Abstract
Introduction
Because of their important roles in disease and excellent “druggability”, kinases have become the second-largest drug target family. The great success of the BCR-ABL inhibitor imatinib in treating CML illustrates the high potential of kinase inhibitor (KI) therapeutics, but also unveiled a major limitation: the development of drug-resistance. This is a significant concern as KIs reach large patient populations for an expanding array of indications.
Areas covered
We provide an up-to-date understanding of the mechanisms through which KIs function, and through which cells can become KI-resistant. We review current and future approaches to overcome KI-resistance, focussing on currently approved KIs and KIs in clinical trials. We then discuss approaches to improve KI efficacy and overcome drug-resistance and novel approaches to develop less drug-resistance prone KI-therapeutics.
Expert opinion
Although drug-resistance is a concern for current KI-therapeutics, recent progress in our understanding of the underlying mechanisms and promising technological advances may overcome this limitation and provide powerful new therapeutics.
Keywords: kinase, imatinib, CML, Bcr, ABL, drug resistance
1. Introduction
By transferring the γ-phosphate from the ATP-cofactor onto diverse substrates, kinases regulate almost every aspect of cellular function, including cell growth, metabolism, proliferation, differentiation, migration, effector functions and death. Perturbed expression, subcellular localization or function of many kinases can cause diseases. Often, this results from inherited or acquired mutations in kinase genes. 164 of 518 human kinase genes are located in genome regions implicated in cancer, another ~80 in regions implicated in other diseases. Cancer “driver” mutations may occur in approximately 120 genes. Among various protein kinase structural domains, “kinase domains” (KD), which harbor both ATP-binding site and catalytic center, are most frequently encoded by cancer genes1, 2. A recent study of 915 human disease-associated kinase mutations implicated 50 kinases in 67 germline-inherited diseases, mainly developmental and metabolic disorders and cancers3. A prominent example for a “kinasopathy” caused by a somatic kinase-gene mutation is the causative role of hyperactive Breakpoint Cluster Region-Abelson kinase (BCR-ABL) fusion proteins in chronic-myelogenous-leukemia (CML, accounts for ≤20% of western adult leukemias) and some acute-lymphoblastic-leukemias (AML)4. Other cancers that are driven by hyperactive or deregulated kinases include non small-cell lung cancer (NSCLC), gastrointestinal stromal tumors (GIST), renal cell carcinoma (RCC), breast cancer (BC), melanoma and thyroid carcinoma (Table 5)1, 5-7.
Table 5.
Exemplary drug-resistance causing point mutations in non-ABL kinases, and drug sensitivity associated EGFR mutations
| Kinase | Mutation | Clinically observed in disease |
Resistant to (or sensitized for, italic) these inhibitors |
Sensitive to these inhibitors |
Topological Location |
Mechanism | Analogous drug-resistant mutations in other kinases |
References |
|---|---|---|---|---|---|---|---|---|
| FLT3 | N676K/D | AML | PKC412 | N-terminal of hinge region | Destabilizes conformation of hinge region, disrupting inhibitor hydrogen-bonds | ABL Q319H | 120, 128, 134 | |
| KIT | V559A | GIST | Imatinib | SH2-KD linker | Secondary resistance mutation. | 6 | ||
| KIT | V654A/E | GIST | Imatinib | Sunitinib | ATP-pocket | Secondary resistance mutation. Intrinsically Imatinib resistant. Reduces surface complementarity with drug, removes hydrophobic contacts with diaminophenyl ring of drug. | 6, 102-105, 107 | |
| KIT | T670I/E | GIST | Imatinib | Sunitinib, PKC412, Sorafenib | Gatekeeper residue | Secondary resistance mutation. Precludes access to ATP-site adjacent Type 2/3 allosteric site, stabilizes hydrophobic spine which stabilizes active kinase conformation58, 59. Potential additional allosteric effects on inhibitory SH3 domain interactions75. See T315I discussion in table 4 for details. | ABL T315I; PDGFRα: T674I; PDGFRβ: T681I; EGFR: T790M; ERBB2: T733I; FGFR1: V561M; RET: V804L/M; FLT3: G697R; c-SRC: T341M; v-SRC: I338 exchange for T341 in c-Src. AURORA-A: T217D. Bold: Most frequently reported. | 79, 102, 103, 105, 107 |
| KIT | S709F | GIST | Imatinib | KI domain | Secondary resistance mutation. | 103 | ||
| KIT | D716N | GIST | Imatinib | KI domain | Secondary resistance mutation. | 103 | ||
| KIT | L783V | GIST | Imatinib | Catalytic loop | Secondary resistance mutation. | 103 | ||
| KIT | C809G | GIST | Imatinib | A-loop | Secondary resistance mutation immediately preceding DFG motif. | EGFRa T854A | 103, 105, 107 | |
| KIT | R815 deletion | GIST | Imatinib | A-loop | Secondary resistance mutation. R815 corresponds to ABL-1b R405 (RA) which forms a salt-bridge with E305 (EαC) in the SFK-like inactive conformation, and in inactive SFKs. | 103, 107 | ||
| KIT | D816H/V/G/E or deletion | GIST | Imatinib, Sunitinib, Sorafenib, PKC412, Dasatinib | A-loop | Secondary resistance mutation. Can occur as primary mutation. D816 stabilizes A-loop in inactive conformation via Hydrogen-bonds to K818 and D819 backbones. Mutation shifts conformational equilibrium to active by disrupting one of these and in part by destabilizing inhibitory JM domain conformation. | PDGFRA D842V (drug-resistant); EGFR L861Q (drug sensitizing); ABL has a 1 AA deletion at this position. | 102-107 | |
| KIT | K818R | GIST | Imatinib | A-loop | Secondary resistance mutation. | ABL M407I/L; EGFR G863D | 103, 104, 107 | |
| KIT | D820A/G/Y/H/E | GIST | Imatinib, Sunitinib | A-loop | Secondary resistance mutation. | PDGFRA D846V | 6, 103, 104, 107 | |
| KIT | N822K/H/Y | GIST | Imatinib, Sunitinib | A-loop | Secondary resistance mutation. Imatinib resistance requires coupling to activating/on cogenic KIT juxtamembrane domain mutant. Also found as primary mutation. | 6, 102-105, 107 | ||
| KIT | Y823D | GIST | Imatinib, Sunitinib | A-loop, corresponds to YA in ABL and SFKs which is autophosphorylated upon activation. This stabilizes the active conformation. KIT Y823D mutation might thus stabilize the active conformation. | Secondary resistance mutation. Also found as primary mutation. | 102-105, 107 | ||
| KIT | A829P | GIST | Imatinib, Sunitinib | A-loop | Secondary resistance mutation. | 102, 104 | ||
| PDGFRA | T674I | HES, CEL | Imatinib | Sorafenib | Gatekeeper residue | Secondary resistance mutation. Precludes access to ATP-site adjacent Type 2/3 allosteric site, stabilizes hydrophobic spine which stabilizes active kinase conformation58, 59. Potential additional allosteric effects on inhibitory SH3 domain interactions75. See T315I discussion in table 4 for details. | ABL T315I; c-KIT: T670I; PDGFRβ: T681I; EGFR: T790M; ERBB2: T733I; FGFR1: V561M; RET: V804L/M; FLT3: G697R; c-SRC: T341M; v-SRC: I338, exchange for T341 in c-Src. AURORA-A: T217D. Bold: Most frequently reported. | 103, 107 |
| PDGFRA | H687Y | GIST | Imatinib | C-lobe, N-terminal of KI region | Secondary resistance mutation. | 6 | ||
| PDGFRA | D842V | GIST | Imatinib, Sorafenib, PKC412 | A-loop | Major PDGFRA mutation. Secondary resistance mutation. Can also occur as primary mutation. | KIT D816H/V/G/E or deletion (drug-resistant); EGFR L861Q (drug sensitizing) ABL has a 1 AA deletion at this position. | 103, 104, 107, 142 | |
| PDGFRA | D846V | GIST | Imatinib | A-loop | Secondary resistance mutation. | KIT D820A/G/Y/H/E | 103 | |
| EGFR | EGFRvIII | Glioblastoma, SCC | Gefitinib, Erlotinib | Irreversible EGFR inhibitors | Deletes 801 bp from extracellular domain | Oncogenic. Not in KD. | 9, 64, 68, 71, 91 | |
| EGFR | V689M | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | SH2-KD-linker | DRUG SENSITIZING. | 68 | |
| EGFR | N700D | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | SH2-KD-linker | DRUG SENSITIZING. | 68 | |
| EGFR | E709A/G | NSCLC | Lapatinib? | G-loop, β-sheet 1 | Clinically associated with increased Gefitinib or Erlotinib sensitivity. E709G associated with Lapatinib resistance in the presence of ERBB2 in a mutagenesis screen. | 64, 68, 88 | ||
| EGFR | E709K/Q | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | G-loop, β-sheet 1 | DRUG SENSITIZING. | 64, 68 | |
| EGFR | G719C/S/A | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | G-loop | DRUG SENSITIZING. G719S is ONCOGENIC. HYPERACTIVE. | 64, 68 | |
| EGFR | S720P | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | G-loop | DRUG SENSITIZING. | ABL G269A/E/R (drug-resistant) | 68 |
| EGFR |
ΔE746-A750/T751/A750(InsRP) /T751(InsA/I or VA)/S752(InsA/V) |
NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | β-sheet 3/αC Helix | DRUG SENSITIZING. ONCOGENIC. HYPERACTIVE. Reduced ATP affinity. Effect may depend on cellular context. ΔE746-A750 may also confer gefitinib resistance. | Overlap with ABL E294K or D295V/G mutations (drug-resistant) | 68 |
| EGFR |
ΔL747/E749(A750P)/A750(InsP) /T751/T751(InsP/S)/S752/752(E746V or P753S)/S752(InsQ)/P753/P753(InsS) |
NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | β-sheet 3/αC Helix | DRUG SENSITIZING. ONCOGENIC. HYPERACTIVE. | Overlap with ABL E294K or D295V/G mutations (drug-resistant) | 64, 68 |
| EGFR | L747S | NSCLC | Gefitinib, Erlotinib; Lapatinib? | β-sheet 3/αC Helix | Various deletions or insertions starting at L747 clinically associated with increased Gefitinib or Erlotinib sensitivity. Associated with Lapatinib resistance in a mutagenesis screen. | ERBB2 L755S | 68, 88, 101 | |
| EGFR | ΔS752-I759 | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | αC Helix | DRUG SENSITIZING. | Overlaps with ABL M297L, E298K, E300K, E301D, F302L (drug-resistant) | 68 |
| EGFR | D761Y | NSCLC | Gefitinib, Erlotinib | αC Helix | 9, 64, 68, 71, 88, 91, 101 | |||
| EGFR | V765A | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | αC Helix | DRUG SENSITIZING. | ABL V308S/I (drug-resistant) | 68 |
| EGFR | S768I | NSCLC | Gefitinib, Erlotinib | αC Helix | ABL E311Q/S (drug-resistant) ERBB2 G776 (Ins VG/C) | 64, 68 | ||
| EGFR | V769L | NSCLC | Gefitinib, Erlotinib. Lapatinib? | αC Helix | Associated with Lapatinib and Erlotinib resistance in a mutagenesis screen. | 68, 88 | ||
| EGFR | D770Y | Lapatinib? | αC Helix | Various insertions starting at D770 are clinically associated with Gefitinib and Erlotinib resistance (see below). D770Y associated with Lapatinib resistance in a mutagenesis screen. | ABL K313R (drug-resistant) | 88 | ||
| EGFR | D770-N771 (ins NPG) | NSCLC | Gefitinib, Erlotinib | αC Helix | ONCOGENIC. HYPERACTIVE. | Overlaps ABL K313R (drug-resistant) | 9, 64, 68, 71, 91 | |
| EGFR | D770-N771 (ins SVQ) | NSCLC | Gefitinib, Erlotinib | αC Helix | Overlaps ABL K313R (drug-resistant) | 68 | ||
| EGFR | D770-N771 (ins G), N771T | NSCLC | Gefitinib, Erlotinib | αC Helix | Overlaps ABL K313R (drug-resistant) | 64, 68 | ||
| EGFR | T783A | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | Hinge region | DRUG SENSITIZING. | 68 | |
| EGFR | T790M | NSCLC | Gefitinib, Erlotinib. Lapatinib? | Irreversible EGFR inhibitors such as WZ4002 (preclinical) | Type 2 allosteric pocket adjacent to ATP binding site. Gatekeeper residue. | Gatekeeper mutation, detected as acquired resistance mutation in ~50% of clinically Gefitinib or Erlotinib resistant patients. May rarely also occur as primary resistance mutation together with a sensitizing mutation. Unlike the ABL T315I mutation, EGFR T790M only mildly affects gefitinib binding but restores ATP affinity, which is often reduced by the primary mutations, to wildtype EGFR levels81. The mutation increases activity and oncogenicity and may play a role in inherited lung cancer susceptibility. | AH drug-resistant: ABL T315I; c-KIT: T670I; PDGFRα: T674I;PDGFRβ: T681I; ERBB2: T733I; FGFR1: V561M; RET: V804L/M; FLT3: G697R; c-SRC: T341M; v-SRC: I338, exchange for T341 in c-Src. AURORA-A: T217D. Bold: Most frequently reported. | 9, 64, 68, 71, 79, 81, 82, 88, 91, 101, 119 |
| EGFR | G796D/R/C | NSCLC | Gefitinib; Erlotinib or CI-1033? | C-lobe, solvent channel | Associated with Erlotinib or CI-1033 resistance in a mutagenesis screen | ABL G340W/E (drug-resistant) | 9, 64, 68, 71, 91 | |
| EGFR | N826S | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | C-lobe | DRUG SENSITIZING. | ABL E371K/G (drug-resistant) | 68 |
| EGFR | A839T | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | C-lobe | DRUG SENSITIZING. | 68 | |
| EGFR | K846R | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | C-lobe | DRUG SENSITIZING. | ABL G391R (drug-resistant) | 68 |
| EGFR | T854A | NSCLC | Gefitinib, Erlotinib | Irreversible inhibitors. | C-lobe | Drug contact site. Also associated with Erlotinib resistance in a mutagenesis screen. | cKIT C809G (drug-resistant) | 88, 101, 143 |
| EGFR | L858R | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | KD-CL, A-loop | DRUG SENSITIZING. ONCOGENIC. HYPERACTIVE. Reduced ATP affinity. | ABL L403M (drug-resistant) | 1, 64, 68, 71, 73 |
| EGFR | L861Q | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | A-loop | DRUG SENSITIZING. | KIT D816H/V/G/E or deletion (drug-resistant); PDGFRA D842V (drug-resistant) ABL has a 1 AA deletion at this position. | 64, 68 |
| EGFR | G863D | NSCLC | Gefitinib, Erlotinib | Gefitinib, Erlotinib | A-loop | DRUG SENSITIZING. | ABL M407I/L (drug-resistant); KIT K818R (drug-resistant) | 68 |
| EGFR | G863S | NSCLC | Lapatinib? | A-loop | G863D clinically associated with increased Gefitinib or Erlotinib sensitivity. G863S associated with Lapatinib resistance in a mutagenesis screen. | ABL M407I/L (drug-resistant); KIT K818R (drug-resistant) | 68, 88 | |
| EGFR | E884K | NSCLC | Gefitinib, Erlotinib | Gefitinib | A-loop | Confers sensitivity to Gefitinib, but resistance to Erlotinib. Disrupts A-loop E884-salt bridge with R958 in C-lobe, may alter substrate interactions and/or A-loop flexibility. | MET E1271K KIT E839K RET E921K | 64, 74, 108 |
| ERBB2 | T733I | Gastric cancer | Lapatinib (in vitro) | G-loop | Oncogenic, found in clinical tumors. | ABL E274(255) K/V | 5 | |
| ERBB2 | L755S | Gastric and breast cancer | Lapatinib (in vitro) | β-sheet 3/αC Helix | Oncogenic, found in clinical tumors | EGFRA L747S | 5 | |
| ERBB2 | G776 (Ins VG/C) | NSCLC | Erlotinib (in vitro) | Irreversible EGFR inhibitors | αC Helix | Constitutively active. | ABL E311Q/S (drug-resistant) EGFR S768I | 9, 64, 68, 71, 91 |
| ERBB2 | T798I | Lapatinib (in vitro) | N-lobe | Gatekeeper residue; found in cell based screen | c-KIT: T670I; PDGFRα: T674I;PDGFRβ: T681I; EGFR: T790M; FGFR1: V561M; RET: V804L/M; FLT3: G697R; c-SRC: T341M; v-SRC: I338, exchange for T341 in c-Src. AURORA-A: T217D. Bold: Most frequently reported. | 5 |
In KIT and EGFR, drug-resistance mutations are usually secondary to primary mutations that hyperactivate the kinase and are oncogenic. Many of these sensitize the kinase to drug inhibition, possibly by shifting the conformational equilibrium towards the active KD conformation which binds with higher affinity to gefitinib and erlotinib71. Due to the large number of potential drug-resistant mutations found in in vitro mutagenesis screens, this table only lists the best characterized, clinically observed examples where a causative role in imatinib resistance has been established. More comprehensive lists of drug-resistance associated mutations in non-ABL kinases can be found in the references listed, and in references therein. Analogous mutations in other kinases were identified based on sequence homology and similar locations in crystal structures of the kinases indicated.
In cancer, mutant kinases frequently act as oncogenes that promote tumor cell survival, proliferation or genomic instability, angiogenesis or cell migration during metastasis3, 8, 9. More recent studies unveiled important disease-promoting kinase roles in immune disorders, organ transplant rejection, glaucoma, cardiovascular, metabolic and neurodegenerative diseases3, 10-12. Many kinases act as key nodes in cellular signaling. Thus, pharmacological modulation of kinase function can alter many physiological and pathological processes in a therapeutically desirable manner. Moreover, kinases are very “druggable”: They are often specifically expressed in targeted tissues, and have specific, often well characterized ATP, substrate, regulatory subunit or ligand binding sites that can be targeted by small-molecules 8, 13. Consequently, kinases have become the second-largest drug target family, with 13 approved kinase inhibitor (KI) drugs (Table 1), ~100 compounds in clinical trials (Tables 2, 3) and many more in preclinical development1, 8, 10-18.
Table 1.
Approved kinase inhibitor drugs
| Compound | Company | Mechanism of inhibition |
Known targets |
Indications | Status | Clinical Resistance observed? |
Recent references |
|---|---|---|---|---|---|---|---|
| Imatinib (STI-571, Gleevec) | Novartis | ATP competitive Type 2 inhibitor, binds inactive conformation | ABL, ARG, KIT, FMS, PDGFR, EGFR, DDR1 | CML, GIST, HES, other cancers | Approved/Phase I-IV. Also in ~100 clinical trials for multiple indication | Yes, Tables 4,5 | 4, 13, 15, 20, 23, 56, 114 |
| Dasatinib (BMS-354825) | Bristol-Myers Squibb | ATP competitive Type 1 inhibitor, binds active and possibly also inactive conformation | ABL, ARG, EGFR, BTK, PDGFR, KIT, SFKs, Ephrins. Inhibits 21 of 22 Imatinib-resistant BCR-ABL mutants. | Imatinib-resistant CML, Ph+ALL; other cancers | Approved/Phase I-III | Yes, Tables 4,5. Preclinical: BCR-ABL L248V, Q252H, G250E, E255K, V299L, T315I (clinical resistance), F317I/C/L/V, possibly others. | 4, 10, 13, 15, 16, 20, 56, 57, 114, 120 |
| Rapamycin (Sirolimus, rapamune) | Wyeth | Allosteric mTOR inhibitor. Rapamycin binds to the cellular FKBP12 protein. The complex or rapamycin alone then competes with phosphatidic acid (PA) binding to the FRB domain of mTOR, preventing PA from facilitating the assembly of mTORC1/2 complexes, which mediate cellular mTOR functions. | mTOR | Approved for transplant rejection, restenosis. In trials for RCC, other cancers, immunosuppression in autoimmune diseases, cardiovascular disease, ADPKD | Approved/Phase I-IV | Yes: (a) Low-dose Rapamycin may primarily inhibit mTORC1 and induce feedback-activation of MAPK signaling and mTORC2 via IGF1R-dependent mechanisms. (b) Many cancers upregulate PLD activity, resulting in augmented PA production which can competitively reduce rapamycin binding to mTOR. | 1, 20, 133 |
| Temsirolimus (CCI-779) | Wyeth | Rapamycin analog allosteric mTOR inhibitor | mTOR | Advanced RCC, Hamartoma syndromes, other cancers | Approved/Phase I-III | 1, 20 | |
| Sunitinib (SU11248) | Pfizer | ATP-competitive inhibitor. | PDGFR, VEGFR, KIT, RET, CSF-1R, FLT3 | RCC, imatinib-refractory GIST, breast, lung, colorectal cancers, AML | Approved/Phase I-IV | 1, 20, 120, 134 | |
| Gefitinib (ZD-1839) | AstraZeneca | ATP-competitive, binds active EGFR. | EGFR (Her1, ErbB-1) | NSCLC; other cancers | Approved/Phase I-IV | Yes, table 5. | 1, 8, 20, 23 |
| Erlotinib (OSI-774) | Genentech/Roche | ATP-competitive inhibitor, binds active EGFR. | EGFR, JAK2 V617F mutant | NSCLC, pancreatic carcinomas; other cancers | Approved/Phase I-IV | Yes, table 5. | 1, 20, 23 |
| Lapatinib (GW572016) | GSK | ATP-competitive inhibitor, binds SKF-like inactive conformation | EGFR (HER2/neu/ERBB2) | Breast cancer; other cancers | Approved/Phase I-IV | 1, 13 | |
| Nilotinib (AMN 107) | Novartis | ATP competitive type 2 inhibitor, targets inactive conformation. May not be imported via hOCT1. | ABL, ARG, PDGFR, KIT, Ephrins | Imatinib-resistant CML; other cancers | Approved/Phase I-IV | Yes, table 4,5. BCR-ABL L248V, G250E, Y253H, E255K/V, E292V, T315I, F359C/V, L384M, L387F etc. Pgp overexpress ion, ABL gene amplification and Lyn activation may contribute. | 4, 13, 16, 20, 56, 121 |
| Fasudil (HA-1077, AT877) | Asahi Kasei Pharma/Sche ring | ATP-competitive inhibitor. | ROCK, MLCK, PKC | Cerebral vasospasm, chronic stable or vasospastic angina; Raynaud's phenomenon, carotid artherosclerosis | Approved/Phase II/III | 10, 20, 135 | |
| Sorafenib (BA 43-9006, Nexavar) | Bayer | ATP-competitive type 2 inhibitor. | BRAF, VEGFR, PDGFRβ, FLT3, KIT | RCC, HCC, AML; thyroid carcinoma, other cancers, vascular diseases and diabetic retinopathies | Approved/Phase I-IV | 1, 7, 8, 13, 20, 35, 89, 120, 134 | |
| Ruboxistaurin (LY333531) | Eli Lily | ATP-competitive inhibitor. | PKCβ, other PKCs | Diabetic retinopathy; explored for diabetic macular edema, diabetic peripheral neuropathy and diabetic nephropathy. | Phase III; FDA issued approvable letter in 2006 with request for additional trial. | 136 | |
| Everolimus (RAD-001, RAD001) | Novartis | Rapamycin analog allosteric mTOR inhibitor | mTOR | Organ transplant rejection; cancers, Hamartoma syndromes, cardiovascular/coronary diseases, PCKD | Approved/Phase I-IV | 20, 133 |
Legend: ADPKD, autosomal-dominant polycystic kidney disease, ALL, acute lymphoblastic leukemia, AML, acute myelogenous leukemia, CML, chronic myelogenous leukemia, GIST, gastrointestinal stromal tumors, HES, hypereosinophilic syndrome, MDS, myelodysplastic syndrome, MM, multiple myeloma, PCKD, polycystic kidney disease, RCC, renal cell carcinoma, HCC, hepatocellular carcinoma, NSCLC, non small-cell lung cancer, Ph+, Philadelphia-chromosome positive, harboring a reciprocal translocation between chromosomes 9 and 22 that generates a breakpoint cluster region (BCR)-Abelson kinase (c-ABL) fusion gene resulting in expression of constitutively active BCR-ABL protein which causes cell transformation in CML15. The table lists currently approved kinase-inhibitor (KI) drugs. Tables 2 and 3 list a selection of compounds in clinical trials. Italic, KIs whose indications include other diseases than cancer. For more detailed discussions and structures of KIs in clinical trials, for many more examples than those listed here, and for discussions of additional potential indications, see4, 8, 10-12, 15-17, 27, 32, 50, 68, 109, 120, 134, 135, 137, 138. For updated clinical trial information of cancer or other therapeutics, visit http://www.cancer.gov/clinicaltrials and http://clinicaltrials.gov20. For an excellent recent discussion of the structural interactions of the most relevant KIs with their targeted kinases, see1.
