Abstract
We have demonstrated that fish oil- and pectin-containing (FO/P) diets protect against colon cancer compared with corn oil and cellulose (CO/C) by upregulating apoptosis and suppressing proliferation. To elucidate the mechanisms whereby FO/P diets induce apoptosis and suppress proliferation during the tumorigenic process, we analyzed the temporal gene expression profiles from exfoliated rat colonocytes. Rats consumed diets containing FO/P or CO/C and were injected with azoxymethane (AOM; 2 times, 15 mg/kg body weight, subcutaneously). Feces collected at initiation (24 h after AOM injection) and at aberrant crypt foci (ACF) (7 wk postinjection) and tumor (28 wk postinjection) stages of colon cancer were used for poly (A)+ RNA extraction. Gene expression signatures were determined using Codelink arrays. Changes in phenotypes (ACF, apoptosis, proliferation, and tumor incidence) were measured to establish the regulatory controls contributing to the chemoprotective effects of FO/P. At initiation, FO/P downregulated the expression of 3 genes involved with cell adhesion and enhanced apoptosis compared with CO/C. At the ACF stage, the expression of genes involved in cell cycle regulation was modulated by FO/P and the zone of proliferation was reduced in FO/P rats compared with CO/C rats. FO/P also increased apoptosis and the expression of genes that promote apoptosis at the tumor endpoint compared with CO/C. We conclude that the effects of chemotherapeutic diets on epithelial cell gene expression can be monitored noninvasively throughout the tumorigenic process and that a FO/P diet is chemoprotective in part due to its ability to affect expression of genes involved in apoptosis and cell cycle regulation throughout all stages of tumorigenesis.
Introduction
Colon cancer continues to be the second highest contributor to cancer deaths in the United States (1). It has been estimated that up to 80% of colon cancers may be preventable by dietary intervention (2). We have demonstrated that diets containing the combination of fish oil and pectin (FO/P)8 result in a lower tumor incidence than diets containing corn oil and cellulose (CO/C) (3). Fish oil is high in (n-3) fatty acids, whereas corn oil is high in (n-6) fatty acids. Pectin is a highly fermentable fiber that yields more butyrate upon microbial fermentation, whereas cellulose is poorly fermented. One of the mechanisms by which FO/P is protective against colon cancer is the induction of apoptosis, a programmed cell death that allows the removal of damaged cells (3–5). Tumor development depends not only on suppression of apoptosis but also on an increase in cell proliferation. We have reported that a FO/P diet also suppresses cell proliferation relative to a CO/C diet (3, 6).
Cell cycle progression is mediated by cyclin dependent kinases (CDK) and cyclins, which are under both transcriptional and post-transcriptional regulation (7). We have demonstrated that fish oil with butyrate increases the expression of Waf1/Cip1, a CDK inhibitor (8). Apoptosis is regulated by multiple routes, including extrinsic and intrinsic pathways as well as the integrins, which control cellular adhesion (9, 10). We have examined the effect of diet on apoptosis at various time points during carcinogenesis (4, 5, 11) and have reported that the expression of bcl2, one of the antiapoptotic factors in the intrinsic pathway, is downregulated in colon cells of rats fed a fish oil-rich diet (12). Therefore, it is important to identify the regulatory relationships among genes during the tumorigenic process to further elucidate the synergistic chemoprotective effects of fermentable fiber and fish oil.
We have developed a noninvasive technique in which intact eukaryotic mRNA can be successfully isolated from exfoliated colonocytes to monitor gene expression profiles (13–16). This novel technique facilitates the determination of changes in gene expression contributing to the regulation of apoptosis and cell proliferation during disease development. In this study, we are using this noninvasive methodology to monitor gene expression at 3 biologically important time points during colon tumorigenesis: initiation, aberrant crypt foci (ACF) formation, and tumor stage. The fecal gene expression results were compared with phenotypic data at the same time points to determine the mechanisms underlying the chemoprotective effects of a FO/P diet.
Materials and Methods
Rats and study.
Male Sprague-Dawley rats (Harlan Teklad) were used to study the chemoprotective effect of FO/P at the initiation, ACF, and tumor stages of colon cancer. The animal use protocol was approved by the Institutional Animal Care and Use Committee of Texas A&M University and conformed to NIH guidelines. Rats were individually housed in a temperature- and humidity-controlled animal facility with a 12-h-light/-dark cycle. After 1 wk of acclimation and 31 d of receiving the experimental diets, rats were injected with azoxymethane (AOM; Sigma; 15 mg/kg body weight). For the initiation stage analyses, 22 rats were killed 24 h after AOM injection. Fecal material was then collected and immediately homogenized in RNA isolation solution for microarray analysis and colon tissue samples were collected and processed as described below. Rats used for the ACF stage (n = 40) were maintained using the same diet and treatment conditions with the exception that animals received a second AOM injection 1 wk after the first injection. Seven weeks after the second AOM injection, rats were killed and colon tissue samples were collected. Rats for the tumor stage analyses (n = 80) were raised using the same diet and treatment conditions as the ACF stage rats, except animals were killed at 31 wk after the second AOM injection. Feces from the tumor stage rats were collected at 7 and 28 wk after the second AOM injection and colon tissue samples were collected at termination.
