Abstract
Low-glycemic index diets and exercise independently improve glucose tolerance and reduce diabetes risk. However, the combined effect of a low-glycemic index diet and exercise on inflammation and glucose metabolism is not known. Therefore, we randomized 28 insulin-resistant adults (age: 66 ± 1 y; BMI: 34.2 ± 0.7 kg⋅m−2) to a 12-wk, low (LGI = 40) or high- (HGI = 80) glycemic index diet plus aerobic exercise (5 d⋅wk−1, 60 min⋅d−1, 80–85% heart ratemax) intervention. All food and fluids were provided during the study. Inflammation was assessed from cytokine (TNFα and IL-6) secretion using peripheral blood mononuclear cells (MNC) stimulated overnight with LPS. Glycemic response was determined following ingestion of a 75-g glucose solution. Fasting blood samples were collected for additional cytokine [TNFα, IL-6, and monocyte chemoattractant protein 1 (MCP-1)] analysis. Both interventions decreased BMI (P < 0.001), fasting plasma glucose (P = 0.01), and insulin (P = 0.02). The glycemic response was reduced only in the LGI group (P = 0.04). Plasma and MNC-derived TNFα secretion were reduced in the LGI group (P = 0.02) but increased in the HGI group (P = 0.02). Secretion of IL-6 from MNC and plasma IL-6 and MCP-1 concentrations were reduced in the LGI group. The change in MNC-derived TNFα (r = 0.43; P = 0.04) and plasma MCP-1 (r = 0.44; P = 0.04) correlated with decreases in the glycemic response. These data highlight the importance of diet composition in the treatment and prevention of inflammation and hyperglycemia. A low-glycemic index diet has antiinflammatory and antidiabetogenic effects when combined with exercise in older, obese prediabetics.
Introduction
Obesity increases the risk of type 2 diabetes and is associated with low-grade inflammation (1–3). There is now a consensus that macrophages infiltrate adipose tissue; promote the production of proinflammatory cytokines, including TNFα, IL-6, and monocyte chemoattractant protein-1 (MCP-1);9 and contribute to the development of inflammation-induced insulin resistance and type 2 diabetes (1, 4–6). Previously, we and others showed that exercise improves insulin sensitivity in obese individuals and that obese individuals secrete more TNFα from peripheral blood mononuclear cells (MNC) compared with lean individuals (4, 5). Further, it has been suggested that postprandial hyperglycemia may be a contributing factor in driving hyperinsulinemia and insulin resistance (4).
Low-glycemic index diets attenuate the prevailing glucose concentrations throughout the day, which may reduce the risk of some chronic diseases and help to improve glucose tolerance (7–9). In line with these observations, Jenkins et al. (9) reported that participants who were counseled to eat a low-glycemic index diet for 6 mo had a reduced hemoglobin A1c and greater glycemic control. Furthermore, we discovered that participants who exercised and reduced the glycemic load in their diet experienced a more marked improvement in insulin sensitivity compared with participants who exercised but maintained a normal glycemic index diet (10). Additionally, several studies have shown that modification of dietary carbohydrate intake can reduce both systemic and adipose tissue production of inflammatory cytokines (11–15).
Several studies have also suggested that exercise alone can reduce inflammatory markers and improve insulin sensitivity (16–18). However, these studies evaluated leisure time activity and did not assess changes in cytokines following a longer term, structured exercise program. Evidence from a small study in healthy volunteers showed that a 6-mo exercise program reduced TNFα production by MNC in healthy adults (19). At present, there are no data on the effects of a well-controlled, clinically relevant lifestyle intervention on proinflammatory cytokine secretion in an at-risk population such as the older obese who are more susceptible to type 2 diabetes and cardiovascular disease. We hypothesized that a lifestyle intervention that included a low-glycemic index diet combined with aerobic exercise would reduce postprandial glucose excursions and thus attenuate the prevailing hyperglycemia that induces low-grade inflammation and cytokine secretion from peripheral MNC.
Methods
Participants.