Table 2.
Selected kinase inhibitor drugs in clinical studies exclusively for cancer indications
| Compound | Company | Mechanism of inhibition |
Known targets |
Indications | Status | Resistance observed? |
Recent references |
|---|---|---|---|---|---|---|---|
| PCI-3276 | Pharmacyclics | BTK | B cell leukemias and lymphomas, NHL | Phase I | 20 | ||
| SF1126 | Semafore Pharmaceuticals | PI3K | Cancer | Phase I | 20 | ||
| GDC-0941 | Genentech | PI3K | Breast cancer, solid cancers, NHL, NSCLC | Phase I | 20 | ||
| BKM120 | Novartis | PI3K | Breast and various other cancers | Phase I | 20 | ||
| PF-04691502 | Pfizer | PI3K/mTOR | Cancer | Phase I | 20 | ||
| PX-866 | Oncothyreon | PI3K | Cancer | Phase I | 20 | ||
| BGT226 | Novartis | PI3K | Cancer, Cowden Syndrome | Phase I-II | 20 | ||
| BEX235 | Novartis | PI3K | Cancer | Phase I | 139 | ||
| XL147 | Exelixis | PI3K | Cancer, lymphoma | Phase I-II | 20 | ||
| XL765 | Exelixis | PI3K/mTOR | Cancer | Phase I-II | 20 | ||
| Perifosine | Memorial Sloan-Kettering Cancer Center | Targets the AKT PH domain and prevents AKT translocation to the plasma membrane. | AKT | Various cancers, Waldenstrom's Macroglobulinemia, chondrosarcomas, GIST, MM, RCC, CLL, colon, pancreatic, ovarian cancer, leukemia, lymphoma | Phase I-III | 20 | |
| Ridaforolimus (AP23573, MK-8669; formerly Deforolimus) | Merck | Rapamycin analog, allosteric mTOR inhibitor | mTOR | Various cancers | Phase I-III | 20 | |
| AZD2014 | AstraZeneca | mTOR | Cancer | Phase I | 20 | ||
| Enzastaurin (LY317615) | Eli Lilly | PKCβ | Various cancers, NSCLC, lymphoma, leukemia, MM, Waldenstrom's Macroglobulinemia | Phase I-III | 20, 135 | ||
| XL-228 | Exelixis | Inhibits BCR-ABL T315I | ABL, AURORA-A, FGFR, IGF1R, SRC | CML, Ph+ ALL, lymphoma, myeloma, solid tumors | Phase I | 16, 20 | |
| AP24534 | Ariad | ATP-competitive type 2 inhibitor. Inhibits BCR-ABL T315I in vitro. | ABL, FGFR, FLT3, VEGFR, KIT | CML, advanced hematologic malignancies | Phase I | 16, 120 | |
| Bosutinib (SKI-606) | Wyeth | ATP competitive inhibitor. Binds intermediate and inactive BCR-ABL conformations. Has efficacy in imatinib-resistant CML at least in short-term studies. Inhibits multiple imatinib-resistant BCR-ABL mutants, but G-loop mutants are partially, and gatekeeper-mutants fully resistant. | ABL, SFKs, CAMK2G, STE20, TEC, KIT, PDGFR | CML, Ph+ ALL, breast cancer | Phase I-III | BCR-ABL T315I, V288L, moderately resistant: E255K/V, G250E. | 16, 20, 27, 56, 121. |
| INNO-406 (NS-187) | Innovive/Cyt Rx | ATP competitive type 2 inhibitor. Higher ABL-affinity than Imatinib. Inhibits several Imatinib-resistant BCR-ABL mutants except T315I. LYN-coinhibition may contribute to its ability to overcome imatinib-resistance. | ABL, LYN, PDGFR, KIT. Not a broad SFK inhibitor. | CML, Ph+ ALL | Phase I/II | BCR-ABL T315I. | 16, 20, 27, 56 |
| AZD0530 | AstraZeneca | ATP competitive inhibitor. | ABL (weak inhibition), SFKs | CML, advanced ovarian cancer, other cancers | Phase I-II | 20, 56 | |
| MK-0457 (VX-680) | Merck | Inhibits BCR-ABL T315I (IC50 ~5 μM). | AURORA-A/B, FLT3, JAK2, ABL (including T315I) | CML, Ph+ ALL, other cancers | Terminated due to cardiac toxicity. | 16, 20, 56 | |
| PHA-739358 | Nerviano Medical Sciences | Inhibits BCR-ABL T315I | AURORA-A/B/C, ABL, FGFR1, RET, TRK | Imatinib-resistant CML, MM, prostate cancer | Phase II | 16, 20, 56 | |
| Axitinib (AG013736) | Pfizer | VEGFR, PDGFR, KIT | Breast cancer, RCC, pancreatic and other cancers | Phase I-III | 20 | ||
| Cediranib (AZD2171) | AstraZeneca | VEGFR | NSCLC, kidney cancer, glioblastoma, colorectal cancer, CNS tumors in children, lung cancer | Phase I-III | 20 | ||
| Vandetanib (ZD6474) | AstraZeneca | VEGFR, EGFR, RET | NSCLC, breast and prostate cancer, thyroid, head and neck carcinoma, glioma | Phase I-III | 7, 20 | ||
| BIBW-2992 | Boehringer Ingelheim | EGFR (HER-2/neu) | NSCLC, breast and prostate cancer, head and neck carcinoma, glioma | Phase I-III | 20 | ||
| Vatalanib (CPG-79787) | Bayer, Schering, Novartis | VEGFR, PDGFR, KIT, FLT-4, FMS | Advanced colorectal cancer, other cancers | Phase I-III | 20, 135 | ||
| Midostaurin (PKC412) | Novartis | ATP-competitive type 1 inhibitor. | PKCs, VEGFR2, PDGFR, FLT3, KIT, MDR | Gleevec-resistant GIST, AML, aggressive systemic mastocytosis (ASM) and mast cell leukemia, relapsed or refractory pediatric leukemia, myelodysplastic syndrome | Phase I-III | Yes | 20, 120, 134, 140 |
| AT9283 | Astex Therapeutics | ABL, AURORA-A/B, FLT3, JAK2/3 | CML, AML, ALL, MDS, NHL, myelofibrosis, solid cancers | Phase I/II | 16, 20 | ||
| KW-2449 | Kyowa Hakko Kirin Pharma | Inhibits BCR-ABL T315I | ABL, AURORA-A, FGFR1, FLT3, VEGFR | CML, AML | Phase I/II terminated (Failure to demonstrate a tolerable dose that had potential for efficacy) | 16, 20, 120, 134 | |
| DCC2036 | Deciphera Pharmaceuticals | Non-ATP competitive allosteric inhibitor. Inhibits BCR-ABL T315I and many other mutants. | ABL, FLT3, KDR, SFKs, TIE2 | CML, Ph+ ALL | Phase I/II | 16, 20 | |
| Tandutinib (MLN518) | Millenium Pharmaceuticals | ATP-competitive | FLT3, PDGFR, KIT | Glioblastoma | Phase I/II | 20, 134 | |
| AC220 | FLT3, KIT, CSF1R/FMS, RET, PDGFR | AML, advanced solid tumors | Phase I/II | 20, 120, 134 | |||
| CHIR-258 (TKI258) | Chiron | FLT3, KIT, FMS, VEGFR, FGFR | MM, AML, refractory MM, renal and prostate cancer, urothelial cancer | Phase I/II | 20, 120 | ||
| LS104 | LymphoSign/Aegera | Non-ATP competitive | JAK2, FLT3 | Refractory/relapsed hematological malignancies | Phase I | 20, 120 | |
| ARRY-142886 (AZD6244) | Array Biopharma/AstraZeneca | MEK1 | Various cancers, leukemia | Phase I-II | 20, 135 |
Legend: See legend to table 1.
Table 3.
Selected kinase inhibitor drugs in clinical studies for non-cancer and sometimes also cancer indications
| Compound | Company | Known targets | Indications | Status | Recent references |
|---|---|---|---|---|---|
| INCB-28050 | Eli Lily, Incyte | JAK1/2 | Rheumatoid arthritis | Phase II | 14, 20 |
| Tasocitinib (CP-690550) | Pfizer | JAK3 | Rheumatoid arthritis, psoriasis, inflammatory bowel disease, transplant rejection | Phase II/III | 14, 20 |
| VX-509 | Vertex | JAK3 | Rheumatoid arthritis | Phase II | 14, 20 |
| VX-702 | Vertex | p38 | Rheumatoid arthritis, cardiovascular disease | Phase II | 10, 14, 20, 135 |
| BMS-582949 | Bristol-Meyers-Squibb | p38 | Rheumatoid arthritis, artherosclerosis, psoriasis | Phase I-II | 10, 14, 20 |
| Fostamatinib disodium (R935788) | AstraZeneca, Rigel | SYK | Rheumatoid arthritis, B-cell lymphoma, immune thrombocytopaenic purpura, peripheral T-cell lymphoma, solid tumours | Phase II | 12, 14, 20 |
| AZD1480 | AstraZeneca | JAK2 | Solid malignancies, primary myelofibrosis, post-polycythaemia vera, essential thrombocythaemia, myelofibrosis | Phase I-II | 20 |
| INCB018424 | AstraZeneca/Incyte | JAK1/2 | MM, myelofibrosis; polycythemia vera; thrombocytosis, rheumatoid arthritis, psoriasis | Phase I-II | 12, 20 |
| GS856553 | GlaxoSmith Kline | p38 | Neuropathic pain, acute coronary syndrome | Phase II | 20 |
| SB-681323 | GlaxoSmith Kline | p38 | Acute lung injury, acute respiratory distress syndrome, COPD, rheumatoid arthritis | Phase I-II | 10, 20, 135 |
| SCIO-469 | Scios/J&J | p38 | MM, rheumatoid arthritis | Phase II | 20, 135 |
| SAR113945 | Sanofi-Aventis | IKKβ | Osteoarthritis | Phase I | 20 |
| Palomid 529 | Paloma Pharmaceuticals | mTORC1/2 | Age-Related Macular Degeneration | Phase I | 20 |
| CAL-263 | Calistoga Pharmaceuticals | PI3Kδ | Allergic Rhinitis | Phase I | 20 |
| CAL-101 | Calistoga Pharmaceuticals | PI3Kδ | Allergic Rhinitis, NHL, CLL, AML, MM | Phase I | 20 |
| KAI-9803 | KAI Pharmaceuticals | PKCδ | Myocardial Infarction | Phase I-II | 20 |
| Lestaurtinib (CEP-701) | Cephalon | FLT3, JAK2, TrkA-C, VEGFR2, PKC | AML, advanced MM, prostate cancer, neuroblastoma, myelofibrosis, essential thrombocythemia, polycythemia vera, psoriasis | Phase I-III | 20, 120, 134 |
| Sotrastaurin (AEB071) | Novartis | pan-PKC | Immunosuppression for transplant rejection, psoriasis, ulcerative colitis | Phase II | 10, 20 |
| Tasocitinib (CP-690550) | Pfizer | JAK3 | Immunosuppression for rheumatoid arthritis, transplant rejection, psoriasis, dry eye disease | Phase II/III | 12, 20 |
| AT9283 | Astex Therapeutics | ABL, AURORA-A/B, FLT3, JAK2/3 | CML, AML, ALL, MDS, NHL, myelofibrosis, solid cancers | Phase I/II | 16, 20 |
| AMG-548 | Amgen | p38 | Rheumatoid arthritis | Suspended after phase I | 10, 135 |
| Doramapimod (BIRB-796) | Boehringer Ingelheim | p38(Type 2 inhibitor) | Psoriasis, rheumatoid arthritis, Crohn's | Development halted after phase II due to liver toxicity | 10, 12, 135 |
| SCIO-323 | JNJ/Scios | p38 | Rheumatoid arthritis | Phase I | 10, 135 |
| Ro 320-1195 | Roche | p38 | Rheumatoid arthritis | Phase I | 10, 135 |
| PH-797804 | Pfizer | p38 | Rheumatoid arthritis, Osteoarthritis | Phase II | 10, 20 |
| Y-39983 | Mitsubishi-Senju | ROCK | Glaucoma, ocular hypertension | Phase II | 10 http://www.senju.co.jp/english/rd/pipeline.html |
| K-115 | Kowa | ROCK | Glaucoma, ocular hypertension | Phase II | 10 http://www.kowa.co.jp/eng/g/rd/pipeline.htm |
| DE-104 | Santen Pharma & Ube Industries | ROCK | Glaucoma, ocular hypertension | Phase I | 10 |
| SAR407899 | Sanofi-Aventis | ROCK | Erectile dysfunction | Phase II completed | 20 |
| VX-745 | Vertex | p38 | Rheumatoid arthritis | Phase II, discontinued due to liver toxicity and brain effects | 12 |
| R-112 | Rigel | Syk | Allergic rhinitis | Phase II stopped since no difference to placebo | 135 |
| SC-80036 | Pfizer | p38 | Rheumatoid arthritis | Phase II - no recent updates | 135 |
| CC-401 | Celgene | JNK1/2 | Immune disorders, transplant rejection, psoriasis, osteoarthritis, diabetes mellitus, myeloid leukemia | Phase I-II - terminated/no recent updates | 20, 135 |
| TAK-715 | Takeda | p38 | Rheumatoid arthritis | Phase II completed | 20, 135 |
Legend: See legend to table 2.
Oncogenic or cancer supporting roles of many deregulated kinases, including ABL-fusion proteins, EGFR, KIT, PDGFR, FMS, VEGFR, FLT3, SRC-family (SFK) or cyclin-dependent kinases (CDKs), spurred the initial development of most KIs as cytostatics (Table 1). The success of the ATP-competitive BCR-ABL KI imatinib19 as a breakthrough CML-therapeutic demonstrates the benefit of this approach4, 12. Surprisingly low toxicity of many KIs and improved target-selectivity have broadened KI-therapeutic indications to include less life-threatening autoimmune diseases, transplant rejection, allergic rhinitis, chronic obstructive pulmonary disease (COPD), osteoarthritis, cardiovascular diseases, neuropathic pain, age-related macular degeneration, glaucoma, erectile dysfunction and other diseases (Tables 1-3)8, 10-12, 14, 15, 20. Many of these disorders are chronic, necessitating live-long drug administration. For these patients, limited KI efficacy and limited target selectivity causing toxic side effects over time remain challenges.
Another important challenge for KI-therapeutics is the development of drug-resistance. This “Achilles heel” may affect kinases more than most other drug targets. One reason is that because of the key roles of many kinases in cell metabolism, survival and function, cells underlie significant selection pressure to compensate for the loss of function of an important kinase. Moreover, kinases show complex intra- and intermolecular interactions with regulatory subunits or ligands that govern extensive structural changes required for activation. Their many interaction interfaces and conformational dynamic provide multiple interference points for mutational or other mechanisms that reduce KI drug-binding or -effect while maintaining sufficient ATP-binding and catalysis for restoring kinase function. In a patient treated with a KI-therapeutic, this can result in the development of drug-resistance through various cell extrinsic and -intrinsic mechanisms (Fig. 4) 9, 16, 17, 21-25. In particular tumor cells, which are genetically unstable, can harbor pre-existing primary, or acquire secondary drug-resistance mechanisms upon KI treatment. The clinically most important mechanism is the accumulation of drug-resistant mutant alleles of the targeted kinase. For example, the outgrowth of tumor cell clones harboring drug-resistant BCR-ABL alleles is the main cause of imatinib resistance in CML patients26. 15-25% of imatinib-treated CML patients have primary resistance, failing to show sustained drug responses altogether. 7-15% develop secondary imatinib-resistance, losing initial responses. Altogether, ~33% may eventually need alternative treatment options. This necessitated the costly and, for some relapsed patients, too late development of “second” and “third generation” BCR-ABL-inhibitors 9, 12, 16, 17, 27. In the past decade, KI drug-resistance has become a common clinical complication affecting multiple cancers, targeted kinases and drugs (Tab. 4,5). Preclinical studies have unveiled drug-resistance mechanisms for many additional kinases28-33. As KIs reach large patient populations for expanding indications, drug-resistance could thus become a major liability that limits the therapeutic use of this otherwise excellent drug class. Here, we critically review this problem and discuss recent advances in overcoming it.
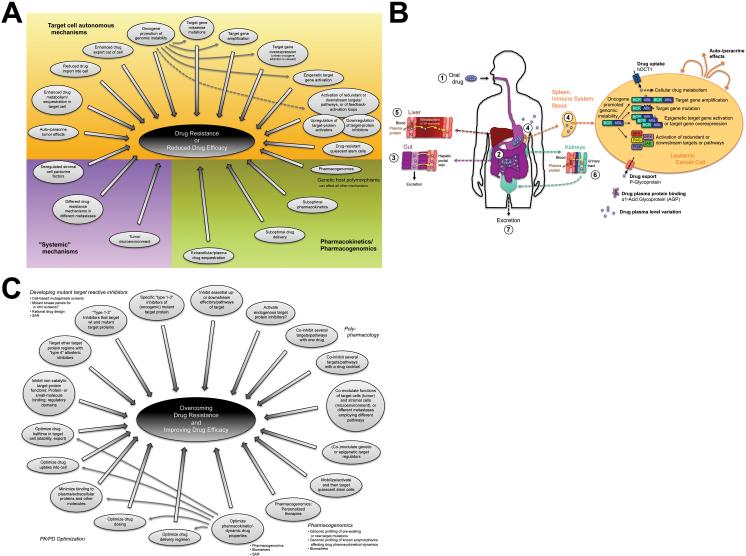
Fig. 4. Mechanisms of kinase inhibitor (KI) drug-resistance and approaches to overcome it.
