Abstract
The objective of the present study was to examine C57BL/6J (B6) mice during extinction conditions, following food training, for rates and patterns of operant behavior that appear similar to behavior maintained by IV cocaine injections. The rationale was to evaluate the potential for false positives in the IV self-administration test using protocols common in studies of knockout mice backcrossed to B6. An additional aim was to assess the influence of food- and drug- associated cues and mouse strain. Mice were allowed to acquire lever pressing reinforced by sweetened condensed milk under a fixed ratio (FR) 1 then FR 2 schedule of reinforcement accompanied by a flashing light. A catheter base was then implanted for simulation of IV self-administration conditions. Mice were allowed to lever press with cues remaining the same as during food training but without further scheduled consequences (i.e., no drug or food reinforcers delivered). All mice sustained lever pressing for several weeks, and over half met commonly used criteria for “self-administration behavior”. Thus B6 mice showed perseveration of a previously reinforced behavior that closely resembled rates and patterns of drug self-administration. This effect in B6 mice was greater than with A/J mice, and the lack of extinction was even more robust in the presence of cocaine-associated cues than with food-associated cues. We suggest that a necessary criteria for positive results in the IV drug self-administration test include an increase in responding when cocaine is made available after extinction with saline self-administration.
Keywords: self-administration, mouse, strain, extinction
Introduction
Intravenous drug self-administration in laboratory animals is a well-established procedure for measuring the reinforcing effects of drugs. As such it represents the “gold standard” of behavioral assays used to evaluate abuse-potential of compounds, investigate the neurobiology of drug abuse and dependence, and test potential therapeutic approaches to substance use disorders. Mice with targeted gene mutations (e.g., transgenic, knockout, knock-in) provide a powerful tool for investigating candidate genes that may be involved in drug abuse in humans. Relative to non-human primates and rats, fewer self-administration studies have been conducted in the mouse species, due mainly to technical challenges such as surgical implantation of intravenous catheters and maintaining catheter patency and animal health for long durations.
Accordingly, experimental approaches are often aimed at obtaining data expeditiously. This may include establishing the operant response with a food reinforcer, prior to catheter implantation surgery (Caine et al., 1999). Unfortunately, it may also include omitting experimental phases that are not always considered essential for proper interpretation of drug self-administration data, such as thorough examination of behavior under extinction conditions. It is not uncommon to find reports using study designs in which mice were not exposed to extinction conditions until the final phase of testing – and then data where collected for vehicle self-administration levels for consecutive sessions until low rates were obtained – or not at all. Remarkably, this is also the case for studies in which the operant response was established using a food reinforcer, followed by cocaine self-administration (using the same cues associated with both reinforcers). This approach poses the concern that responding attributed to the reinforcing effects of cocaine could be due, in part or entirely, to persistent responding maintained by the food-associated cues (i.e., conditioned reinforcement). Examples of this type of experimental design are fairly common in the literature, including from respected laboratories, and appear also in high-profile journals (Rocha et al. 1998; Ripley et al. 2002; Ward et al. 2009; Thanos et al. 2010). We and others have observed that extinction of an acquired operant response can be particularly protracted in mice, relative to e.g., rats or monkeys (Hironaka et al. 2004; Thomsen and Caine 2006; Pañeda et al. 2009; Thomsen et al. 2009b; Ward et al 2009). We have further noted that even without prior food training, some mutant mice initially emitted high rates of responding (perhaps maintained by presumed “neutral” cues associated with the response), but subsequently failed to maintain cocaine self-administration (Thomsen et al., 2009a,b).
The first experiment in the present investigation was designed to test the hypothesis that a commonly used strain of mice for behavioral and gene-targeting studies, the C57BL/6J (hereafter referred to as B6), can maintain high levels of operant responding for extended periods of time, which are virtually indistinguishable from cocaine self-administration behavior. Rather than designing the experiment to maximize such behavior, we sought to replicate experimental conditions that have been used in the literature. We chose a study published in a high-profile journal (Rocha et al. 1998), in which a positive result in the mouse self-administration test, in a B6-based mutant mouse line, contradicted many previously published findings in other species and/or behavioral procedures (Caine, 1998). The experimental parameters used in the present study were matched as closely as possible to the methods used in that report.
In a second experiment, we evaluated potential strain differences in cue reactivity and latency to extinction, testing B6 mice alongside A/J mice in cocaine self-administration or food-maintained behavior. The A/J strain was selected for two reasons. Firstly, context associated with IV cocaine was shown to produce much more pronounced conditioned behavior in B6 mice than in A/J mice in a direct comparison using a locomotor assay (Mead et al., 2002). Secondly, recombinant B6 x A/J mouse lines are available, providing avenues to investigate further the genetic basis for potential strain differences in extinction or cue reactivity (Boyle and Gill, 2001).
