Abstract
Purpose of review
Asthma and allergic diseases are common and disproportionately affect racial and ethnic minorities. Large-scale research efforts and the expense committed to multiple genome-wide association studies (GWAS) have led to the identification of numerous susceptibility loci for the allergic diseases, but few successes have been reported in populations that are not of European ancestry.
Recent findings
Of the more than two dozen GWAS’s for asthma and allergic disease performed to date, very few have included racial/ethnic minorities. Lessons learned from the studies conducted so far suggest that the GWAS approach must include considerations unique to the ancestral populations represented in the sample, population stratification due to admixture, and recognition that the current coverage of common variants both in the public database and on commercially available SNP chips is inadequate to detect true genetic associations among ethnic/racial groups.
Summary
Advancements in the GWAS technology for identifying genes relevant to asthma and allergic disease among underrepresented ethnic and racial who suffer most will facilitate the identification and confirmation of validated genetic risk factors that are both unique to minority groups as well as confirm risk factors that are generic to the population at large.
Keywords: genomewide association studies (GWAS), ethnicity, population stratification, admixture
Introduction
Allergic diseases are complex phenotypes wherein the interplay between genetic factors and environmental exposures has significant influence on susceptibility and disease prognosis. Over a decade of genetic mapping and positional cloning have revealed evidence suggesting linkage to over two dozen loci for asthma alone, and association studies have identified a multitude of variants associated with traits associated with allergic disease (asthma, allergic rhinitis, atopic dermatitis). With the emergence of genome-wide association studies (GWAS), the candidate gene approach is being replaced by a more unbiased approach to search for genes controlling risk to complex diseases, including atopy. In GWAS, multiple haplotype tagging SNPs (htSNP) from continental reference populations (i.e., the HapMap project1) theoretically allow for detection of associations to potentially causal variants, and have successfully broadened the scope of gene discovery for many complex traits, including allergic disease. For asthma alone, nearly a dozen reports of associations using GWAS have been published, but nearly all of these have been for European ancestry populations. Of the few that have been performed in minority populations, results are inconsistent with results from GWAS’s performed in European-ancestry cohorts, suggesting that populations of different ethnicities, such as those of African descent, may carry unique susceptibility loci. Of concern, however, is that the efficiency of htSNPs chosen from one population (e.g., European), and originally believed to be sufficient for detection of true associations, is questionable in their representation of other continental races (e.g., African) and thereby especially important in GWAS of admixed populations (e.g., African American). This review explores the findings to date from GWAS’s performed in ethnically diverse populations to identify polymorphisms that confer risk to asthma and its associated, ‘atopy-related’ traits in ethnically diverse populations. It further interrogates the pitfalls with the current GWAS technology, and considers the impact of anticipated advancements in the field, making it possible to broaden the focus of genetic studies of complex traits from populations of European-ancestry to better represent minority populations.
Does Genetic Variation Account for Ethnic Disparities in Allergic Disease?
It is well-accepted that allergic diseases, especially asthma, are disproportionately high and continue to increase among many ethnic minorities2. For example, the death rate due to asthma among African Americans is 4-6 times higher than among whites3, and certain Hispanic populations (which also share African ancestry), especially Puerto Ricans, have an even higher asthma prevalence and greater morbidity and mortality than African Americans4-6. For other underrepresented minority groups, fewer studies have been conducted to accurately compare prevalence and morbidity, but disparities are likely. Pacific Islanders (i.e., native Hawaiians), have historically suffered from more widespread and severe asthma than other ethnic groups living in Hawaii7, and among children 0-14 years old, asthma prevalence is 22% in Hawaiians/part-Hawaiians, compared to 14% in Filipino, 12% in Chinese, and 8% in Japanese and whites (http://hawaii.gov/health/statistics/hhs/hhs_07/index.html). The National Survey of Children’s Health, a telephone survey in 2003–2004 of a national random sample of parents and guardians which included over 100,000 children ages 0-17 years, is one of the few large epidemiological studies to include Native Americans and collect data on prevalence of allergic disease. In this survey, disparities in prevalence of asthma and allergies were observed when comparing white, African American, Latino, Asian/Pacific Islander, Native American, and multiracial children8. Specifically, Native American children were more likely to be asthmatic (14.2% vs. 11.5%, P= 0.0001) and have skin-related (i.e., eczema) allergies (12.0% vs. 9.2%, P= 0.0001) compared to whites.
Outside of the U.S., asthma prevalence is high and continues to increase in African countries and countries with African admixture (i.e., Latin America). In the most recent ISAAC report, prevalence continues to increase in the Caribbean country of Barbados specifically9. In the Asthma Insights and Reality in Latin America (AIRLA) survey, over 2,000 adults or children in 11 Latin American countries, including Colombia and Brazil, were evaluated, and this report concludes asthma is both underdiagnosed and poorly managed10. What has become increasingly clear, at least among U.S. populations, is that the striking racial and ethnic disparities in disease prevalence for common disorders, including asthma and allergies, cannot be explained entirely by environmental, social, cultural, or economic factors, and genetic factors are likely at play11.