(A) Generalized mechanisms that can reduce KI drug efficacy or cause drug-resistance. Pharmacokinetic (PK) factors such as drug sequestration in extracellular space or plasma, suboptimal delivery, absorption, tissue penetration, enhanced metabolic turnover, clearance and excretion primarily affect drug cellular availability and efficacy (Fig. 4A,B)65. In pre-clinical models, drug-binding to plasma α1-acid glycoprotein (AGP) reduced drug delivery into cancer cells. However, the clinical relevance is unclear. Imatinib-release by the AGP competitive binder erythromycin could provide an avenue to overcome this potential problem9, 16, 22. Pharmacogenomic factors can profoundly affect almost every PK/pharmacodynamic (PD) property of a drug9. For example, polymorphisms in the drug-importer OCT-1 or the drug exporters MDR-1/P-glycoprotein (Pgp) and ABCG2 may affect imatinib, gefitinib or erlotinib pharmacokinetics and toxicity, although conflicting data render the clinical relevance unclear9, 16, 24. Drug-resistance can be caused by target-cell extrinsic “systemic” mechanisms, or by a broad array of target cell autonomous/intrinsic mechanisms. In addition to those discussed in the text, the upregulation of cellular target protein activators, or the downregulation of target-protein inhibitors might contribute to drug-resistance or reduced drug efficacy9, 16, 21-24, 66, 67. (B) Schematic depiction of factors influencing KI efficacy or drug-resistance, exemplified by ABL-inhibitors9, 16. To maximize ease of use and patient compliance, drugs (blue stars) are preferably applied orally as tablets/capsules (1). In the gut (2), they are released and enter the blood via the hepatic portal vein (3). Unabsorbed drugs are excreted in the faeces. KIs enter their target cells (cells of the immune system in the case of ABL-inhibitors) via import-proteins, including the organic cation importer hOCT1 in case of imatinib (4). Cellular sequestration or metabolism may affect cellular drug efficacy. KIs are exported by ATP binding-cassette transporter families B and G2, including AGP in case of imatinib. Illustrated by BCR-ABL, several cell-intrinsic, auto- and paracrine effects can cause drug-resistance, discussed in detail in the text. Many of them are facilitated by the genomic instability of tumor cells. A major role of target gene missense mutations complements contributions by target-gene amplification, overexpression or epigenetic activation, or by the deregulation of redundant or downstream pathways. A recent review of KI PK properties65 suggests that overall, KIs reach their maximum plasma levels relatively fast, have an unknown absolute bioavailability, are extensively distributed and highly bound to plasma-proteins such as AGP in the blood (5,6). KIs are primarily metabolized (red stars) in the liver by cytochrome P450 (CYP) 3A4 (5) and excreted primarily via the biliary-fecal route (3). Only a minor fraction is eliminated with the urine (6,7). In a small study, elevated CYP3A activity and production of the therapeutically active metabolite CGP74588 associated with higher imatinib molecular responses147. CYP1A1 induction by cigarette smoke may decrease erlotinib exposure. No significant contribution of CYP3A4/5 polymorphisms to KI efficacy/toxicity has been reported 9. Finally, the interesting KI ability to inhibit some of their own metabolizing enzymes and transporters renders steady-state metabolism and drug-drug interactions complex and unpredictable65. (C) General approaches to improve drug efficacy and overcome drug-resistance. For details, see text and9, 15, 21-24, 65, 67, 109-112.
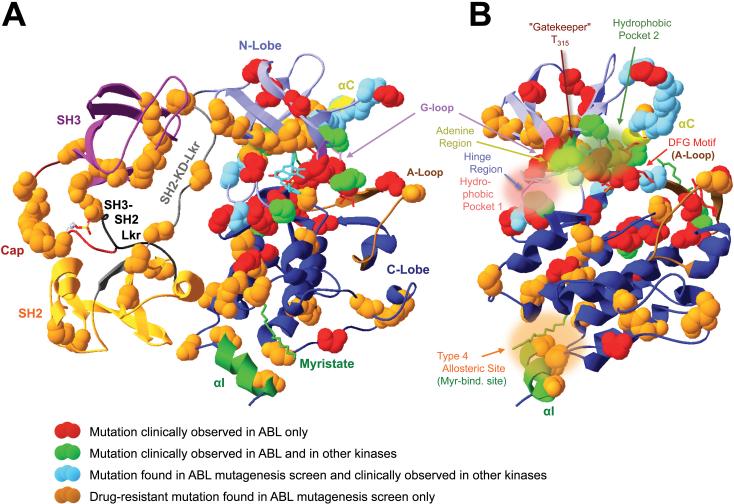
Table 4.
Drug-resistance causing point mutations in ABL
| ABL mutation Numbering based on48 |
Clinically observed? |
Strong resistance |
Resistant to these inhibitors |
Topological location |
Mechanism | Analogous mutations in other kinases |
References | |
|---|---|---|---|---|---|---|---|---|
| ABL1b (1OPL numbering, Fig. 5) |
ABL1a (clinical numbering) |
|||||||
| E57K | E38K | Imatinib | Cap | Cap structure in this region unknown. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. | 48 | |||
| A64G/V | A45G/V | Imatinib | Cap | Cap structure in this region unknown. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. | 48 | |||
| K70Q | K51Q | Imatinib | Cap | Forms water-mediated Hydrogen-bond network with S222, L232 and H233 on the SH2 domain surface in autoinhibited 2FO0 whose mutational perturbation might perturb auto-inhibitory Cap-SH2 domain interactions39. K70 also participated in hydrophobic packing interactions with W67 that may stabilize the cap39. Indeed, K70 mutation activates c-ABL similarly as SH3-domain release or prevention of myristoylation39, 42. | 48 | |||
| E71K | E52K | Imatinib | Cap | Unclear per 2FO0 analysis. Cap implication in c-ABL autoinhibition47 may suggest disruption of autoinhibitory Cap interactions. E71A mutation only very mildly activated ABL42. | 48 | |||
| N72D/T | N53D/T | Imatinib | Cap | Unclear per 2FO0 analysis. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. N72A mutation only very mildly activated ABL42. | 48 | |||
| A75V | A56V | Imatinib | Cap | Unclear per 2FO0 analysis. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. | 48 | |||
| G76E | G57E | Imatinib | Cap | Unclear per 2FO0 analysis. Could possibly facilitate formation of bend that allows cap to interact with SH2-SH2-KD-linker region. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. | 48 | |||
| P77R | P58R | Imatinib | Cap | Unclear per 2FO0 analysis. Could possibly facilitate formation of bend that allows cap to interact with SH2-SH2-KD-linker region. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. | 48 | |||
| E79K | E60K | Imatinib | Cap | Unclear per 2FO0 analysis. Cap implication in c-ABL autoinhibition39, 41, 47 may suggest disruption of autoinhibitory Cap interactions. | 48 | |||
| N83Y | N64Y | Imatinib | SH3 | Unclear per 1OPL analysis. | 48 | |||
| V86G | V67G | Imatinib | SH3 | In conserved FVALYD motif. Unclear per 1OPL analysis. | 48 | |||
| A87E/V | A68E/V | Imatinib | SH3 | In conserved FVALYD motif. Unclear per 1OPL analysis. | 48 | |||
| Y89D/H/N | Y70D/H/N | Imatinib | SH3 | In conserved FVALYD motif. Unclear, but faces SH2-KD linker in 1OPL (Fig. 2B,C) and may possibly contact SH2-KD linker K23848, mutations might perturb autoinhibitory SH3/SH2-KD linker/N-lobe interactions. | 48 | |||
| D90N | D71N | Imatinib | SH3 | In conserved FVALYD motif. Unclear, but close to SH2-KD linker in 1OPL (Fig. 2B,C). Hyperactivates autophosphorylation. | 48 | |||
| W129C | W110C | Imatinib | SH3 | W129 is critical for ABL-type poly-proline peptide binding. Hyperactivates autophosphorylation. W129 faces the SH2-KD linker in 1OPL (Fig. 2B,C), mutations might thus perturb autoinhibitory SH3/SH2-KD linker/N-lobe interactions but could also perturb inhibitory interactions with PAG or other proteins. | 48 | |||
| V130L | V111L | Imatinib | SH3 | Unclear per 1OPL analysis. | 48 | |||
| P131Q | P112Q | Imatinib | SH3 | Unclear, but faces SH2-KD linker in 1OPL (Fig. 2B,C), mutations might perturb autoinhibitory SH3/SH2-KD linker/N-lobe interactions. P131 mutation increases ABL transforming activity, induces its relocalization from nucleus to cytoplasm, disrupts inhibitory PAG/MSP23-interactions and increased drug-resistance in cells. However, unphosphorylated ABL P131L is rendered imatinib-hypersensitive. Thus, effects of P131 mutation depend on the ABL activation state and cellular context. | 48, 95 | |||
| S132N | S113N | Imatinib | SH3 | Unclear per 1OPL analysis. | 48 | |||
| V138G | V119G | Imatinib | SH3-SH2 linker | Unclear per 1OPL analysis. | 48 | |||
| S140R | S121R | Imatinib | SH3-SH2 linker | Unclear per 1OPL analysis. Oncogenic. S140I mutation increased ABL activity, suggesting that the mutants disrupt SH3-SH2-linker rigidity which is important for ABL autoinhibition42. | SRC linker mutation augmented activity43. | 48 | ||
| S167C | S148C | Imatinib | SH2 | Unclear per 1OPL/2FO0 analysis. Possibly false positive48. | 48 | |||
| S176T | S157T | Imatinib | SH2 | Unclear per 1OPL/2FO0 analysis. Possibly false positive48. | 48 | |||
| S206F/P | S187F/P | Imatinib | SH2 | Unclear per 1OPL/2FO0 analysis. | c-SRC T218-mutation in specificity determining SH2 domain EF loop, disrupts inhibitory C-terminal phospho-Y527 binding48. | 48 | ||
| V224E | V205E | Imatinib | SH2 | Unclear per 1OPL/2FO0 analysis. Possibly false positive48. | 48 | |||
| A236G | A217G | Imatinib | SH2-KD linker | Unclear per 1OPL/2FO0 analysis. | 48 | |||
| K238E | K219E | Imatinib | SH2-KD linker | Unclear per 1OPL analysis. Might contact SH3 domain Y8948, abrogation of this interaction could destabilize inactive conformation. | 48 | |||
| T243A | T224A | Imatinib | SH2-KD linker | Hydrogen-bonds backbone carbonyl of P315 in ABL-1b N-lobe in loop between αC-helix and β-sheet 4 at the N-lobe/SH2-KD linker/SH3 interface in 1OPL (Fig. 2B,C). Might also pack against Q319 in KD-NL. Thus, T243 mutation might destabilize these auto-inhibitory interactions. | 48 | |||
| P249L | P230L | Imatinib | SH2-KD linker | Per 1OPL/2FO0 structures, packs with aromatic side-chains of F91, W118 and W129 in the SH3 domain. The P249 backbone carbonyl is part of a network of electrostatic interactions involving N113, H114 and N115 in the SH3 domain. All these interactions surrounding the SH2-KD linker “kink” introduced by P249 are likely to mediate SH3 domain binding to the SH2-KD linker close to the N-lobe of the KD (Fig. 2B,C). The P249L mutation might perturb these interactions by removing the kink, destabilizing the autoinhibited ABL conformation. Indeed, P242E/P249E double mutation activates ABL42. | 48 | |||
| M256I | M237I | yes, low frequency | KD-NL | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. | 16 | |||
| M263V/I | M244V/I | yes, low-medium frequency | Imatinib | KD-NL: G-loop | Indirect: May increase ABL entropy or destabilize distorted G-loop conformation of inactive ABL, disfavoring Imatinib binding. In N-lobe, M244 is important for packing against G-loop. The mutations may also reduce drug binding by affecting the relative binding free energy (RBFE) contributions of non G-loop residues including the DFG F38299. | 16, 48, 56, 76, 78 | ||
| L267R/V | L248R/V | yes, high frequency | + | Imatinib, Dasatinib, Nilotinib | KD-NL: G-loop | Imatinib contact site. Reduced topological fit for Imatinib; destabilizes inactive conformation | 4, 10, 13, 15, 16, 20, 48, 56, 57, 78, 98, 114, 118, 120, 121 | |
| G269A | G250A | yes, high frequency | Imatinib | KD-NL: G-loop | unclear | EGFRA S720P | 16, 56 | |
| G269E/R * | G250E/R * | yes, low-medium frequency | + | Imatinib, Dasatinib, Nilotinib | KD-NL: G-loop | Indirect: Stabilize active or other conformation that disfavors Imatinib binding, or destabilize the distorted G-loop conformation typical of inactive ABL. Here, the G-loop forms a cage around the Imatinib pyridine and pyrimidine rings that bind the adenosine-site of the ATP pocket. G250E/R mutation might possibly stabilize the active conformation by introducing novel electrostatic side-chain interactions77. | 4, 10, 13, 15, 16, 20, 48, 56, 57, 76-78, 114, 120, 121 | |
| Q271H/R/E | Q252H/R/E | yes, low-medium frequency | + | Imatinib, Dasatinib | KD-NL: G-loop | Indirect: Destabilize inactive conformation, possibly by destabilizing the distorted G-loop conformation typical of inactive ABL. | 4, 10, 13, 15, 16, 20, 48, 56, 57, 76, 78, 114, 120 | |
| Y272H/C/F * | Y253H/C/F * | yes, high frequency | + | Imatinib, Nilotinib | KD-NL: G-loop | Imatinib contact site. Mutations destabilize inactive conformation, in part by removing water-mediated Hydrogen-bond with N322 side-chain that folds G-loop down into distorted conformation that increases surface complementarity with the drug and is typical of inactive ABL. Y253H/C also remove an aromatic ππ-interaction with Imatinib. Finally, the mutations may disrupt Y253 Hydrogen-bonds with D363 and R362 in the SFK-like inactive conformation, destabilizing it40. Interestingly, HDX-MS data suggest that the conformational mutation effects on myristoylated ABL may be small although Y253H activates myristoylated ABL75. The mutations may also prevent Y253 phosphorylation, which may inhibit T315A mutant ABL oncogenicity with unclear effects on catalysis92. |
4, 13, 16, 20, 40, 44, 48, 56, 75-77, 92, 95, 118, 121 | |
| E274K/V * | E255K/V * | yes, low-medium frequency | + | Imatinib, Dasatinib, Nilotinib | KD-NL: G-loop | Indirect: Destabilize inactive conformation, possibly by destabilizing the distorted G-loop conformation typical of inactive ABL. Disrupt a β1 K247-β2 E255 SB that is conserved in 58 kinases and stabilizes the G-loop, and an intra-β2 E255-Y257 Hydrogen-bond that has been suggested to be specific for the distorted G-loop conformation of inactive ABL but can also be found in crystal structures representing active KDs34. MD analyses suggest that abrogation of these interactions increases G-loop and overall N-lobe flexibility34, 99, although HDX-MS data suggest that the conformational mutation effects on myristoylated ABL may be small75. The mutations may also reduce drug binding through electrostatic contributions to the relative binding free energy (RBFE) of G-loop and non G-loop residues including the DFG F38299. Variable effects on ABL kinase activity depending on construct used for expression. Abrogation of the analogous SB also reduces SFK catalytic activity through similar mechanisms34. | ERBB2 T733I | 4, 10, 13, 15, 16, 20, 34, 44, 56, 57, 75-77, 92, 93, 95, 99, 114, 118, 120, 121 |
| Y276C | Y257C | Imatinib | KD-NL, G-loop | May disrupt intra-β2 E255-Y257 Hydrogen-bond that has been suggested to be specific for the distorted G-loop conformation of inactive ABL but can also be found in crystal structures representing active KDs34. May also prevent Y257 phosphorylation, which may otherwise promote catalytic activity in particular of T315I mutant ABL. Reduced Y257F activity and oncogenicity suggest that the intra-β2 Hydrogen-bond and/or Y257 phosphorylation are important for catalysis92. | 48, 78 | |||
| E277D | E258D | Imatinib | KD-NL | 48 | ||||
| S284T/I | S265T/I | Imatinib | KD-NL | 48 | ||||
| L285M/V | L266M/V | Imatinib | KD-NL | 48 | ||||
| V287/289 A | V268/270 A | Imatinib | KD-NL | 48 | ||||
| A288V | A269V | Imatinib | KD-NL | Imatinib contact site. | 48 | |||
| E294K | E275K | Imatinib | KD-NL | Overlaps with EGFRA deletions ΔE746-A750/T751/A750(InsR P)/T751(InsA/I or VA)/S752(InsA/V) and ΔL747-/E749(A750 P)/A750(In sP)/T751/T 751(InsP/S)/S752/S752(E746V or P753S)/S752(InsQ)/P7 53/P753(InsS) | 48 | |||
| D295V/G | D276V/G | yes, low frequency | Imatinib | KD-NL: αC-helix | Side-chain Hydrogen-bonds A-loop backbone amides in SFK-like inactive ABL structure. Hence, these mutations may destabilize the SFK-like inactive structure40. | Mutation of αC-helix residues alters A-loop interactions, destabilizing the SFK inactive conformation141. | 16, 40, 48 | |
| M297L | M278L | Imatinib | KD-NL: αC-helix | May disrupt SFK-like inactive ABL conformation40. | Overlaps with EGFRA deletion ΔS752-I759 | 40, 48 | ||
| E298K | E279K | + | Imatinib | KD-NL: αC-helix | Overlaps with EGFRA deletion ΔS752-I759 | 48 | ||
| E300K | E281K | (+) | Imatinib | KD-NL: αC-helix | Overlaps with EGFRA deletion ΔS752-I759 | 48 | ||
| E301D | E282D | Imatinib | KD-NL: αC-helix | Hyperactivates autophosphorylation. | Overlaps with EGFRA deletion ΔS752-I759 | 48 | ||
| F302L | F283L | Imatinib | KD-NL: αC-helix | Overlaps with EGFRA deletion ΔS752-I759 | 48 | |||
| L303F | L284F | Imatinib | KD-NL: αC-helix | 48 | ||||
| K304N | K285N | Imatinib, Nilotinib | KD-NL: αC-helix | 118 | ||||
| V308S/I | V289S/I | yes, low frequency | Imatinib | KD-NL: αC-helix | Imatinib contact site. | EGFRA V765A | 48,16 | |
| M309L/T | M290L/T | Imatinib | KD-NL: αC-helix | Imatinib contact site, undergoes van der Waals interactions with drug that may be altered by mutation. Component of the hydrophobic spine that stabilizes active kinase conformations (see T334/T315 gatekeeper mutant)58. Mutation could affect this. | 44, 48 | |||
| K310E/R | K291E/R | Imatinib | KD-NL | 48 | ||||
| E311Q/V | E292Q/S/V | yes, low-medium frequency | Imatinib, Nilotinib | KD-NL | unclear | EGFRA S768I; overlaps ERBB2 G776 (InsVG/C) | 4, 13, 16, 20, 48, 56, 121 | |
| K313R | K294R | Imatinib | KD-NL: SH3 and SH3/SH 2-KD linker contact in N-lobe | Disrupts N-lobe SB with E117 in SH3 domain41, possibly disrupting inhibitory SH3/SH2-KD-linker/KD interactions and destabilizing inactive ABL conformation based on the 1OPL structure (Fig. 2B,C). K313 also contributes to a hydrophobic crevice binding the SH2-KD linker Y245 in the auto-inhibited conformation. Disruption of this interaction by the bulkier R313 might contribute to destabilizing the inactive conformation, similar to the activating effect of Y245 phosphorylation41. | EGFRA D770Y; overlaps EGFRA insertions D770-N771 (ins NPG/SVQ/G) | 48 | ||
| Q319H | Q300H | Imatinib | KD-NL: SH3/SH 2-KD linker contact in N-lobe | May disrupt SB with Y245 in SH2-KD linker and thereby inhibitory KD/SH3 interactions based on 1OPL structure (Fig. 2B,C). Q319 might also pack against T243 in SH2-KD linker. Hyperactivates autophosphorylation. Oncogenic. | c-SRC Q327-mutants; FLT3 N676K/D | 48 | ||
| L320F | L301F | Imatinib | KD-NL: SH3/SH 2-KD linker contact in N-lobe | Component of the hydrophobic spine that stabilizes active kinase conformations (see T334/T315 gatekeeper mutant)58. Mutation could affect this. | 48 | |||
| V323G | V304G | yes, low frequency | KD-NL | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. | 16 | |||
| F330I/L/V | F311I/L/V | yes, low-medium frequency | Imatinib | KD-NL | Indirect: Destabilizes inactive conformation. Hyperactivates autophosphorylation. | 16, 48, 56, 78 | ||