Methods
Subjects and housing
Ten male B6 (C57BL/6J) mice were used for Experiment 1. Ten male mice each of the B6 and A/J strains were used for Experiment 2 using cocaine as the reinforcer, and an additional eight mice of each strain were used for Experiment 2 using food as the reinforcer. There was some attrition in Experiment 2: One A/J mouse in the food group died of unknown causes before the experiments were initiated, and four B6 mice and three A/J mice in the cocaine group were excluded due to catheter failure or poor health before completion of the experiment. All mice were acquired at 6 weeks of age from the Jackson Labs (Bar Harbor, ME), and were acclimated to the housing facilities at least 7 days before experiments were initiated. During this time they were also handled, and were anesthetized briefly once for s.c. implantation of an identification microchip. Animals were kept on a 12-h light/dark cycle at ~22 °C and ~55% humidity, housed individually. Water and standard rodent chow (rodent diet 5001, PMI Feeds, Inc., St. Louis, MO) were freely accessible in the home cage. For enrichment, nesting material and small enclosures were provided (for details, see Thomsen and Caine, 2005). All testing was conducted during the light phase of the circadian cycle, Monday through Friday. All procedures were carried out in accordance with NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Operant conditioning apparatus
Mouse operant conditioning chambers (ENV-300, Med-Associates, Georgia, VT) were used, and have been described in detail (Caine et al. 1999; Thomsen and Caine, 2005). Briefly, each chamber contained either two ultra-sensitive mouse levers (ENV-310M, Med-Associates; Experiment 1), or nose-poke holes containing a yellow cue light (ENV-313M, Med-Associates; Experiment 2). Cue lights were present above each lever but were never illuminated during Experiment 1. Centered between the levers was a plate into which liquid food could be delivered from a syringe pump. A liquid swivel mounted on a balance arm was used for simulated or actual intravenous drug delivery in the freely moving animals. Each apparatus was enclosed in a sound-attenuating cubicle equipped with a syringe pump for reinforcer deliveries, a fan (providing ventilation and background noise), and a 3-Watt white stimulus light (ENV-221, Med-Associates). The light was turned on at the beginning of each session, signaling reinforcer availability, and was also used for visual cues during Experiment 1.
Experiment 1
Food training
Mice were first introduced to the operant conditioning chambers with a food reinforcer available (33% sweetened condensed milk in water) under an FR 1 schedule of reinforcement (no postreinforcer timeout, [TO] i.e., continuous reinforcement). Each reinforcer delivery was accompanied by flashing of the stimulus light throughout the pump activation time (3.42 sec). Half the mice were assigned to left lever active, and half to right lever active (chosen randomly). Responses on the inactive lever had no scheduled consequences. Sessions lasted 1 h or until 20 reinforcers were earned, whichever occurred first. Criteria for acquisition of lever pressing behavior were: a minimum of 15 reinforcers earned per session and 75% of responses on the active lever. Once criteria were met, the FR was increased to 2 until mice again met the above criteria for three consecutive sessions. The active and inactive levers were then reversed, under the FR 1 schedule of reinforcement until at least 15 reinforcers were earned (all mice met this step in one session), followed by the FR 2 schedule until criteria were met again for three consecutive sessions.
Sham catheter implantation and extinction conditions
Surgical methods were based on catheter implantation techniques that have been described in detail (Thomsen and Caine, 2005), except that the jugular vein was left intact for the present study. Specifically, a modified catheter base (i.e., the silicone catheter itself was removed to reduce irritation) was implanted s.c. above the midscapular region under oxygen/sevoflorane vapor anesthesia. Two days after the surgery, mice were again introduced to the operant conditioning chambers and the catheter base was attached to a liquid swivel and counterbalanced arm via a flexible tether to replicate intravenous drug self-administration conditions. One active lever was pseudorandomly assigned to each mouse (half on each side). Responses on the active lever resulted in a 5-s activation of the empty syringe pump and flashing of the light, under an FR 1 TO 30-s schedule of reinforcement. The light was off during TO periods. Responses on the active lever during the TO period, and responses on the inactive lever at any time, were recorded but had no scheduled consequences. Sessions started with the delivery of one non-contingent “reinforcer” (flashing stimulus light), and lasted 3 h or until 20 “reinforcers” (flashing stimulus light presentations) were earned, whichever occurred first. Criteria for acquisition of “self-administration” behavior were: at least 15 “reinforcers” earned per session, at least 75% of responses on the active lever, and no more than 20% variation in the number of “reinforcers” earned over 3 consecutive sessions. Once those criteria were met, the FR was increased to 2, the session length was shortened to 90 min, and unlimited reinforcers were available for each session. Criteria for stable behavior under the FR 2 schedule were no more than 20% variation in the number of “reinforcers” earned over 3 consecutive sessions.
Experiment 2
Catheter implantation and maintenance
Surgical methods as described in detail elsewhere (Thomsen and Caine, 2005). Briefly, a catheter (Silastic tubing 0.2 mm inner diameter, 0.4 mm outer diameter) was inserted 1.2 cm into the jugular vein and delicately anchored to the vein. The catheter ran s.c. to the base located above the midscapular region. The mice were allowed 7 days recovery, during which 0.02 ml of 0.9% saline containing heparin (30 USP units/ml) and antibiotic (cefazoline, 67 mg/ml) was infused daily through the catheter to forestall clotting and infection. After the post-operative recovery period, catheters were flushed with saline containing heparin immediately before and after self-administration sessions, and the free end of the cannula guide was kept closed at all times. Catheter patency was confirmed before initiation of cocaine self-administration then weekly by the infusion of 0.02–0.03 ml of 1% brevital in saline. Loss of muscle tone and clear signs of anesthesia within 3 sec. of infusion indicated catheter patency.
