Abstract
T cell immunotherapy is a promising strategy to treat cancer. The aim of this study was to investigate a new approach to deliver selective T cell activation against tumor cells. We have developed a bi-specific fusion protein that recognizes tumor cells through an NK activating receptor NKG2D and stimulates T cells through an anti-CD3 single chain fragment (scFv-NKG2D). In vitro, scFv-NKG2D engages both T cells and tumor cells, resulting in T cells producing IFN-γ and cytotoxicity against NKG2D ligand-positive tumor cells. In vivo, expression of scFv-NKG2D by tumor cells (NKG2D ligand-positive) significantly reduced tumor burden and, in some case, led to tumor-free survival. In vivo administration of scFv-NKG2D also significantly promoted survival in a murine lymphoma model. In addition, tumor-free mice were resistant to rechallenge with cognate tumor cells, suggesting the generation of a host specific immunological memory response. Host adaptive immunity (including γδ T cells) was required for scFv-NKG2D-mediated therapeutic efficacy. ScFv-NKG2D also inhibited the growth of NKG2D-ligand negative B16F10 tumors, reduced the percentage of myeloid-derived suppressor cells as well as regulatory T cells and increased T cell infiltration, suggesting that scFv-NKG2D target these immune suppressive cells. In summary, these results indicate that scFv-NKG2D represents a promising multi-tumor targeting reagent to induce anti-tumor immunity.
Keywords: Cancer immunotherapy, CD8 T cells, NKG2D, chimeric protein, myeloid suppressor, regulatory T cells
Introduction
T cells can play an important role in controlling tumor growth and prolonging survival in cancer patients (1). However, it is difficult to mount and sustain effective tumor-specific T-cell responses due to weak immunogenicity of tumor antigens, clonal deletion of high affinity T cells, low frequency of antigen-specific T cells, down-regulation of MHC molecules, existence of immune suppressor cells and tumor-derived immunosuppressive molecules (2–4). Although adoptive transfer of T cells can partially restore anti-tumor immunity, in vitro generation of large numbers of tumor-specific T cells for treatment still remains a difficult task.
Alternatively, T-cell-mediated anti-tumor immunity can be achieved using bi-specific T cell-engagers, which bind to a surface target antigen on cancer cells and to CD3 on T cells (5). This strategy allows T cells to recognize tumor cells and become activated irrespective of their T-cell receptor specificity and their activation by antigen presenting cells. Recent results from a clinical trial with a CD19/CD3-bispecific antibody showed promise as a cancer treatment (5, 6).
Natural killer (NK) cells attack tumor and virally infected cells in the absence of MHC restriction, utilizing a combination of multiple activating receptors. One of these activating receptors is NKG2D. The ligands for NKG2D receptor include Rae-1, Mult-1 and H60 in mouse; MICA/B and RAET1, also called UL-16 binding proteins (ULBPs), in human, which are preferentially expressed on tumor cells but not on most normal tissues(7–9). NKG2D ligands represent ideal targets for immunotherapeutic approaches because they are selectively over-expressed on many types of tumor cells as well as on tumor-associated suppressor cells (10, 11). Therefore, the NKG2D receptor-NKG2D ligand system provides a relatively specific system for immune cells to recognize tumor cells and the tumor microenvironment. In this study, we describe a novel anti-tumor strategy based on a bi-specific T-cell engager (scFv-NKG2D), in which a single chain variable fragment (scFv) of anti-CD3ε was fused to the extracellular domain of NKG2D receptor. Our hypothesis was that scFv-NKG2D would bind NKG2D ligand-positive tumor cells via NKG2D and activate T cells via anti-CD3ε scFv portion, leading to elimination of the tumor and induction of host anti-tumor immunity. Because many types of tumor cells (leukemia, lymphoma, colon, breast, prostate, and ovarian cancers, among others) and immune suppressor cells (myeloid-derived suppressor cells and regulatory T cells) express NKG2D ligands (9–11), scFv-NKG2D targeting provides a means to engage T cells against multiple types of tumor cells as well as local tumor immune suppressor cells.