| T334I/S/G/N * | T315I/S/G/N * | yes, high frequency (primarily T315I) | + | Imatinib, Dasatinib, Nilotinib | KD-NL: AS2/3 “gatekee per” | Accounts for 15-20% of Imatinib-resistant CML cases. Imatinib contact site. Large side-chain sterically hinders drug access to AS2/3 without significantly impairing ATP binding. Also removes a hydrogen-bond to Imatinib without significantly impairing ATP binding. HDX-MS data suggest enhanced Imatinib-binding site flexibility consistent with reduced structural organization. Recent structural analyses suggest that gatekeeper mutations stabilize a “hydrophobic spine” linking gatekeeper residue and the A-loop YA, characteristic of active kinase conformations58, 59. HDX-MS data also suggest enhanced flexibility within the SH3 domain RT-loop of the mutant kinases, possibly indicating distant allosteric effects of the gatekeeper mutation that might reduce inhibitory SH3-KD interactions, or Abl-binding to other proteins75. Consistent with perturbations of the inactive ABL conformation, T315I mutation activates myristoylated ABL75. | c-KIT: T670I; PDGFRα: T674I; PDGFRβ: T681I; EGFR: T790M; ERBB2: T733I; FGFR1: V561M; RET: V804L/M; FLT3: G697R; c-SRC: T341M; v-SRC: I338, exchange for T341 in c-Src. AURORA-A: T217D. Bold: Most frequently reported. | 4, 13, 16, 20, 22, 26, 48, 56, 58, 75-77, 79, 118, 121 |
| E335D | E316D | Imatinib | KD-NL | 48 | ||||
| F336L/V | F317L/V | yes, low-high frequency | Imatinib, Dasatinib | KD-hinge region | Imatinib contact site. Undergoes ππ and van der Waals interactions with drug. Mutations may alter these and reduce topological fit with Imatinib without significantly impairing ATP binding. | 4, 10, 13, 15, 16, 20, 48, 56, 57, 76, 78, 114, 120 | ||
| G340W/E | G321W/E | yes, low frequency | Imatinib | KD-CL | Imatinib contact site. Hyperactivates autophosphorylation. | EGFRA G796D/R/C | 16, 48 | |
| N350S | N331S | Imatinib | KD-CL, SH2 contact | 48 | ||||
| V357G | V338G | Imatinib | KD-CL, SH2 contact | V357Q mutation mildly increased ABL activity42. Suggests that V357G might destabilize autoinhibitory SH2-KD interactions. | 48 | |||
| V358A/G | V339A/G | Imatinib | KD-CL, SH2 contact | Faces SH2 domain, possibly involved in inhibitory interactions. | 48 | |||
| M362T | M343T | yes, low-medium frequency | Imatinib | KD-CL, SH2 contact | Indirect: May increase ABL entropy, disfavoring Imatinib binding, possibly by interfering with inhibitory KD-SH2-domain interactions through introduction of a polar side-chain in a hydrophobic interface involving the SH2-domain A-loop based on the 1OPL structure (Fig. 2B,C). | 48,16, 56, 76, 78 | ||
| A363V | A344V | Imatinib | KD-CL, SH2 contact | 48 | ||||
| Q365H | Q346H | Imatinib | KD-CL, SH2 contact | Faces SH2 domain, possibly involved in inhibitory interactions. | 48 | |||
| M370T/I * | M351T/I * | yes, high frequency | Imatinib | KD-CL, SH2 contact region | Indirect: May increase ABL entropy, disfavoring Imatinib binding, possibly by affecting kinase hydrophobic core packing. In 1OPL, not directly involved in SH2 contacts. | 16, 48, 56, 76-78 | ||
| E371K/G | E352K/G | yes, low frequency | Imatinib | KD-CL, SH2 contact | Faces SH2 domain, possibly involved in inhibitory interactions. | EGFRA N826S | 16, 48 | |
| Y372H | Y353H | yes, low frequency | KD-CL | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. | 16 | |||
| E374G/D | E355G/D | yes, low (D) - high (G) frequency | Imatinib | KD-CL | Unclear. Possibly second-site mutant associated with F317L. Potential indirect effect destabilizing inactive conformation. E355G removes Hydrogen-bonds that help hold helix E against C-terminal domain and may contribute to Imatinib N-methyl-piperazine binding site78. | 16, 56, 76, 78 | ||
| F378A/C/V * | F359A/C/V * | yes, high frequency (V) | Imatinib, Nilotinib | KD-CL | Reduces topological fit with Imatinib without significantly impairing ATP binding. May also destabilize SFK-like inactive conformation.40 | 4, 13, 16, 20, 40, 48, 56, 76, 78, 121 | ||
| I379F | I360F | Imatinib | KD-CL | Imatinib contact site. | 48 | |||
| A385D | A366D | Imatinib | KD-CL | 48 | ||||
| V390A | V371A | yes, low frequency | KD-CL | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. | 16 | |||
| G391R | G372R | Imatinib | KD-CL | EGFRA K846R | 48 | |||
| E392K/Q | E373K/Q | yes, low frequency | Imatinib | KD-CL | 16, 48 | |||
| V398E/A/I | V379E/A/I | yes, low-medium frequency | Imatinib | KD-CL, A-loop | Indirect: May increase ABL entropy, disfavoring Imatinib binding | 16, 48, 76, 78 | ||
| F401L | F382L | yes, low frequency | Imatinib | KD-CL, A-loop | F of DFG motif, undergoes van der Waals interactions with Imatinib that may be altered by its mutation44, 78. Second site mutation associated with M343T, relevance unclear. F401A catalytically inactive, may disrupt a Mg2+-binding triad. Component of the hydrophobic spine that stabilizes active kinase conformations (see T334/T315 gatekeeper mutant)58. Mutation could affect this. | 16, 44, 48, 76, 78 | ||
| L403M | L384M | Imatinib, Nilotinib | KD-CL, A-loop | EGFRA L858R | 4, 13, 16, 20, 48, 56, 121 | |||
| L406M/F | L387M/F | yes, low frequency | Imatinib, Nilotinib | KD-CL, A-loop | Second site mutation, relevance unclear. Potential indirect effect destabilizing inactive conformation, possibly via altered packing to G-loop Y253 and G25478. May also disrupt SFK-like inactive ABL conformation40. | 4, 13, 16, 20, 40, 56, 76, 78, 121 | ||
| M407I/L | M388I/L | yes, low-medium frequency | Imatinib | KD-CL, A-loop | Indirect: May increase ABL entropy, disfavoring Imatinib binding | KIT K818R; EGFR G863D | 48, 56 | |
| T408A | T389A | yes, low frequency | KD-CL, A-loop | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. | 16 | |||
| H415R/P | H396R/P | yes, low-high frequency | (+) | Imatinib | KD-CL, A-loop | Indirect: Destabilizes inactive conformation of A-loop. | 16, 48, 56, 76-78 | |
| G417R | G398R | Imatinib | KD-CL, A-loop | 48 | ||||
| S436Y | S417Y | yes, low frequency | KD-CL | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. Could affect kinase hydrophobic core packing and remove Hydrogen-bond to K419 side-chain. | 16, 78 | |||
| Y459C | Y440C | Imatinib | KD-CL | 48 | ||||
| E469K | E450K | Imatinib | KD-CL | 48 | ||||
| L470M | L451M | Imatinib | KD-CL | 48 | ||||
| E478K | E459K | yes, low frequency | KD-CL | Found in PTK-inhibitor treated CML or Ph+ ALL patients. Unclear whether resistance-conferring. | 16, 78 | |||
| G482D | G463D | Imatinib | KD-CL | 48 | ||||
| M491I | M472I | Imatinib | KD-CL | 48 | ||||
| R492L | R473L | Imatinib | KD-CL | 48 | ||||
| F505S | F486S | yes, low-medium frequency | (+) | Imatinib | KD-CL | Indirect: May increase ABL entropy, disfavoring Imatinib binding, possibly by affecting kinase hydrophobic core packing. | 16, 48, 56, 78 | |
| E513A | E494A | Imatinib | KD-CL: SH2 contact | The E513 side-chain carboxyl group Hydrogen-bonds with S152 in the SH2 domain41. The mutation disrupts this interaction and might thereby destabilize the autoinhibited conformation. | 48 | |||
| E518K/I | E499K/I | Imatinib | KD-CL: SH2 contact | 48 | ||||
| I521M | I502M | Imatinib | KD-CL: MBS α-helix αI'41 | Mutation might reduce myristate-binding mediated inactivation, but I521D mutants only very mildly activated ABL42. | 48 | |||
| E528D | E509D | Imatinib | KD-CL: MBS α-helix αI'41 | 48 | ||||
Legend: SB, salt bridge, IC, type 2 inhibitor-binding inactive conformation, SIC, SFK-like inactive conformation, AC, active conformation, NL, N lobe, CL, C lobe, GL, G-loop, AL, A-loop, DC, drug contact, HX-MS, hydrogen exchange mass spectrometry75, HP, hydrophobic drug binding pocket (Fig. 5), AS2/3, type 2 allosteric drug binding site (Fig. 3), AS4, type 4 allosteric drug binding site, AS, adenine site, αC, αC helix, CR, catalytic residue, RBFE, relative binding free energy, KD, kinase domain, SH2, SH2-domain, SH3, SH3 domain, MBS, myristate binding site, es, electrostatic, hy, hydrophobic. Bold
In terms of frequency, mutations at these six positions account for 60-70% of all Imatinib-resistant ABL mutations found56. For mutations where the underlying mechanism is indicated as “Unclear per 2FO0 analysis”, we analyzed the 2FO0 crystal structure of auto-inhibited human ABL for obvious, direct involvement of the wildtype residue in autoinhibitory ABL domain or linker interactions whose mutational disruption could possibly explain drug-resistance, but found no convincing evidence for such direct involvement. This does not preclude the possibility of more indirect effects of these mutations on ABL-inhibitor interactions. Analogous mutations in other kinases were identified based on sequence homology and similar locations in crystal structures of the kinases indicated.
2. Small Molecules Can Inhibit Kinases Through Diverse Mechanisms
2.1 Kinase structural features and conformational plasticity
All canonical kinases share a typical kinase domain (KD) fold (Fig. 1) 1, 8, 34. An N-terminal N-lobe and a C-terminal C-lobe flank an ATP and substrate binding active site at the interlobe cleft. The N-lobe is mainly composed of β-sheets. It anchors and orients the ATP. The two most N-terminal β-strands flank a glycine-rich loop (G-loop, also termed phosphate-binding loop or P-loop) that binds and positions the ATP properly for γ-phosphate transfer to the substrate 34. The predominantly α-helical C-lobe primarily binds the substrate and initiates phosphotransfer. N- and C-lobe are connected by a “hinge” whose backbone forms critical hydrogen-bonds with the ATP-adenosine (Fig. 3). Binding of ATP and substrate closes the interlobe cleft through hinge-mediated N- and C-lobe juxtaposition, facilitating γ-phosphate transfer.
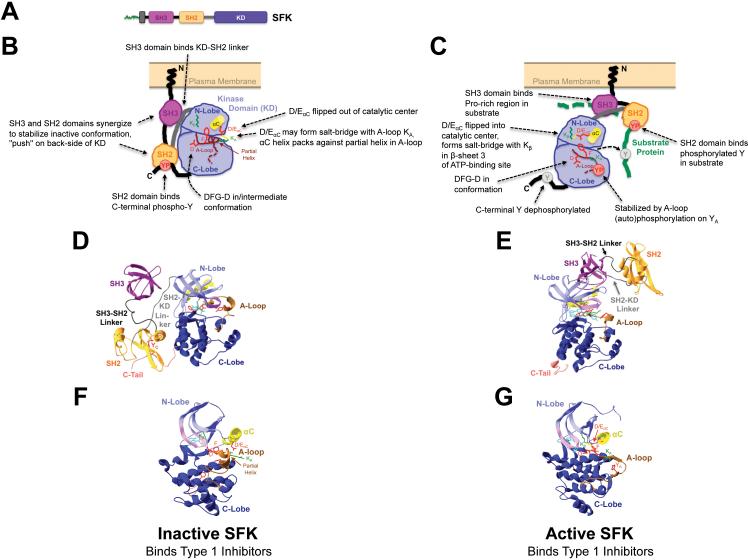
Fig. 1. Conformational changes mediating Src-family protein tyrosine kinase (SFK) activation.
(A) SFK primary structure depicting conserved domains. Green, myristoyl- or farnesyl-conjugated N-terminus. SH3, SH2, KD, Src-homology 3, 2 or KD, respectively. (B-G) Schematic depictions (B,C) and crystal structures (D-G) of the inactive (B,D,F) or active (C,E,G) SFK tertiary structures. The crystal structures shown are hHck-SH2-SH3-PP1 (Pdb accession number 1QCF, D,F)144, hc-Src-des-methyl-Imatinib (1Y57, E)145 and hLck-Furanopyrimidine (2OF2, G)146. (F,G) Kinase domains only. Highlighted in (B-G) and annotated in (B,C) are typical characteristics of the inactive and active SFK conformations, respectively. All structures were rendered and colored in Swiss-PdbViewer (www.expasy.org/spdbv). Domains and interdomain linker regions are indicated and color-coded. Bordeaux, SH3 domain; black, SH3-SH2 interdomain linker; orange, SH2 domain; gray, SH2-KD linker, light blue, KD N-lobe with αC helix (yellow) and G-loop (pink); dark blue, C-lobe with activation (A)-loop (brown); salmon, C-terminal tail (C-Tail). Also indicated are key amino acid (AA) side-chains involved in catalysis, or whose orientation differs markedly among the different conformations in Src or ABL family kinases. Red, D and F of the A-loop DFG motif, D/EαC within the αC helix which forms a salt-bridge with conserved Kβ (green) in N-lobe β-sheet 3 in active SFKs, YA in the A-loop which is auto-phosphorylated into YP (red sphere in C) in active kinases, YC in the C-terminus which is phosphorylated into YP (red sphere in B) by Csk and binds to the SH2-domain in inactive SFKs. Also shown is A-loop KA (green) which may form a salt-bridge with D/EαC in the “αC-out” conformation of inactive SFKs (B,D,F) and of ABL in the SFK-like inactive structure (Fig. 2H). Cyan, bound ATP-competitive inhibitor.
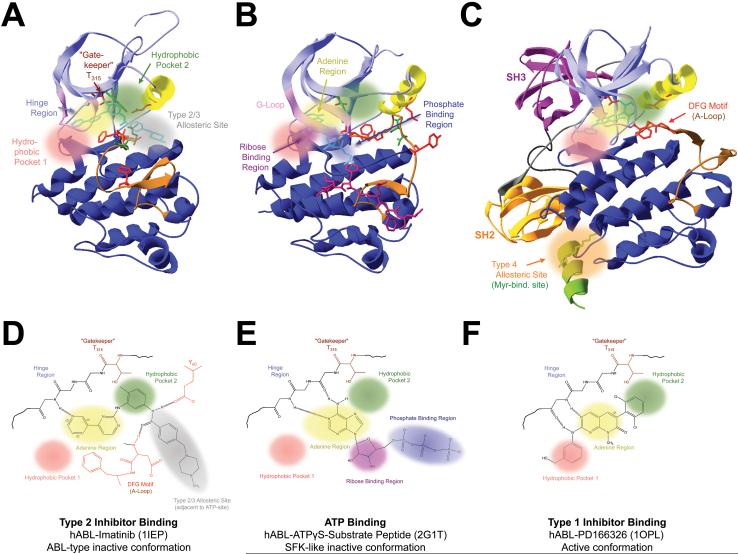
Fig. 3. Types and structural features of small-molecule inhibitor binding sites in ABL/Arg-family protein tyrosine kinases.
Shown are (A-C) the crystal structures and (D-F) schemes of the compound-bound ATP-binding sites of (A,D) the type 2 inhibitor imatinib-bound human (h) ABL kinase domain (KD, 1IEP)97, (B,E) the ATPγS/substrate peptide bound hABL KD in the SFK-like inactive conformation (2F1T)40 and (C,F) the type 1 inhibitor PD166326-bound hABL KD in the active conformation (1OPL)41. Domains and structural features are color-coded as in fig. 1,2. Colored spheres highlight the positions of the following key sites involved in inhibitor-interactions: Hydrophobic pocket 1 (pink) or 2 (green), ATP-adenine binding region (yellow), ATP-ribose binding region (violet), ATP-triphosphate binding region (blue), type 2/3 allosteric site (gray) and the myristate-binding region as an example for a type 4 allosteric site remote from the ATP-binding region (orange). Also indicated are the hinge region, which forms conserved hydrogen-bonds (dashed lines) with the ATP-adenine or adenine-analogous moieties of ATP-competitive inhibitors, the T315 “gatekeeper” residue which can control access to hydrophobic pocket 2 and type 2/3 allosteric site, and characteristic hydrogen-bonds between imatinib and residues in αC-helix and in the DFG-motif at the beginning of the A-loop1, 8, 13, 35, 50.
Recent data suggest that kinases exist in a dynamic equilibrium of multiple different conformations (Fig. 1,2). The transition from inactive to catalytically active conformations involves characteristic conformational changes in several conserved structural elements that harbor amino acid (AA) residues directly involved in catalysis, or in stabilizing inactive vs. active conformations. Studies of SFKs (Fig. 1), ABL (Fig. 2) and other kinases have provided mechanistic insight into how these conformational changes control kinase function 8, 35-44. Briefly, the activation loop (commonly termed A-loop) in the C-lobe often occludes the catalytic domain in inactive kinases. A-loop auto- or heterologous phosphorylation induces or stabilizes conformational changes, permitting ATP/substrate-access to the catalytic site, and allowing the acidic D-side-chain in a conserved N-terminal DFG (in some cases DLG) AA consensus motif within the A-loop to contact an ATP-coordinating metal ion 8.
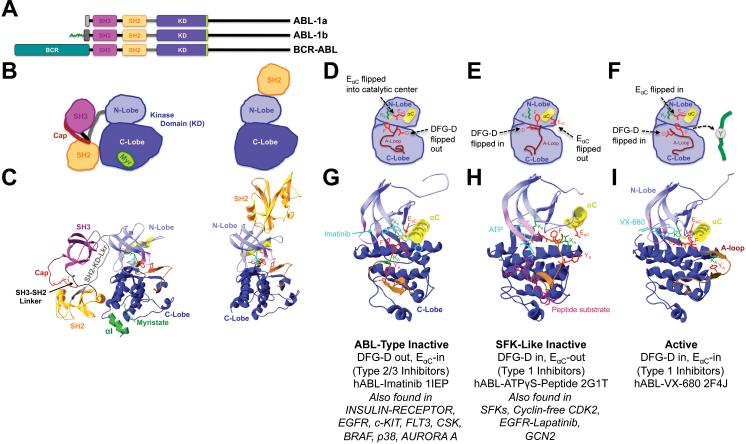
Fig. 2. Conformational changes mediating ABL/Arg-family protein tyrosine kinase activation.
(A) ABL and oncogenic BCR-ABL primary structures depicting conserved domains. Green, myristoyl-conjugated N-terminus only present in ABL1b (ABL-1b) due to alternative splicing. SH3, SH2, KD, Src-homology 3, 2 or KD, respectively41. (B,C) Schemes (B) and crystal structures (C) of myristate-bound, autoinhibited full length hAbl-PD166326 (Pdb accession number 2FO0, left)39, or hAbl-P16 (1OPL chain B, right)41, which illustrates potential N-lobe/SH2 domain interactions in active ABL. Domains and features are indicated and color-coded as in fig. 1. Cayenne, N-terminal cap region (Cap) folding back over and interacting with the SH2 domain through a phosphorylated serine39. Green, myristate (Myr) binding site including myristate moiety and involving a unique αI helix. The region between Cap and Myristate is disordered and harbors a deletion39. (D-I) Schematic depictions (D-F) and crystal structures (G-I) of the following complexes: (D,G) hABL-Imatinib (1IEP)97 in the type 2 inhibitor-binding ABL-type inactive conformation. (E,H) hABL-ATPγS-substrate peptide (2G1T)40 in the type 1 inhibitor-binding competent SFK-like inactive conformation. (F,I) hABLVX-680 (2F4J)87 in the type 1 inhibitor-bound active conformation. The active conformation most likely results from synergy between a H396P mutation, which destabilizes the ABL-type inactive conformation, and binding of VX-680, which favors the active conformation through hydrogen-bonding and steric effects. VX-680 binds Abl in a mode that accommodates the T315I “gatekeeper” mutation87. Cyan, bound ATP-analog. Bordeaux stick model, substrate peptide in (H). Key characteristics of each conformation and other kinases for which the respective conformation has been reported are summarized underneath the respective structures35.
In inactive SFKs, the A-loop forms a partial helix that interacts with N-lobe helix αC (short for α helix C45). This interaction includes an electrostatic salt-bridge between a conserved basic A-loop KA and a conserved D/EαC that is enabled by D/EαC-orientation out of the catalytic center (D/EαC-out conformation). In active SFKs, D/EαC is flipped into the catalytic site (D/EαC-in conformation) and salt-bridges with Kβ of the N-lobe. The DFG D-side-chain is oriented into the catalytic site in active SFKs (DFG-D-in conformation) and intermediately oriented in inactive SFKs whose non-helical A-loop does not bind αC (Fig. 1). The active A-loop conformation is stabilized by phosphorylation of conserved YA. SFK-activation involves complex interactions of their non-catalytic domains (Fig. 1)36, 37. SH2 (Src homology 2) domain-binding to a Csk/Chk-phosphorylated C-terminal YC, and SH3 domain-binding to the SH2/KD-linker cause αC-flipping into the out-position and stabilize the inactive conformation 46. YC-dephosphorylation, SH2- and SH3-domain binding to other ligands usually in the substrate, or mutagenesis of interacting domain/linker-residues disassemble these inhibitory intramolecular interactions, causing conformational changes and SFK activation that is further stabilized by A-loop phosphorylation. Recent data suggest that not all inhibitory interactions may need to be dissolved for SFK-activation 36, 37.
Similar interactions control ABL, whose two splice-variants ABL-1a and -1b harbor differing N-termini (Fig. 2)37. The N-terminus of ABL-1b is myristoylated. In contrast to the SFKs, the ABL inactive conformation is stabilized through phospho-Y independent SH2 domain/C-lobe binding. Besides SH3 domain-SH2/KD-linker interactions, the N-terminal cap region may fold over the SH2 domain and allow the ABL-1b N-terminal myristoyl-moiety to bind to a specific site in the C-lobe. Mutagenesis data suggest that these interactions are auto-inhibitory (Tab. 4)39, 41, 47, 48. ABL-activation likely involves their disruption, SH2 domain-translocation to an N-lobe interaction, and A-loop phosphorylation (Fig. 2). The ABL KD can adopt two different inactive conformations: A SFK-like inactive EαC-out, DFG-D-in configuration may possibly represent a transitional intermediate between active ABL (EαC-in, DFG-D-in) and an ABL-type inactive structure where EαC is flipped into the catalytic site, but DFG-D is flipped out and the A-loop is in an inactive conformation. Examples for both inactive conformations exist in other kinases (Fig. 2). Energetic constraints may cause differential representation of the various structures in a dynamic equilibrium. They provide distinct physicochemical environments that can be targeted by KIs. Indeed, the clinical success of several KIs relies on their abilities to bind and stabilize distinct kinase conformations 19.