Acquisition of cocaine self-administration or food-maintained behavior
Mice were randomly assigned to either cocaine or food, and were allowed to acquire the nose-poke response reinforced by 1 mg/kg/infusion cocaine or liquid food (vanilla-flavored Ensure® nutrition drink) under an FR 1 TO-20s schedule of reinforcement, with no prior training. Sessions lasted 3 h or until 30 reinforcers were earned, whichever occurred first. The house light and the cue light in the right (active) nose poke hole were illuminated at the start of the session and signaled reinforcer availability. The right cue light was turned off at the onset of reinforcer delivery and remained off during the postreinforcer TO period. Responses in the left hole were recorded but had no scheduled consequences. Acquisition criteria were at least 15 reinforcers earned per session and response rates varying by no more than 20% over 3 consecutive sessions, with at least 75% of responses in the active hole. The average response rate at criteria was recorded and use as the baseline to determine extinction criteria in each mouse.
Extinction and reintroduction of the cues
For the extinction phase, saline was substituted for cocaine, and water was substituted for liquid food, in the respective groups. During the extinction phase, all lights remained off, and all other conditions remained as in the acquisition phase. Extinction criteria were met when the response rate decreased to ≤30% of the acquisition level. Once criteria were met in a given mouse, the cues were reintroduced in a single session, so that all experimental conditions were the same as the acquisition phase, with the exception that responding resulted in delivery of saline or water instead of cocaine or food. This experimental design was chosen so that presentation of the cue was not dependent upon a response being made, to avoid the potential confound that latency to the first response may vary between the two strains. In other words, there were both response-independent cues (house and cue lights on at session start) and response-contingent cues (cue light off for 20 s).
Data analysis
Number of sessions to criteria and response rates were compared between strains using the two-tailed unpaired-sample t-test, and response rates at extinction criteria were compared to the cues tests within subjects using the two-tailed paired-sample t-test; the effect of cues was also compared between strains as a percentage of extinction rates. Strain comparisons were made for the food group only due to the low number of A/J mice that acquired cocaine self-administration.
Results
Experiment 1
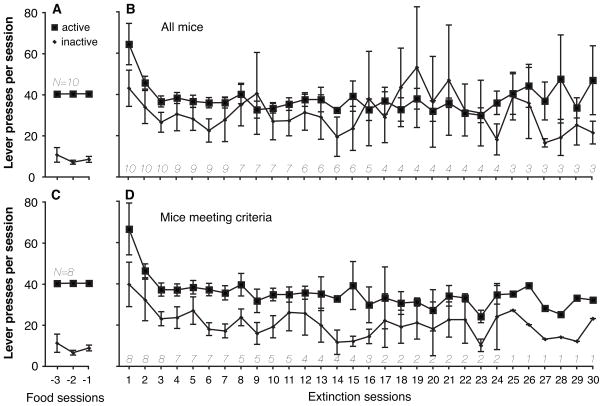
All ten mice acquired the lever pressing response reinforced by sweetened condensed milk, and required on average 9.3±2.2 sessions to meet acquisition criteria. The entire food-training period, including reversal learning, required on average 20.1±2.2 sessions. Once lever-pressing behavior was robust (i.e., over 5 responses) for a single session in a mouse, all of the mice earned all available reinforcers in almost every session thereafter throughout the food training. Active and inactive lever presses during the last three food training sessions (under an FR 2 schedule of reinforcement) are shown in Figure 1A.
Figure 1.
Active and inactive lever presses during the last three food training sessions (FR 2, no TO; panels A and C) and during the first 30 sessions of extinction (FR 1 TO 30-sec; panels B and D). The two phases were separated only by surgery and 2 days of recovery (i.e., no training or testing). Upper panels show data for all mice; lower panels only the 8 mice which met “acquisition of self-administration” criteria. Data are group means, bars represent one standard error of the mean. Group sizes are indicated in italic in each panel. Note that a maximum of 20 reinforcers could be earned during the food training sessions, which accounts for the tight distribution of active responses in this phase.
Following the catheter base implantation, all ten mice maintained high levels of lever pressing under the FR 1 schedule of stimulus light presentations (with no food or drug reinforcers), and eight of the ten met the “self-administration” criteria for this phase. The two mice that did not meet criteria failed to do so not by emitting too few responses, but by emitting more than 25% responses on the inactive lever (i.e., did not meet at least 75% active-lever pressing criterion for three consecutive sessions). Those two mice continued to earn all available reinforcers in almost every session until the experiment was terminated (77 and 81 sessions, respectively). Active and inactive lever presses during the FR 1 phase of the extinction conditions are shown in Figure 1B, truncated at 30 sessions. In mice that did meet all criteria for “self-administration”, inactive responses were relatively high (see Fig. 1B), and it took on average 16.1±5.1 sessions before criteria were met. Figure 1C–D shows the same data as Figure 1A–B, but excluding the two mice that failed to meet criteria.