Diets.
Rats were assigned to receive a diet containing either FO/P or CO/C as previously described (3). All diets contained oils at 15% by weight and 30% of energy. The 2 lipid sources differed in fatty acid composition; fish oil contained higher amounts of EPA [20:5 (n-3)] and DHA [22:6 (n-3)] than CO, which had higher amounts of linoleic acid [18:2 (n-6)]. The fish oil diet included 3.5 g corn oil/100 g diet to prevent essential fatty acid deficiency. The amount of fiber in the diet was 6% by weight, which is equivalent to 30 g/d for humans. Fiber sources had differences in fermentability; pectin is highly fermentable, whereas cellulose is poorly fermented. Citrus pectin was obtained from Danisco Cultor and cellulose was provided by Harlan Teklad. Corn oil and bulk vacuum-deodorized menhaden fish oil were obtained from Degussa. The antioxidant levels in the diets were balanced by including 15 mg d-α-tocopherol, 14 mg d-γ-tocopherol, and 5 mg tertiary butylhydroquinone/100 g diet in the FO/P diet and 19 mg tertiary butylhydroquinone/100 g diet in the CO/C diet. Rats were provided with fresh diet daily to prevent lipid oxidation and consumed food and water ad libitum.
Tissue collection.
Rats were killed by CO2 overdose and cervical dislocation. The colon was resected and 1 cm of the distal colon was fixed in 4% paraformaldehyde (PFA) and another 1 cm of distal colon was used for 70% ethanol fixation. At the ACF stage, the remaining colon was used for ACF scoring. Tissues from the tumor stage were evaluated for tumor incidence.
RNA isolation from fecal samples.
To enrich the level of eukaryotic mRNA in the fecal samples, poly (A)+ RNA was isolated from total RNA using oligo(dT) cellulose micro spin columns and the mTRAP Maxi kit (Active Motif) (13). Fecal poly (A)+ RNA isolation was followed by DNase treatment and aliquots were analyzed on an Agilent Bioanalyzer 2100 to assess mRNA quality and quantity. The remaining sample was used for microarray analyses.
Microarray data acquisition.
Fecal poly (A)+ RNA was used to monitor gene expression using CodeLink Rat Whole Genome Arrays (Applied Microarray) containing 35,129 gene probes. cRNA synthesis was performed using between 10 and 100 ng of fecal poly (A)+ RNA. Briefly, reverse transcriptase and a T7-oligo(dT) primer were used for first-strand cDNA and DNA polymerase was used for second-strand cDNA generation. After in vitro transcription incorporating biotinylated nucleotides, purified and fragmented cRNA was hybridized to a Rat Whole Genome Bioarray in an Innova 4080 shaking incubator (New Brunswick) at 300 rpm. After hybridization, the arrays were processed as previously described (11). Images of processed arrays were captured on an Axon GenePix Scanner.
High multiplicity ACF assay.
To determine whether the FO/P diet was able to suppress formation of early preneoplastic lesions of colon cancer (aberrant crypts) compared with CO/C, we collected colon samples from rats 7 wk after the 2nd AOM injection. Colons were opened and placed flat within folded Whatman #1 paper, followed by fixation in 70% ethanol for 24 h. To identify aberrant crypts, tissue was stained in a 0.5% solution of methylene blue for 45 s. The total number of high multiplicity ACF (HM ACF) (foci containing 4 or more aberrant crypts) were counted using a 40× objective (17).
Colon cancer incidence.
Colons from rats killed at 31 wk after the second AOM injection were used to determine tumor incidence. Tumors were counted and tumor-bearing tissues were fixed in 4% PFA for 4 h and embedded in paraffin blocks for histological examination. Tumor sections (4 μm) were stained with hematoxylin and eosin and tumors were classified as adenomas or adenocarcinomas (3).
In situ apoptosis.
Apoptosis was measured by terminal deoxynucleotidyl transferase mediated UTP-biotin nick end labeling of fragmented pieces of DNA using 4-μm sections of PFA-fixed, paraffin-embedded tissue. Apoptotic cells with condensed chromatin, apoptotic bodies, and intense brown staining were counted in 50 crypt columns for each rat. The apoptotic index was calculated as 100 × the mean number of apoptotic cells per crypt column divided by the total number of cells per crypt column (3).
Colonocyte proliferation.
Cell proliferation was measured using the proliferating cell nuclear antigen assay. Sections (4 μm) of 70% ethanol-fixed, paraffin-embedded tissue were incubated with proliferating cell nuclear antigen monoclonal antibody (Signet Laboratories). Sections were incubated with biotinylated anti-mouse IgG (Vector Lab) and then stained with diaminobenzidine tetrahydrochloride (Sigma) and counterstained with hematoxylin. Twenty-five crypt columns were counted per rat. The number of cells per crypt column and the proportion of proliferating cells per crypt column were determined.
qRT-PCR confirmation of fecal microarray data.