2.2 Compounds can employ different mechanisms to perturb kinase function
KIs can competitively target protein, small-molecule ligand, substrate or ATP-binding sites. Compound-binding to allosteric sites can inhibit kinases through conformational effects1, 8, 13, 49, 50. Large interfaces mediating very strong interactions make small-molecule interference with protein-protein interactions difficult despite some recent progress49-51. Traditional screens for small-molecule KIs yielded primarily ATP-competitive compounds that bind to the ATP-binding site. Achieving high target kinase affinities and inhibitory potencies proved relatively easy. Consequently, most approved and clinically explored current compounds are ATP-competitors (Tab. 1-3). However, kinase domains need to bind ATP and orchestrate the stereo-selective ATP γ-phosphoryl transfer to nucleophilic residues in the substrate. This places major constraints on shape and physicochemical environment of the ATP-binding site. Consequently, its shape and key molecular AA interactions with ATP-atoms, in particular the ribose and triphosphate moieties, are strongly conserved among many kinases. Paucity of un-conserved physicochemical features makes it difficult to develop highly selective ATP-competitors that only inhibit a targeted kinase. Although a moderate lack of selectivity can sometimes be exploited to “poly-target” several kinases that contribute to a pathology (Tab. 1,2)15, 22, 52, 53, it can also cause side effects or toxicity. Fortunately, the resolution of over 755 kinase/inhibitor complex crystal structures54, extensive biochemical and genetic analyses, and refined rational, structure and quantitative structure-activity-relationship (QSAR)-based design approaches have recently allowed the development of more selective KIs1, 8, 9, 13, 35, 55.
Most current KIs employ one of five KD binding-modes (Fig. 3)1, 8, 13, 35, 50. Type 1 KIs (T1KIs) including the ABL-inhibitors Dasatinib, PD166326 or MK-0457/VX-680 (Fig. 2,3; Tab. 1,2)4, 13, 16, 56, 57 compete with ATP for binding to the ATP-binding site. They employ similar KD interactions as the ATP adenine, including 1-3 hinge hydrogen-bonds. Their selectivity and potency can be increased through additional interactions with two hydrophobic pockets (HP1/2) flanking the adenine-site (Fig. 3C,F)13, 35. T1KIs bind and inhibit active and inactive kinase conformations, including the SFK-like inactive conformation (Fig. 1,2)35, 40.
Type 2 KIs (T2KIs) including imatinib, gefitinib, nilotinib and sorafenib (Tab. 1)8, 13 are indirectly ATP-competitive. They harbor a distinct moiety that interacts with HP2 and an adjacent hydrophobic type 2/3 allosteric site that is generated by A-loops in the DFG-D-out position and specific for ABL/KIT-like inactive kinase conformations (Fig. 3A,D)13, 35. T2KI binding may involve DFG-D-out specific DFG and YαC interactions. T2KIs have improved potency and selectivity, because HP2 and type 2/3 allosteric site are not involved in ATP-binding and hence less conserved. This and their ability to induce and/or stabilize the ABL-type inactive KD conformation (Fig. 2,3)44 make T2KIs powerful therapeutics. T2KIs may extend into the ATP-adenine region and form hinge hydrogen-bonds. This property can be rationally added to improve potency35. The ABL/KIT-like inactive conformation was observed in ABL, insulin-receptor, p38, BRAF, EGFR, HDR, KIT, CSK, FLT3 and AURORA-A, but is energetically unfavorable in certain other kinases including SFKs and cyclin-dependent kinases (CDKs)35. This allows augmented T2KI selectivity, exemplified by the ~2000-fold higher Imatinib affinity for ABL over SFKs35, 38, 56. Access to HP2 and type 2/3 allosteric site is controlled by a “gatekeeper” residue between adenine-site and HP2 (Fig. 3). In many kinases, a small gatekeeper side-chain allows KI binding. Gatekeeper mutations such as ABL-1a T315I (Tab. 4) that introduce bulky side-chains are a main cause of KI-resistance through several mechanisms, including steric hindrance of drug access to both sites13, 58, 59.
Recently developed type 3 cSRC KIs (T3KIs) exclusively bind the type 2/3 allosteric site 60. Like T2KIs, they stabilize the inactive kinase conformation. Their fusion to T1KI-scaffolds can generate T2KIs through “hybrid design”35, 61.
Attempts to overcome type 1/2 KI drug-resistance in ABL and other kinases have recently provided ATP-noncompetitive “type 4” allosteric KIs (T4KIs) . These bind kinase regions outside the ATP-pocket, often in remote locations (Fig. 3C)13, 49, 50, 60. Because these sites are less conserved, T4KIs can have high target-selectivity. Examples are GNF-2/5 inhibitors (Fig. 3)13, 55, 62, 63. GNF-2/5 or, likely, myristate-binding to the ABL myristate binding-site inhibit catalysis by stabilizing the inactive conformation and causing conformational changes at the ATP-site through mechanisms that involve SH2/SH3 domain interactions (Fig. 2,3). Interestingly, SRC also possesses a C-lobe myristate-site but is not inhibited by myristate or GNF-2/562. Additional allosteric inhibitors have been developed for mTOR, AKT, MEK, IKK, CHK and CAMKII55. Intriguingly, allosteric kinase activators also exist13.
Another KI-type are covalent inhibitors (cKI), including five EGFR-inhibitors in clinical trials. These bind covalently to nucleophilic cysteines in the active site and irreversibly inhibit ATP-binding or activity13, 64. Cysteines near the ATP-pockets of ~200 human kinases provide opportunities to broadly explore the potential benefit of high cKI potency, and the potential liability of covalently modifying unanticipated targets13.
3. Mechanisms of Kinase Inhibitor Drug-resistance
Many factors can contribute to pre-existing/primary or acquired/secondary KI-resistance (Fig. 4A,B). Target-cell extrinsic mechanisms include well-established pharmacokinetic (PK) factors that primarily affect drug efficacy9, 16, 22, 65. Another factor is the tumor microenvironment. EGFR inhibitors likely inhibit angiogenesis both by inhibiting tumor cell VEGF production, and by inhibiting EGFR signaling in surrounding endothelial cells9. Stromal cell paracrine HGF secretion may promote gefitinib-resistance in EGFR-mutant NSCLC66.
Pharmacogenomic factors including gene polymorphisms can cause considerable variation in drug efficacy and toxicity. They can contribute to primary drug-resistance and may necessitate individually optimized dosing regimen9, 65. For example, EGFR polymorphisms affect EGFR expression, gefitinib sensitivity and toxicity, possibly contributing to variations in EGFR-inhibitor clinical efficacy between Asian versus Caucasian lung cancer patient populations9.
3.1 Acquired drug-resistance involves primarily target cell intrinsic mechanisms
Cell-intrinsic drug-resistance mechanisms include target gene amplification, overexpression or epigenetic activation, upregulation/activation of redundant or downstream signaling effectors, or secondary missense mutations in the targeted kinase which reduce drug-affinity or -effect (Fig. 4A,B) 9, 16, 21-24, 66, 67. BCR-ABL overexpression due to gene amplification occurred in some imatinib-resistant CML patients 16, 26. Elevated histone-deacetylase (HDAC) and reduced histone-acetyltransferase activities in imatinib-resistant CML cells and synergistic pro-apoptotic effects of KIs and HDAC-inhibitors suggest that altered epigenetic modifications can contribute to imatinib-resistance. It will be interesting to explore the clinical benefit of this approach16.
Augmented redundant/downstream signaling can result from upregulation of positive or downregulation of negative effectors. Upregulation of BCR-ABL downstream effectors including SFKs (Lyn), PI3K, JAK/STAT or Ras/Erk-pathways was found in imatinib-resistant CML cell subsets 16, 22, 56. Although ABL-independent SFK signaling may contribute to imatinib-resistance, SFK-binding may also stabilize the BCR-ABL active conformation. ABL-phosphorylation by SFKs may reduce imatinib-sensitivity, possibly through allosteric effects16. Therefore, the ability of dasatinib to overcome imatinib-resistance in CML may sometimes include SFK-inhibition in addition to the inhibition of many ABL mutants. In GIST cells, AXL-upregulation and subsequent AKT activation may contribute to imatinib-resistance. PI3K-activation via oncogenic PIK3CA mutation, PTEN-loss or MET-amplification and ERBB3-signaling can confer EGFR-KI-resistance9, 21. PTEN-downregulation in ~70% of NSCLC may contribute to gefitinib/erlotinib-hyposensitivity9, 68. This provides a rationale for evaluating co-inhibition of targeted kinase and upregulated effectors clinically9. However, if target-effectors participate in signaling loops, their (co-)inhibition can cause complications: mTORC1-inhibitors promoted AKT activation and potentially tumor growth by downregulating PI3K feedback-inhibition 9.
Leukemic stem cells (LSCs) may play an important role in KI-resistance in CML, and in the importance of disease-stage for prognosis24, 67. Their quiescence, or environmental survival signals in the stem-cell niche, may render LSC-viability BCR-ABL-independent, causing ABL-inhibitor resistance 24. LSC-subsets harboring drug-resistant mutations can thus provide a reservoir of drug-resistant CML cell precursors despite a complete cytogenetic response to KI-treatment24.
Finally, tumor cell genetic instability might facilitate the emergence of different drug-resistance mechanisms in different metastases in a patient, seriously complicating attempts to overcome drug-resistance. One NSCLC patient had one metastasis with MET-amplifications and another one with an EGFR-T790M mutation69. Both mechanisms can cause gefitinib/erlotinib-resistance.
Most relapsing CML patients show acquisition of one or more of >50 different missense mutations in the BCR-ABL KD, or BCR-ABL oncogene-amplification (Table 4, Fig. 4,5)13, 21, 25, 26, 56. Clinical studies have implicated FLT3, KIT, PDGFRA, EGFR or ERBB2 mutations in KI-resistance in various cancers (Table 5). Intriguingly, many of these mutations are located in similar positions in the different kinases, and may engage similar mechanisms (Tables 4,5; Fig. 5). Below, we discuss how studying the most important mutations advanced our mechanistic understanding of KI-resistance and enabled the development of less resistance-prone therapeutics.
Fig. 5. Topological locations of known drug-resistance causing point mutations in ABL.
Shown is the crystal structure of myristate-bound, autoinhibited full length hAbl-PD166326 (Pdb accession number 2FO0)39. (A) shows the entire structure including regulatory subunit interactions. (B) shows the KD only, oriented as those in fig. 2,3. Domains, structural features and kinase-inhibitor interaction sites are labeled and color-coded as in fig. 1-3. The colored spheres indicate the positions of drug-resistant point mutations clinically observed in ABL only (red), clinically observed in ABL and other kinases (light green), observed preclinically in ABL and clinically in other kinases (light blue), or only observed preclinically in ABL to date (brown, tab. 4,5).
3.2 Oncogenic and drug-resistance mutations occur in hotspots
For ABL, oncogenic mutation usually relies on translocations like the Philadelphia Chromosome. These cause expression of fusion proteins like BCR-ABL, which are hyperactive due to loss of the auto-inhibitory ABL1b myristate-moiety, and to dimerization through the fused domain1. In contrast, oncogenic mutation of EGFR, which contrary to ABL does not require A-loop phosphorylation for activity1, can result from deletions 64, 68. Moreover, EGFR overexpression (observed in 62% of NSCLC cases) and hyperstimulation by the secreted ligands EGF and TGFα in auto-/paracrine feedback-loops can confer growth advantages to tumors and promote metastasis. Biological EGFR-antagonists act primarily by disrupting these loops68. 50-80% of EGFR-mutant NSLCs respond to the small-molecule KIs gefitinib or erlotinib68. KI-treated NSCLC patients showed an accumulation of small deletions, insertions or point mutations in the EGFR-KD that often positively associate with tumor-sensitivity to KIs (Table 5)64, 68, 70. Their use as clinical markers for patient selection strongly increased TKI response rates, although ≤20% of mutation-carrying patients are gefitinib-resistant64, 70. The topological locations of several primary EGFR-mutations overlap with those of KI-resistant ABL-mutations. Yet, many do not alter EGFR-KI binding-affinities64. Instead, they may hyperactivate EGFR and augment/prolong downstream signaling. However, some data are conflicting, and primary mutations can reduce ATP-affinity68, 71. The precise mechanism through which they increase drug sensitivity is still unclear but may vary with cellular context and genetic background. It likely involves destabilization of inactive, or stabilization of active kinase conformations, oncogene addiction, where tumor growth/survival become dependent on the presence of a specific EGFR-mutant, and stronger KI-inhibition of pro-survival than pro-apoptotic EGFR-mutant signaling64, 68, 71-73. The resulting “oncogenic shock” may explain why KIs often have maximal efficacy against cancers that harbor deregulated target-kinase alleles9.
Other EGFR-mutations variably reduce drug binding or -efficacy (Table 4). Again, several have analogous drug-resistant ABL-mutations. Further complicating the issue, some EGFR-mutations including E884K confer gefitinib-sensitivity but erlotinib-resistance in NSCLC74. Similarly to EGFR, clinical ERBB2, KIT or PDGFR KI-resistance mutations often occur secondary to oncogenic mutations and involve topologically analogous residues to drug-resistance mutations in ABL or other kinases (Table 5).
Comparing imatinib-resistant ABL point-mutations identified in a cell-based mutagenesis screen48 with clinically resistance-associated mutations in BCR-ABL (Table 4), FLT3, EGFR, ERBB2, PDGFRA, KIT and FMS (Table 5, Fig. 5) unveils several conserved mutational “hot spots” within the KD. They usually harbor missense mutations, although small deletions and insertions can also occur 3, 13, 16, 22, 26, 55, 68. Most drug-resistance mutations arise in protein regions involved in drug interactions (HP1/2, adenine-region, type 2/3 allosteric site, G-loop), or in the transitions between active and inactive kinase conformations (G-loop, A-loop, αC-helix, Fig. 1-3). To cause drug-resistance, a mutation must impair drug binding or the involved conformational changes more than ATP-binding and catalysis. Consequently, directly ATP-interacting residues of hinge or ATP-phosphate binding region are infrequently involved (Fig. 3). Mutation of directly drug- but not ATP-binding residues loosens drug binding selectively. Examples are ABL1b-L267(248 in ABL1a, the common clinical nomenclature), Y272(253), V308(289), M309(290), T334(315), F336(317), G340(321), F401(382), FLT3-N676 or KIT-V654 (Table 4,5). Additional clinical mutations occur throughout the N-lobe and in several C-lobe locations including the substrate-binding site (Fig. 5, Tables 4,5). Structural and often sequence conservation among analogous drug-resistant mutations in different kinases suggest conserved mechanisms. Hence, the lessons learned from studying drug-resistance in CML and other cancers can likely be applied to other kinasopathies. We therefore next discuss the key principles identified and their relevance for overcoming KI-resistance.
3.2.1 The gatekeeper mutation
Besides drug-binding residues, small side-chains that sterically accommodate drugs can be mutated into bulky side-chains that hinder drug access. A prominent example is the aforementioned ABL1a-T315I mutation of the small T315 “gatekeeper” residue between adenine-site and HP2 (Fig. 3). This “gatekeeper mutation” remains resistant to most currently approved ABL-inhibitors, including imatinib, dasatinib and nilotinib (Tables 1,4). Replacement of the small T315 side-chain by a bulky isoleucine side-chain sterically blocks T1/2KI-access to HP2 and type 2/3 allosteric site without impairing ATP-binding. This was initially considered the main mechanism through which T315I causes imatinib-resistance, besides removal of a T315-imatinib hydrogen-bond. However, recent results showed that gatekeeper-mutation stabilizes a “hydrophobic spine” linking gatekeeper-residue and A-loop YA in active kinases (Fig. 1,2) 58, 59. This destabilizes the T2KI-binding inactive, but stabilizes the ATP-binding active conformation, causing re-gained catalysis, drug-resistance and increased transforming potential58. Hydrogen-deuterium-exchange mass-spectrometric (HDX-MS) analyses confirmed that gatekeeper-mutation enhanced imatinib-binding site conformational flexibility. They also suggest enhanced SH3 domain RT-loop flexibility, possibly indicating distant allosteric effects that might reduce inhibitory SH3-KD interactions, or Abl-binding to other proteins 75. Consistent with a perturbed inactive conformation, T315I-mutation activated myristoylated ABL, thought to be auto-inhibited75.
A small gatekeeper residue is conserved in many kinases. Its mutation in KIT, PDGFRA, EGFR and ERBB2 causes KI-resistance (Tables 4,5)13, 16, 22, 25, 26, 48, 55, 56, 58, 75-79. Importantly, gatekeeper mutants are the most frequent clinical BCR-ABL, KIT, PDGFRα and EGFR drug-resistance mutants 13. This demonstrates the clinical importance of the general mechanism to cause drug-resistance through distributed allosteric effects that can affect remote regions within the KD or even in other domains. Overcoming gatekeeper-mutation induced drug-resistance clinically is extremely difficult. This may reflect the “dominant” effect of stabilizing the active kinase conformation and obstructing drug access along with the enhanced transforming potential of the mutation58. Moreover, ABL-T315I might promote drug-resistance in neighboring cells through KI-induced paracrine IL-3 release, although the clinical relevance is unclear 80. Erkhyperactivation in KI-treated ABL-T315I mutant cells might suggest additional contributions of upregulated downstream-signaling (Fig. 4)24.
Stabilization of the active conformation, the different mode of EGFR-deregulation and the usually secondary occurrence of KI-resistance mutations following primary mutations in the same cell might explain why EGFR-T790M gatekeeper-mutation only mildly affects gefitinib-binding, but restores the often reduced ATP-affinity of primary EGFR-mutants like L858R to wildtype-EGFR levels81. T790M increases EGFR activity and oncogenicity, occurs in ~50% of KI-resistant NSCLC patients, can occur as a primary resistance-mutation and might contribute to inherited lung cancer susceptibility 25, 64, 68, 70, 82.
3.2.2 G-loop mutations
The G-loop binds ATP and, sometimes, substrate or other parts of the kinase (Fig. 1-3). This flexible clamp anchors and orients the ATP α/β-phosphates to properly position the γ-phosphate for transfer onto the substrate, may stabilize the catalytic transition-state, controls nucleotide affinity/specificity and γ-phosphoryl transfer-rate 34, 54, 83, 84. All canonical protein kinases harbor the conserved G-loop consensus motif G-2x-1G0x1x2G3. G0 is most conserved34. X1/2 comprise the turn. The glycines provide conformational flexibility, allow tight ATP “contouring”, precluding water-access and non-productive ATP-hydrolysis, and enable backbone hydrogen-bonds with ATP-phosphates. G-2 and G0 mutation impairs ATP binding and/or catalysis34. Analysis of 532 nonredundant protein kinase/inhibitor-complex crystal-structures unveiled additional conservation of a hydrophobic X-3, aromatic X2 and hydrophobic X554. X-3/5 interact nonpolarly with ATP-adenine and many ATP-competitive inhibitors 54, 85. Many KIs also interact with G-2, G0 and X-1. Typically, inhibitors contact 2-4, maximally 8 G-loop residues through mostly hydrophobic interactions 54. 11 structures showed KI hydrogen-bonds with X254. Moreover, 58 kinases including SFKs and ABL share a X-4/X+4 salt-bridge across their G-loops 34. Its mutational disruption in SFKs impairs ATP-binding and catalysis by augmenting the flexibility of G-loop and other catalytically important KD-regions, including hinge and αC-helix 34. This suggests important G-loop conformational interactions with other KD-regions.
Usually, the G-loop-conformation is extended (Fig. 1, 2H, 3B). However, several structures of inactive and active ABL (Fig. 2G/I; 3A/C), p38α, FGFR1, CK2α1, JNK3, AURORA-A, ROCK1 and CDK5 show a distorted G-loop with altered X-3/-2 dihedral angles 54. This “kinked” G-loop was suggested to represent inactive kinase conformations, and to increase surface-complementary with drugs. It may be induced or stabilized by T2KIs 44, 54, 77, 86. However, kink-preservation in T1KI-bound ABL in the active conformation (Fig. 2I, 3C)87 may argue against a specific kink-role in inactive conformations.
The G-loop is a hotspot for primary oncogenic or drug sensitizing mutations in EGFR, ERBB2 (Tab. 5), and BRAF (R461I, I462S, G463V/E, G465A/E/V, G468A/E) 1, 5, 9, 64, 68, 71, 88-91. Many disrupt interactions that stabilize the inactive conformation, causing hyperactivity and likely oncogene addiction 1, 71, 73. For example, oncogenic BRAF G-loop or A-loop mutations may disrupt inhibitory G-loop/A-loop interactions, besides variably affecting ATP-binding 90.
Some of the clinically most important kinase drug-resistance mutations also occur in the G-loop (Tab. 4,5, Fig. 5)34, 54, 56, 83. In particular, G-loop mutations that destabilize inactive or stabilize active conformations, or remove direct drug interactions cause clinical resistance to T2KIs. In CML-patients, G-loop mutations represent ≤48% of all imatinib-resistant BCR-ABL mutations. They associate with a particularly poor prognosis 16, 22, 76-78. Different G-loop mutations occur with different frequencies and confer different levels of imatinib-resistance. Consistent with the very similar ABL and BRAF inactive KD structures90, the imatinib-resistant BCR-ABL G-loop Y253F and E255K mutations (clinical ABL1a nomenclature) may be oncogenic48, 92, 93.
E255K/V and Y257C disrupt an electrostatic triad involving the K247-4, E255+4 and Y257+6 side-chains. It incorporates the conserved X-4/X+4 salt-bridge 34, 77, 78 and was suggested to be specific for the kinked G-loop of inactive ABL77, 78. E255K/V and Y257C imatinib-resistance suggests that the triad is required for imatinib-binding. Its disruption might reduce imatinib-binding by destabilizing the kinked conformation, decreasing G-loop surface-complementarity with imatinib, and contributing to distributed allosteric effects that destabilize the inactive BCR-ABL conformation 44, 56, 76-78, 94-96. However, preservation of the K-4/E+4 salt-bridge in active ABL87, 97, inactive and active SFKs (Hck, protein data bank (PDB) accession number 1AD5; Src 2SRC, 2OIQ, 3EL8, 1FMK, 1Y57; Lck 2OF2, 3LCK; Lyn 2ZV8, 2ZVA, 2ZV9), and the strongly reduced activity of salt-bridge disrupted SFKs 34 suggest broader salt-bridge/triad roles beyond inactive conformations. Indeed, E255K/V mildly reduced BCR-ABL inhibition by dasatinib, which binds active and inactive conformations 57, 98.