Twenty-eight older, obese, previously sedentary adults (age 66 ± 1 y; BMI 34.2 ± 0.7 kg/m2) were recruited from the local community to undergo a 12-wk diet and exercise training intervention. All volunteers underwent a medical history, physical exam, oral glucose tolerance test, and complete blood profile (lipid profile and hepatic/renal/hematological function tests). Female participants were postmenopausal and were not using hormone replacement therapy. Prior physical activity levels were recorded using the Minnesota Leisure Time Physical Activity questionnaire (20); volunteers were deemed sedentary if their leisure time activity was <300 kcal/d (1255 kJ/d). Participants were required to be weight stable for at least the previous 6 mo. Individual energy requirements were determined by indirect calorimetry (21). The Cleveland Clinic Institutional Review Board approved the study and all participants provided signed, informed, written consent in accordance with guidelines for the protection of human participants.
Intervention.
Participants were randomized to 1 of 2 groups: either a low-glycemic index diet plus exercise (LGI: n = 13; 5 male, 8 female) or high-glycemic index diet plus exercise (HGI: n = 15; 8 male, 7 female). All volunteers undertook 60 min of aerobic exercise 5 d/wk for 12 wk at ~85% of the maximum heart rate obtained during an incremental maximal aerobic exercise test (VO2max test). Every session was fully supervised by an exercise physiologist. All meals for the 12-wk intervention were provided to participants. Diets were designed by a registered dietitian as previously described (22). The dietary macronutrient composition (including fiber) was matched between groups (Table 1); however, the LGI participants received a diet with a mean glycemic index of 40 units, whereas participants in the HGI group consumed foods with a mean glycemic index of 80 units. Adherence to the diet was determined via daily food container weigh backs plus a weekly counseling session with the study dietitian. Dietary analysis was performed using Nutritionist Pro software (Axxya Systems).
TABLE 1.
Composition of the study diets1
| Study diet | LGI | HGI |
| GI,2a.u. | 40.3 ± 0.4 | 80.2 ± 1.0* |
| GL, a.u. | 102 ± 9 | 218 ± 24* |
| EI, kJ/d | 7400 ± 678 | 7890 ± 803 |
| Carbohydrate, g/d | 248 ± 23 | 272 ± 29 |
| Carbohydrate, % energy | 55.8 ± 0.1 | 57.7 ± 0.6 |
| Fat, g/d | 56 ± 5 | 60 ± 6 |
| Fat, % kJ | 31.9 ± 0.3 | 31.8 ± 0.3 |
| Protein, g/d | 77 ± 7 | 81 ± 8 |
| Protein, % energy | 17.4 ± 0.4 | 17.0 ± 0.1 |
| Fiber, g/d | 28 ± 3 | 28 ± 3 |
Data are mean ± SEM, = 13 (LGI) or 15 (HGI). *Different from LGI, P < 0.05.
GI, glycemic index; GL, glycemic load; a.u., arbitrary units; EI, energy intake.
Inpatient control period.
Pre- and postintervention assessments of body composition, aerobic fitness, glucose tolerance, and MNC were performed during a 3-d in-patient stay in the Clinical Research Unit. During this period, participants received a weight maintenance eucaloric diet (total kcal/d = resting metabolic rate × 1.2; 55% carbohydrate, 28% fat, 17% protein). Postintervention metabolic measures were performed within 16 h after the last exercise bout and the participant’s meal the night before testing was matched to the study diet.
Body composition.
Height and body weight were measured by standard techniques (23). Whole body adiposity [fat mass (FM) and fat-free mass (FFM)] was measured by DXA (model iDXA; Lunar).
Glycemic response.
The glucose response to ingestion of a 75-g glucose solution (Azer Scientific) was assessed after an overnight fast pre- and postintervention. Following baseline draws, the solution was ingested and blood samples were subsequently drawn at 30, 60, 90, 120, and 180 min. Plasma glucose was determined immediately on a YSI 2300 STAT Plus analyzer (YSI Life Sciences). The samples were stored at −80°C for cytokine and substrate analysis.
MNC isolation and culturing.