A Dearth of Validated Genetic Determinants for Asthma and Allergic Disease among Ethnic Minorities
Asthma and its associated trait ‘atopy’ were perhaps some of the first complex diseases for which a strong genetic basis was established12-14. Nearly a dozen genome-wide linkage screens have been performed on asthma and its associated phenotypes15-25, which have identified 10 chromosomal regions giving multiple reports of linkage. From several of these family-based genomewide linkage screens, six novel genes were identified by further positional cloning25-30.
The huge research efforts and expense committed to asthma genetics from candidate gene and linkage studies have changed the perception about the etiology of asthma and allergic disease, including a new appreciation for the role of innate as well as adaptive immune-response genes, and the potential importance of the epithelial barrier and its defense mechanisms in asthma 14, 31. However, genomewide approaches have the advantage of being completely unbiased from a genetic perspective, and have emerged as a powerful approach for detecting novel genetic variants with plausible effect sizes for a plethora of complex traits, including asthma. To date, nearly 600 GWAS’s have been published on a plethora of complex traits 32.
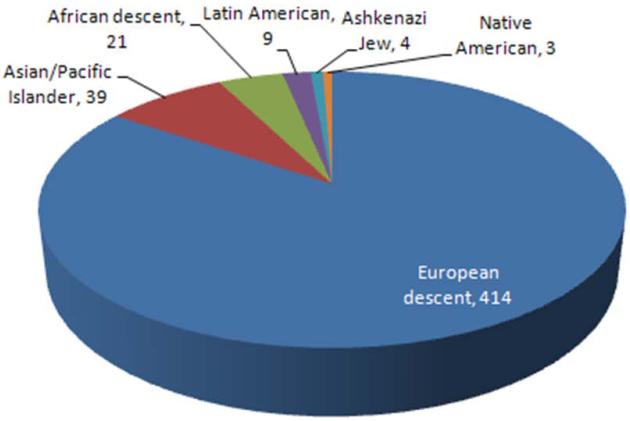
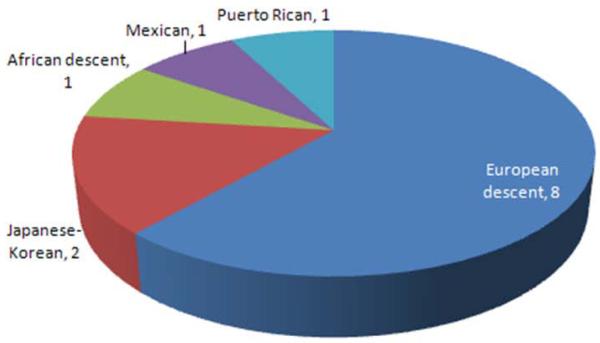
Notably, however, the GWAS’s performed so far have focused primarily on European ancestry populations as the discovery population, with very limited replication in non-European groups. In a review of 570 references from “A Catalog of Published Genome-Wide Association Studies” (http://www.genome.gov/gwastudies/), as of March, 2010, there have only been 72 studies that included cohorts from non-European populations (Figure 1, panel A). Within the asthma community, 13 groups have published results from their GWAS on asthma; however, of the asthma GWAS’s completed to date, only one focused on African Americans33 and two focused on Hispanics 34, 35 (Figure 1, panel B). To date, one GWAS has been published on AD in a German sample36, replicated in an independent German group, and a second, European-based GWAS is in the replication phase. A GWAS focusing on total IgE as the primary outcome37 leveraged the GABRIEL consortium (http://www.gabriel-fp6.org/public/index.htm), the European sample selected for asthma (currently 10,000 asthma cases and 16,000 non-asthmatic controls) from which the first results from a GWAS for asthma were generated.
Figure 1.
Summary of GWAS by ethnicity of the discovery population (as of March, 2010). Panel A: All GWAS’s published in the “Catalog of Published Genome-Wide Association Studies” (http://www.genome.gov/gwastudies/) according to ethnicity/race; Panel B: Published asthma GWAS’s to date according to ethnicity/race.
The initial GABRIEL report demonstrated strong association between asthma and markers near the ORMDL3 gene on chromosome 17q21 (P<10−12) 38, and has been widely replicated in several European ancestry39-43, Asian44, 45 and Hispanic46, 47 populations. However, the SNPs significantly associated in the discovery population were not associated in nearly 3,500 African Americans from Philadelphia48, an African American sample from San Francisco/Oakland area46, or African American and African Caribbean populations from Baltimore/Washington, D.C. and Barbados, respectively33. The San Francisco group did report significant association between a different ORMD3 SNP (rs9894164) and asthma among African Americans, but this marker was not on the original commercial SNP chip, underscoring the challenges in replicating SNP-for-SNP findings from GWAS performed in European populations. In the first asthma GWAS focusing exclusively on populations of African descent, replication at a SNP-for-SNP level was not observed across multiple independent populations; however, three genes (CTNNA3, DPP10 and KCNMA1) that showed evidence for association in the African-ancestry discovery samples were replicated in a European population using a gene-based approach33. Conclusions drawn from this study include the possibility that subtle differences in ancestry between populations can lead to a failure to replicate associations for individual SNPs, even when gene-based evidence does exist.