Four differentially expressed genes of known function and robust sample size from the microarray platform were selected for validation by qRT-PCR using an ABI 7900HT. These genes were B4galt1 for the 24-h time point, Musdhl and Pdgfa for the 7-wk time point, and Id3 for the 28-wk time point. In addition, we selected 3 other nondifferentially highly expressed genes with relevance to colon cancer (Ctsb, Tff3, and Txn1) (18–20). cDNA was synthesized from 2 ng fecal poly (A)+ RNA and amplified using Ovation PicoSL WTA RNA amplification System (NuGen Technologies). PCR was performed using SYBR Green PCR master mix (Applied Biosystems). Primer sequences are shown in Supplemental Table 1 (Integrated DNA Technologies). Data are presented as the ratio of the expression level in FO/P-fed rats to that of CO/C-fed rats.
Statistical analyses.
Gene expression data for the fecal samples were normalized using the 2-stage, semiparametric normalization method of Liu et al. (21), which is specifically designed for data generated from partially degraded mRNA. Data were analyzed in SAS using a linear mixed model ANOVA procedure to evaluate the diet effect (FO/P vs. CO/C) at each time point. To correct for multiple testing, a false discovery rate (22) was applied. All genes that were differentially expressed (false discovery rate, P < 0.05) between diets from each time point were used for functional categorization and pathway analysis based on gene ontology (GO) (Database for Annotation, Visualization, and Integrated Discovery Bioinformatics Resources) (23). By importing the list of all differentially expressed genes, this program identified GO categories showing enrichment for genes in the list and the probability that the GO categories were being significantly affected by diet and time. We chose to study GO categories within the term “biological process” with a filter of enrichment P < 0.05.
Phenotypic data were analyzed using ANOVA to determine the effect of diet (FO/P vs. CO/C) on apoptosis, proliferation, and HM ACF. Colon tumor incidence was analyzed by chi square analysis and reported as the percentage of rats bearing tumors. Values reported are LSmean ± SEM.
Results
The goal of this study was to monitor the protection provided by the combined FO/P diet in terms of changes in global patterns of intestinal gene expression at the initiation, promotion, and tumor stages. Gene expression was monitored using microarray procedures and the resulting data were compared with phenotypic data at each of the 3 time points to determine whether the patterns of expression identified by GO analysis were predictive of changes in disease phenotypes detected in these rats.
Initiation stage.
At the initiation stage of colon tumorigenesis, FO/P resulted in higher levels of apoptosis in the colonic crypt compared with CO/C rats (P = 0.024; Table 1). Although there was no significant diet effect on the expression of genes involved in apoptosis 24 h after AOM injection, there was a lower expression of cell adhesion genes (B4galt1, Smoc1, and Scarb2) in FO/P rats compared with CO/C rats at this time point (Supplemental Table 2).
TABLE 1.
Phenotypes at initiation (24 h after AOM injection) and at ACF (7 wk postinjection) and tumor (28 wk postinjection) stages of colon cancer in rats fed FO/P or CO/C diets1
| Item | CO/C | FO/P |
| 24 h | ||
| Apoptotic index, % | 4.36 ± 0.17 (11) | 5.59 ± 0.21* (11) |
| 7 wk | ||
| HM ACF, n | 14.1 ± 1.81 (15) | 5.78 ± 1.16* (15) |
| Proliferative zone, % | 66.4 ± 0.66 (15) | 62.8 ± 0.62* (15) |
| Apoptotic index, % | 0.09 ± 0.04 (15) | 0.27 ± 0.07* (15) |
| Total cells, n/crypt | 36.1 ± 0.24 (15) | 33.0 ± 0.24* (15) |
| 31 wk | ||
| Colon tumor incidence, % | 26.8 (41) | 10.3* (39) |
| Apoptotic index, % | 0.33 ± 0.20 (19) | 0.73 ± 0.31* (20) |
Data are LSmean ± SEM ( ). *Different from CO/C, P < 0.05.
ACF stage.
Rats receiving the FO/P diet had fewer HM ACF than did rats receiving the CO/C diet (P = 0.0002) (Table 1). In addition, the extent of the proliferative zone was lower in rats receiving the FO/P diet compared with those fed the CO/C diet (P = 0.0001) (Table 1). Relative to observations from rats consuming the CO/C diet, rats consuming the FO/P diet had an elevated apoptotic index (P = 0.027) (Table 1). The smaller number of cells in the crypt in FO/P group (P = 0.0001) (Table 1) likely was due to both suppression of cell proliferation and induction of apoptosis. In contrast to the initiation stage, at the HM ACF stage, there were 602 genes that were differentially (false discovery rate, P < 0.05) expressed as a function of diet. Upon completion of GO analyses, 80 biological process categories were found to be significantly enriched (Supplemental Table 3). Among the 80 clusters, 5 were directly involved with cell cycle regulation (GO:0007049 cell cycle, GO:0022402 cell cycle process, GO:0000074 regulation of progression through cell cycle, GO:0051726 regulation of cell cycle, and GO:0000079 regulation of cyclin-dependent protein kinase activity). Because 4 of these categories are a subset of the parent category of cell cycle (GO:0007049), we focused on the parent category to include the maximum number of differentially expressed genes. The FO/P diet yielded almost uniformly lower levels of expression of both cell cycle promoters and suppressors in this cell cycle category (Table 2).