MNC isolation and culture was performed as previously described (4, 5, 24). Briefly, MNC were isolated via Histopaque-1077 density gradient centrifugation, washed, and resuspended in RPMI (Sigma) and seeded in coated cell culture plates (5 × 106cells/well). The cells were stimulated with 1 pg/L LPS and incubated (humidified, 5% CO2, 37°C) for 24 h. Cell supernatants were collected (10,000 × g for 2 min) and stored at −80°C for subsequent cytokine analysis.
Biochemical analyses.
All samples were measured in duplicate and each participant’s pre- and postintervention samples were batch analyzed. Plasma insulin was determined via RIA (Millipore) and both plasma and MNC-derived TNFα were determined by ELISA (Invitrogen). Plasma IL-6 and MCP-1 were measured via ELISA (Bio-Rad).
Statistics.
Between-group (LGI vs. HGI) comparisons were analyzed using 2-way (group × time) repeated-measures ANOVA and Bonferroni post hoc tests were applied to significant group × time interactions. Baseline values for each variable were compared between groups using Student’s t tests. In the event of a significant t statistic, baseline values were used as a covariate in the 2-way repeated-measures ANOVA. Bivariate correlation analyses were used to identify relationships between variables. Significance was accepted at P < 0.05. Analyses were carried out using StatView for Windows 5.0.1 (SAS Institute) and all data are expressed as mean ± SEM.
Results
Diet and exercise.
Dietary analysis shows that diets for both groups were matched with respect to macronutrient composition, including fiber, but the glycemic index was markedly different (Table 1). The glycemic responses to the study diets were confirmed and previously reported (25). Diet and exercise compliance was high (~97%). Exercise was performed at 83.2 ± 0.5% of maximal heart rate, and following the study there was an increase in VO2 max in both groups [LGI, pre: 38.4 ± 1.3, post: 42.9 ± 2.9; HGI, pre: 39.6 ± 1.4, post: 45.7 ± 1.8 mL/(kg−1 FFM⋅min−1); P < 0.001]. Baseline and postintervention VO2 max values did not differ between the groups.
Body composition.
Groups were well matched for BMI prior to the onset of the intervention (P = 0.41). Poststudy, both groups significantly reduced body weight and they did not differ in the amount of weight loss achieved (Table 2). There was a small but significant decrease in FFM in both groups (Table 2). In contrast, whole body FM was markedly reduced in both groups (Table 2). Truncal fat was also reduced following both interventions (Table 2). FFM, FM, and truncal fat did not differ between the groups at the end of the interventions.
TABLE 2.
Participant characteristics, body composition, glucose, and insulin responses to glucose in older, obese adults who consumed a LGI or HGI diet for 12 wk1
| LGI |
HGI |
ANOVA2 |
|||
| Characteristics | Pre | Post | Pre | Post | Time |
| Males, females, n | 7/6 | — | 8/7 | — | — |
| Age, y | 67 ± 1 | — | 66 ± 1 | — | — |
| Weight, kg | 94.6 ± 4.0 | 88.1 ± 3.4 | 97.9 ± 4.1 | 88.3 ± 4.1 | <0.0001 |
| BMI kg·m−2 | 34.2 ± 1.0 | 31.9 ± 1.1 | 34.8 ± 1.0 | 31.5 ± 1.0 | <0.0001 |
| FM, kg | 44.8 ± 2.0 | 38.5 ± 1.9 | 43.5 ± 2.1 | 35.1 ± 2.6 | 0.001 |
| FFM, kg | 49.8 ± 2.8 | 49.4 ± 2.5 | 56.9 ± 3.5 | 55.3 ± 3.3 | 0.004 |
| Plasma glucose, mmol·L−1 | 6.2 ± 0.2 | 5.3 ± 0.2 | 5.6 ± 0.1 | 5.2 ± 0.1 | <0.001 |
| Plasma insulin, pmol·L−1 | 140 ± 19.2 | 87.0 ± 10.8 | 106 ± 25.2 | 57.6 ± 6.0 | 0.004 |
| Glucose AUC, mol·L−1·3 h | 1.63 ± 0.12 | 1.42 ± 0.08 | 1.36 ± 0.06 | 1.31 ± 0.07 | 0.02 |
| Insulin AUC, nmol·L−1·3 h | 149 ± 0.4 | 81.1 ± 0.2 | 102 ± 0.2 | 51.1 ± 0.1 | 0.0005 |
| HbA1c, % | 5.8 ± 0.1 | 5.6 ± 0.1 | 5.4 ± 0.1 | 5.5 ± 0.1 | 0.45 |
Data are mean ± SEM, = 13 (LGI) or 15 (HGI).