In one of the few observations of SNP-for-SNP replication of a GWAS for asthma, Hakonarson and colleagues observed significant associations between variants in the gene encoding DENND1B in a large sample of European Americans (P=7.8×10−8), which replicated in independent European-ancestry samples49. Replication was also found in a combined African American case-control group, but the association was with a different allele and the estimate OR was in the opposite direction. This ‘flip-flop’ phenomenon across different ethnic groups may reflect differences in genetic background and/or differences in linkage disequilibrium (LD) across populations, especially when non-causal ‘tag’ markers are tested50 (as is typical in GWAS using commercial chips). These observations similarly underscore the complexities of drawing firm conclusions from marker panels in distinct sub-populations, and the need to test beyond simple SNP-SNP replication.
The Complexities of Ethnically Admixed Populations in Genetic Association Studies
Genetic structure and substructure occurs when a population is comprised of more than one or more ethnic/racial groups representing different ancestries. Ethnic or racial admixture is particularly relevant for genetic studies in the U.S. and elsewhere in the Americas, where the unique population history has been characterized by a mixture of European ancestry with West Africans, indigenous groups (northern and southern Native Americans, as in the case of Mestizos in Central and South America), and a multitude of peoples from other distinct geographical regions. A serious problem arises when the disease of interest (e.g., asthma, atopy) is more prevalent in a particular group (or groups) within the population, because any alleles that are more common among the minority group(s) of interest will tend to be associated with the disease, even if completely unlinked to the disease-causing locus. This phenomenon is referred to as ‘population stratification’, and the consequent confounding due to population stratification can lead to spurious associations between genetic markers, especially in population-based (i.e., case-control) studies, and can limit generalizability of results to the broader population 51-53.
While matching the self-reported ethnic backgrounds of cases and controls in study design is one possibility, it is still possible to have hidden, or >cryptic= stratification 54. GWAS has accelerated the development of statistical approaches toward detecting stratification and controlling for ancestral differences in association testing. Currently there are a variety of algorithms that employ non-hierarchical cluster analysis or principal component analysis (PCA) for these statistical approaches 55, 56 . Correction for population stratification is typically performed using information on either: (1) markers for which allele frequencies differ substantially between ancestral populations referred to as ‘ancestry informative markers’ (AIMs); or (2) leveraging the large number of markers available in GWAS studies where it is easy to argue most are in fact unlinked to the true susceptibility loci for the disease under consideration 55, 57-61.
African Americans represent a racial/ethnic group that is both disproportionately affected by asthma and allergic disease (as described above) and a classic admixed population. To this end, ancestry estimation in this group has been extensively pursued. Relatively early studies demonstrated that African Americans are comprised of ~18%-20% European ancestry, but within the U.S. this proportion can vary regionally 62. African Caribbean populations are believed to be similar in terms of genetic ancestry, but using a relatively modest panel of AIMs, Torres and colleagues estimated the West African component to be higher (up to 90%) among certain West Indian groups 63.
In the first GWAS focused on populations of African descent, 417 AIMs were used combined with similar genotypic data from 210 unrelated individuals drawn from the HAPMAP data resource (60 YRI, 60 CEU, and 120 CHB/JPT) to define “ancestral populations” while estimating admixture in the ~2,000 study samples. Cases and controls were shown to be highly admixed with a balanced estimated proportion of African genes of 72.2 and 72.4%, respectively 33. It was further demonstrated that, after expanding this analysis to a large subset (~14,000 SNPs) of the full panel of autosomal SNPs from the GWAS chip (>620K) and also including two East African (Maasai and Luhya) populations from the newest version of HapMap, Phase III, the AIMs were just as good for detecting substructure larger numbers of random markers 64. These approaches for a variety of populations expected to have prominent African admixture component, as illustrated in Figure 2, show the highest proportion of YRI ancestry in the African Americans, Barbadians and Jamaicans (73-88%), and lower proportions in the Brazilian and Colombian data (38-50%).
Figure 2.