Molecular dynamics simulations suggest that E255K/V might augment the flexibility of G-loop and other N-lobe regions34, 99. E255K/V and M244V/I may moreover affect the relative-binding-free-energy contributions of other G-loop and non G-loop residues, or the electrostatic charge-distribution within the G-loop 99. However, small E255-mutation effects on ABL-inhibition by some T2KIs 94, small conformational effects in HDX-MS analyses 75 and variable E255K/V effects on ABL kinase-activity depending on expressed construct and assay conditions probably indicate important contributions by the complex intra-molecular domain interactions and covalent modifications regulating ABL in cells to the physiological consequences of E255K/V-mutation (Fig. 3) 34, 44, 92, 93, 95.
Besides disrupting the G-loop triad, Y257C also prevents Y257-phosphorylation. Reduced Y257F activity and oncogenicity suggest that triad and/or Y257-phosphorylation are catalytically important 48, 78, 92. However, G-loop phosphorylation inhibits catalysis in several kinases including ABL, disfavoring ATP- or substrate-binding. Y253F oncogenicity supports a potential inhibitory role for Y253-phosphorylation 85, 92, 100. Clearly, more studies are required to discern the specific Y253 versus Y257 roles in ABL-catalysis versus KI-binding.
Surprisingly few drug-resistant G-loop mutations were clinically observed in other kinases (Tab. 5). The Y253F/E255K analogous positions in PDGFR and KIT already harbor F or K residues, respectively22, 78. Thus, destabilization of the inactive conformation by ABL-Y253F/E255K need not translate to other kinases. Indeed, disruption of the K-4/E+4 salt-bridge in SFKs reduced catalysis and might increase N-lobe conformational dynamics more than in ABL34. Thus, G-loop salt-bridge disruption can reduce catalysis, or cause drug-resistance or even oncogenicity. It will be interesting to determine how it affects catalysis and KI-interactions in other kinases sharing the G-loop salt-bridge 34. Interestingly, ERBB2 lacks the G-loop salt-bridge but harbors lapatinib-resistant T733I at the ABL-E255K/V-analogous position (Tab. 4,5) 5. The otherwise drug-sensitizing EGFR-E709A/G associated with lapatinib-resistance in the presence of ERBB2 in vitro 64, 68, 88. Altogether, G-loop mutation can have complex and diverse effects on catalysis and drug-interactions. This reflects the G-loop importance in controlling kinase conformational dynamics, ATP and inhibitor interactions.
3.2.3 αC helix mutations
The αC helix of the N-lobe undergoes major conformational changes between inactive and active kinase conformations (Fig. 2,3)8, 35, 36, 38-44. In active SFKs, active or ABL-type inactive ABL, the conserved EαC is oriented into the ATP-site and salt-bridges with Kα in β-sheet 3 of the N-lobe. This is required for catalysis 1. In inactive SFKs and SFK-like inactive ABL, EαC is flipped out and may salt-bridge with A-loop KA, stabilizing the inactive conformation. Several KIs interact with αC residues. αC-interactions cause higher ABL affinity for nilotinib over imatinib 1. Not surprisingly, αC is another hotspot for inhibitor-resistant mutations (Tab. 4,5, Fig. 5). ABL-V289S/I (ABL1a nomenclature) alters an imatinib contact-site. It is topologically analogous to drug-sensitizing EGFRA-V765A 16, 48. EGFR-deletions N-terminal of αC likely destabilize the inactive conformation. Most act drug-sensitizing/oncogenic 68, 71, 72. Interestingly, some may reduce ATP-affinity and confer gefitinib-resistance (Tab. 5). Various deletions or insertions starting at EGFR-L747 clinically associate with increased gefitinib/erlotinib sensitivity 68, 88, 101. In contrast, several αC point mutations including S768I/V769L associate with drug-resistance in EGFR and have ABL/ERBB2 analogs (Tab. 5) 5, 64, 68, 88. Similar to the G-loop, αC-mutations may cause drug-sensitization, oncogenic activation and/or drug-resistance primarily by destabilizing inactive kinase conformations, besides abrogating drug interactions.
3.2.4 A-loop mutations
The main mutational hotspot in the C-lobe is the A-loop. Like G-loop and αC helix, it undergoes major conformational changes between inactive and active conformations (Fig. 2,3,5; Tab 4,5) 8, 35, 36, 38-44. Several A-loop mutations have analogs in multiple kinases (Tab. 4,5, Fig. 5). A recurring motif among 6 clinical imatinib-resistant ABL A-loop mutations are indirect effects that disfavor drug-binding by increasing entropy or destabilizing the inactive conformation. F382L (ABL1a numbering) mutates the DFG-F and might affect the hydrophobic spine that stabilizes the active conformation besides reducing imatinib-interactions 16, 44, 48, 76, 78. L387M/F may alter A-loop packing to G-loop Y253/G254 78.
KIT-R815 corresponds to ABL1b-R405 (RA), which salt-bridges with E305 (EαC) in the SFK-like inactive conformation and in inactive SFKs. KIT-R815-deletion might thus destabilize inactive conformations. KIT-D816 and analogous PDGFRA-D842 mutation may cause drug-resistance by abrogating hydrogen-bonds that stabilize inactive conformations. The positionally analogous EGFR-L861Q is drug-sensitizing 102-107. KIT-Y823 corresponds to ABL/SFK-YA. By mimicking YA-phosphorylation, KIT-Y823D might stabilize the active or destabilize the inactive conformation, reducing T2KI binding 102-105, 107.
L858R represents ~41% of oncogenic EGFR mutations. L858R disrupts A-loop partial-helix hydrophobic interactions with αC and G-loop F723 that stabilize the inactive conformation and may reduce ATP-affinity 1, 64, 68, 71, 73. The analogous ABL-L403M is imatinib-resistant. EGFR-E884K confers gefitinib-sensitivity, but erlotinib-resistance. It disrupts an A-loop salt-bridge with C-lobe R958, may alter substrate-interactions and/or A-loop flexibility 64, 74, 108.
Finally, BRAF A-loop mutants within and C-terminal of the DFG motif (D593V, F594L, G595R, L596V/R, T598I, V599E/D/K/R, K600E) destabilize the inactive conformation by disrupting hydrophobic G-loop interactions 89, 90. Thus, most A-loop mutations disrupt interactions that stabilize inactive kinase conformations.
3.3 In vitro mutagenesis unveiled additional drug-resistance mutations
An important lesson taught by the mutation hotspots is that most drug-resistance-mutations destabilize conformations of high, or stabilize conformations of low inhibitor affinity. The frequent contribution of distributed and remote allosteric/conformational effects explains their common resistance against several different KIs. Following these considerations, other kinase-regions involved in functionally important structural/allosteric interactions might be additional mutational hotspots. Candidates that stabilize inactive kinase conformations are the SH3 domain/SH2-KD linker/N-lobe interactions in SFKs and ABL, SFK SH2 domain/phospho-YC interactions, phospho-Y independent ABL SH2 domain/C-lobe interactions and myristate-binding to the ABL C-lobe (Fig. 1,2) 39, 41-43, 48. Indeed, cell-based mutagenesis-screens have unveiled KI-resistance mutations in all these interfaces and in the ABL-cap whose SH3/SH2-domain interactions have been implicated in ABL-autoinhibition (Fig. 5, Tab. 4) 39, 41, 47, 48, 62. Several of the mutations activate ABL in vitro. Drug-resistance mutations outside of the ABL KD have not yet been reported clinically, possibly due to a KD-bias in genotyping. An interesting question is whether such mutations account for some of the ~50% of imatinib-resistant CML-patients lacking known ABL-mutations 24.
Mutations in the SFK SH3-SH2 domain linker or SH2 domain counteracted inhibitory interactions 43, 48. Drug-resistant KIT-V559A6, 103, 107, and drug-sensitizing EGFR-V689M/N700D68 map to interaction sites with non-KD regions (Tab. 5). It will be interesting to explore KD-extrinsic mutations in cancers involving these kinases.
Finally, abrogation of inhibitory SH2 domain/C-lobe interactions, allosteric effects and direct drug binding-site alterations may underlie drug-resistance caused by C-lobe mutations including ABL1b-V357(ABL1a 338)G, M362(343)T, F378(359)A/C/V and M370(351)T/I, which represents ≤20% of clinical imatinib-resistance (Tab. 4)16, 22, 40, 42, 48, 56, 76, 78. ABL-I521(502)M and E528(509)D in myristate binding-site helix αI’ might reduce myristate-inhibition. However, clinical data are unavailable 41, 42, 48. Drug-sensitizing (N826S, A839T, K846R) and resistant (G796D/R/C, T854A) EGFR C-lobe mutations often have positional ABL-analogs (Tab. 4,5, Fig. 5).
Altogether, drug-sensitizing and drug resistance-mutations frequently employ allosteric/conformational effects on remote catalytic or drug-binding regions. They can occur within the KD or in other kinase regions mediating functionally important structural interactions.
4. Overcoming Kinase Inhibitor Drug-Resistance
Overcoming the different drug-resistance mechanisms is a major challenge for developing KIs into safe, effective drugs. Because of their particular clinical importance, we here focus on strategies to tackle drug-resistant point mutations. Additional approaches are discussed briefly and summarized in Fig. 4C. Several excellent recent reviews discuss details 9, 21-23, 109.
The optimization of drug pharmacokinetic/pharmacodynamic properties is well established 65, 110. Importantly, maximized short-term kinase inhibition by high-affinity KIs with 3-5 hr half-lifes (dasatinib, nilotinib) may be preferable to continuous inhibition by long half-life TKIs (imatinib), yielding irreversible target-cell death while reducing toxicity and, possibly, drug-resistance 17, 24, 111, 112.
Pharmacogenomic profiling using improved biomarkers can be combined with quantitative structure-pharmacokinetic/pharmacodynamic relationship analyses to optimize drugs or treatment regimen for different patient populations or individuals 110, 113. It can also unveil which drug-resistance mechanisms exist in a patient, and allow one to select the most efficacious treatment. Systemic drug-resistance mechanisms (Fig. 4A) may require polypharmacologic drug combinations that co-target different mechanisms in a tumor, its microenvironment or in different metastases53. For example, antibodies that block secreted HGF in gefitinib-resistant EGFR-mutant NSCLC 66 could be co-administered with EGFR-KIs.
Cell-autonomous target-kinase gene amplification or overexpression might be addressed through increased KI-doses, or by co-targeting kinase and enzymes mediating its overexpression, exemplified by the synergistic pro-apoptotic KI and HDAC-inhibitor effects in CML cells 16. Co-targeting might also overcome drug-resistance due to upregulation of target downstream-effectors 9. Beyond co-administering several drugs, appropriately poly-targeted drugs could simplify treatment regimen and reduce toxicity due to combined off-target effects of several drugs. The broad target spectrum of most approved or clinically evaluated KI cancer-therapeutics may in some cases underlie their high efficacy (Tab. 1,2)15, 114. For example, dasatinib's ability to overcome certain cases of imatinib-resistance in CML may involve SFK co-inhibition (Tab. 1). Broad target spectra can moreover allow to use one drug for multiple indications, exemplified by imatinib efficacy in CML, GIST, HES and other diseases through inhibition of BCR-ABL, KIT, PDGFR or other targets 15. The toxicity of various pleiotropic KIs is surprisingly low and acceptable at least for life-threatening cancer-indications15.
Finally, mobilizing leukemic stem cells (LSCs) could render this dormant reservoir of drug-resistance mutations sensitive to cytostatics. Interestingly, triptolide might promote apoptosis of drug-resistant CML cell quiescent precursors 115. However, preventing the co-ablation of normal hematopoietic precursors remains a challenge 24, 67.
4.1 Improving kinase-inhibitors
Various approaches can yield compounds that inhibit drug-resistant mutant kinases (Fig. 4C). Key is the identification of drug-resistant kinase-mutations in patient samples or through mutagenesis screens (Tab. 4,5) 48, 56, 98, 116-118. Their expression in cell-based or in vitro assay systems, including differential cytotoxicity-screens, allowed the identification of compounds which inhibit first-generation-KI resistant mutant kinases 27, 33, 55, 62, 63, 119. Rational approaches have combined quantitative structure-activity-relationships and improved mechanistic understanding of drug-resistance mutations to design such compounds. This resulted in multiple second/third generation ABL, KIT, EGFR and PDGFR KIs in clinical studies (Tab. 1,2). Even more are in pre-clinical development. They include improved T1/2KIs where additional kinase-interactions, often employing the type 2/3 allosteric site or non-conserved ATP-site residues, improve target-affinity, allosteric T4KIs 50, and covalent KIs. Other compounds inhibit kinase-interactions with regulatory proteins. The efficacy of several compounds may rely on the poly-targeted inhibition of multiple kinases.
Among several particularly instructive examples, the approved T1KI dasatinib inhibits ABL and other kinases including SFKs 4, 13, 15, 16, 56, 57, 114, 120. Some of these contribute to imatinib-resistance (Tab. 1). Dasatinib binds adenine-site and HP2, and has 325-fold higher BCR-ABL affinity than imatinib. It binds both active and inactive ABL, and 21 imatinib-resistant ABL-mutants except G-loop, gatekeeper and a few others. Clinical trials showed advantages over imatinib particularly in low imatinib-responders. However, second-line dasatinib treated patients can accumulate secondary dasatinib-resistant ABL-mutations. Other contributing factors and adverse effects possibly related to PDGFR-inhibition are discussed elsewhere 16, 17, 20, 56, 109, 121. The efficacy of T1KIs against imatinib-resistant ABL at least partially relies on the destabilization of the type-2-inactive ABL-conformation by the mutations, which increases the representation of T1KI-susceptible active ABL-conformations. Thus, targeting conformations enriched in drug-resistant mutants may overcome drug-resistance. Co-inhibition of SFKs and other kinases contributing to imatinib-resistance may contribute variably.
Another approach improves target-affinity over first-generation drugs. The rationally designed nilotinib targets the type-2-inactive conformation of ABL and other kinases excluding SFKs with higher affinity than imatinib (Tab. 1). It inhibited 32 imatinib-resistant ABL-mutants but not G-loop, gatekeeper and others. Clinical trials showed efficacy in imatinib-resistant patients. However, nilotinib-resistance can develop through incompletely understood mechanisms, including novel ABL-mutants, MDR-1/Pgp drug exporter overexpression or LYN-hyperactivation (Fig. 4). LYN-involvement can confer dasatinib-sensitivity, providing a rationale for nilotinib/dasatinib co-administration 16, 17, 19, 20, 56, 109, 116, 121.
Resistance of G-loop and gatekeeper-mutants limits the use of many first/second generation drugs 17. The recent development of compounds that inhibit these recalcitrant alleles thus represents an important milestone. Understanding their inhibition-mechanisms teaches important lessons for designing improved KI-therapeutics. Among compounds in clinical trials (Tab. 2), the rationally designed pleiotropic type-2 SFK/ABL-KI AP24534 inhibits ABL-T315I and even compound imatinib-resistant mutants. AP24534 accommodates a bulky gatekeeper-residue through a juxtaposed carbon-carbon triple-bond whose “flat” structure prevents steric interference with the gatekeeper side-chain122. AURORA-kinases control mitosis and can act oncogenic. AURORA-inhibition causes apoptosis. By inhibiting proliferation and promoting death of cancer cells, and through co-inhibition of ABL, SFKs and other kinases, AURORA-KIs may overcome imatinib-resistance. Examples which inhibit gatekeeper-mutant ABL are XL-228, PHA-739358 and MK-0457(VX-680). Compared to imatinib, MK-0457 forms stronger hinge-interactions and avoids clashes with the enlarged T315I-side-chain 4, 16, 27, 56, 120. The non-ATP-competitive DCC2036 allosterically inhibits gatekeeper- and other ABL-mutants by binding to “switch-pockets” mediating active-inactive conformation transitions 4, 16.
Preclinical compounds include the type-4 allosteric GNF-2/5 class, which binds the BCR-ABL myristate-pocket and stabilizes the inactive conformation 13, 55, 62, 63. GNF-5/imatinib combination had some efficacy against BCR-ABL-T315. GNF-2/5 alone had little effect but inhibited most clinical BCR-ABL mutants 55, 62. The substrate-competitive ON012380 potently inhibited BCR-ABL-T315I and LYN 27, 49, 56.
The T2KIs GNF-7123 and HG-7-85-01122 inhibit BCR-ABL, KIT or PDGFR gatekeeper-mutants by bridging ATP- and type-2-hydrophobic site through a linker that accommodates large gatekeeper-residues. Compound 14 binds the inactive DDFG-out conformation, disrupts the hydrophobic spine and inhibits BCR-ABL-T315I 58. DSA-compounds bind inactive ABL and SFK conformations and potently inhibit both kinase-families, including G-loop and gatekeeper-mutant ABL, in part through altered adenine-site interactions 94. Thus, gatekeeper-mutation overcoming KIs need not bind the active kinase conformation stabilized by the mutation, but can act by stabilizing the energetically disfavored gatekeeper-mutant inactive conformation.
Another KI-class that can overcome drug-resistance through very high potency are irreversible inhibitors that covalently bind their targets 13, 64, 68. Examples are the EGFR/ERBB2-inhibitors HKI-292/CL-387785 64, 68, 119. Several irreversible KIs are in clinical trials68. A challenge is to minimize toxicity due to co-inhibition of the wildtype allele of the targeted mutant kinase. Encouragingly, differential screens have yielded compounds that inhibit drug-resistant EGFR-mutants 100-fold more potently than wildtype-EGFR 119.
These examples illustrate that introducing affinity-enhancing interactions with kinases, increasing sterical compatibility with mutant binding pockets, or targeting allosteric binding sites or mechanisms can yield improved KIs that may overcome drug-resistance 1, 50, 123. Moreover, indirect mechanisms can be exploited: Omacetaxine causes apoptosis through various mechanisms, including Mcl-1 downregulation and caspase-activation. Clinical trials may suggest efficacy in imatinib-resistant CMLs including gatekeeper-mutants4, 16. Targeting chaperones including HSP-90 with the clinically-explored IPI504 or 17-AAG promotes degradation of oncogenic and KI-resistant kinases including EGFR-L858R/T790M 21, 68.
5. Summary
Due to their major roles in disease and excellent druggability, kinases have become the second-largest drug-target class. As larger patient populations were exposed to KIs, drug-resistance emerged as an important clinical problem particularly in cancer where tumor cell genetic instability facilitates mutagenesis to cause kinase oncogenic activation, drug sensitization or resistance. Both drug sensitizing and resistance-conferring mutations are frequently located in kinase-domain regions that undergo profound conformational changes as kinases transition between active and inactive conformations. Drug-sensitizing mutations often destabilize inactive kinase conformations, causing oncogenic hyperactivation and oncogene addiction that enhances drug sensitivity. Drug-resistance mutations often alter drug-kinase interactions, or contribute to distributed allosteric effects that stabilize inhibitor-nonbinding, or destabilize inhibitor-binding conformations. Thus, drug-sensitization and drug-resistance can involve similar mechanistic principles. They often affect conserved structural features or residues in different kinases. Maximizing sensitization while avoiding resistance is a major therapeutic challenge. Drug-resistance caused by structural/allosteric alterations is often more difficult to overcome than resistance caused by disruption of direct kinase-drug interactions which may be restored by adding functional groups to the compound. The most promising, broadly applicable strategy to overcome allosteric drug-resistance mechanisms is to allosterically induce and stabilize inactive kinase conformations. Indeed, promising results have recently been achieved with allosteric kinase-inhibitors.
6. Expert Opinion
Mutational disruption of a conserved G-loop salt-bridge in BCR-ABL causes imatinib-resistance in CML-patients. The analogous SFK-mutation impairs catalysis and causes autoimmune-glomerulonephritis in mice 34. Hence, the molecular and genetic mechanisms causing KI-resistance or kinasopathies intersect. It will be interesting to examine whether other drug-resistance mutations can cause kinasopathies, or whether kinase-mutations underlying non-cancer diseases 3 can cause KI-drug resistance.
Multiple approaches have been devised to overcome drug-resistance (Fig. 4C). However, clinical data demonstrating their efficacy in patients are largely missing. Optimized dosing-regimen can sometimes improve imatinib-efficacy 17, 24. Second-generation drugs like dasatinib or nilotinib can overcome certain cases of imatinib-resistance17. However, many imatinib-resistant BCR-ABL mutants remain resistant to these drugs (Tab. 1). Moreover, the sequential treatment of CML-patients with different ABL-inhibitors can cause the emergence of additional resistance mutations, or of compound mutations with increased transforming potential in the same cell21. Despite the recently discovered abilities of allosteric and covalent KIs to inhibit even recalcitrant gatekeeper-mutant kinases and very promising results in preclinical models, it remains to be seen whether poly-targeted compounds or drug-cocktails that co-inhibit multiple drug-resistant target mutants and/or other resistance-mechanisms (Fig. 4) will fully eradicate a tumor including LSCs22, 52, 53. Similar approaches have proven very powerful in treating AIDS. An obvious potential liability is toxicity. Moreover, it remains challenging to develop compounds that selectively inhibit mutant oncogenic or drug-resistant kinases but leave the wildtype kinases unaffected to avoid toxicity. Encouragingly, differential cytotoxicity screens and rational approaches have yielded compounds that inhibit drug-resistant EGFR mutants 100-fold more potently than wildtype-EGFR119.