TABLE 2.
Differential gene expression in feces-derived exfoliated cells from rats at 7 wk after AOM injection (ACF stage)1
| GenBank accession2 | Gene symbol | Description | Relative expression, FO/P / CO/C |
| Cell cycle promoters | |||
| BF388494 | App | Amyloid β (a4) precursor protein | 0.40 |
| NM_012923 | Ccng1 | Cyclin G1 | 0.44 |
| BI294914, BE113451 | Ccnk | Cyclin K | 0.69 |
| BQ206043 | Ccnl1 | Cyclin l1 | 0.31 |
| AI411332 | Ccnt2_predicted | Cyclin T2 (predicted) | 0.55 |
| AA819214 | Cdc34_predicted | Cell division cycle 34 homolog (S. cerevisiae) (predicted) | 0.51 |
| AW532478 | Gfi1b_predicted | Growth factor independent 1B (predicted) | 0.64 |
| NM_053347 | Nde1 | Nuclear distribution gene E homolog 1 (A. nidulans) | 0.54 |
| AA685941 | Pafah1b1 | Platelet-activating factor acetylhydrolase, isoform ib, α subunit 45 kda | 0.56 |
| NM_012801 | Pdgfa | Platelet derived growth factor, α | 0.50 |
| BI395817 | Ruvbl1 | Ruvb-like protein 1 | 0.56 |
| Cell cycle suppressors | |||
| NM_001005902 | Abtb1 | Ankyrin repeat and BTB (POZ) domain containing 1 | 0.40 |
| AY351678 | Cdkn1c | Cyclin-dependent kinase inhibitor 1C (P57) | 0.48 |
| BF406173 | Ddit3 | DNA-damage inducible transcript 3 | 0.46 |
| BQ191258 | Dst_predicted | Dystonin (predicted) | 0.39 |
| NM_053484 | Gas7 | Growth arrest specific 7 | 0.49 |
| NM_032080 | Gsk3b | Glycogen synthase kinase 3 β | 0.53 |
| AW916463.1 | Pms2_predicted | Postmeiotic segregation increased 2 (S. cerevisiae) (predicted) | 3.23 |
| AW526814 | Rbl2 | Retinoblastoma-like 2 (p130) | 0.61 |
| NM_031745.2 | Clip1 | CAP-GLY domain containing linker protein 1 | 0.32 |
| BI297192, NM_001024796.1 | Spin1 | Spindlin 1 | 0.41 |
All < 0.05, indicating that expression differed between groups.
GO:0007049.
Tumor stage.
Similar to the reduction of early preneoplastic lesion numbers, colon tumor incidence evaluated 31 wk after the second AOM injection was lower in FO/P rats than in CO/C rats (Table 1). Part of the protection against tumor formation may be attributable to the enhanced apoptotic index in the FO/P rat colons compared with those from rats consuming CO/C (Table 1), which was elevated at all 3 stages of the tumorigenic process.
At the tumor stage, 81 genes were differentially expressed in response to diet and 13 biological processes were identified by GO analysis as being enriched. Of the 13 categories, 6 were associated with apoptosis (Supplemental Table 4). Among the remaining 75 differentially expressed genes, we identified 16 genes known to be involved in signal transduction and tumor development, progression, and invasion (Table 3).
TABLE 3.
Differential gene expression in feces-derived exfoliated cells from rats at 28 wk after AOM injection (tumor stage)1
| GenBank accession | Gene symbol | Description | Relative expression, FO/P / CO/C | Function | Membrane related2 |
| Apoptosis from GO category | |||||
| NM_181386 | Tmem23 | Transmembrane protein 23 | 0.55 | Antiapoptosis | Y |
| BI302754, NM_013058 | Id3 | Inhibitor of DNA binding 3 | 2.16 | Proapoptosis | |
| NM_031054 | Mmp2 | Matrix metallopeptidase 2 | 0.69 | Proapoptosis, tumor invasion | Y |
| AA999104 | Fem1b | Feminization 1 homolog b (C. elegans) (predicted) | 0.39 | Proapoptosis | |
| BF542507 | Hipk2 | Homeodomain interacting protein kinase 2 (predicted) | 0.10 | Proapoptosis | |
| NM_017017 | Hgf | Hepatocyte growth factor | 0.55 | Antiapoptosis | |
| Signal transduction | |||||
| CF113820 | Mtmr4 | Myotubularin related protein 4 (predicted) | 3.88 | TGFβ signaling | |
| NM_019268 | Slc8a1 | Solute carrier family 8 (sodium/calcium exchanger), member 1 | 0.41 | Calcium singaling, proapoptosis | Y |