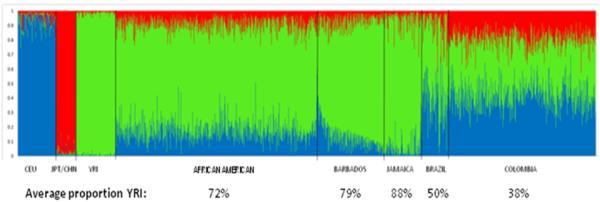
Heterogeneity of African admixture in five populations. African Americans (N= 906), GRAAD Barbadians (N = 294), Jamaicans (N = 174), Brazilians (N= 119) and Colombians (N=668), relying on autosomal SNPs that qualify as ancestry informative SNP markers (AIMs; 416, 416, 79, 79 and 52 AIMs, respectively) with absolute differences >0.4 in allele frequencies between pairs of putative parental populations (Utah residents with ancestry from northern and western Europe, or ‘CEU’, indicated in blue; Asians collectively represented by Japanese in Tokyo, Japan, or ‘JPT’/Han Chinese in Beijing, China, or ‘CHB’, indicated in red; and Yoruba in Ibadan, Nigeria, or ‘YRI’, indicated in green). Color combinations (blue, red, green) for the five test populations reflect the degree of admixture of the three parental populations. The semi-parametric Bayesian algorithm implemented in STRUCTURE (v2.2; http://pritch.bsd.uchicago.edu/software) was used to infer both mean and individual West African, Northern European and Asian ancestry proportions in these five populations along with the Phase II HapMap groups.
Admixture and the impact of population stratification are not limited to overtly admixed populations, as demonstrated by Tian and colleagues, who demonstrated a spurious correlation between SNPs in strong linkage disequilibrium with a candidate gene for rheumatoid arthritis in a set of rheumatoid arthritis cases recruited from multiple U.S. sites compared to a group of self-identified European Americans recruited in New York city 65. In this study, the investigators used a set of putative European substructure AIMs (ESAIMs), and demonstrated that the large differences in allele frequency for a gene initially showing a strong association with RA was actually due to the difference in allele frequency among different European subpopulation groups, whereby the largest allele frequency difference was between predominantly Irish controls from New York city and cases of Northern, Central, and Eastern European descent. These studies together underscore the importance of considering population stratification in any population-based genetic association study, perhaps most importantly in populations with a known strong admixture component.
Technological Limitations: Coverage on Commercially-Available GWAS SNP Chips and Power for GWAS
The GWAS approach is predicated on the notion that data cataloged in the International HapMap Project, combined with more accurate approaches in selecting tagging markers, will be sufficiently dense to capture most of the common variation in the human genome. As described elsewhere, HapMap was initially generated from 269 DNA samples representing four biogeographical groups. This has recently been expanded to 1,115 individuals from 11 populations 66. However, the commercial enterprises that create the chips have, for the most part, relied upon the European ancestry reference panel as a proxy for all continental groups. Not surprisingly, the GWAS approach is most successful in situations where the genetic variants evaluated are selected from populations most representative of the study population at hand (i.e., European Americans genotyped on markers largely selected from the CEU HapMap sample). Next generation sequencing technologies are rapidly driving the costs of assessing variation at every site in the genome down at a somewhat constant rate, and these may eventually replace commercially available genomewide platforms and at the very least compete with the price of capturing just a fraction of variable sites (e.g., via high density SNP arrays). Nevertheless, currently, the cost of a whole genome sequence is still prohibitive, so using SNP arrays will, for the immediate future, continue to be the most cost-effective strategy for GWAS.
A recently published perspective on the scientific value of GWAS67 suggested currently available commercial chips do capture the bulk of common genetic variation, and SNPs with large effects may have already been discovered, implying there is no need for additional genomewide scans, and focus should therefore shift towards a detailed search for rare variants. However, it is difficult to argue most important SNPs have already been discovered, if what constitutes one of the largest and most genetically heterogeneous continental populations – Africans - is so severely underrepresented in the GWAS literature. Second, while the collective findings of GWAS do support the notion that most identified genetic risk factors only account for a small fraction of observed heritability of most complex diseases and typically have an effect size <1.5, it is probable that multiple rare causal variants are more likely to be found in African ancestry populations and further argues for greater focus on this ethnic group.
In a recent analysis by co-investigator Bhangale and colleagues66, 76 genes were resequenced in unrelated HapMap samples (24 YRI, 23 CEU) and they examined coverage of all genetic variation data using various commercial genotyping arrays and Phase II HapMap SNPs to capture common variants in these 76 genes. In direct comparisons of coverage performance of the commercial arrays compared to the SeattleSNP-based coverage at r2≥0.8, the commercial chip with the highest proportion of YRI SNPs only provided an estimated 55% coverage.