MATERIALS AND METHODS
Mice and cell lines
C57BL/6 (B6, wildtype, wt) were purchased from NCI (Frederick, MD). TCRα−/− mice (B6 background) were obtained from Dr. William R. Green (Dartmouth medical School). Rag1−/− mice (B6 background) were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred in the animal facility of Dartmouth College. Animals used in experiments were between 7 and 12 weeks of age. All experiments were conducted according to protocols approved by Dartmouth College’s Institutional Animal Care and Use Committee. Anti-mouse CD3ε hybridoma 145.2C11 was obtained from ATCC (Manassas, VA). H-2Kb-restricted B3Z T cell hybridoma cells which recognize the OVA epitope were obtained from Dr. Nilabh Shastri (University 257–264 of California at Berkley). Upon activation, B3Z cells express the LacZ gene (12). Murine colon cancer MC-38 cells (H-2b) were obtained from Dr. Richard J. Barth (Dartmouth Medical School). Mouse T cell line lymphoma RMA and RMA/RG, ovarian cancer cells ID8 and melanoma B16F10 have been described previously (13–15). Mastocytoma cell line P815/Rae1 was generated by retroviral transduction of P815 cells (H-2d) with a mouse NKG2D ligand Rae1. RMA and B16F10 cells are NKG2D ligand-negative, whereas RMA/RG, P815/Rae1, ID8 and B3Z cells are NKG2D ligand-positive. Packaging cells PT67 (ATCC) and ovarian cancer ID8 cells were grown in Dulbecco's modified Eagle medium (DMEM) with a high glucose concentration (4.5 g/liter) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, Utah), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM pyruvate, 10 mM Hepes, 0.1 mM non-essential amino acids and 50 μM 2-mercaptoethanol. All other cell lines were cultured in RPMI plus the same supplements as in DMEM.
Construction of scFv-NKG2D
Variable regions of both heavy (VH, aa 1-136) and light chains (VL, without signal peptide, aa 21–128) were PCR amplified using cDNA derived from 145.2C11 hybridoma. Extracellular domain of murine NKG2D was amplified using the full length NKG2D cDNA as template (16). To make anti-CD3ε scFv, VH and VL were joined with a 15-amino acid glycine (G)-serine (S) linker (G4S)3 (three repeats of GGGGS). All PCR reactions were performed using a high-fidelity DNA polymerase Phusion™ (New England Biolabs, Ipswich, MA). All oligos were synthesized by either Integrated DNA Technologies (Coralville, IA) or Sigma-Genosys (Woodsland, TX). ScFv-NKG2D was created by joining anti-CD3ε scFv to the extracellular domain of mouse NKG2D (aa 90-232) with a second (G4S)3 linker. A histidine tag (6xHis) was added at the C-terminus to facilitate protein purification. The fusion gene was then cloned into a retroviral vector pFB-neo (Stratagene, Palo Alto, CA). A negative control fusion gene scFv-HuNKG2D was constructed by joining the scFv with the extracellular domain of human NKG2D gene.
Production of scFv-NKG2D protein
ScFv-NKG2D proteins were expressed in retroviral vector-stably transduced B16F10 cells according to our previous protocols(16, 17). Briefly, B16F10 cells were retrovirally transduced with vectors that were generated from scFv-NKG2D-stably transfected packaging cell PT67 for 5 rounds. Stable B16F10 cell lines with amplified expression of scFv-NKG2D (either human or mouse version) were selected with G418 (1.5 mg/ml) for 14 days. The resulting stable B16F10 lines (B16F10/scFv-mNKG2D and B16F10/scFv-HuNKG2D) were then cultured in serum-free media (293 SFM II, Invitrogen, Carlsbad, CA). Supernatants were collected every 48 h and were subjected to affinity chromatography using HisTrap™ columns (GE Healthcare Bio-Sciences, Piscataway, NJ) according to the manufacturer’s instructions. Eluted fractions were then concentrated and desalted using Amicon Ultra columns (30K MWCO, Millipore, Billerica, MA). Purified scFv-NKG2D proteins were resuspended in PBS, filtered (0.22 μm) and stored in -20°C. The integrity of scFv-NKG2D protein was determined by SDS-PAGE, followed by staining with SYPRO® orange (Invitrogen) and visualized using a Typhoon 9400 imager (GE Healthcare). Concentration of scFV-NKG2D was quantitated with ImageJ software (US National Institutes of Health; http://rsb.info.nih.gov/nih-image/Default.html).