Effects of group and time × group were not significant, ≥ 0.05.
Cytokine and glycemic responses.
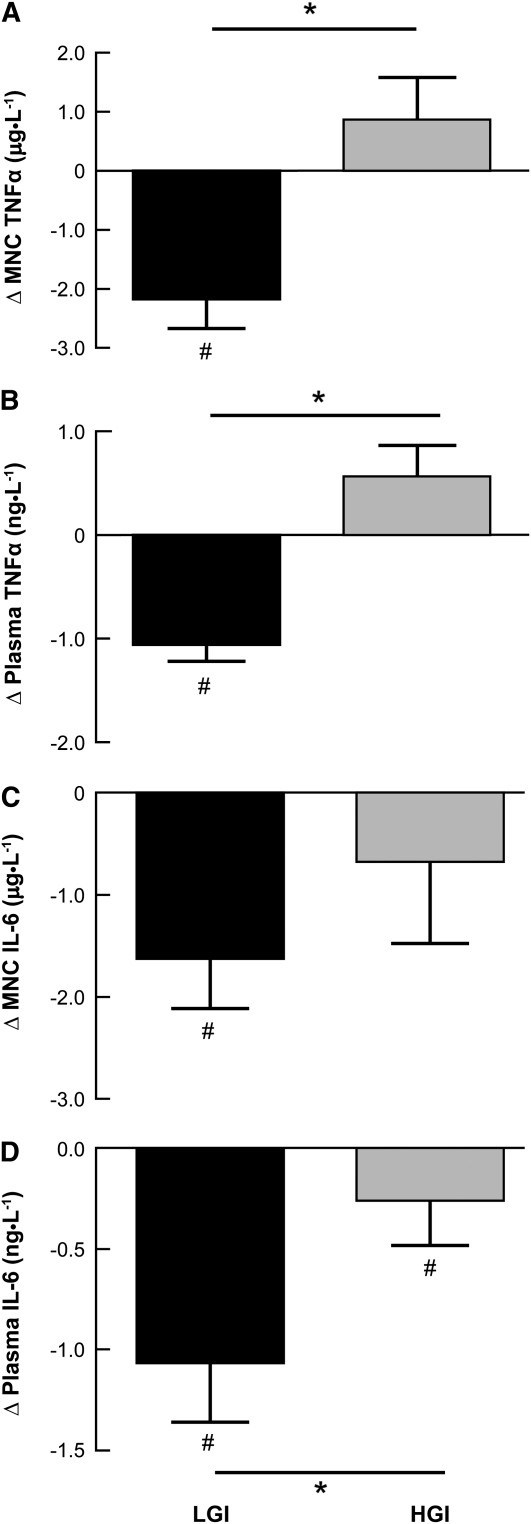
Both lifestyle interventions reduced fasting plasma glucose and insulin (P < 0.05) (Table 2). However, oral glucose tolerance (AUC) was improved only in the LGI group. The plasma insulin concentration decreased in response to oral glucose (P < 0.001) (Table 2). Both MNC (P = 0.002) and plasma-derived TNFα (P = 0.001) were lower in the LGI group, whereas plasma TNFα concentrations (P = 0.08) and secretion from MNC (P = 0.06) tended to be higher in the HGI group. There was also a difference in the change in TNFα responses for the LGI and HGI groups with respect to both MNC (P = 0.004) and plasma (P = 0.004) (Fig. 1A,B). In addition, we observed a significant decrease in IL-6 secretion from MNC (P = 0.02) following the LGI intervention but no change in the HGI group; the groups did not differ (Fig. 1C). However, the plasma IL-6 concentration was lower (P = 0.04) in both groups after the interventions, a change that was more pronounced in the LGI group (P = 0.01) (Fig. 1D). Further, MCP-1 concentrations were reduced by 33% in the LGI group (21.8 ± 6.3 vs. 15.2 ± 3.1) following the 12-wk intervention (P = 0.02); the HGI group did not change (20.6 ± 4.6 vs. 21.8 ± 4.9 ng/L) and the changes tended to differ between the groups (P = 0.07).