Recent advances in genome sequencing technologies have enabled the pharmacogenomic profiling of patients as a powerful approach to optimize treatments2, 9, 113, 124. By profiling patients for oncogenic and drug-resistance mutations as genomic biomarkers, it becomes possible to select the most efficacious drugs or drug-combinations for a given patient, to monitor the potential emergence of additional drug-resistance mechanisms and to quickly modify the therapy accordingly. Clinical evaluation of this approach is ongoing9, 113. A clinically observed complication is the independent occurrence of different resistance-mechanisms in different metastases. For example, a gefitinib/erlotinib-resistant patient showed EGFR gatekeeper-mutation in one, and MET gene-amplification in another metastasis69. This demonstrates the need to analyze multiple different tumor cells or metastases in each patient. Due to the low frequencies of some genetic alterations, broad application of this approach will require cost-effective high-throughput techniques that can efficiently be implemented in the clinic. While whole-genome sequencing costs are dropping rapidly, substantial technological development will be required to make profiling of deregulated signaling pathways or epigenetic alterations affordable for personalized treatments. Novel techniques including mass spectrometry-based flow cytometry provide promising approaches125.
Recently developed high-throughput platforms for the simultaneous, multiplexed profiling of large compound libraries against multiple kinases22, 53, 126 not only facilitate selectivity-profiling but can also be used to test drug-efficacy against multiple different kinase-mutants. This will accelerate the development of multi-targeted drugs and polypharmacologic drug cocktails that inhibit multiple drug-resistant kinase mutants or co-pathogenic pathways. However, this approach is limited to known drug-resistance mechanisms, which are often only identified once a drug is on the market.
The development of compounds that potently and safely inhibit existing and previously unknown drug-resistance mechanisms that may emerge in a treated patient remains a challenge. Drawing on lessons learned from ABL and other clinically targeted kinases (Tab. 4,5), we consider two approaches particularly powerful to overcome it. First, cell based and in vitro mutagenesis-screens can broadly identify kinase mutations and possibly additional KI-resistance mechanisms (Tab. 3, Fig. 5) and have been conducted for BCR-ABL, EGFR, FLT3, BRAF, MEK1, PI3K-p110α and AURORA-kinases29-31, 33, 48, 56, 116-118, 127-129. Microfluidic single-cell arrays might allow such screens in a highly cost-effective, large-scale format130. Encouraging this approach, the need to preserve catalytic activity, cofactor, substrate and other interactions limits the mutation spectrum a kinase can “employ” to become drug-resistant. However, low recovery frequencies of drug-resistant mutants indicate a need for more efficient mutagenesis methods. Moreover, even allosteric type 4 inhibitors are susceptible to drug-resistance55, 62. One reason is that the large conformational plasticity of kinases opens many opportunities to alter inhibitor-interactions through mutations in many different locations even remote from catalytic sites (Fig. 5). Thus, KI-resistance will likely remain a clinical problem even for advanced compounds. Although mutagenesis-screens are powerful tools to identify drug-resistance mechanisms, limited efficiency, cost, logistics and time considerations make complementing approaches desirable.
Second, computational advances such as heterogeneous computing, improved supercomputer clusters, internet-based cloud, distributed and grid computing such as the Open Science or Worldwide LHC Computing Grids that leverage enormous computing powers at low cost, and large-capacity storage systems now reaching Exabyte capacities131 may eventually enable the combination of in silico mutagenesis-screens with large-throughput structural modeling and molecular dynamics simulations to predict most drug-resistance causing mutations for any given kinase and inhibitor. Structural genomics approaches including virtual screening could then be used to rationally design compounds which inhibit most or all of the predicted mutants132. As our understanding of intra- and intercellular signaling networks advances and techniques to model them improve, similar “systems” approaches could possibly be envisioned to in silico model compound effects on other drug-resistance mechanisms (Fig. 4) identified via pharmacogenomic profiling.
In an integrated approach, mutagenesis-screen and simulation results could be used to prioritize compounds for experimental evaluation in in vitro, cell-based and ultimately whole animal models. Compared to traditional approaches, where drug-resistance mechanisms are first identified in patients treated with first-generation drugs, then studied and the results used to develop second and third generation drugs, the integrated approach could identify the most relevant drug-resistance mechanisms at the beginning of the drug development process, and yield drugs with minimized potential to cause drug-resistance “up-front”. The potential benefit for patients and health-care system could be major.
Article Highlights Box.
Because of their important roles in many diseases and great “druggability”, kinases have become the second largest drug target family, with 13 approved kinase-inhibitor (KI) drugs, about 100 compounds in clinical trials and many more in preclinical development.
Because many kinases have key and essential roles in cell metabolism, survival and function, cells underlie significant selection pressure to compensate for the loss of function of an important kinase. In a patient treated with a KI-therapeutic, this can cause drug-resistance through various cell extrinsic and -intrinsic mechanisms. In particular, genetically unstable tumor cells can harbor pre-existing, or accumulate drug-resistant mutant alleles of targeted kinases.
As KI drugs reach large patient populations for an expanding number of less life-threatening indications, the development of drug-resistance could become a major liability that limits the therapeutic use of KIs. Here, we critically discuss this problem and highlight important recent advances in overcoming KI drug-resistance.
Analyses of over 800 kinase crystal structures and advances in the analysis of solution structure dynamics have unveiled that kinases exist in a dynamic equilibrium of many different active and inactive conformations. This demonstrated the pivotal role of allosteric mechanisms in controlling kinase activation. Physiologically, these are governed by interactions of kinase regulatory and adaptor protein subunits, and by covalent modifications at key regulatory sites.
Among multiple different mechanisms, the mutational introduction of missense mutations in the coding sequence accounts for most clinically observed cases of KI drug-resistance. This is prominently illustrated by an ~33% relapse rate in imatinib-treated chronic myelogenous leukemia patients. Most of these mutations occur in “hotspots” that participate directly in the conformational mechanisms which regulate kinase activation.
Structural and biochemical studies showed that kinase missense mutations primarily cause drug-resistance by abrogating specific molecular interactions with the inhibitor, or by introducing distributed allosteric effects that destabilize conformations with high, or stabilize conformations with low inhibitor affinity.
KIs can target protein interaction sites involved in kinase regulation, sites mediating allosteric regulation of a kinase, substrate or ATP-cofactor binding sites. Traditional screens for KIs have primarily yielded ATP-competitive compounds. More recently, attempts to improve selectivity and potency and to overcome drug-resistance have unveiled allosteric KIs as very promising compounds. They can target allosteric sites adjacent to, or remote from the ATP-binding site. In addition, covalently binding KIs can provide high potency and inhibit drug-resistant mutant kinases.
Current strategies for overcoming KI drug-resistance focus on the development of compounds that inhibit known drug-resistant mutant kinases, and on poly-targeted compounds or compound-cocktails that simultaneously inhibit several parallel or downstream pathways and mutant kinases mediating drug-resistance. Combined with pharmacogenomic profiling to determine the precise mechanisms and mutations present in a patient, the efficacy of these polypharmacologic approaches can be optimized in personalized therapies, albeit at a possibly high cost.
The development of compounds that potently and safely inhibit existing and previously unknown drug-resistance mechanisms that may emerge in a treated patient and cause relapse remains a challenge. Recent technological advances may eventually allow one to combine saturating mutagenesis screens with the computational modeling of compound interactions with all possible mutant variants of a targeted kinase. Compared to traditional approaches, where drug-resistance mechanisms are first identified in patients treated with first-generation drugs, then studied and the results used to develop improved drugs, this integrated approach could identify the most relevant drug-resistance mechanisms at the beginning of the drug development process, and yield improved drugs with minimized potential to cause drug-resistance “up-front”.
Acknowledgements
This is manuscript #20919 from The Scripps Research Institute. We apologize to our colleagues for citing reviews in lieu of many important original references due to limitations to the number of references allowed.
Declaration of Interest
K.S. is supported by NIH grants AI070845 and GM088647, and by Scholar Award 1440-11 from The Leukemia & Lymphoma Society. The authors declare no financial conflicts of interest.
Non-standard abbreviations
- AA
amino acid
- A-loop
activation loop
- ADPKD
autosomal-dominant polycystic kidney disease
- ALL
acute lymphoblastic leukemia
- AML
acute myelogenous leukemia
- cKI
covalent kinase inhibitor
- CML
chronic myelogenous leukemia
- G-loop
glycine-rich loop, identical with P-loop
- GIST
gastrointestinal stromal tumors
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HDX-MS
hydrogen-deuterium-exchange mass-spectrometry
- HES
hypereosinophilic syndrome
- HP1/2
hydrophobic pocket 1/2
- HTS
high-throughput screening
- KD
kinase domain
- KI
kinase inhibitor
- LSC
leukemic stem cell
- MDS
myelodysplastic syndrome
- MM
multiple myeloma
- NBP
nucleotide-binding protein
- NSCLC
non small-cell lung cancer
- P-loop
phosphate-binding loop, identical with G-loop
- PCKD
polycystic kidney disease
- Ph+
Philadelphia-chromosome positive
- PK
pharmacokinetics
- PTK
protein tyrosine kinase
- RCC
renal cell carcinoma
- SFK
SRC-family protein-tyrosine kinase
- TKI
tyrosine kinase inhibitor, T1/2/3/4KI, type 1/2/3/4 KI
Footnotes
Declaration of interest
K Sauer has received an NIH grant AI070845 and GM088647 and a Scholar Award 1440-11 from the Leukemia and Lymphoma Society, the Scripp Research Institute.
Bibliography
- 1.Johnson LN. Protein kinase inhibitors: contributions from structure to clinical compounds. Q Rev Biophys. 2009 Feb;42(1):1–40. doi: 10.1017/S0033583508004745. [DOI] [PubMed] [Google Scholar]
- 2**.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007 Mar 8;446(7132):153–8. doi: 10.1038/nature05610. [References 2 and 3 identified multiple mutations in kinase genes that are associated with diseases, providing a strong rationale for pursuing kinases as therapeutic targets for many indications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahiry P, Torkamani A, Schork NJ, Hegele RA. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Nat Rev Genet. 2010 Jan;11(1):60–74. doi: 10.1038/nrg2707. [DOI] [PubMed] [Google Scholar]
- 4.Quintas-Cardama A, Kantarjian H, Cortes J. Imatinib and beyond--exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol. 2009 Sep;6(9):535–43. doi: 10.1038/nrclinonc.2009.112. [DOI] [PubMed] [Google Scholar]
- 5.Trowe T, Boukouvala S, Calkins K, Cutler RE, Jr., Fong R, Funke R, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008 Apr 15;14(8):2465–75. doi: 10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 6.Lim KH, Huang MJ, Chen LT, Wang TE, Liu CL, Chang CS, et al. Molecular analysis of secondary kinase mutations in imatinib-resistant gastrointestinal stromal tumors. Med Oncol. 2008;25(2):207–13. doi: 10.1007/s12032-007-9014-2. [DOI] [PubMed] [Google Scholar]
- 7.Puxeddu E, Romagnoli S, Dottorini ME. Targeted therapies for advanced thyroid cancer. Current Opinion in Oncology. 9000;Publish Ahead of Print:10.1097/CCO.0b013e328340cf94. [DOI] [PubMed]
- 8.Cuny GD. Kinase inhibitors as potential therapeutics for acute and chronic neurodegenerative conditions. Curr Pharm Des. 2009;15(34):3919–39. doi: 10.2174/138161209789649330. [DOI] [PubMed] [Google Scholar]
- 9.Janne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009 Sep;8(9):709–23. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 10.Rokosz LL, Beasley JR, Carroll CD, Lin T, Zhao J, Appell KC, et al. Kinase inhibitors as drugs for chronic inflammatory and immunological diseases: progress and challenges. Expert Opin Ther Targets. 2008 Jul;12(7):883–903. doi: 10.1517/14728222.12.7.883. [DOI] [PubMed] [Google Scholar]
- 11.Won J, Lee GH. T-cell-targeted signaling inhibitors. Int Rev Immunol. 2008 Jan-Apr;27(1-2):19–41. doi: 10.1080/08830180701798976. [DOI] [PubMed] [Google Scholar]
- 12.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009 Jun;8(6):480–99. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature reviews. 2009 Jan;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opar A. Kinase inhibitors attract attention as oral rheumatoid arthritis drugs. Nat Rev Drug Discov. 2010 Apr;9(4):257–8. doi: 10.1038/nrd3155. [DOI] [PubMed] [Google Scholar]
- 15.Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol. 2009 Apr;10(4):356–60. doi: 10.1038/ni.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology Am Soc Hematol Educ Program. 2009:461–76. doi: 10.1182/asheducation-2009.1.461. [DOI] [PubMed] [Google Scholar]
- 17.Valent P. Standard treatment of Ph+ CML in 2010: how, when and where not to use what BCR/ABL1 kinase inhibitor? Eur J Clin Invest. 2010 Jul 2; doi: 10.1111/j.1365-2362.2010.02328.x. [DOI] [PubMed] [Google Scholar]
- 18.Ihle NT, Powis G. Inhibitors of phosphatidylinositol-3-kinase in cancer therapy. Mol Aspects Med. 2010 Apr;31(2):135–44. doi: 10.1016/j.mam.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996 May;2(5):561–6. doi: 10.1038/nm0596-561. [This was the first publication of imatinib (STI-571, Gleevec), the first breakthrough CML therapeutic.] [DOI] [PubMed] [Google Scholar]
- 20**.NIH 2010 http://clinicaltrials.gov. [An excellent, interactive webpage that allows one to search for ongoing and completed clinical trials in many countries.]
- 21.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008 Feb;18(1):73–9. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Daub H, Specht K, Ullrich A. Strategies to overcome resistance to targeted protein kinase inhibitors. Nat Rev Drug Discov. 2004 Dec;3(12):1001–10. doi: 10.1038/nrd1579. [DOI] [PubMed] [Google Scholar]
- 23.Buschbeck M. Strategies to overcome resistance to targeted protein kinase inhibitors in the treatment of cancer. Drugs R D. 2006;7(2):73–86. doi: 10.2165/00126839-200607020-00002. [DOI] [PubMed] [Google Scholar]
- 24.La Rosee P, Hochhaus A. Molecular pathogenesis of tyrosine kinase resistance in chronic myeloid leukemia. Curr Opin Hematol. 2010 Mar;17(2):91–6. doi: 10.1097/MOH.0b013e3283366be1. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurty R, Maly DJ. Biochemical Mechanisms of Resistance to Small-Molecule Protein Kinase Inhibitors. ACS Chemical Biology. 2010;5(1):121–38. doi: 10.1021/cb9002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001 Aug 3;293(5531):876–80. doi: 10.1126/science.1062538. [This was the first identification of molecular and genetic mechanisms causing imatinib-resistance in patients. It laid the basis for further investigation of kinase-inhibitor drug resistance mechanisms, and for the development of improved therpaeutics.] [DOI] [PubMed] [Google Scholar]
- 27.Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007 Oct;6(10):834–48. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 28.Marmorstein R. Anticipating drug resistance in the MAP kinase pathway. Pigment Cell Melanoma Res. 2010 Feb;23(1):7–9. doi: 10.1111/j.1755-148X.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girdler F, Sessa F, Patercoli S, Villa F, Musacchio A, Taylor S. Molecular basis of drug resistance in aurora kinases. Chem Biol. 2008 Jun;15(6):552–62. doi: 10.1016/j.chembiol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20411–6. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Zunder ER, Knight ZA, Houseman BT, Apsel B, Shokat KM. Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110 alpha. Cancer Cell. 2008 Aug 12;14(2):180–92. doi: 10.1016/j.ccr.2008.06.014. [This interesting study describes a screen in yeast which for the first time revealed drug-sensitizing and drug-resistance mutations in PIK3CA encoding the p110α subunit of PI3-kinase, the most frequently mutated kinase in human cancer. The results suggest that PI3K-inhibitors, an intensively pursued drug class, may be prone to drug-resistance. The study also unveiled a surprising paucity of gatekeeper mutations in PIK3CA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitzen JJ, de Jonge MJ, Verweij J. Aurora kinase inhibitors. Crit Rev Oncol Hematol. 2010 Feb;73(2):99–110. doi: 10.1016/j.critrevonc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Warmuth M, Kim S, Gu XJ, Xia G, Adrian F. Ba/F3 cells and their use in kinase drug discovery. Curr Opin Oncol. 2007 Jan;19(1):55–60. doi: 10.1097/CCO.0b013e328011a25f. [DOI] [PubMed] [Google Scholar]
- 34*.Barouch-Bentov R, Che J, Lee CC, Yang Y, Herman A, Jia Y, et al. A Conserved Salt Bridge in the G Loop of Multiple Protein Kinases Is Important for Catalysis and for In Vivo Lyn Function. Molecular Cell. 2009;33(1):43–52. doi: 10.1016/j.molcel.2008.12.024. [This interesting study describes the effects of mutationally disrupting a salt-bridge that is conserved among the G-loops of 58 eukaryotic kinases. In BCR-ABL, its abrogation is a major cause of clinical imatinib-resistance. The analogous mutation in SFKs severely reduces catalytic activity. Strikingly, this causes autoimmune-glomerulonephritis in mice. Hence, the molecular mechanisms causing drug-resistance and disease intersect.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006 Jul;2(7):358–64. doi: 10.1038/nchembio799. [This is an in-depth review of rational approaches to design type-2-allosteric kinase inhibitors. It provides many compound structures to illustrate the power of fragment-based hybrid-design strategies.] [DOI] [PubMed] [Google Scholar]
- 36.Engen JR, Wales TE, Hochrein JM, Meyn MA, 3rd, Banu Ozkan S, Bahar I, et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008 Oct;65(19):3058–73. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pawson T, Kofler M. Kinome signaling through regulated protein-protein interactions in normal and cancer cells. Curr Opin Cell Biol. 2009 Apr;21(2):147–53. doi: 10.1016/j.ceb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Shan Y, Seeliger MA, Eastwood MP, Frank F, Xu H, Jensen MO, et al. A conserved protonation-dependent switch controls drug binding in the Abl kinase. Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):139–44. doi: 10.1073/pnas.0811223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagar B, Hantschel O, Seeliger M, Davies JM, Weis WI, Superti-Furga G, et al. Organization of the SH3-SH2 unit in active and inactive forms of the c-Abl tyrosine kinase. Mol Cell. 2006 Mar 17;21(6):787–98. doi: 10.1016/j.molcel.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 40*.Levinson NM, Kuchment O, Shen K, Young MA, Koldobskiy M, Karplus M, et al. A Src-like inactive conformation in the abl tyrosine kinase domain. PLoS Biol. 2006 May;4(5):e144. doi: 10.1371/journal.pbio.0040144. [Reference 40 for the first time described a SFK-like inactive conformation in ABL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagar B, Hantschel O, Young MA, Scheffzek K, Veach D, Bornmann W, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003 Mar 21;112(6):859–71. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 42.Hantschel O, Nagar B, Guettler S, Kretzschmar J, Dorey K, Kuriyan J, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003 Mar 21;112(6):845–57. doi: 10.1016/s0092-8674(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 43.Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001 Apr 6;105(1):115–26. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 44**.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000 Sep 15;289(5486):1938–42. doi: 10.1126/science.289.5486.1938. [References 39, 41, 42, 44 and 46 unveiled the intricate structural and conformational mechanisms regulating ABL activation through domain-interactions and covalent modifications. They provided a basis to mechanistically understand how many drug-resistance mutations act.] [DOI] [PubMed] [Google Scholar]
- 45.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. Faseb J. 1995 May;9(8):576–96. [PubMed] [Google Scholar]
- 46.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002 May 3;109(3):275–82. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 47.Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002 Jan 25;108(2):247–59. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 48**.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003 Mar 21;112(6):831–43. doi: 10.1016/s0092-8674(03)00190-9. [This paper reports the first broad mutagenesis screen to identify drug-resistant kinase mutants. It unveiled multiple mutations in ABL, many of which are identical to clinically observed mutations.] [DOI] [PubMed] [Google Scholar]
- 49.Kirkland LO, McInnes C. Non-ATP competitive protein kinase inhibitors as anti-tumor therapeutics. Biochem Pharmacol. 2009 May 15;77(10):1561–71. doi: 10.1016/j.bcp.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Bogoyevitch MA, Fairlie DP. A new paradigm for protein kinase inhibition: blocking phosphorylation without directly targeting ATP binding. Drug Discov Today. 2007 Aug;12(15-16):622–33. doi: 10.1016/j.drudis.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Arkin MR, Whitty A. The road less traveled: modulating signal transduction enzymes by inhibiting their protein-protein interactions. Curr Opin Chem Biol. 2009 Jun;13(3):284–90. doi: 10.1016/j.cbpa.2009.05.125. [DOI] [PubMed] [Google Scholar]