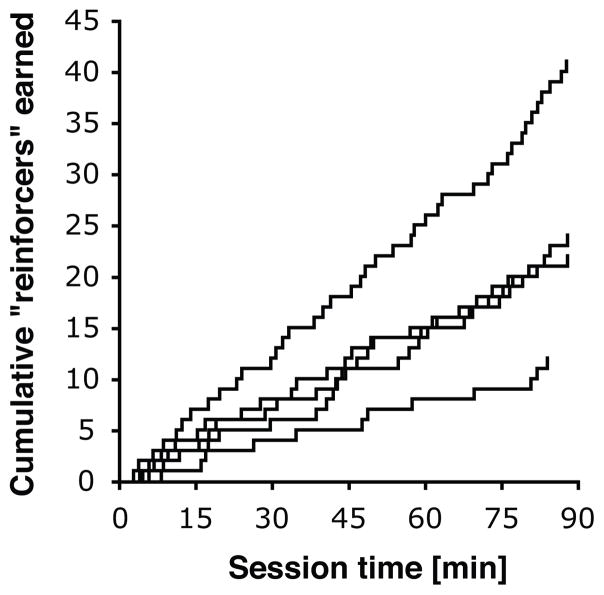
Of the eight mice that met criteria for the FR phase, seven completed the final experimental phase (i.e., FR 2 schedule of stimulus light presentations with no food or drug reinforcers). One mouse was excluded because the skin became dehisced/retracted from the catheter base (concomitant with a sharp decrease in responding), 7 sessions into the FR 2 phase. Of the seven mice, five met criteria for stable behavior, after on average 7.0±3.3 sessions under this schedule. Figure 2 shows cumulative records for the last of the three sessions after first meeting all “self-administration” criteria in each of those five mice. Responding was maintained at comparable high levels in the mice that met criteria, for a minimum of 24 sessions and up to 45 sessions after transition to the FR 2 schedule (i.e., until termination of the experiment, data not shown). Responding in the remaining two mice that were tested under this phase, which did not meet criteria, decreased to low levels during this phase. Figure 3 shows cumulative records from the prior report upon which the present experiment was based (Rocha et al., 1998, reprinted with permission). Thus, we obtained comparable data using the same training methods with B6 mice but with no cocaine injections delivered during the final phase of testing.
Figure 2.
Cumulative records of conditioned reinforcers earned under the final extinction conditions, FR 2 schedule of reinforcement, in the five mice that met “self-administration” criteria. Each line represents one mouse, for the last of the three consecutive “criterion” sessions. Sessions terminated at 90 min.
Figure 3.
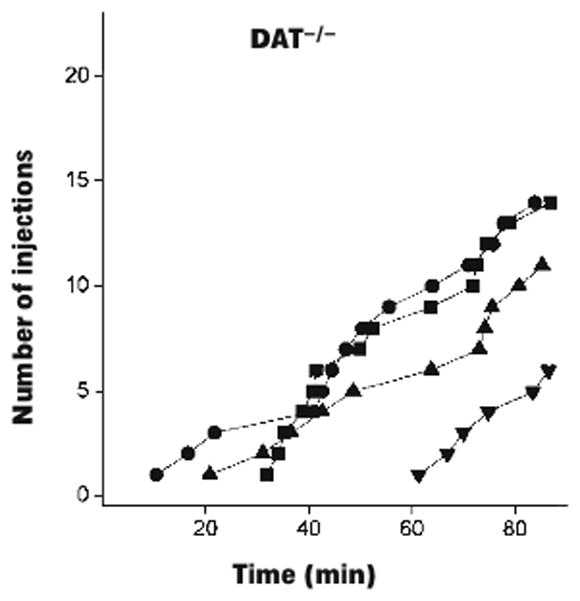
Data from the study upon which the present conditions were based. Cumulative records of cocaine self-administration under the FR 2 schedule of reinforcement, in wild-type and dopamine transporter-deficient knockout (DAT−/−). Each line represents one mouse. Note that maximum of the vertical axis is different from Figure 2. Reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Rocha et al., 1998), copyright (1998).