The limitations of the current technology are especially apparent in data that are emerging from the “1000 Genomes” project (http://www.1000genomes.org), an international research consortium which aims to create the most detailed catalog to date of human genetic variation and provide a resource that will be useful for human disease studies. Samples from about 2,000 individuals from ~22 populations are being sequenced with the goal of finding nearly all DNA sequence variants. Relying on Phase 1 data, which includes the greatly expanded whole genome sequencing of the original four continental populations represented in HapMap, >17 million SNPs from 181 samples have been identified, which includes >9M novel discovery SNPs. In other words, ~54% SNPs were not previously reported in the public database (Mathias, Qin, Abacasis, unpubl data). As illustrated in Figure 3, in the YRI data specifically, there are 12,068,821 SNPs identified in 50 samples, of which 5,953,505 were novel SNPs. Moreover, in an assessment of the overlap in variation between the four HAPMAP populations, each population has a set of unique SNPs (0.51 – 5.65 million), with the largest representation of unique variation in the YRI samples, with 5.65 million unique SNPs.
Figure 3.
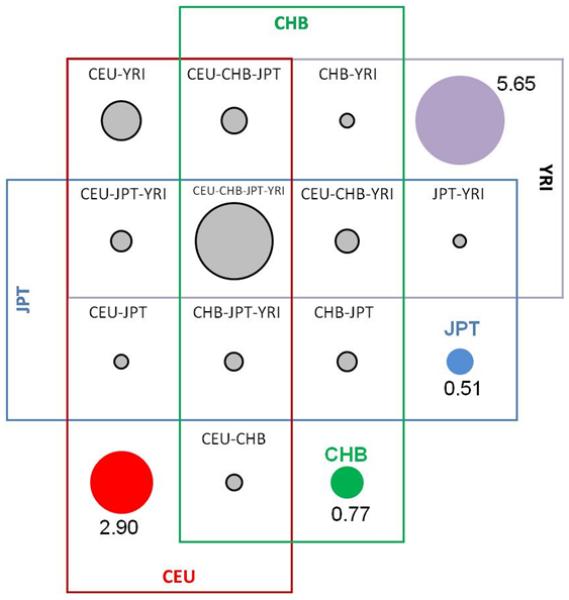
A graphical representation of the overlap in 17million SNPs between four HAPMAP populations according to Phase 1 data from the 1000 Genomes Project (August, 2009). Purple SNPs are unique to YRI, red SNPs are unique to CEU, blue SNPs are unique to JPT, and green SNPs are unique to CHB. Adapted from HapMap (http://www.hapmap.org/).
Conclusion
Despite the major advancements in the identification of novel genes associated with asthma and allergic disease through GWAS, few studies have been conducted in non-European populations. GWAS in ethnically and racially diverse populations demands consideration of characteristics unique to underrepresented cohorts, including population heterogeneity due to admixture. A critical limitation in conducting GWAS on ethnically and racially diverse samples arises because commercially available chips were constructed using SNPs identified in European derived samples and the density of these SNP panels does not provide adequate coverage. New technologies are on the horizon that promise to more accurately capture the genetic diversity of all continental populations, and advance the field.
Acknowledgements
The author wishes to thank Dr. Candelaria Vergara, and Nicholas Rafaels, Tanda Murray and Pat Oldewurtel for technical assistance. A special thanks to Drs. Rasika A. Mathias, Zhaohui Qin, and Gonçalo Abecasis who generated critical preliminary data and contributed to important discussions. The author gratefully acknowledges the contributions of the Genomic Research on Asthma in the African Diaspora (GRAAD) consortium in generating much of the data used in this review.
Funding Disclosure: The author was supported in part by the Mary Beryl Patch Turnbull Scholar Program and by NIH HL087699.
Footnotes
The author has no conflicts of interest to report.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altshuler D, Brooks LD, Chakravarti A, Collins FS, Daly MJ, Donnelly P. A haplotype map of the human genome. Nature. 2005;437:1299–320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph CL, Williams LK, Ownby DR, Saltzgaber J, Johnson CC. Applying epidemiologic concepts of primary, secondary, and tertiary prevention to the elimination of racial disparities in asthma. J Allergy Clin Immunol. 2006;117:233–40. doi: 10.1016/j.jaci.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIAID . Asthma: A Concern for Minority Populations. National Institutes of Health; 2001. [Google Scholar]
- 4.Carter-Pokras O, Gergen P. Reported asthma among Puerto Rican, Mexican-American, and Cuban Children: 1982 through 1984. American Journal of Public Health. 1993;83(4):580–2. doi: 10.2105/ajph.83.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homa DM, Mannino DM, Lara M. Asthma Mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban Heritage, 1990-1995. American Journal of Respiratory and Critical Care Medicine. 2000;161(2):504–9. doi: 10.1164/ajrccm.161.2.9906025. [DOI] [PubMed] [Google Scholar]
- 6.Burchard EG, Avila PC, Nazario S, Casal J, Torres A, Rodriguez-Santana JR, et al. Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma. American Journal of Respiratory and Critical Care Medicine. 2004;169(3):386–92. doi: 10.1164/rccm.200309-1293OC. [DOI] [PubMed] [Google Scholar]
- 7.Massey DG, Hope BE, Fournier-Massey G. Asthma in Hawaii: a tradition of excess mortality. J Asthma. 1997;34:113–7. doi: 10.3109/02770909709075655. [DOI] [PubMed] [Google Scholar]
- 8 *.Flores G, Tomany-Korman SC. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121:e286–98. doi: 10.1542/peds.2007-1243. One of the most comprehensive epidemiological surveys of asthma and allergic disease to include all racial and ethnic groups in the U.S.