Flow cytometry
To determine whether scFv-NKG2D binds to CD3ε, RMA cells were stained with scFv-NKG2D (0.01-1μg/ml), followed by staining with PE-labeled anti-mouse NKG2D mAb (CX5, eBioscience, San Diego, CA) or isotype control mAb. In a blocking experiment, RMA cells were pre-incubated with anti-CD3ε (145.2C11, eBioscience, 0.01–1μg/ml) at room temperature for 15 mins prior to staining with scFv-NKG2D. Rae1 expression was determined by flow cytometry using APC labeled pan anti-Rae1 mAb (Clone 186107, R&D systems, Minneapolis, MN) Infiltration of CD4+ and CD8+ T cells, T cell activation (CD69 expression), myeloid-derived suppressor cells (CD11b+F4/80+Gr1+) and regulatory T cells (CD4+Foxp3+) in tumors were determined by flow cytometry after digestion of excised established tumors using cocktails of DNAse and collagenase according to our previous protocol (18). All samples were preincubated with FcR block antibody (anti-mouse CD16/CD32) to reduce nonspecific staining. Cell fluorescence was monitored using an Accuri cytometer (Ann Arbor, MI). Flow cytometry analysis was performed using either Accuri or FlowJo software (Ashland, OR).
Cytokine production by T cells
To determine whether scFv-NKG2D can engage both T cells and tumor cells and lead to T cell activation, spleen cells were stimulated with ConA and IL-2 for 4 days before co-culture with tumor cells with or without scFv-NKG2D (50 ng/ml) for 24 h. Amounts of IFN-γ in supernatants were analyzed with ELISA. For tumor cells grown in suspension, co-culture with pre-activated murine primary T cells (105) was performed in round-bottom 96-well plates at a ratio of 1:1, whereas adherent tumor cells (2.5×104) were co-cultured with T cells in flat-bottom plates. Tumor cells were irradiated (120 Gys) before use. Cell-free supernatants were collected after 24 hr. Twenty-four-hour supernatants were assayed for IFN-γ by ELISA using Duoset ELISA kits (R&D systems).
Cytotoxicity assay
Lysis of target cells was determined by a 5-hr 51Cr release assay as previously described (16, 17).
BetaRed staining
The LacZ activity in B3Z cells was determined by using a BetaRed™ β-Galactosidase assay kit (EMD bioscience, San Diego, CA,) according to the manufacturer’s instruction.
Tumor inoculation
For the determination of effects of local expression of scFv-NKG2D on MC-38 or B16F10 tumor growth, either WT or scFv-NKG2D-transduced tumor cells (5×105) were injected s.c. into the shaved right flank of recipient mice. Tumors were then measured every two days using a caliper, and tumor areas were calculated. Mice were sacrificed when tumor burden became excessive. Animals were regarded as tumor free when no tumor was found four weeks after inoculation. For the rechallenge experiments, mice were inoculated with 105 WT MC-38 cells on the shaved left flank. In the systemic lymphoma model, B6 mice were injected with 105 of RMA/RG cells via tail veins in 400μl of HBSS. For treatment with recombinant scFv-NKG2D proteins (either mouse or human NKG2D fusion protein), mice were administered i.v. with 5 μg scFv-NKG2D on days 5, 7 and 9 post-tumor inoculations. Mice were monitored closely and sacrificed when moribund signs were observed.
Statistical analysis
Differences between groups were analyzed using the student’s t test. Values p<0.05 were considered significant. Kaplan-Meier survival curves were plotted and analyzed using Prism software (GraphPad Software, San Diego, CA).