Experiment 2
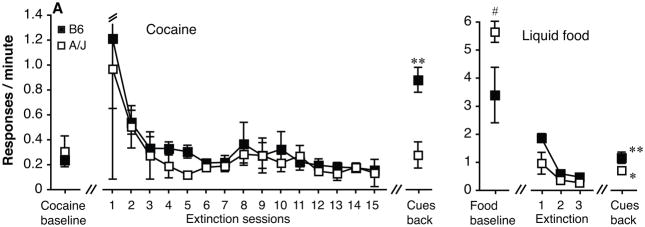
All six B6 mice acquired cocaine self-administration, after on average 7.2±1.6 sessions, while only two of the seven A/J mice met self-administration criteria, after 6 and 36 sessions, respectively. Response rates at acquisition are shown in Figure 4A, leftmost point. Extinction of cocaine-reinforced nose-poking appeared if anything more protracted than in our previous investigation using B6 or B6-backcrossed wild-type mice (see Table 1, sessions to extinction to <80% and <50% of baseline). For extinction down to <30% of the baseline response rate, the criterion used in the present investigation, cocaine-trained B6 mice took on average 26.5±4.7 sessions. The two cocaine-trained A/J mice met criteria at 19 and 22 sessions, respectively. The rightmost point in Figure 4 shows response rates when the cues were reintroduced. Reintroduction of the light cue increased responding dramatically in the cocaine-trained B6 mice (p < 0.001 vs. extinction level; over 370% of cocaine self-administration levels and over 1700% of extinction levels) and to a lesser degree in the two A/J mice (less than 100% of baseline levels). Due to the general failure of the A/J mice to acquire cocaine self-administration, statistical strain comparisons were limited to the food group.
Figure 4.
Response rates in the active nose poke hole in B6 and A/J mice at acquisition criteria (mean of 3 sessions), during the first 15 sessions of extinction (light cues absent), and after reintroduction of the reinforcer-associated light cues (one session), in mice trained with cocaine (A) or liquid food (B) as the reinforcer. Data are group means, bars represent one standard error of the mean. Group sizes: cocaine: B6, N=6, A/J, N=2; food: B6, N=8, A/J, N=7. *p<0.05, **p<0.001 vs. response rate at extinction criteria. #p<0.05 vs. B6 mice.
Table 1.
Summary of previous methods and outcomes in cocaine self-administration studies
| Experimental history of food training | Manipulanda | Mouse strain, sex | Extinguished ≤14 sessions? | Sessions to criteria | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food | Ext. | Food/water alternation | Dilutions | Cues during extinction | <80% | <50% | ||||
| Yes | Yes | Yes | Yes (x2) | Solid light | Nose pokes | 129S6/SvEvTac, male | 100%, N=29 | 2.6±0.3 | nd | Thomsen & Caine, 2006 |
| Yes | Yes | Yes | Yes (x2) | Solid light | Nose pokes | 129X1/SvJ, male | 96%, N=27 | 2.6±0.2 | nd | Thomsen & Caine, 2006 |
| Yes | Yes | Yes | Yes (x2) | Solid light | Nose pokes | C57BL/6J, male | 89%, N=28 | 5.3±1.2 | nd | Thomsen & Caine, 2006 |
| Yes | Yes | Yes | Yes (x1) | Solid light | Nose pokes | C57BL/6J-backcrossed wild-type, male+female |
94%, N=18 | 3.4±0.8 | 4.7±1.1 | Thomsen et al., 2005 |
| Yes | Yes | No | Yes (x2) | Solid light | Nose pokes | C57BL/6J-backcrossed | 100%, N=13 | 1.9±0.2 | 2.1±0.3 | Thomsen et al., 2009a |
| (x1) | wild-type, male+female | 71%, N=7 | 8.6±2.0 | - | Thomsen et al., 2009b | |||||
| Yes | Yes | No | No | Solid light | Nose pokes | Swiss Webster, male | Yes (97%, N=32) | 4.7±0.7 | 5.0±0.7 | Thomsen et al., 2010 |
| Yes | Yes | No | No | Solid light | Nose pokes | BALB/cByJ, male | Yes (100%, N=9) | 3.7±0.9 | 3.7±0.9 | Thomsen et al., submitted |
| Yes | No | No | No | Flashing light | Levers | C57BL/6J, male | 0%, N=10 | - | - | This investigation |
| Naïve (no food training) | Solid light | Nose pokes | C57BL/6J-backcrossed | 100%, N=16 | 3.3±0.7 | 3.5±1.0* | Thomsen et al., 2005 | |||
| wild-type, male+female | 100%, N=11 | 1.3±0.1 | 1.6±0.2 | Thomsen et al., 2009a | ||||||
| 81%, N=16 | 8.3±1.1 | nd | Thomsen et al., 2009b | |||||||
| Naïve (no food training) | No cues | Nose pokes | C57BL/6J, male | 83%, N=6 | 9.3±2.7 | 12.5±3.1 | This investigation | |||
In all but the present Experiment 1, observations refer to extinction when saline was substituted following cocaine self-administration.
2 mice were not extinguished to 50%, and for this value N=14; nd: not determined.
In the food group, all mice acquired the behavior: all B6 mice took 5 sessions to meet criteria, while the A/J mice required on average 9.0±0.7 sessions (p < 0.001 vs. B6). Despite their more rapid acquisition, average food-maintained rates at criteria were lower in the B6 than in the A/J mice (p < 0.05, see Figure 4B, leftmost point). The food-trained mice met extinction criteria much faster than the cocaine-trained mice, on average 2.6±1.1 sessions in the B6 mice and 1.1±0.1 sessions in the A/J mice (not significant, p = 0.08). Reintroducing the light cues in the food-trained mice produced a significant increase in response rates in the B6 mice (p < 0.001 vs. extinction), and in the A/J mice (p < 0.05 vs. extinction). As for cocaine, the percent increase was larger in the B6 mice than in the A/J mice (35% vs. 14%, p < 0.01). It is also noteworthy that the cue produced a much less pronounced effect in the food-trained mice than in the cocaine-trained mice.