| AW918423 | Dupd1 | Dual specificity phosphatase and pro isomerase domain containing 1 (predicted) | 0.46 | MAPK signaling | |
| CB749439 | Ppp1r7 | Protein phosphatase 1, regulatory (inhibitor) subunit 7 | 0.61 | MAPK signaling | |
| CB611690 | Mfn1 | Mitofusin 1 | 0.53 | Antiapoptosis | Y |
| NM_053788 | Stx1a | Syntaxin 1A (brain) | 0.57 | Proapoptosis | Y |
| NM_001002835 | Smoc1 | SPARC-related modular calcium binding protein 1 | 0.54 | Positive regulation of cell-substrate adhesion | Y |
| NM_019378 | Snip | SNAP25-interacting protein | 0.70 | Negative regulation of cell adhesion | |
| NM_053346 | Nrn1 | Neuritin | 0.65 | Hypoxia-induced genes | Y |
| NM_130410 | Il23a | Interleukin 23, α subunit p19 | 0.56 | Immune response | |
| NM_017020 | Il6ra | Interleukin 6 receptor, α | 0.52 | Immune response | Y |
| NM_031089 | Pthr2 | Parathyroid hormone receptor 2 | 0.41 | Cell proliferation | Y |
| Tumor | |||||
| CO562407 | Cyp2s1 | Cytochrome P450, family 2, subfamily s, polypeptide 1 | 0.62 | Metabolism | Y |
| NM_001009605 | Brms1 | Breast cancer metastasis-suppressor 1 | 2.60 | Cancer metastasis | |
| BI299377 | Rbbp6 | Retinoblastoma binding protein 6 | 2.03 | Tumor suppressor | |
| NM_020302 | Adam3 | A disintegrin and metalloprotease domain 3 (cyritestin) | 0.58 | Tumor invasion | Y |
All < 0.05, indicating that expression differed between groups
Y indicates genes included in the membrane category (GO:0016020).
qRT-PCR confirmation of fecal microarray.
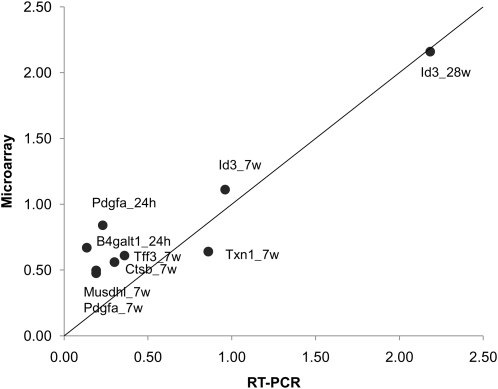
To validate the fecal microarray data, we performed qRT-PCR on select genes using the same fecal poly (A)+ RNA isolates. The regression between fecal microarray and qRT-PCR results demonstrate a reasonable degree of similarity in the pattern of expression for the 7 genes selected for this comparison (R2 = 0.87) (Fig. 1).
FIGURE 1.
Correlation of fecal microarray and qRT-PCR results within a subset of genes. Data are expressed as the level of gene expression from feces-derived exfoliated cells of FO/P rats relative to CO/C rats (R2 = 0.87).
Discussion
Most chemoprevention studies are targeted to a single time point in the carcinogenic process. We used a noninvasive technique that permits the isolation of eukaryotic mRNA from exfoliated colonocytes in fecal material (13–16) to monitor temporal changes in gene expression. The purpose of the current study was to determine how diet influences the expression of genes at 3 discreet stages of tumorigenesis and if the differences were reflective of the changes in phenotypes measured at those time points.
At the initiation stage of colon tumorigenesis, there were only 3 annotated genes, which were differentially expressed as a function of diet. The low number of diet-induced differentially expressed genes at the initiation stage was not unexpected, because we have previously shown a relatively small number of differentially expressed genes between diets 12 h after AOM injection (11). The few differences between the diets could be explained by the extensive effect of AOM on the colonic epithelium, which prevented detection of diet effects on gene expression. These 3 genes are all involved in maintaining cell adhesion and it is known that cell adhesion to basement membranes prevents cell death (9), suggesting that the FO/P diet would facilitate apoptosis induction. The lower relative expression of cell adhesion-related genes in cells after AOM injection likely contributes to the ability of FO/P rats to effectively eliminate cells with DNA damage (4, 24). Whether the elimination is through the induction of apoptosis or by cell sloughing (9, 25), these changes in gene expression would explain some of the chemoprotection provided by a FO/P diet relative to the effects observed with a CO/C diet.