FIGURE 1.
Changes in MNC (A,C) and plasma (B,D) TNFα (A,B) and IL-6 (C,D) in older, obese adults who consumed a LGI or HGI diet for 12 wk. Data are means ± SEM for the change in pre- to postintervention values, LGI (n = 13), HGI (n = 15). #Change pre- to postintervention, P < 0.05; *LGI and HGI differ, P < 0.05.
Regression analyses.
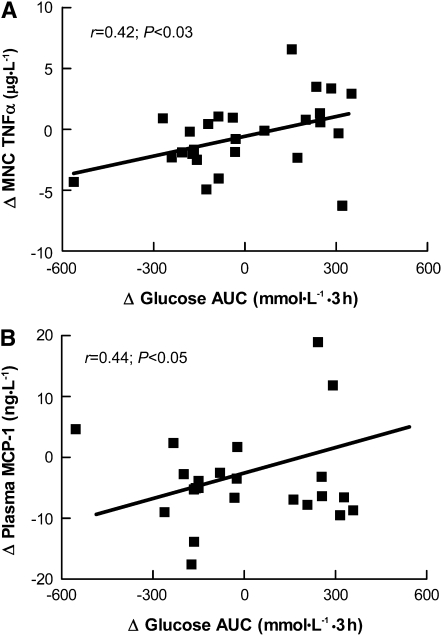
Decreases in TNFα secretion from MNC were correlated with the reduced glycemic response (Fig. 2A) and tended to be associated with the fasting glucose concentration (r = 0.32; P = 0.09). There was also a positive correlation between the change in TNFα secretion from MNC and plasma TNFα (r = 0.49; P = 0.04). There was a significant correlation between changes in MNC-derived IL-6 and total FM (r = 0.51; P = 0.02) as well as truncal fat (r = 0.60; P = 0.003). Further, changes in MCP-1 and IL-6 were positively associated (r = 0.48; P = 0.03) as were the changes in MCP-1 and the glycemic response following glucose ingestion (Fig. 2B).
FIGURE 2.
Correlations between the change in glycemic response and the changes in TNFα secretion from MNC (A) and plasma MCP-1 concentrations (B) in older, obese adults who consumed a LGI or HGI diet for 12 wk. Data are pre- minus postintervention values, n = 28.
Discussion
Our data show for the first time, to our knowledge, that a lifestyle intervention that includes a low-glycemic index diet combined with aerobic exercise attenuates cytokine production in older, insulin-resistant adults, suggesting a reversal of the effects of obesity on inflammation. In line with these findings, we noted that the decrease in TNFα secretion was associated with the improvement in glucose tolerance. Further, the low-glycemic diet and exercise intervention decreased circulating TNFα, IL-6, and MCP-1. Collectively, these findings suggest that attenuation of glycemia via a low-glycemic index diet and exercise regulates proinflammatory cytokine release and control of hyperglycemia. In contrast, a high-glycemic index diet attenuates improvements in postprandial glycemia and inflammation that usually occur after exercise interventions.