Table 1 shows a summary of some previous cocaine self-administration investigations in mice in our laboratory, comparing times to extinction criteria relative to strain and Experimental history. Thus we have found extinction of cocaine self-administration to be significantly protracted in B6 mice relative to 129X1/SvJ mice or 129S6/SvEvTac mice, with a history of food training that included extinction, a series of daily food/water alternations, and two series of food dilutions in water (Table 1 and Thomsen and Caine, 2006). In wild-type mice of various mutant strains all backcrossed extensively to the B6 strain, latencies to extinction varied between lines, but were relatively constant regardless of whether the mice acquired cocaine self-administration behavior with no prior experimental history or were food-trained (see Table 1, Thomsen et al., 2005 see Table 1, Thomsen et al., 2009a and 2009b). As an additional measure, the percentage of mice meeting extinction criteria within 14 sessions is indicated (although all mice eventually extinguished, then re-established responding in those studies). While extinction with water substitution was always included, food/water alternations and/or food dilution series were not included in all investigations; their omission had no clear effect on latencies to extinction, although no formal comparison within one mouse line has been made. In fact, dose-effect functions between wild-type lines appear remarkably constant, with peak numbers of reinforcers earned averaging 69.6±15.2, 69.0±14.1, and 69.8±19.9 in three different investigations using wild-type mice bred at three different facilities, and saline values averaging 27.9±6.5, 27.6±4.7, and 39.8±8.4 (N=15, N=4, N=12, food-trained wild-type mice only, from Thomsen et al., 2005, 2009a,b, respectively). Further, dose-effect functions from naïve and food-trained animals were comparable in both studies using both groups (Thomsen et al., 2009a,b). Finally, using only acquisition and extinction of food-maintained behavior prior to cocaine self-administration, Swiss-Webster mice yielded comparable saline levels (27.9±3.3 infusions earned per session), and a higher peak relative to the B6-based wild-type strains (112.0±13.5 reinforcers/session, N=31, all mice used in Thomsen et al., 2010).
Discussion
In the present study we evaluated lever-pressing behavior in C57BL/6J mice under extinction conditions following food training, replicating previously published methods for evaluating IV cocaine self-administration, but without IV cocaine injections. We found that all mice maintained high levels of lever-pressing behavior for several weeks of testing, suggesting either a lack of extinction of lever pressing in the absence of the food reinforcers, or conditioned reinforcing effects of the stimulus lights previously associated with food delivery, or a combination of the two. Furthermore, eight of ten mice tested met the pre-set criteria for “acquisition of self-administration”, indicating that even with relatively stringent criteria of lever selection and stability over sessions, the experimental design was prone to a high level of false positive results. The patterns of responding obtained under the final schedule under extinction conditions were stable through the session (as opposed to “extinction burst” followed by a decline in responding), and were similar to the cocaine self-administration data reported in the original publication upon which the present study was based (Rocha et al. 1998). An alternative interpretation to a lack of extinction or a high level of conditioned reinforcement could be that the cues themselves maintained lever pressing. Indeed, operant responding maintained by visual stimuli has been reported in rats, monkeys and mice (Marx et al. 1955; Kish 1955; Stewart 1960; Baron and Kish 1962; Blatter and Schultz 2006; Cain et al. 2006; Olsen and Winder 2009). Those observations included acquisition, and maintenance over weeks, of lever pressing reinforced by a combination of pump sound and flashing light stimuli in experimentally naïve C57BL/6J mice, in patterns similar to those reported here (Olsen and Winder 2009). Taken together, those observations support the view that conditioned reinforcement or cue-induced behavior can be virtually indistinguishable from cocaine self-administration behavior in mice, highlighting the need for careful vehicle control and other extinction phases in all drug self-administration experiments, particularly with inbred mice on a C57BL/6J background.