At the HM ACF stage, we found that 602 genes were differentially expressed as a function of diet. This suggests that as carcinogenesis progresses to this stage, the ability of diet to affect gene expression and thereby provide a chemoprotective effect is enhanced, as reflected by the number of phenotypic changes that are detected (i.e. apoptosis, proliferative zone, and HM ACF). GO analysis of the 602 differentially expressed genes revealed that cell cycle regulation was affected by a FO/P diet compared with CO/C. Changes in the expression of genes involved in cell cycle regulation are critical to the promotion of colon carcinogenesis (26). The cyclin-CDK complex initiates the phosphorylation of Rb, stimulating the regulation of cell cycle progression (7). Relative to the expression in the CO/C rats, expression of cyclin G1, K, L1, and T2_predicted as well as cdc34_predicted was lower in rats consuming the FO/P diet. Rbl2 is known to control progression from G0 into the G1 phase of the cell cycle and its expression was lower in rats consuming a diet containing FO/P compared with a diet containing CO/C. However, the phosphorylation of Rbl2 is needed to release E2F and activate the cell cycle. FO/P resulted in lower levels of expression of CDK and GSK3b, which is a reported kinase of Rbl2 (27) and is expected to reduce the degree of phosphorylation of Rbl2. Another promoter of cell cycle activity is Ruvbl1, which is upregulated in human colon cancers. Ruvbl1 enhances the transcription of Wnt target genes by interacting with β-catenin (28). In this experiment, the FO/P diet downregulated the expression of Ruvbl1 compared with the CO/C diet, which suggests the FO/P diet may inhibit Wnt signaling. These findings suggest that the FO/P diet could suppress the uncontrolled cell proliferation that occurs in colon cancer cells at the ACF stage in part by modulating the expression of genes that are essential for cell cycle progression.
In addition to the suppression of cell proliferation, we previously reported on the cell-specific expression of O6-methylguanine DNA methyltransferase (MGMT, DNA repair enzyme). The expression of MGMT in fish oil-fed rats was 4-fold higher than corn oil-fed rats in areas of colon crypts where apoptosis typically occurs (4). Pms2 is known to be involved in DNA mismatch repair systems and mutation of Pms2 is documented to cause hereditary nonpolyposis colorectal cancer (29). In the current study, we found that the expression of Pms2_predicted was 3-fold higher in FO/P rats than in CO/C rats. We also observed that FO/P significantly enhanced apoptosis compared with CO/C (Table 1). Therefore, the FO/P diet may facilitate removal of DNA-damaged cells by increasing DNA repair and apoptosis.
At the tumor stage, the modulation of expression of several genes involved in apoptosis occurred in concert with the induction of apoptosis in the FO/P rats. Of the 6 differentially expressed genes involved in the apoptosis pathway, Mmp2 expression increased in the colon of CO/C tumor-bearing rats. This is noteworthy because Mmp2 has been implicated in colon cancer invasion (30). Expression of Id3, an apoptosis inducer (31), was higher in FO/P rats than in CO/C rats, whereas Tmem23 and Hgf, both apoptosis inhibitors (32, 33), were lower in FO/P rats compared with CO/C rats. This pattern of gene expression is consistent with the apoptotic phenotype observed across all time points, indicating at a molecular level why the FO/P diet is more effective in promoting apoptosis than the CO/C diet.
The expression of 11 genes (Slc8a1, Dupd1, Ppp1r7, Mfn1, Stx1a, Smoc1, Snip, Nrn1, Il23a, Il6ra, and Pthr2) involved in several signal transduction pathways was downregulated in FO/P rats compared with CO/C rats, suggesting that FO/P is capable of attenuating multiple signaling pathways at the tumor stage. For example, Mfn1, which is a transmembrane GTPase, is one of the genes downregulated by FO/P compared with CO/C. Mfn1 mediates mitochondrial fusion and elevated expression of Mfn1 has been demonstrated to increase the resistance of cells to death stimuli (34). With regard to this mechanism of action, 11 genes in the membrane category (GO:0016020) were differentially expressed at the tumor stage (indicated by “Y” in Table 3). This may be explained by the incorporation of DHA, a bioactive component of fish oil, into both plasma and mitochondrial membranes (35). Indeed, we and others have demonstrated that (n-3) PUFA promote an oxidation-reduction imbalance in the intestine (36–39). Recently, we demonstrated that DHA promotes mitochondrial oxidative stress and increases mitochondrial Ca2+ levels, which directly induce apoptosis in colonocytes (40, 41).
Tumor-related genes involved in tumor formation, progression, and invasion were favorably modulated by FO/P consumption compared with CO/C. For example, the expression of Cyp2s1 was downregulated by FO/P compared with CO/C. This gene encodes for one of the cytochrome P450 superfamily members and plays a pivotal role in the oxidative metabolism of xenobiotics such as carcinogens. A study designed to identify markers of colon cancer prognosis demonstrated that the expression of Cyp2s1 was significantly higher in primary colon cancers than in normal colon tissue (42). Adam3, an indicator of tumor invasion, was also downregulated in FO/P rats compared with CO/C (43, 44). In contrast, Brms1, a tumor suppressor gene, was upregulated by FO/P compared with CO/C. Similarly, FO/P rats had twice the level of Rbbp6 (also designated P2P-R) expression as CO/C rats and overexpression of this gene results in mitotic arrest at prometaphase and mitotic apoptosis (45).
Existing studies with the AOM model of colon carcinogenesis have reported gene expression at discreet points in the tumorigenic process (46, 11). Those studies also found changes in expression of genes involved in cell adhesion (46), apoptosis, or cell cycle (11). The unique contribution of the current study is that we found that diet differentially affects apoptotic genes, depending upon the stage of tumorigenesis. Therefore, we have discovered time-specific effects of diet that contribute to chemoprotection against colon cancer. Interestingly, although the FO/P diet promoted apoptosis at all 3 time points, it differentially affected gene expression at each stage. These data are consistent with recent observations (47), indicating a clear time-dependent, divergent regulation of gene expression signatures in response to the fatty acid content of the diet.