- 9.Pearce N, Ait-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62:758–66. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GINA GIfA AJAf, editor. Global strategy for asthma management and prevention. 2008 doi: 10.1183/09031936.00138707. www.ginasthma.com [DOI] [PubMed]
- 11.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proc Am Thorac Soc. 2007;4:58–68. doi: 10.1513/pats.200607-146JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes KC. Genetics and epidemiology. Curr Opin Allergy Clin Immunol. 2001;1:383–5. doi: 10.1097/01.all.0000011049.37817.c2. [DOI] [PubMed] [Google Scholar]
- 13.Barnes KC. Atopy and asthma genes - Where do we stand? European Journal of Allergy and Clinical Immunology. 2000;55:803–17. doi: 10.1034/j.1398-9995.2000.00123.x. [DOI] [PubMed] [Google Scholar]
- 14.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006 doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 15.Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, et al. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383:247–50. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- 16.CSGA The Collaborative Study on the Genetics of Asthma: A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nature Genetics. 1997;15(4):389–92. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 17.Ober C, Cox NJ, Abney M, Di Rienzo A, Lander ES, Changyaleket B, et al. Genome-wide search for asthma susceptibility loci in a founder population. The Collaborative Study on the Genetics of Asthma. Human Molecular Genetics. 1998;7(9):1393–8. doi: 10.1093/hmg/7.9.1393. [DOI] [PubMed] [Google Scholar]
- 18.Malerba G, Trabetti E, Patuzzo C, Lauciello MC, Galavotti R, Pescollderungg L, et al. Candidate genes and a genome-wide search in Italian families with atopic asthmatic children. Clinical and Experimental Allergy. 1999;29(Suppl 4):27–30. [PubMed] [Google Scholar]
- 19.Wjst M, Fischer G, Immervoll T, Jung M, Saar K, Rueschendorf F, et al. A genome-wide search for linkage to asthma. Genomics. 1999;58(1):1–18. doi: 10.1006/geno.1999.5806. German Asthma Genetics Group. [DOI] [PubMed] [Google Scholar]
- 20.Dizier MH, Besse-Schmittler C, Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J, et al. Genome screen for asthma and related phenotypes in the French EGEA study. American Journal of Respiratory and Critical Care Medicine. 2000;162(5):1812–8. doi: 10.1164/ajrccm.162.5.2002113. [DOI] [PubMed] [Google Scholar]
- 21.Ober C, Tsalenko A, Parry R, Cox NJ. A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. American Journal of Human Genetics. 2000;67(5):1154–62. doi: 10.1016/s0002-9297(07)62946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokouchi Y, Nukaga Y, Shibasaki M, Noguchi E, Kimura K, Ito S, et al. Significant evidence for linkage of mite-sensitive childhood asthma to chromosome 5q31-q33 near the interleukin 12 B locus by a genome-wide search in Japanese families. Genomics. 2000;66(2):152–60. doi: 10.1006/geno.2000.6201. [DOI] [PubMed] [Google Scholar]
- 23.Laitinen T, Daly MJ, Rioux JD, Kauppi P, Laprise C, Petays T, et al. A susceptibility locus for asthma-related traits on chromosome 7 revealed by genome-wide scan in a founder population. Nature Genetics. 2001;28(1):87–91. doi: 10.1038/ng0501-87. [DOI] [PubMed] [Google Scholar]
- 24.Hakonarson H, Bjornsdottir US, Halapi E, Palsson S, Adalsteinsdottir E, Gislason D, et al. A Major Susceptibility Gene for Asthma Maps to Chromosome 14q24. American Journal of Human Genetics. 2002;71(3) doi: 10.1086/342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418(6896):426–30. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 26.Allen M, Heinzmann A, Noguchi E, Abecasis G, Broxholme J, Ponting CP, et al. Positional cloning of a novel gene influencing asthma from chromosome 2q14. Nature Genetics. 2003;35(3):258–63. doi: 10.1038/ng1256. [DOI] [PubMed] [Google Scholar]
- 27.Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, et al. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–4. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- 28.Nicolae D, Cox NJ, Lester LA, Schneider D, Tan Z, Billstrand C, et al. Fine mapping and positional candidate studies identify HLA-G as an asthma susceptibility gene on chromosome 6p21. American Journal of Human Genetics. 2005;76:349–57. doi: 10.1086/427763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi E, Yokouchi Y, Zhang J, Shibuya K, Shibuya A, Bannai M, et al. Positional identification of an asthma susceptibility gene on human chromosome 5q33. Am J Respir Crit Care Med. 2005;172:183–8. doi: 10.1164/rccm.200409-1223OC. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Leaves NI, Anderson GG, Ponting CP, Broxholme J, Holt R, et al. Positional cloning of a quantitative trait locus on chromosome 13q14 that influences immunoglobulin E levels and asthma. Nature Genetics. 2003;34(2):181–6. doi: 10.1038/ng1166. [DOI] [PubMed] [Google Scholar]
- 31.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 32 **.Hindorff LA, Junkins HA, Mehta JP, Manolio TA. [accessed S, ed, 2009];A catalog of published genome-wide association studies. Available at http://www.genome.gov/26525384. The most comprehensive and up to date summary of all published GWAS’s.