RESULTS
Construction and expression of scFv-NKG2D
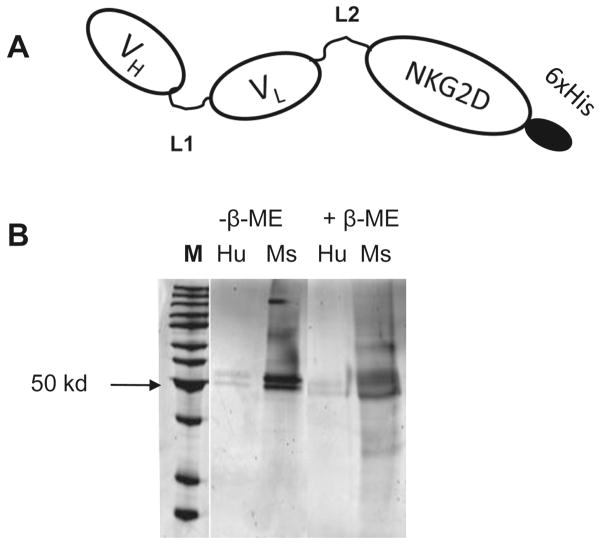
ScFv-NKG2D was made by joining anti-CD3ε scFv to the extracellular domain of NKG2D receptor. The structure of scFv-NKG2D is shown in Fig. 1. Two 15-aa (G4S)3 linkers were inserted between VH and VL as well as between scFv and NKG2D to allow flexible interactions among scFv-NKG2D, CD3ε and NKG2D ligands. Human NKG2D was also used to make a control fusion protein scFv-huNKG2D since human NKG2D receptor has minimal cross-reactivity with mouse NKG2D ligands. In scFv, the antibody Fc fragment is removed. Therefore, binding of these fusion proteins to FcR-positive cells (such as macrophages, B cells, neutrophils, dendritic cells and endothelial cells via the Fc region) is eliminated, resulting in less non-tumor associated T cell activation. As shown in Fig.1B, purified scFv-NKG2D proteins were monomers at sizes around 50 kD under both native and reducing conditions, which were in line with the expected sizes of 44 kD.
Fig 1. Structure of scFv-NKG2D.
(A) Anti-CD3ε VH and VL are linked with a G4S linker (L1) and fused to the extracellular domain (Ex) of NKG2D receptor with the second G4S linker (L2) in between. For the convenience of protein purification, a histidine tag (6 repeats of histidine) was added at the C-termini. (B) scFv-NKG2D proteins express as monomers. Transiently transfected 293T cells that were cultured in serum-free media (Invitrogen) were used to express scFv–NKG2D fusion proteins. Both human (Hu, −ve ctrl) and mouse extracellular NKG2D (Ms) portions were fused to anti-mouse CD3ε scFv. Recombinant proteins were purified through nickel columns. The sizes and purity of protein samples were checked by SDS-PAGE either in native (without β-ME) or reducing conditions (with β-ME). The expressed scFv-NKG2Ds showed sizes around 50 kD, which are similar to expected 44 kD.
ScFv-NKG2D fusion protein binds to both CD3ε and NKG2D ligands
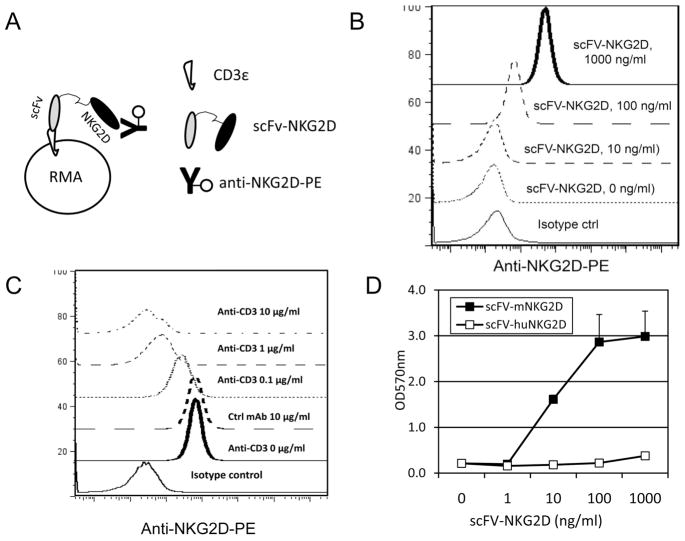
To determine whether scFv-NKG2D fusion protein preserves the binding affinity and specificity of the parental anti-CD3ε 145-2C11 antibody, CD3+NKG2D− T cell lymphoma RMA cells were stained with scFv-NKG2D (0.01–1 μg/ml), followed by staining with anti-NKG2D-PE (Fig. 2A). As shown in Fig. 2B, scFv-NKG2D binds to CD3ε in a dose-dependent manner. In addition, pre-incubation of RMA cells with 145-2C11 mAb (0.1–10 μg/ml) could block the binding of the scFv-NKG2D fusion protein to RMA cells in a dose-dependent manner, indicating the specific recognition of CD3ε molecule by scFv-NKG2D (Fig. 2C).