We have demonstrated the feasibility of monitoring gene expression over time using an mRNA-based noninvasive technique. This makes it possible to determine the mechanisms whereby a chemopreventive diet may inhibit colon carcinogenesis as well as to monitor human disease progression and identify critical time points for potential diet intervention. In this study, we identified differentially expressed genes involved in apoptosis and/or cell proliferation at 3 time points during colon carcinogenesis. At the initiation stage, there were few differential effects of diet on gene expression. At the promotion stage, the expression of many more genes was affected by diet, suggesting this stage is more susceptible to the FO/P dietary intervention. GO pathways enriched at this stage include cell proliferation. However, at the tumor stage, the main gene expression pathway affected was principally associated with apoptosis. Consequently, the central mechanism by which FO/P produces a chemoprotective effect is through changes in gene expression that enhance cell cycle regulation and apoptosis throughout tumorigenesis.
Supplementary Material
Acknowledgments
N.D.T., L.A.D., R.S.C., and J.R.L. designed the research; Y.C., J.C.M., N.D.T., and S.S.T. conducted the research; Y.C., H.K., J.W., N.W., M.V., and R.J.C. analyzed the data; Y.C. wrote the paper; and J.R.L. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by National Space Biomedical Research Institute, National Aeronautics and Space Administration (NASA) NCC 9-58 (J.R.L., N.D.T.), NASA NAG-9-1523 (J.R.L., N.D.T.), and NIH CA59034 (R.S.C.), CA129444 (R.S.C.), CA74552 (N.W.), CA90301 (R.J.C.), CA61750 (J.R.L.), and CA057030 (R.J.C.).
Supplemental Tables 1–4 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: AOM, azoxymethane; CDK, cyclin dependent kinase; CO/C, corn oil and cellulose; FO/P, fish oil and pectin; GO, gene ontology; HM ACF, high multiplicity aberrant crypt foci; PFA, paraformaldehyde.
Literature Cited
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49 [DOI] [PubMed] [Google Scholar]
- 2.Cummings JH, Bingham SA. Diet and the prevention of cancer. BMJ. 1998;317:1636–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–30 [DOI] [PubMed] [Google Scholar]
- 4.Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers Prev. 2000;9:819–26 [PubMed] [Google Scholar]
- 5.Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr. 1998;128:491–7 [DOI] [PubMed] [Google Scholar]
- 7.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–60 [DOI] [PubMed] [Google Scholar]
- 8.Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, Chapkin RS, Lupton JR. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis. 2008;29:1415–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–38 [DOI] [PubMed] [Google Scholar]
- 10.Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg. 2006;191:517–26 [DOI] [PubMed] [Google Scholar]
- 11.Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Wang N, Lupton JR, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong MY, Chapkin RS, Davidson LA, Turner ND, Morris JS, Carroll RJ, Lupton JR. Fish oil enhances targeted apoptosis during colon tumor initiation in part by downregulating Bcl-2. Nutr Cancer. 2003;46:44–51 [DOI] [PubMed] [Google Scholar]
- 13.Davidson LA, Jiang YH, Lupton JR, Chapkin RS. Noninvasive detection of putative biomarkers for colon cancer using fecal messenger RNA. Cancer Epidemiol Biomarkers Prev. 1995;4:643–7 [PubMed] [Google Scholar]
- 14.Davidson LA, Aymond CM, Jiang YH, Turner ND, Lupton JR, Chapkin RS. Non-invasive detection of fecal protein kinase C betaII and zeta messenger RNA: putative biomarkers for colon cancer. Carcinogenesis. 1998;19:253–7 [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Ivanov I, Dougherty ER, Hartman TJ, Lanza E, Bobe G, Colburn NH, Lupton JR, Davidson LA, et al. Noninvasive detection of candidate molecular biomarkers in subjects with a history of insulin resistance and colorectal adenomas. Cancer Prev Res (Phila). 2009;2:590–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapkin RS, Zhao C, Ivanov I, Davidson LA, Goldsby JS, Lupton JR, Mathai RA, Monaco MH, Rai D, et al. Noninvasive stool-based detection of infant gastrointestinal development using gene expression profiles from exfoliated epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;298:G582–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–65 [DOI] [PubMed] [Google Scholar]
- 18.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51 [DOI] [PubMed] [Google Scholar]
- 19.Guzinska-Ustymowicz K, Zalewski B, Kasacka I, Piotrowski Z, Skrzydlewska E. Activity of cathepsin B and D in colorectal cancer: relationships with tumour budding. Anticancer Res. 2004;24:2847–51 [PubMed] [Google Scholar]
- 20.Babyatsky M, Lin J, Yio X, Chen A, Zhang JY, Zheng Y, Twyman C, Bao X, Schwartz M, et al. Trefoil factor-3 expression in human colon cancer liver metastasis. Clin Exp Metastasis. 2009;26:143–51 [DOI] [PubMed] [Google Scholar]