- 33 **.Mathias RA, Grant AV, Rafaels N, Hand T, Gao L, Vergara C, et al. A genome-wide association study on African-ancestry populations for asthma. Journal of Allergy and Clinical Immunology. 2010;125:336–46. doi: 10.1016/j.jaci.2009.08.031. The first published GWAS on asthma focused exclusively on populations of African descent as the discovery populations, deomonstrating the importance of a gene-centric approach in GWAS when comparing diverse ancestral populations.
- 34 **.Hancock DB, Romieu I, Shi M, Sienra-Monge JJ, Wu H, Chiu GY, et al. Genome-wide association study implicates chromosome 9q21.31 as a susceptibility locus for asthma in mexican children. PLoS Genet. 2009;5:e1000623. doi: 10.1371/journal.pgen.1000623. One of only two published GWAS’s to date focused on Hispanic populations.
- 35 **.Choudhry S, Taub M, Mei R, Rodriguez-Santana J, Rodriguez-Cintron W, Shriver MD, et al. Genome-wide screen for asthma in Puerto Ricans: evidence for association with 5q23 region. Hum Genet. 2008;123:455–68. doi: 10.1007/s00439-008-0495-7. One of only two published GWAS’s to date focused on Hispanic populations.
- 36 *.Esparza-Gordillo J, Weidinger S, Folster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596–601. doi: 10.1038/ng.347. The only published GWAS on AD to date.
- 37 *.Weidinger S, Gieger C, Rodriguez E, Baurecht H, Mempel M, Klopp N, et al. Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus. PLoS Genet. 2008;4:e1000166. doi: 10.1371/journal.pgen.1000166. The only published GWAS on total serum IgE to date.
- 38.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression are determinants of susceptibility to childhood asthma. Nature. 2007;448:470–3. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 39.Bouzigon E, Corda E, Aschard H, Dizier MH, Boland A, Bousquet J, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359:1985–94. doi: 10.1056/NEJMoa0806604. [DOI] [PubMed] [Google Scholar]
- 40.Madore AM, Tremblay K, Hudson TJ, Laprise C. Replication of an association between 17q21 SNPs and asthma in a French-Canadian familial collection. Hum Genet. 2008;123:93–5. doi: 10.1007/s00439-007-0444-x. [DOI] [PubMed] [Google Scholar]
- 41.Sleiman PM, Annaiah K, Imielinski M, Bradfield JP, Kim CE, Frackelton EC, et al. ORMDL3 variants associated with asthma susceptibility in North Americans of European ancestry. J Allergy Clin Immunol. 2008;122:1225–7. doi: 10.1016/j.jaci.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 42.Tavendale R, Macgregor DF, Mukhopadhyay S, Palmer CN. A polymorphism controlling ORMDL3 expression is associated with asthma that is poorly controlled by current medications. J Allergy Clin Immunol. 2008;121:860–3. doi: 10.1016/j.jaci.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Bisgaard H, Bonnelykke K, Sleiman PM, Brasholt M, Chawes B, Kreiner-Moller E, et al. Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. Am J Respir Crit Care Med. 2009;179:179–85. doi: 10.1164/rccm.200809-1436OC. [DOI] [PubMed] [Google Scholar]
- 44.Hirota T, Harada M, Sakashita M, Doi S, Miyatake A, Fujita K, et al. Genetic polymorphism regulating ORM1-like 3 (Saccharomyces cerevisiae) expression is associated with childhood atopic asthma in a Japanese population. J Allergy Clin Immunol. 2008;121:769–70. doi: 10.1016/j.jaci.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 45.Leung TF, Sy HY, Ng MC, Chan IH, Wong GW, Tang NL, et al. Asthma and atopy are associated with chromosome 17q21 markers in Chinese children. Allergy. 2009;64:621–8. doi: 10.1111/j.1398-9995.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 46 **.Galanter J, Choudhry S, Eng C, Nazario S, Rodriguez-Santana JR, Casal J, et al. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med. 2008;177:1194–200. doi: 10.1164/rccm.200711-1644OC. The only study to date to demonstrate replication of association between variants in the ORMDL3 gene and asthma in both Hispanic and African American samples, but further demonstrating the difficulties in SNP-for-SNP replication in that significant association between an ORMD3 SNP and asthma in a locus distinct from the original report.