Fig 2. scFv-NKG2D can engage both T cells and tumor cells.
(A) Schematic demonstration of binding assay of scFv-NKG2D to T cells. A T cell lymphoma cell line RMA (105, CD3+ NKG2D−), which does not express ligands for NKG2D, was stained with scFV-NKG2D (0.01–1 μg/ml) followed by staining with anti-NKG2D-PE. Samples were analyzed with an Accuri C6 flow cytometer. (B) Surface levels of NKG2D on RMA cells after staining with scFv-NKG2D are shown. (C) Anti-CD3 mAb can competitively block the binding of scFv-NKG2D to CD3 molecule in a dose-dependent manner. To confirm that scFv-NKG2D binds to CD3 molecule, RMA cells were pre-incubated with anti-CD3 mAb (0.1–10 μg/ml) prior to staining with scFv-NKG2D. (D) ScFv-NKG2D can activate T cells after cross-linking both CD3 and NKG2D ligands. CD3+ and NKG2D ligand Mult1+ T cell hybridoma B3Z cells (106/ml) were cultured in the presence of scFV-NKG2D (0.01–1 μg/ml) for 24 h. T cell activation (β-galactosidase activity) was analyzed using a BetaRed analysis as described in Methods and Materials. Human NKG2D-scFv fusion protein (scFv-HuNKG2D) was used as a negative control since human NKG2D receptor does not cross-react with mouse NKG2D ligands.
To examine whether scFv-NKG2D was capable of binding simultaneously to both T cells via scFv and tumor cells via NKG2D (Fig. 2D), a mouse T hybridoma B3Z was used. B3Z T cells express both CD3ε and an NKG2D ligand Mult1 (data not shown). We hypothesized that if scFv-NKG2D binds to CD3ε and Mult1 on B3Z cells simultaneously, cross-linking of CD3ε molecule will lead to activation of T cells. Since B3Z cells contain the IL-2 promoter-controlled LacZ gene as a reporter, after activation, B3Z cells will express LacZ (β-galactosidase). The ability of scFv-NKG2D to stimulate Mult1+ B3Z cells was evaluated by measurement of the OD570nm or OD550nm values after addition of a beta-red substrate. As expected, incubation of B3Z cells with scFv-NKG2D, but not with control molecule scFv-HuNKG2D (human), led to cell activation in a dose-dependent manner, indicating that the scFv-NKG2D can engage both T and tumor cells via NKG2D ligands. Based on the data (Fig. 2D), we estimate the ED50 of scFv-NKG2D for activating T cells was about 10 ng/ml.