The glycemic index provides a measure of the blood glucose response to individual foods in a meal. High-glycemic diets have been linked to disease (26, 27), whereas low-glycemic diets are thought to be protective (13, 14), due primarily to reduced postprandial glucose excursions (28). Previously, we showed that 12 wk of exercise improves insulin sensitivity in older, obese individuals (10, 29) and that the magnitude of improvement was enhanced by a low-glycemic index diet (10). One mechanism that may contribute to this effect is related to the ability of the MNC to use glucose not only for glycolysis, but also for production of NADPH. NADPH is oxidized and results in the generation of reactive oxygen species, activating the NF-κB pathway and resulting in increased TNFα (30–32). Circulating TNFα released from both adipose tissue and MNC bind to TNFα receptors, inducing activation of serine kinases that can stimulate transcription of inflammatory genes, leading to an increase in inflammatory protein production within target tissues, including muscle, adipose, and the liver (33, 34). This establishes a positive feed-forward loop that further amplifies inflammation and insulin resistance. In line with these observations, we found a correlation between plasma glucose and TNFα, suggesting that when glycemia is normalized, the stimulus to produce TNFα is reduced. These findings are supported by both in vivo (35) and in vitro (36) studies that induce hyperglycemia. This is also in agreement with our data from the HGI group, where there was an increase in TNFα production with no improvement in glucose tolerance. Further, these results are consistent with recent work by Kallio et al. (11) that showed differential modulation of proinflammatory cytokine production is dependent on the type of carbohydrate consumed. Despite the fact that both groups in the current study exercised and that physical activity can reduce plasma TNFα (16–18), our data suggest that plasma glucose concentrations are the primary factor driving the changes in TNFα and highlight the importance of using a low-glycemic index diet to modulate glucose excursions throughout the day.
Increased IL-6 has been noted in adipose tissue and plasma of type 2 diabetics and in the circulation of obese persons. The increase in IL-6 may be related to both adipose tissue mass and hyperglycemia (35, 37–39). Following the 12-wk intervention, we detected a decrease in both plasma and MNC-derived IL-6 in the LGI group as well as a reduction in plasma IL-6 in the HGI group. Further, both groups reduced truncal fat and total FM following the 12 wk, which coincided with reduced IL-6 from MNC. In obesity, hypertrophied adipocytes are largely responsible for the secretion of IL-6 (40); thus, the change in body fat is likely to have been partially responsible for the reduced plasma IL-6 concentration. This is a favorable metabolic outcome in that IL-6 has been shown to reduce both mRNA and protein expression of glucose transporter 4 protein, leading to reduced glucose uptake (41, 42). Thus, attenuation of IL-6 should improve glucose uptake, normalizing plasma glucose concentrations. Indeed, for both groups, fasting plasma glucose and IL-6 were reduced; however, there was no correlation between changes in these 2 variables. This is not completely unexpected, because IL-6 is regulated not only by hyperglycemia, but largely by physical activity and weight loss. Recently, Christiansen et al. (43) and Dekker et al. (44) reported decreases in plasma IL-6 following 12-wk lifestyle interventions that produced similar weight loss to that achieved in the current report. Further, results from the Health, Aging and Body Composition Study (16) found that participants who engaged in physical activity ≥ 180 min/wk also reduced plasma IL-6. For our exercise protocol, participants exercised for 300 min/wk at a moderate/high intensity; thus, the change in IL-6 measured in this study may be reflective of a combination of factors: reduced plasma glucose, decreases in body weight and body fat, and increased physical activity. It is important to highlight that the change in plasma IL-6 was greater in the LGI group and that IL-6 secretion from MNC was reduced only in the LGI group. Although not correlative, this may be reflective of the changes in plasma glucose and therefore the inflammatory stimuli on MNC to secrete IL-6. The disparity between the change in MNC and plasma-derived IL-6 for the 2 groups (LGI and HGI) is most likely due to the complex interaction between MNC and adipose tissue, as well as the combined effects of weight loss, physical activity, and altered blood glucose, and the subsequent influences on cytokine production.
In support of these observations, we found that MCP-1 was reduced following the LGI intervention and observed an association between the change in MCP-1 and the change in plasma glucose. MCP-1 is a chemokine, which, analogous to IL-6, is secreted from hypertrophied adipocytes and plays a crucial role in the recruitment of MNC into tissues (45). MNC are the primary secretors of cytokines and infiltration/differentiation of MNC into macrophages in adipose tissue is presumed to have unique inflammatory properties compared with resident macrophages (46). Thus, the decrease in MCP-1 in the LGI group that corresponded to changes in plasma glucose is likely the result of a reduced stimulus to recruit MNC into adipose tissue (i.e. improved glucose tolerance) and in turn a halting of the cycle of cytokine production and activation of MCP-1. This is a favorable adaptation in that in vitro studies show that MCP-1 treatment reduces glucose uptake in both adipocytes and myocytes (47, 48) and thus, along with IL-6 and TNFα, may also contribute to insulin resistance. Classically, MCP-1 exerts paracrine or autocrine effects in adipose tissue rather than having a direct systemic pathogenic role (40). Thus, the decrease in MCP-1 in the LGI group is suggestive of improvement in adipose tissue function.