- 47.Wu H, Romieu I, Sienra-Monge JJ, Li H, del Rio-Navarro BE, London SJ. Genetic variation in ORM1-like 3 (ORMDL3) and gasdermin-like (GSDML) and childhood asthma. Allergy. 2009;64:629–35. doi: 10.1111/j.1398-9995.2008.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flory JH, Sleiman PM, Christie JD, Annaiah K, Bradfield J, Kim CE, et al. 17q12-21 variants interact with smoke exposure as a risk factor for pediatric asthma but are equally associated with early-onset versus late-onset asthma in North Americans of European ancestry. J Allergy Clin Immunol. 2009;124:605–7. doi: 10.1016/j.jaci.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 49 **.Sleiman PMA, Imielinski M, Bradfield JP, Annaiah K, Willis-Owen S, Rafaels NM, et al. Variants of DENND1B associated with asthma in children. New Engl J Med. 2010;362:36–44. doi: 10.1056/NEJMoa0901867. One of the only GWAS’s in asthma to demonstrate replication of a finding in an African American sample; however, the association was with a different allele and the estimate odds ratio was in the opposite direction, illustrating the ‘flip-flop’ phenomenon which is not uncommon across different ethnic groups.
- 50.Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. Am J Hum Genet. 2007;80:531–8. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchini J, Cardon LR, Phillips MS, Donnelly P. The effects of human population structure on large genetic association studies. Nat Genet. 2004;36:512–7. doi: 10.1038/ng1337. [DOI] [PubMed] [Google Scholar]
- 52.Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, Patterson N, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388–93. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 53.Clayton DG, Walker NM, Smyth DJ, Pask R, Cooper JD, Maier LM, et al. Population structure, differential bias and genomic control in a large-scale, case-control association study. Nat Genet. 2005;37:1243–6. doi: 10.1038/ng1653. [DOI] [PubMed] [Google Scholar]
- 54.Ewens WJ, Spielman RS. The transmission/disequilibrium test: history, subdivision, and admixture. American Journal of Human Genetics. 1995;57:455–64. [PMC free article] [PubMed] [Google Scholar]
- 55.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55(4):997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 58.McKeigue PM, Carpenter JR, Parra EJ, Shriver MD. Estimation of admixture and detection of linkage in admixed populations by a Bayesian approach: application to African-American populations. Annals of Human Genetics. 2000;64(2):171–86. doi: 10.1017/S0003480000008022. [DOI] [PubMed] [Google Scholar]
- 59.Reich DE, Goldstein DB. Detecting association in a case-control study while correcting for population stratification. Genetic Epidemiology. 2001;20(1):4–16. doi: 10.1002/1098-2272(200101)20:1<4::AID-GEPI2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 60.Schork NJ, Fallin D, Thiel B, Xu X, Broeckel U, Jacob HJ, et al. The future of genetic case-control studies. Advances in Genetics. 2001;42:191–212. doi: 10.1016/s0065-2660(01)42023-2. [DOI] [PubMed] [Google Scholar]
- 61.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, et al. Estimating African American admixture proportions by use of population-specific alleles. American Journal of Human Genetics. 1998;63(6):1839–51. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benn-Torres J, Bonilla C, Robbins CM, Waterman L, Moses TY, Hernandez W, et al. Admixture and Population Stratification in African Caribbean Populations. Ann Hum Genet. 2006 doi: 10.1111/j.1469-1809.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 64 *.Murray T, Beaty TH, Mathias RA, Rafaels N, Grant AV, Faraque MU, et al. African and Non-African Admixture Components in African Americans and an African Caribbean Population. Genetic Epidemiology. 2010 doi: 10.1002/gepi.20512. In Press. An in-depth report on the extent to which additional and valuable information is captured when including many random autosomal markers versus a fewer number of select ancestry informative markers (AIMs) to interrogate the ancestral background of pouplation-based and family-based samples selected for asthma.
- 65 **.Tian C, Plenge RM, Ransom M, Lee A, Villoslada P, Selmi C, et al. Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet. 2008;4:e4. doi: 10.1371/journal.pgen.0040004. Demonstrates the potentially profound impact of cryptic population stratification in European ancestral populations.
- 66 **.Bhangale TR, Rieder MJ, Nickerson DA. Estimating coverage and power for genetic association studies using near-complete variation data. Nat Genet. 2008;40:841–3. doi: 10.1038/ng.180. A critical report illustrating the limitations of commercially available GWAS chips in capturing true associations in non-European populations.
- 67 *.Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696–8. doi: 10.1056/NEJMp0806284. A succinct review of the power of GWAS as well as its limitations and the potential impact of new technologies in combination with or in addition to GWAS.