ScFv-NKG2D activates primary T cells by producing IFN-γ and exerting cytotoxicity
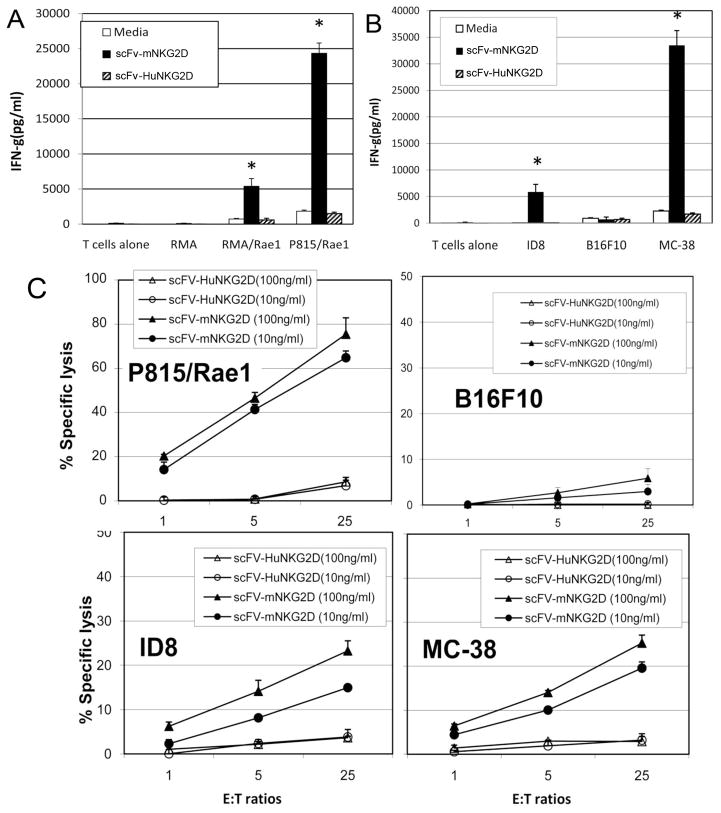
To investigate whether scFv-NKG2D stimulates primary T cells, we examined IFN-γ production and cytotoxicity of T cells against tumor cells in the presence of scFv-NKG2D. ConA and IL-2 (25U/ml) pre-activated T cells were used as effector cells. As shown in Fig. 3A&B, in the presence of scFv-NKG2D (50 ng/ml), T cells produced significant amounts of IFN-γ after coculture with NKG2D ligand-positive cells (RMA/Rae1, P815/Rae1 and ID8) but not with ligand-negative RMA and B16F10 cells indicating that scFv-NKG2D allows T cells to functionally recognize NKG2D ligand-bearing tumor cells. In contrast, control protein scFv-HuNKG2D does not significantly stimulate T cells to produce IFN-γ in the presence of NKG2D ligand positive tumor cells. These data indicate that anti-CD3ε scFv itself has minimal ability to activate T cells under these conditions. T-cell cytotoxicity in the presence of scFv-NKG2D against various tumor cells was also determined. As shown in Fig. 3C, T cells were able to lyse NKG2D ligand-positive target cells (P815/Rae1, ID8 and MC-38) but not the ligand-negative cell line B16F10 in vitro in the presence of scFv-NKG2D. Similar to IFN-γ production, no significant killing was observed when control protein scFv-huNKG2D was used. In addition, we also demonstrated that scFv-NKG2D did not affect NK cell-mediated cytotoxicity against tumor cells despite the facts that incubation of high concentration (1μg/ml) of scFv-NKG2D with tumor cells reduced surface NKG2D ligand expression (Supplemental Fig. 1) .
Fig 3. T cells respond to NKG2D ligand positive cells by producing IFN-γ and exerting cytotoxicity in the presence of scFv-NKG2D.
Bulk spleen cells were stimulated with ConA (1ug/ml) and IL-2 (25 U/ml) before co-culture with irradiated tumor cells for 24 h. Both suspension (A) (105) and adherent tumor cells (B) (2.5×104) were co-cultured with ConA-stimulated spleen cells (105) in 96 well plates. Fusion protein scFv-huNKG2D (in which mouse NKG2D was replaced with the extracellular domain of human NKG2D) is used as negative controls. The final concentrations of scFv-NKG2D are 50 ng/ml. IFN-γ amounts in the supernatants were analyzed with ELISA. Results are shown in mean + SD. These data show that the expression of NKG2D ligands on tumor cells is required for induction of IFN-γ production. *: P<0.01 (scFv-mNKG2D vs ctrl). (C) ConA-stimulated T cells were co-cultured with NKG2D ligand-positive P815/Rae1 (mastocytoma), ID8 (ovarian cancer) and MC-38 (colon cancer) cells and –negative B16F10 (melanoma) at E:T ratios of 1:1 to 25:1 in the presence of scFv-mNKG2D (solid symbols) or control scFv-HuNKG2D (open symbols) of for 5h. The specific lysis was determined by Cr51-assay. The scFv-NKG2D proteins were added at concentrations of 10 and 100ng/ml. Results are shown in mean + SD of triplicates.