A major strength of this study was the control of diet and exercise throughout the 12-wk intervention. Participants were fed all meals, snacks, and beverages and each exercise session was supervised by an exercise physiologist to ensure compliance and attention to workload. However, there are some limitations, such as the lack of dietary control groups, i.e. participants consuming either a low-or high-glycemic index diet without exercise. However, other studies that have examined diet alone have produced equivocal results (12, 49). Further, our previous work with an uncontrolled diet suggested that there were synergist effects on reductions in diabetes risk when exercise and a low-glycemic index diet were combined (10). In addition, individuals randomized to the LGI group tended to have higher baseline concentrations of insulin and glucose; however, they were not significantly different compared with participants in the HGI group. Nonetheless, this could contribute to the greater changes observed after the LGI intervention.
From the data provided herein, regulation of cytokine release is multi-factorial but largely dependent on plasma glucose concentrations. This study provides strong evidence that consumption of a low-glycemic index diet and normalization of plasma glucose can halt the vicious cycle of hyperglycemia-insulinemia, which contributes to insulin resistance, adipose tissue dysfunction, and, ultimately, proinflammatory cytokine production. The present study was a highly controlled dietary intervention and confirmed our previous observation that a low-glycemic index diet improved glucose tolerance and provides novel evidence that a low-glycemic index diet can reduce the inflammation that is now classically associated with obesity. The lack of change in both glucose tolerance and cytokine secretion following the HGI confirms the importance of diet and, more specifically, the contribution of a low-glycemic index diet to controlling glucose excursions throughout the day. Although the HGI group did show improvements in plasma insulin, these changes are likely related to the exercise and weight loss and not to diet, because we have previously shown the same effect with a similar exercise intervention without a dietary component (22).
In conclusion, adherence to a low-glycemic index diet in combination with aerobic exercise can reverse the effects of obesity on proinflammatory cytokine production in older, obese adults. Our data suggest that the hyperglycemia that typically accompanies obesity and insulin resistance is a driving force behind elevated cytokine production, which can further accelerate the positive feed-forward loop of inflammatory cytokine production leading to chronic diseases such as type 2 diabetes.
Acknowledgments
We thank Christine Marchetti for her help with the implementation of the study. We also thank our clinical research coordinator, Julianne Filion, for her excellent nursing and organizational assistance. J.P.K. and H.B. designed the research; K.R.K., J.M.H., T.P.J.S., A.J.P.M., M.C., and J.P.K. conducted the research; K.R.K., J.M.H., T.P.J.S., M.R., H.B., and J.P.K. analyzed the data; K.R.K. and J.P.K. wrote the manuscript; and J.P.K. obtained funding for the study and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grant RO1 AG12834 (J.P.K.) and in part by the NIH, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio. K.R.K. was supported by NIH grant T32 DK007319 and J.M.H. was supported by NIH grant T32 HL007887.
Abbreviations used: FFM, fat-free mass; FM, fat mass; HGI, high-glycemic index diet and exercise, LGI, low-glycemic index diet and exercise; MCP-1, monocyte chemoattractant protein 1; MNC, mononuclear cell.
