [PubMed] [Google Scholar]
- 18.Healy JI, Goodnow CC. Positive versus negative signaling by lymphocyte antigen receptors. Annual Review of Immunology. 1998;16:645–670. doi: 10.1146/annurev.immunol.16.1.645. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc. Natl Acad. Sci. USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christopher CG. Balancing Immunity, Autoimmunity, and Self-tolerance. Annals of the New York Academy of Sciences. 1997;815:55–60. doi: 10.1111/j.1749-6632.1997.tb52044.x. [DOI] [PubMed] [Google Scholar]
- 21.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Molecular Immunology. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Gross AJ, Lyandres JR, Panigrahi AK, Prak ETL, DeFranco AL. Developmental Acquisition of the Lyn-CD22-SHP-1 Inhibitory Pathway Promotes B Cell Tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning FMaJL. BAFF: A Fundamental Survival Factor for B Cells. Nature Reviews Immunology. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 24.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 25.Huggins J, Pellegrin T, Felgar RE, Wei C, Brown M, Zheng B, Milner ECB, Bernstein SH, Sanz I, Zand MS. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 2007;109:1611–1619. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, Cagliuso M, Quinti I, Carsetti R. CpG Drives Human Transitional B Cells to Terminal Differentiation and Production of Natural Antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 27.Good KL, Bryant VL, Tangye SG. Kinetics of Human B Cell Behavior and Amplification of Proliferative Responses following Stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 28.Tangye SG, Avery DT, Hodgkin PD. A Division-Linked Mechanism for the Rapid Generation of Ig-Secreting Cells from Human Memory B Cells. J Immunol. 2003;170:261–269. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 30.Diz R, McCray SK, Clarke SH. B Cell Receptor Affinity and B Cell Subset Identity Integrate to Define the Effectiveness, Affinity Threshold, and Mechanism of Anergy. J Immunol. 2008;181:3834–3840. doi: 10.4049/jimmunol.181.6.3834. [DOI] [PubMed] [Google Scholar]
- 31.Bell SE, Goodnow CC. A selective defect in IgM antigen receptor synthesis and transport causes loss of cell surface IgM expression on tolerant B lymphocytes. EMBO J. 1994;13:816–826. doi: 10.1002/j.1460-2075.1994.tb06324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson J, Nemazee D. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J. Exp. Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G Double-Deficient Mice Have Massively Increased B1 Cell Numbers and Develop Systemic Autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 34.Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Current Opinion in Immunology. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double- transgenic model. J. Exp. Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu H-B, Cyster JG. Reduced Competitiveness of Autoantigen-Engaged B Cells due to Increased Dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 37.Fields ML, Metzgar MH, Hondowicz BD, Kang S-A, Alexander ST, Hazard KD, Hsu AC, Du Y-Z, Prak EL, Monestier M, Erikson J. Exogenous and Endogenous TLR Ligands Activate Anti-Chromatin and Polyreactive B Cells. J Immunol. 2006;176:6491–6502. doi: 10.4049/jimmunol.176.11.6491. [DOI] [PubMed] [Google Scholar]
- 38.Adams E, Basten A, Goodnow CC. Intrinsic B-Cell Hyporesponsiveness Accounts for Self-Tolerance in Lysozyme/Anti-Lysozyme Double-Transgenic Mice. Proceedings of the National Academy of Sciences. 1990;87:5687–5691. doi: 10.1073/pnas.87.15.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 40.Yokochi T, Holly RD, Clark EA. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982;128:823–827. [PubMed] [Google Scholar]
- 41.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 System of T Cell Costimulation. Annual Review of Immunology. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 42.Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B Lymphocytes as APC for Naive T Lymphocytes: Dependence on CD40 Ligand/CD40. J Immunol. 2000;164:688–697. doi: 10.4049/jimmunol.164.2.688. [DOI] [PubMed] [Google Scholar]
- 43.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B Lymphocytes Are Critical Antigen-Presenting Cells for the Initiation of T Cell-Mediated Autoimmune Diabetes in Nonobese Diabetic Mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 44.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 46.Cappione A, III, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappione AJ, Pugh-Bernard AE, Anolik JH, Sanz I. Lupus IgG VH4.34 Antibodies Bind to a 220-kDa Glycoform of CD45/B220 on the Surface of Human B Lymphocytes. J Immunol. 2004;172:4298–4307. doi: 10.4049/jimmunol.172.7.4298. [DOI] [PubMed] [Google Scholar]
- 48.Isnardi I, Ng Y-S, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E. Complement receptor 2/CD21-negative human naive B cells mostly contain autoreactive unresponsive clones. Blood. 2010 doi: 10.1182/blood-2009-09-243071. blood-2009-2009-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, Peake J, King C, French MA, Engelhard D, Al-Hajjar S, Al-Muhsen S, Magdorf K, Roesler J, Arkwright PD, Hissaria P, Riminton DS, Wong M, Brink R, Fulcher DA, Casanova J-L, Cook MC, Tangye SG. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.