ScFv-NKG2D suppresses growth of NKG2D ligand-positive tumor cells in vivo and induces a host memory response
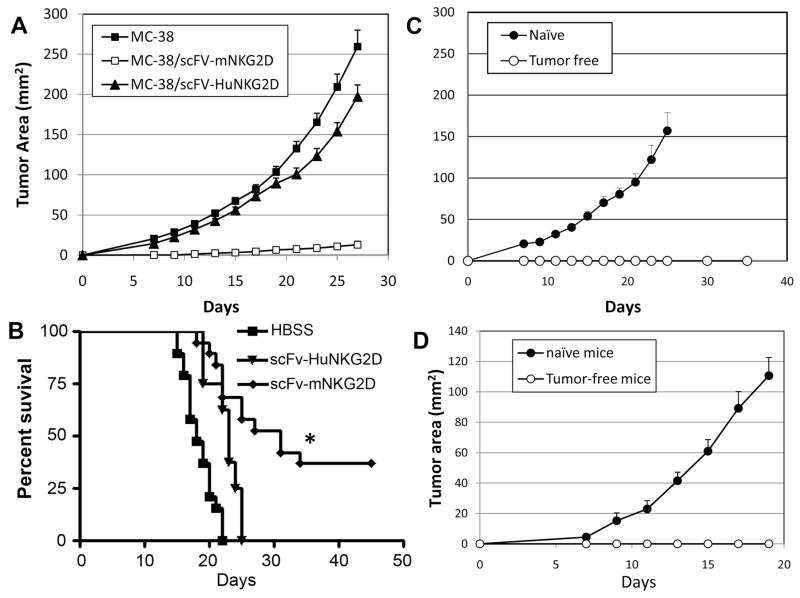
Having shown that T cells could react against NKG2D ligand-positive tumor cells in vitro after engagement with scFv-NKG2D, we determined the therapeutic potential of scFv-NKG2D in vivo. First, colon cancer MC-38 cells that stably secreted scFv-mNKG2D (supplemental Fig. 2) were tested for growth in vivo after subcutaneous injection. This design represents a localized scenario for scFv-NKG2D treatment that could be produced by exogenous gene transfer. MC-38 cells expressing scFv-human NKG2D fusion protein (scFv-HuNKG2D) were used as negative control. There was no significant difference between wildtype and scFv-NKG2D-expressing MC-38 cells to grow in vitro (data not shown). As shown in Fig. 4A, scFv-mNKG2D expression by MC-38 tumor cells significantly reduced or prevented tumor growth compared to wildtype and scFv-HuNKG2D-expressing MC-38 tumor cells. Fifty nine percent of B6 mice (13 out of 22) receiving MC-38/scFv-mNKG2D tumor cells were tumor-free after 30 days. In a second and more stringent model, lymphoma-bearing mice that had been given NKG2D ligand-positive T cell lymphoma RMA/RG cells i.v .were treated with 3 doses of either recombinant scFv-mNKG2D (5 μg per dose, i.v.) or scFv-HuNKG2D (specificity control). As shown in Fig. 4B, systemic administration of scFv-mNKG2D significantly improved survival of tumor-bearing mice. The median survival increased from 18 days (HBSS) to 31 days (P<0.01); whereas treatment with control molecule scFv-HuNKG2D only lead to median survival of 23 days. In addition, 39% (7/18) of the scFv-mNKG2D treated lymphoma-bearing mice were tumor-free after 45 days. As for toxicity, the animals treated with scFv-mNKG2D proteins did not show any overt evidence of inflammatory damage (i.e., ruffled hair, hunchback, or diarrhea) suggesting there was no overt toxicity (data not shown).