Literature Cited
- 1.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–30 [DOI] [PubMed] [Google Scholar]
- 2.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–81 [PubMed] [Google Scholar]
- 3.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez F, Minium J, Rote NS, Kirwan JP. Altered tumor necrosis factor alpha release from mononuclear cells of obese reproductive-age women during hyperglycemia. Metabolism. 2006;55:271–6 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-alpha release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5336–42 [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-a in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost G, Leeds A, Trew G. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic index diet. Metabolism. 1998;47:1245–51 [DOI] [PubMed] [Google Scholar]
- 8.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22:10–8 [DOI] [PubMed] [Google Scholar]
- 9.Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–53 [DOI] [PubMed] [Google Scholar]
- 10.Kirwan JP, Barkoukis H, Brooks LM, Marchetti CM, Stetzer BP, Gonzalez F. Exercise training and dietary glycemic load may have synergistic effects on insulin resistance in older obese adults. Ann Nutr Metab. 2009;55:326–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallio P, Kolehmainen M, Laaksonen DE, Pulkkinen L, Atalay M, Mykkanen H, Uusitupa M, Poutanen K, Niskanen L. Inflammation markers are modulated by responses to diets differing in postprandial insulin responses in individuals with the metabolic syndrome. Am J Clin Nutr. 2008;87:1497–503 [DOI] [PubMed] [Google Scholar]
- 12.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–11 [DOI] [PubMed] [Google Scholar]
- 13.Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, Miller CK, Bobe G, Colburn NH, et al. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140:60–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botero D, Ebbeling CB, Blumberg JB, Ribaya-Mercado JD, Creager MA, Swain JF, Feldman HA, Ludwig DS. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring). 2009;17:1664–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolever TM, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, Bjorck I, Brighenti F, Brown R, et al. Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr. 2008;87:S247–57 [DOI] [PubMed] [Google Scholar]
- 16.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–104 [DOI] [PubMed] [Google Scholar]
- 17.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–64 [DOI] [PubMed] [Google Scholar]
- 18.Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048–54 [DOI] [PubMed] [Google Scholar]
- 19.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–7 [DOI] [PubMed] [Google Scholar]
- 20.Taylor HL, Jacobs J. DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55 [DOI] [PubMed] [Google Scholar]
- 21.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon TP, Haus JM, Kelly KR, Cook M, Watanabe R, Barkoukis H, Kirwan JP. A low glycemic index diet combined with exercise reduces insulin resistance and suppresses compensatory hyperinsulinemia and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirwan JP, Krishnan RK, Weaver JA, del Aguila LF, Evans WJ. Human aging is associated with altered TNF-a production during hyperglycemia and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2001;281:E1137–43 [DOI] [PubMed] [Google Scholar]
- 25.Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr. 2009;90:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50:14–21 [DOI] [PubMed] [Google Scholar]
- 27.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–56 [DOI] [PubMed] [Google Scholar]
- 28.Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87:S269–74 [DOI] [PubMed] [Google Scholar]
- 29.Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab. 2009;296:E1269–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–3 [DOI] [PubMed] [Google Scholar]
- 31.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622 [DOI] [PubMed] [Google Scholar]
- 32.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–71 [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S53–5 [DOI] [PubMed] [Google Scholar]
- 34.Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S6–11 [DOI] [PubMed] [Google Scholar]
- 35.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72 [DOI] [PubMed] [Google Scholar]
- 36.Morohoshi M, Fujisawa K, Uchimura I, Numano F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996;45:954–9 [DOI] [PubMed] [Google Scholar]
- 37.Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–9 [DOI] [PubMed] [Google Scholar]
- 38.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–51 [DOI] [PubMed] [Google Scholar]
- 39.Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124:495–502 [DOI] [PubMed] [Google Scholar]
- 40.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol. 2010;318:69–78 [DOI] [PubMed] [Google Scholar]
- 41.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84 [DOI] [PubMed] [Google Scholar]
- 42.Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311:372–9 [DOI] [PubMed] [Google Scholar]
- 43.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise-training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects. A 12-week randomized intervention-study. Am J Physiol Endocrinol Metab. 2010;298:E824–31 [DOI] [PubMed] [Google Scholar]
- 44.Dekker MJ, Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R, Robinson LE. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56:332–8 [DOI] [PubMed] [Google Scholar]
- 45.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8 [DOI] [PubMed] [Google Scholar]
- 46.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23 [DOI] [PubMed] [Google Scholar]
- 47.Sell H, Dietze-Schroeder D, Kaiser U, Eckel J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology. 2006;147:2458–67 [DOI] [PubMed] [Google Scholar]
- 48.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010;92:366–74 [DOI] [PubMed] [Google Scholar]