We have previously reported on some mutant strains in which a very few mice met criteria for acquisition of drug self-administration, but represented a minority relative to most mice of the same experimental group (Caine et al., 2007; Thomsen et al., 2009a). It is quite possible that cue-induced behaviors accounted for some or most of those observations. In other words, we believe that we have obtained false positive self-administration in our own studies of mutant mice that were backcrossed to C57BL/6J. However, in such cases we have found additional criteria of extinction upon saline substitution, and, most importantly, increased responding above saline self-administration levels when cocaine was again made available, to be useful in determining whether cocaine reliably served as a positive reinforcer or not (Thomsen et al., 2009a,b). Importantly, cues remained the same during saline extinction as during cocaine self-administration in all those investigations. As summarized in the Results and in Table 1, extinction as well as dose-dependent cocaine self-administration (with saline always included in a Latin-square design of dose presentation) was obtained in all cases, using food-trained mice or mice with no prior training, including in B6 mice and B6-backcrossed wild-type mice in our laboratory. While we found both latency to extinction and the shape and position of the dose-effect function could vary with strain, the amount of training in the food phase (e.g., presence or absence of food/water alternations or dilution ranges) did not appear to affect those outcomes systematically. Further, mice that acquired cocaine self-administration without prior training showed latencies to extinction comparable to food-trained mice. Latencies to extinction and dose-effect functions also did not differ between mice trained and tested with levers vs. nose pokes as the manipulandum (B6 strain; Caine et al., 1999). Thus we believe important factors in minimizing resistance to extinction include strain and early and sufficient exposure to extinction conditions, while other details of training history appear of lesser impact. Another strategy to avoid carrying over effects of cues associated with food training, which is commonly used in rats, is to introduce cue lights or tones only in the cocaine self-administration phase, but not at the food-training stage. Because the response is still the same, one might argue extinction of food-maintained behavior is still advisable in those cases.
It is worth noting that the experimental design used by Rocha and colleagues (1998), and replicated here, in training the mice to reverse behavior from one lever to the other, probably increased the likelihood that mice would sample both levers under extinction conditions. This design therefore arguably reduced the risk of “false positive” self-administration by decreasing the likelihood that mice would allocate a greater proportion of responding (e.g., at least 75%) to the “active” lever under extinction conditions. However, many studies report assigning one active manipulandum throughout the study, thus decreasing the likelihood that mice will emit high response levels on the inactive manipulandum under extinction conditions. In those cases, it would be even more difficult to discern lack of extinction, conditioned reinforcement, or visual-stimuli maintained behavior from actual drug self-administration behavior. Experimental designs in which a single manipulandum is used (with no inactive manipulandum at all) represent an extreme of this situation, in which response selection cannot be used as an additional criterion to evaluate the nature of the behavior. Commendably, the experimental design used by Rocha and colleagues (1998) featured both active and inactive manipulanda as well as transition to an FR 2 schedule of reinforcement. The latter appeared to precipitate extinction of lever pressing in some subjects (though only two of seven) in the present experiment. Thus, designs involving increased response requirements may facilitate extinction of previously reinforced behaviors and lower the risk of false-positive self-administration.
In a second experiment, we evaluated potential strain differences in extinction of cocaine self-administration and food-maintained behavior, as well as reactivity to cues previously associated with cocaine or food reinforcement. The cocaine-trained B6 mice showed strong cue reactivity, showing active response rates 17 times that of extinction conditions, and lower cue reactivity in the food-trained group. The A/J mice generally failed to acquire cocaine self-administration, limiting the conclusions that can be drawn from the extinction and cue reactivity data in this strain, as only 2 mice completed those phases. Low reinforcing effects of cocaine in A/J mice relative to B6 mice are in agreement with previously reported lower locomotor activating effects of i.p. or IV administered cocaine in A/J mice than in B6 mice (Schuster et al., 1977; Boyle and Gill, 2001; Mead et al., 2002; Downing et al., 2003; Thomsen et al., manuscript submitted), although this difference in sensitivity does not generalize to all behavioral or physiological effects (Golden et al., 2001; Mead et al., 2002). In the mice that did self-administer cocaine in the present investigation, cocaine intake was comparable to the B6 mice, but cocaine-associated cues reinstated responding to a much lower level than in the B6 mice. Smaller effects of cocaine-associated cues in a self-administration assay (if confirmed in larger samples) would be in agreement with more modest conditioned activity in a cocaine-associated context in A/J mice compared to B6 mice, following repeated IV cocaine administration (Mead et al., 2002). Thus our preliminary cocaine data, taken together with the lower cue-induced reinstatement of responding in A/J mice than in B6 mice in the food group and with previous findings, suggest lower cue reactivity in the A/J strain relative to the B6 strain. Cue-induced reinstatement of lever pressing previously reinforced with 0.75 mg/kg/infusion cocaine has also been reported in 129X1/SvJ mice, producing approximately a doubling in active responses relative to extinction conditions (Highfield et al., 2002). In CD1 mice trained to self-administer 1.0 mg/kg/infusion cocaine, reintroducing the cues increased lever pressing 3- or 4-fold above extinction levels (Soria et al., 2008). Previous investigations have shown cue-induced reinstatement of behavior previously maintained by foods, including Ensure, in B6 mice, comparable to the data reported here (Ward et al., 2007). With no prior food-training, B6 mice trained to self-administer 0.3 mg/kg/infusion cocaine reinstated lever pressing roughly 3-fold following extinction when cues were reintroduced, and mice trained with 0.03 mg/kg/infusion nicotine showed just under a doubling in responding (Contet et al., 2010). B6 mice trained to self-administer 0.1 mg/kg/infusion methamphetamine reinstated lever pressing roughly 7- or 8-fold following extinction when cues were reintroduced (Yan et al., 2007). Interestingly, responding maintained by a visual stimulus alone could also be reinstated in B6 mice (Contet et al., 2010). While few direct comparisons have been made, and most studies have used single doses of drugs, the above findings collectively suggest the degree of cue-induced reinstatement may vary with mouse strain, and also suggests the possibility that it may parallel reinforcer strength.