- 21.Liu LY, Wang N, Lupton JR, Turner ND, Chapkin RS, Davidson LA. A two-stage normalization method for partially degraded mRNA microarray data. Bioinformatics. 2005;21:4000–6 [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Statistical Methodological). 1995;57:289–300 [Google Scholar]
- 23.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 24.Hong MY, Chapkin RS, Wild CP, Morris JS, Wang N, Carroll RJ, Turner ND, Lupton JR. Relationship between DNA adduct levels, repair enzyme, and apoptosis as a function of DNA methylation by azoxymethane. Cell Growth Differ. 1999;10:749–58 [PubMed] [Google Scholar]
- 25.Chao C, Jamshidi-Parsian A, Wang WW, McMasters KM. Colorectal cancer cell adhesion attenuates Ad-E2F–1 mediated apoptosis. J Surg Res. 2003;113:81–7 [DOI] [PubMed] [Google Scholar]
- 26.Polyak K, Hamilton SR, Vogelstein B, Kinzler KW. Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am J Pathol. 1996;149:381–7 [PMC free article] [PubMed] [Google Scholar]
- 27.Litovchick L, Chestukhin A, DeCaprio JA. Glycogen synthase kinase 3 phosphorylates RBL2/p130 during quiescence. Mol Cell Biol. 2004;24:8970–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauscher JC, Loddenkemper C, Kosel L, Grone J, Buhr HJ, Huber O. Increased pontin expression in human colorectal cancer tissue. Hum Pathol. 2007;38:978–85 [DOI] [PubMed] [Google Scholar]
- 29.Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, Rosen CA, Haseltine WA, Fleischmann RD, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75–80 [DOI] [PubMed] [Google Scholar]
- 30.Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int J Cancer. 2003;107:541–50 [DOI] [PubMed] [Google Scholar]
- 31.Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol. 1998;18:2371–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu JX, Morii E, Liu Y, Nakamichi N, Ikeda J, Kimura H, Aozasa K. High tolerance to apoptotic stimuli induced by serum depletion and ceramide in side-population cells: high expression of CD55 as a novel character for side-population. Exp Cell Res. 2007;313:1877–85 [DOI] [PubMed] [Google Scholar]
- 33.Kitamura S, Kondo S, Shinomura Y, Kanayama S, Miyazaki Y, Kiyohara T, Hiraoka S, Matsuzawa Y. Met/HGF receptor modulates bcl-w expression and inhibits apoptosis in human colorectal cancers. Br J Cancer. 2000;83:668–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Chan DC. New insights into mitochondrial fusion. FEBS Lett. 2007;581:2168–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–25 [DOI] [PubMed] [Google Scholar]
- 36.Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Wang N, Spinka CM, Carroll RJ, Turner ND, et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J Nutr. 2004;134:3233–8 [DOI] [PubMed] [Google Scholar]
- 37.Latham P, Lund EK, Johnson IT. Dietary n-3 PUFA increases the apoptotic response to 1,2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis. 1999;20:645–50 [DOI] [PubMed] [Google Scholar]
- 38.Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, Lupton JR, Chapkin RS. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan YY, Zhan Y, Aukema HM, Davidson LA, Zhou L, Callaway E, Tian Y, Weeks BR, Lupton JR, et al. Proapoptotic effects of dietary (n-3) fatty acids are enhanced in colonocytes of manganese-dependent superoxide dismutase knockout mice. J Nutr. 2009;139:1328–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–8 [DOI] [PubMed] [Google Scholar]
- 41.Kolar SS, Barhoumi R, Callaway ES, Fan YY, Wang N, Lupton JR, Chapkin RS. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca(2+) accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G935–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumarakulasingham M, Rooney PH, Dundas SR, Telfer C, Melvin WT, Curran S, Murray GI. Cytochrome p450 profile of colorectal cancer: identification of markers of prognosis. Clin Cancer Res. 2005;11:3758–65 [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Lin QD, Di W. Suppression of human ovarian carcinoma metastasis by the metastasis-suppressor gene, BRMS1. Int J Gynecol Cancer. 2006;16:522–31 [DOI] [PubMed] [Google Scholar]
- 44.Arribas J, Bech-Serra JJ, Santiago-Josefat B. ADAMs, cell migration and cancer. Cancer Metastasis Rev. 2006;25:57–68 [DOI] [PubMed] [Google Scholar]
- 45.Gao S, Scott RE. P2P-R protein overexpression restricts mitotic progression at prometaphase and promotes mitotic apoptosis. J Cell Physiol. 2002;193:199–207 [DOI] [PubMed] [Google Scholar]
- 46.Xiao R, Badger TM, Simmen FA. Dietary exposure to soy or whey proteins alters colonic global gene expression profiles during rat colon tumorigenesis. Mol Cancer. 2005;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kachroo P, Ivanov I, Davidson LA, Chowdhary BP, Lupton JR, Chapkin RS. Classification of diet-modulated gene signatures at the colon cancer initiation and progression stages. Dig Dis Sci. 2011; DOI 10.1007/s10620-011-1652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.