- 52.Fojo T. Commentary: Novel therapies for cancer: why dirty might be better. Oncologist. 2008 Mar;13(3):277–83. doi: 10.1634/theoncologist.2007-0090. [DOI] [PubMed] [Google Scholar]
- 53.Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nature reviews. 2010 Feb;10(2):130–7. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel RY, Doerksen RJ. Protein Kinase-Inhibitor Database: Structural Variability of and Inhibitor Interactions with the Protein Kinase P-Loop. J Proteome Res. 2010 Aug 3; doi: 10.1021/pr100662s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Adrian FJ, Jahnke W, Cowan-Jacob SW, Li AG, Iacob RE, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010 Jan 28;463(7280):501–6. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nature reviews. 2007 May;7(5):345–56. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 57**.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004 Jul 16;305(5682):399–401. doi: 10.1126/science.1099480. [This study first reported the ability of dasatinib to inhibit multiple imatinib-resistant BCRABL mutants.] [DOI] [PubMed] [Google Scholar]
- 58**.Azam M, Seeliger MA, Gray NS, Kuriyan J, Daley GQ. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat Struct Mol Biol. 2008 Oct;15(10):1109–18. doi: 10.1038/nsmb.1486. [This interesting study showed that gatekeeper-mutations activate kinases by stabilizing a hydrophobic spine that stabilizes the active conformation, changing our mechanistic understanding of these important drug-resistance mutations. The study also showed that compounds which maximize complementarity with the dismantled spine can inhibit gatekeeper-mutant BCR-ABL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kornev AP, Haste NM, Taylor SS, Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17783–8. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Simard JR, Kluter S, Grutter C, Getlik M, Rabiller M, Rode HB, et al. A new screening assay for allosteric inhibitors of cSrc. Nat Chem Biol. 2009 Jun;5(6):394–6. doi: 10.1038/nchembio.162. [Complementing rational design and differential screening approaches, this paper describes a novel assay to identify allosteric kinase inhibitors that selectively stabilize inactive kinase conformations. It relies on the covalent attachment of fluorophors to the A-loop, which enables monitoring of the conformational changes as kinases transition between active and inactive conformations.] [DOI] [PubMed] [Google Scholar]
- 61.Getlik M, Grutter C, Simard JR, Kluter S, Rabiller M, Rode HB, et al. Hybrid compound design to overcome the gatekeeper T338M mutation in cSrc. J Med Chem. 2009 Jul 9;52(13):3915–26. doi: 10.1021/jm9002928. [DOI] [PubMed] [Google Scholar]
- 62.Fabbro D, Manley PW, Jahnke W, Liebetanz J, Szyttenholm A, Fendrich G, et al. Inhibitors of the Abl kinase directed at either the ATP- or myristate-binding site. Biochim Biophys Acta. 2010 Mar;1804(3):454–62. doi: 10.1016/j.bbapap.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 63*.Adrian FJ, Ding Q, Sim T, Velentza A, Sloan C, Liu Y, et al. Allosteric inhibitors of Bcrabl-dependent cell proliferation. Nat Chem Biol. 2006 Feb;2(2):95–102. doi: 10.1038/nchembio760. [This study shows how a differential cytotoxicity screen yielded allosteric inhibitors with higher potency for BCR-ABL than wildtype ABL.] [DOI] [PubMed] [Google Scholar]
- 64.Irmer D, Funk JO, Blaukat A. EGFR kinase domain mutations - functional impact and relevance for lung cancer therapy. Oncogene. 2007 Aug 23;26(39):5693–701. doi: 10.1038/sj.onc.1210383. [DOI] [PubMed] [Google Scholar]
- 65.van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009 Dec;35(8):692–706. doi: 10.1016/j.ctrv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008 Nov 15;68(22):9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 67.Burchert A. Roots of imatinib resistance: a question of self-renewal? Drug Resist Updat. 2007 Aug-Oct;10(4-5):152–61. doi: 10.1016/j.drup.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nature reviews. 2007 Mar;7(3):169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 69.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007 May 18;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 70.Rosell R, Viteri S, Molina MA, Benlloch S, Taron M. Epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in advanced nonsmall-cell lung cancer. Curr Opin Oncol. 2010 Mar;22(2):112–20. doi: 10.1097/CCO.0b013e32833500d2. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys. 2008;37:353–73. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006 Jun 16;125(6):1137–49. doi: 10.1016/j.cell.2006.05.013. [This study showed that similar to CDKs and SFKs, the EGFR kinase domain can exist in an auto-inhibited conformation. EGFR activation results from its disruption by A-loop mutation or the formation of an asymmetric dimer in which the C-lobe of one EGFR-subunit plays a cyclin-like activating role for the other EGFR-subunit. This provides a mechanistic explanation for the importance of dimerization in EGFR-activation.] [DOI] [PubMed] [Google Scholar]
- 73.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007 Mar;11(3):217–27. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choong NW, Dietrich S, Seiwert TY, Tretiakova MS, Nallasura V, Davies GC, et al. Gefitinib response of erlotinib-refractory lung cancer involving meninges--role of EGFR mutation. Nat Clin Pract Oncol. 2006 Jan;3(1):50–7. doi: 10.1038/ncponc0400. quiz 1 p following 7. [DOI] [PubMed] [Google Scholar]
- 75*.Iacob RE, Pene-Dumitrescu T, Zhang J, Gray NS, Smithgall TE, Engen JR. Conformational disturbance in Abl kinase upon mutation and deregulation. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1386–91. doi: 10.1073/pnas.0811912106. [This study used hydrogen exchange mass spectrometry to experimentally analyze the conformational effects of drug-resistant ABL-mutations. This technique is a valuable complement to static crystal structures and molecular dynamics simulations for proteins whose size precludes NMR analyses of solution structures.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002 Aug;2(2):117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 77.Cowan-Jacob SW, Fendrich G, Floersheimer A, Furet P, Liebetanz J, Rummel G, et al. Structural biology contributions to the discovery of drugs to treat chronic myelogenous leukaemia. Acta Crystallogr D Biol Crystallogr. 2007 Jan;63(Pt 1):80–93. doi: 10.1107/S0907444906047287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cowan-Jacob SW, Guez V, Fendrich G, Griffin JD, Fabbro D, Furet P, et al. Imatinib (STI571) resistance in chronic myelogenous leukemia: molecular basis of the underlying mechanisms and potential strategies for treatment. Mini Rev Med Chem. 2004 Mar;4(3):285–99. doi: 10.2174/1389557043487321. [DOI] [PubMed] [Google Scholar]
- 79.Carlomagno F, Guida T, Anaganti S, Vecchio G, Fusco A, Ryan AJ, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004 Aug 12;23(36):6056–63. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Joha S, Idziorek T, Corm S, Hetuin D, Philippe N, et al. BCR-ABL mutants spread resistance to non-mutated cells through a paracrine mechanism. Leukemia. 2008 Apr;22(4):791–9. doi: 10.1038/leu.2008.3. [DOI] [PubMed] [Google Scholar]
- 81.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005 Dec;37(12):1315–6. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 83.Bossemeyer D. The glycine-rich sequence of protein kinases: a multifunctional element. Trends Biochem Sci. 1994 May;19(5):201–5. doi: 10.1016/0968-0004(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 84.Madhusudan, Akamine P, Xuong NH, Taylor SS. Crystal structure of a transition state mimic of the catalytic subunit of cAMP-dependent protein kinase. Nat Struct Biol. 2002 Apr;9(4):273–7. doi: 10.1038/nsb780. [DOI] [PubMed] [Google Scholar]
- 85.Liao JJ. Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. J Med Chem. 2007 Feb 8;50(3):409–24. doi: 10.1021/jm0608107. [DOI] [PubMed] [Google Scholar]
- 86.Zhou T, Parillon L, Li F, Wang Y, Keats J, Lamore S, et al. Crystal structure of the T315I mutant of AbI kinase. Chem Biol Drug Des. 2007 Sep;70(3):171–81. doi: 10.1111/j.1747-0285.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 87.Young MA, Shah NP, Chao LH, Seeliger M, Milanov ZV, Biggs WH, 3rd, et al. Structure of the kinase domain of an imatinib-resistant Abl mutant in complex with the Aurora kinase inhibitor VX-680. Cancer Res. 2006 Jan 15;66(2):1007–14. doi: 10.1158/0008-5472.CAN-05-2788. [DOI] [PubMed] [Google Scholar]
- 88.Avizienyte E, Ward RA, Garner AP. Comparison of the EGFR resistance mutation profiles generated by EGFR-targeted tyrosine kinase inhibitors and the impact of drug combinations. Biochem J. 2008 Oct 15;415(2):197–206. doi: 10.1042/BJ20080728. [DOI] [PubMed] [Google Scholar]
- 89.Halilovic E, Solit DB. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr Opin Pharmacol. 2008 Aug;8(4):419–26. doi: 10.1016/j.coph.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 90.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004 Mar 19;116(6):855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 91.Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009 Jun;6(6):352–66. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
- 92.Skaggs BJ, Gorre ME, Ryvkin A, Burgess MR, Xie Y, Han Y, et al. Phosphorylation of the ATP-binding loop directs oncogenicity of drug-resistant BCR-ABL mutants. Proc Natl Acad Sci U S A. 2006 Dec 19;103(51):19466–71. doi: 10.1073/pnas.0609239103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griswold IJ, MacPartlin M, Bumm T, Goss VL, O'Hare T, Lee KA, et al. Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006 Aug;26(16):6082–93. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seeliger MA, Ranjitkar P, Kasap C, Shan Y, Shaw DE, Shah NP, et al. Equally potent inhibition of c-Src and Abl by compounds that recognize inactive kinase conformations. Cancer Res. 2009 Mar 15;69(6):2384–92. doi: 10.1158/0008-5472.CAN-08-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roumiantsev S, Shah NP, Gorre ME, Nicoll J, Brasher BB, Sawyers CL, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10700–5. doi: 10.1073/pnas.162140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vajpai N, Strauss A, Fendrich G, Cowan-Jacob SW, Manley PW, Grzesiek S, et al. Solution conformations and dynamics of ABL kinase-inhibitor complexes determined by NMR substantiate the different binding modes of imatinib/nilotinib and dasatinib. J Biol Chem. 2008 Jun 27;283(26):18292–302. doi: 10.1074/jbc.M801337200. [DOI] [PubMed] [Google Scholar]
- 97.Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res. 2002 Aug 1;62(15):4236–43. [PubMed] [Google Scholar]
- 98.Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3395–400. doi: 10.1073/pnas.0409770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee TS, Potts SJ, Kantarjian H, Cortes J, Giles F, Albitar M. Molecular basis explanation for imatinib resistance of BCR-ABL due to T315I and P-loop mutations from molecular dynamics simulations. Cancer. 2008 Apr 15;112(8):1744–53. doi: 10.1002/cncr.23355. [DOI] [PubMed] [Google Scholar]
- 100.Squire CJ, Dickson JM, Ivanovic I, Baker EN. Structure and inhibition of the human cell cycle checkpoint kinase, Wee1A kinase: an atypical tyrosine kinase with a key role in CDK1 regulation. Structure. 2005 Apr;13(4):541–50. doi: 10.1016/j.str.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009 Jul;10(4):281–9. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1542–7. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008 Sep;53(3):245–66. doi: 10.1111/j.1365-2559.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- 104.Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008 Nov 20;26(33):5352–9. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishida T, Kanda T, Nishitani A, Takahashi T, Nakajima K, Ishikawa T, et al. Secondary mutations in the kinase domain of the KIT gene are predominant in imatinib-resistant gastrointestinal stromal tumor. Cancer Sci. 2008 Apr;99(4):799–804. doi: 10.1111/j.1349-7006.2008.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts KG, Odell AF, Byrnes EM, Baleato RM, Griffith R, Lyons AB, et al. Resistance to c-KIT kinase inhibitors conferred by V654A mutation. Mol Cancer Ther. 2007 Mar;6(3):1159–66. doi: 10.1158/1535-7163.MCT-06-0641. [DOI] [PubMed] [Google Scholar]
- 107.Fletcher JA, Rubin BP. KIT mutations in GIST. Curr Opin Genet Dev. 2007 Feb;17(1):3–7. doi: 10.1016/j.gde.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 108.Tang Z, Jiang S, Du R, Petri ET, El-Telbany A, Chan PS, et al. Disruption of the EGFR E884-R958 ion pair conserved in the human kinome differentially alters signaling and inhibitor sensitivity. Oncogene. 2009 Jan 29;28(4):518–33. doi: 10.1038/onc.2008.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee F, Fandi A, Voi M. Overcoming kinase resistance in chronic myeloid leukemia. Int J Biochem Cell Biol. 2008;40(3):334–43. doi: 10.1016/j.biocel.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Mager DE. Quantitative structure-pharmacokinetic/pharmacodynamic relationships. Adv Drug Deliv Rev. 2006 Nov 30;58(12-13):1326–56. doi: 10.1016/j.addr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010 Feb;95(2):232–40. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Snead JL, O'Hare T, Adrian LT, Eide CA, Lange T, Druker BJ, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009 Oct 15;114(16):3459–63. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petak I, Schwab R, Orfi L, Kopper L, Keri G. Integrating molecular diagnostics into anticancer drug discovery. Nat Rev Drug Discov. 2010 Jul;9(7):523–35. doi: 10.1038/nrd3135. [DOI] [PubMed] [Google Scholar]
- 114*.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008 Jan;26(1):127–32. doi: 10.1038/nbt1358. [This intriguing study provides interaction maps for 38 kinase inhibitors across a panel of 317 kinases representing >50% of the predicted human protein kinome.] [DOI] [PubMed] [Google Scholar]
- 115.Mak DH, Schober WD, Chen W, Konopleva M, Cortes J, Kantarjian HM, et al. Triptolide induces cell death independent of cellular responses to imatinib in blast crisis chronic myelogenous leukemia cells including quiescent CD34+ primitive progenitor cells. Mol Cancer Ther. 2009 Sep;8(9):2509–16. doi: 10.1158/1535-7163.MCT-09-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.von Bubnoff N, Manley PW, Mestan J, Sanger J, Peschel C, Duyster J. Bcr-Abl resistance screening predicts a limited spectrum of point mutations to be associated with clinical resistance to the Abl kinase inhibitor nilotinib (AMN107). Blood. 2006 Aug 15;108(4):1328–33. doi: 10.1182/blood-2005-12-010132. [DOI] [PubMed] [Google Scholar]
- 117.Bradeen HA, Eide CA, O'Hare T, Johnson KJ, Willis SG, Lee FY, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006 Oct 1;108(7):2332–8. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ray A, Cowan-Jacob SW, Manley PW, Mestan J, Griffin JD. Identification of BCR-ABL point mutations conferring resistance to the Abl kinase inhibitor AMN107 (nilotinib) by a random mutagenesis study. Blood. 2007 Jun 1;109(11):5011–5. doi: 10.1182/blood-2006-01-015347. [DOI] [PubMed] [Google Scholar]
- 119.Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009 Dec 24;462(7276):1070–4. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weisberg E, Sattler M, Ray A, Griffin JD. Drug resistance in mutant FLT3-positive AML. Oncogene. 2010 Jul 12; doi: 10.1038/onc.2010.273. [DOI] [PubMed] [Google Scholar]
- 121.Redaelli S, Piazza R, Rostagno R, Magistroni V, Perini P, Marega M, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009 Jan 20;27(3):469–71. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 122.Weisberg E, Choi HG, Ray A, Barrett R, Zhang J, Sim T, et al. Discovery of a small-molecule type II inhibitor of wild-type and gatekeeper mutants of BCR-ABL, PDGFRalpha, Kit, and Src kinases: novel type II inhibitor of gatekeeper mutants. Blood. 2010 May 27;115(21):4206–16. doi: 10.1182/blood-2009-11-251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi HG, Ren P, Adrian F, Sun F, Lee HS, Wang X, et al. A type-II kinase inhibitor capable of inhibiting the T315I “gatekeeper” mutant of Bcr-Abl. J Med Chem. 2010 Aug 12;53(15):5439–48. doi: 10.1021/jm901808w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007 Mar;39(3):347–51. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 125.Maecker HT, Nolan GP, Fathman CG. New technologies for autoimmune disease monitoring. Curr Opin Endocrinol Diabetes Obes. 2010 Aug;17(4):322–8. doi: 10.1097/MED.0b013e32833ada91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goldstein DM, Gray NS, Zarrinkar PP. High-throughput kinase profiling as a platform for drug discovery. Nat Rev Drug Discov. 2008 May;7(5):391–7. doi: 10.1038/nrd2541. [DOI] [PubMed] [Google Scholar]
- 127.von Bubnoff N, Barwisch S, Speicher MR, Peschel C, Duyster J. A cell-based screening strategy that predicts mutations in oncogenic tyrosine kinases: implications for clinical resistance in targeted cancer treatment. Cell Cycle. 2005 Mar;4(3):400–6. doi: 10.4161/cc.4.3.1560. [DOI] [PubMed] [Google Scholar]
- 128.von Bubnoff N, Engh RA, Aberg E, Sanger J, Peschel C, Duyster J. FMS-like tyrosine kinase 3-internal tandem duplication tyrosine kinase inhibitors display a nonoverlapping profile of resistance mutations in vitro. Cancer Res. 2009 Apr 1;69(7):3032–41. doi: 10.1158/0008-5472.CAN-08-2923. [DOI] [PubMed] [Google Scholar]
- 129.Yu Z, Boggon TJ, Kobayashi S, Jin C, Ma PC, Dowlati A, et al. Resistance to an irreversible epidermal growth factor receptor (EGFR) inhibitor in EGFR-mutant lung cancer reveals novel treatment strategies. Cancer Res. 2007 Nov 1;67(21):10417–27. doi: 10.1158/0008-5472.CAN-07-1248. [DOI] [PubMed] [Google Scholar]
- 130*.Faley SL, Copland M, Wlodkowic D, Kolch W, Seale KT, Wikswo JP, et al. Microfluidic single cell arrays to interrogate signalling dynamics of individual, patient-derived hematopoietic stem cells. Lab Chip. 2009 Sep 21;9(18):2659–64. doi: 10.1039/b902083g. [This methodological paper describes chip-based microfluidic three-color cell viability assays that detected differential responses of normal and CML stem/progenitor cells to dasatinib. This technique could allow analyses of compound-effects on rare leukemic stem cells. It might also open the way to large-scale profiling of multiple compounds against large panels of cells harboring known or predicted drug-resistant kinase mutants.] [DOI] [PubMed] [Google Scholar]
- 131**.Schadt EE, Linderman MD, Sorenson J, Lee L, Nolan GP. Computational solutions to large-scale data management and analysis. Nat Rev Genet. 2010 Sep;11(9):647–57. doi: 10.1038/nrg2857. [This interesting review discusses novel techniques for the large-scale, multi-dimensional analysis of different cell types and signaling events therein. They could transform biomedical research and in particular allow one to broadly and simultaneously determine the impact of specific genes, mutations or compounds on many different signaling pathways in many different cell types.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marsden BD, Knapp S. Doing more than just the structure-structural genomics in kinase drug discovery. Curr Opin Chem Biol. 2008 Feb;12(1):40–5. doi: 10.1016/j.cbpa.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 133.Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle. 2009 Apr 1;8(7):1026–9. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009 Feb-Apr;12(1-2):8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Klebl BM, Muller G. Second-generation kinase inhibitors. Expert Opin Ther Targets. 2005 Oct;9(5):975–93. doi: 10.1517/14728222.9.5.975. [DOI] [PubMed] [Google Scholar]
- 136.Danis RP, Sheetz MJ. Ruboxistaurin: PKC-beta inhibition for complications of diabetes. Expert Opin Pharmacother. 2009 Dec;10(17):2913–25. doi: 10.1517/14656560903401620. [DOI] [PubMed] [Google Scholar]
- 137.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009 Jul;8(7):547–66. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 138.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010 Aug;9(8):643–60. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 139.Xue Q, Hopkins B, Perruzzi C, Udayakumar D, Sherris D, Benjamin LE. Palomid 529, a novel small-molecule drug, is a TORC1/TORC2 inhibitor that reduces tumor growth, tumor angiogenesis, and vascular permeability. Cancer Res. 2008 Nov 15;68(22):9551–7. doi: 10.1158/0008-5472.CAN-08-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140**.(NCI) NCI NCI Clinical Trial Search Webpage. 2010 [Webpage] [cited; Available from: http://www.cancer.gov/clinicaltrials. [An excellent, interactive webpage that allows one to search for cancer clinical trials.]
- 141**.Gonfloni S, Weijland A, Kretzschmar J, Superti-Furga G. Crosstalk between the catalytic and regulatory domains allows bidirectional regulation of Src. Nat Struct Biol. 2000 Apr;7(4):281–6. doi: 10.1038/74041. [References 141 and 43 used structural data to show how intramolecular domain-interactions orchestrate SFK function.] [DOI] [PubMed] [Google Scholar]
- 142.Lierman E, Michaux L, Beullens E, Pierre P, Marynen P, Cools J, et al. FIP1L1-PDGFRalpha D842V, a novel panresistant mutant, emerging after treatment of FIP1L1-PDGFRalpha T674I eosinophilic leukemia with single agent sorafenib. Leukemia. 2009 May;23(5):845–51. doi: 10.1038/leu.2009.2. [DOI] [PubMed] [Google Scholar]
- 143.Bean J, Riely GJ, Balak M, Marks JL, Ladanyi M, Miller VA, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008 Nov 15;14(22):7519–25. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schindler T, Sicheri F, Pico A, Gazit A, Levitzki A, Kuriyan J. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol Cell. 1999 May;3(5):639–48. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- 145.Cowan-Jacob SW, Fendrich G, Manley PW, Jahnke W, Fabbro D, Liebetanz J, et al. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure. 2005 Jun;13(6):861–71. doi: 10.1016/j.str.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 146.Martin MW, Newcomb J, Nunes JJ, Bemis JE, McGowan DC, White RD, et al. Discovery of novel 2,3-diarylfuro[2,3-b]pyridin-4-amines as potent and selective inhibitors of Lck: synthesis, SAR, and pharmacokinetic properties. Bioorg Med Chem Lett. 2007 Apr 15;17(8):2299–304. doi: 10.1016/j.bmcl.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 147.Green H, Skoglund K, Rommel F, Mirghani RA, Lotfi K. CYP3A activity influences imatinib response in patients with chronic myeloid leukemia: a pilot study on in vivo CYP3A activity. Eur J Clin Pharmacol. 2010 Apr;66(4):383–6. doi: 10.1007/s00228-009-0772-y. [DOI] [PubMed] [Google Scholar]