In conclusion, we have shown that B6 mice trained to lever-press for a food reinforcer can maintain high levels of operant behavior for several weeks under extinction conditions. We showed that the lever-pressing behavior was typically stable over time in each mouse, under an FR 1 or an FR 2 schedule of reinforcement, and that patterns of responding closely resembled patterns of cocaine self-administration behavior previously reported under comparable conditions. We further found that B6 can show very high rates of responding when exposed to cues previously paired with cocaine, and that this effect was much lower in intensity for food-associated cues. B6 mice may also be more cue reactive than some other strains, including the A/J. Thus the inclusion of extinction training, as well as careful measures of vehicle-maintained self-administration at appropriate times when dose-effect functions are evaluated, are essential parts of experimental designs aimed at avoiding false positives in the IV self-administration test. Finally, we recommend including the observation of increased responding when cocaine is again made available following extinction with saline as a necessary criterion for a positive result in the IV drug self-administration test, particularly for mice having a B6 background.
Acknowledgments
We thank Joon Ying Boon and Jennifer Dohrmann for expert technical assistance.
Support
Portions of this work were supported by a NARSAD Young Investigator Award (MT) an Eleanor and Miles Shore/Harvard Medical School fellowship (MT), and National Institute on Drug Abuse (NIH) DA027825 (MT), DA012142 and DA02752.
Footnotes
The authors declare no conflict of interest.
References
- Baron A, Kish GB. Low-intensity auditory and visual stimuli as reinforcers for the mouse. J Comp Physiol Psychol. 1962;55:1011–3. doi: 10.1037/h0044286. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K. Sensitivity of AXB/BXA recombinant inbred lines of mice to the locomotor activating effects of cocaine: a quantitative trait loci analysis. Pharmacogenetics. 2001;11:255–64. doi: 10.1097/00008571-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Blatter K, Schultz W. Rewarding properties of visual stimuli. Exp Brain Res. 2006;168:541–6. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Processes. 2006;73:360–6. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB. Cocaine abuse: Hard knocks for the dopamine hypothesis? Nat Neurosci. 1998;1:90–92. doi: 10.1038/335. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 1999;147:22–4. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel K, Berkowitz J, Gold L, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of cocaine self-administration in dopamine D1 receptor knockout mice. The Journal of Neuroscience. 2007;27:13140–50. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Whisler KN, Jarrell H, Kenny PJ, Markou A. Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacology (Berl) 212:283–99. doi: 10.1007/s00213-010-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing C, Rodd-Henricks K, Marley RJ, Dudek BC. Genetic variation in the psychomotor stimulant properties of cocaine in Mus musculus. Psychopharmacology (Berl) 2003;167:159–66. doi: 10.1007/s00213-003-1387-0. [DOI] [PubMed] [Google Scholar]
- Golden GT, Ferraro TN, Smith GG, Snyder RL, Jones NL, Berrettini WH. Acute cocaine-induced seizures: differential sensitivity of six inbred mouse strains. Neuropsychopharmacology. 2001;24:291–9. doi: 10.1016/S0893-133X(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology (Berl) 2002;161:417–24. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- Hironaka N, Ikeda K, Sora I, Uhl GR, Niki H. Food-reinforced operant behavior in dopamine transporter knockout mice: enhanced resistance to extinction. Ann N Y Acad Sci. 2004;1025:140–5. doi: 10.1196/annals.1316.018. [DOI] [PubMed] [Google Scholar]
- Kish GB. Learning when the onset of illumination is used as reinforcing stimulus. J Comp Physiol Psychol. 1955;48:261–4. doi: 10.1037/h0040782. [DOI] [PubMed] [Google Scholar]
- Marx MH, Henderson RL, Roberts CL. Positive reinforcement of the bar-pressing response by a light stimulus following dark operant pretests with no after effect. J Comp Physiol Psychol. 1955;48:73–6. doi: 10.1037/h0045062. [DOI] [PubMed] [Google Scholar]
- Mead AN, Katz JL, Rocha BA. Intravenous cocaine-induced activity in A/J and C57BL/6J mice: behavioral sensitization and conditioned activity. Neuropharmacology. 2002;42:976–86. doi: 10.1016/s0028-3908(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–94. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pañeda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J Neurosci. 2009;29:4155–61. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology. 2002;43:1258–68. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–7. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–90. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology (Berl) 2008;199:593–603. doi: 10.1007/s00213-008-1184-x. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–93. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Habibi R, Michaelides M, Patel UB, Suchland K, Anderson BJ, et al. Dopamine D4 receptor (D4R) deletion in mice does not affect operant responding for food or cocaine. Behav Brain Res. 2010;207:508–11. doi: 10.1016/j.bbr.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005;Chapter 9(Unit 9):20. doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 2006;184:145–54. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009a;29:1087–92. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009b;331:204–11. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, Fink-Jensen A, Caine SB. Attenuation of cocaine’s reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332:959–69. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–55. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]