Fig 4. scFv-NKG2D treatment reduces the growth NKG2D ligand-positive tumors in vivo and induces memory responses.
(A) Mouse colon cancer MC-38 cells were genetically modified with a retroviral vector containing either scFv-mNKG2D (□, n=22) or control molecule scFv-HuNKG2D (▲, n=12) and then injected s.c. (5×105) into right flanks of B6 mice on day 0. Only 9 of 22 mice developed tumors, whereas in HBSS-treated groups (■, n=19) in which wt MC-38 cells were given, all 19 mice developed tumors. The tumor areas are pooled data from four independent experiments. (B) Intravenous administration of scFv-NKG2D promotes survival in a systemic lymphoma model. Treatment of RMA/RG (105, i.v., day 0) tumor-bearing mice with 3 doses of scFv-mNKG2D ( , 5μg, i.v. n=19) on days 5, 7 and 9 significantly enhanced survival compared to HBSS (■, n=19) or control molecule scFv-HuNKG2D (n=8). Data is presented in Kaplan-Meier survival curves. *: p<0.002. (C) Tumor free mice (○) in the MC-38/scFV-NKG2D group (shown in panel A) and age-matched naïve mice (■) were re-challenged with wild type MC-38 cells (105) s.c. into the left flanks. (D) Tumor free mice (○) in the scFv-NKG2D-treated RMA lymphoma model (shown in panel B) and age-matched naïve mice (●) were re-challenged with wild type RMA cells (104) s.c. into the left flanks. The tumor areas are represented as Mean + SEM. The error bars represent SEM.
To determine whether treatment with scFv-NKG2D induced a host memory response against wild-type tumor cells, scFv-NKG2D-treated mice that had remained tumor-free after 45 days were challenged with either wildtype MC-38 (105) or RMA (NKG2D ligand-negative, 104) cells on their left flank. These tumor-free mice were resistant to a subsequent challenge of the same type of tumor cells; whereas all control naïve mice had large tumors after 3 weeks (Fig. 4C&D). This observation indicated that scFv-NKG2D treatment induced hosts to generate specific immunological memory against tumor antigens.
Host adaptive immunity is involved in scFv-NKG2D-mediated anti-tumor efficacy
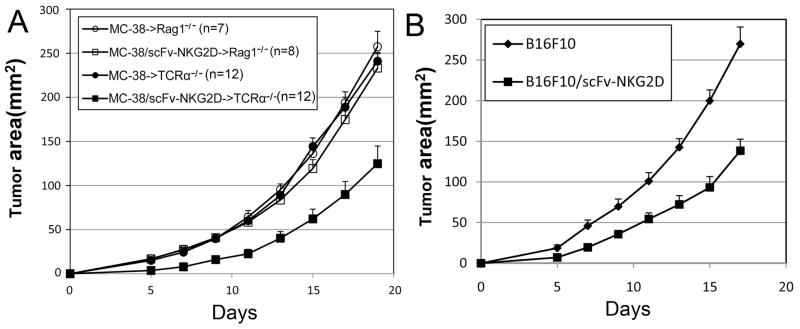
Since scFv-NKG2D protein uses T cells to target tumor cells, it is expected that the absence of T cells would render scFv-NKG2D treatment ineffective in reducing tumor growth. To test this hypothesis, either wildtype MC-38 or scFv-NKG2D-expressing MC-38 cells (MC-38/scFv-NKG2D) were inoculated (at a dose of 5×105 cells) subcutaneously into B6.RAG1−/− mice, which lack T and B lymphocytes. As expected, both wildtype and MC-38/scFv-NKG2D cells grew progressively in B6.RAG1−/−mice (Fig.5A). Thus, lymphocytes play an obligate role in scFv-NKG2D-mediated anti-tumor growth in vivo.
Fig 5. Host adaptive immune cells are involved in scFv-NKG2D-mediated tumor rejection and local expression of scFv-NKG2D also reduces NKG2D ligand-negative tumor growth.
(A) Either wt (circle) or scFv-NKG2D-expressing (square) MC-38 cells were injected s.c. (5×105) into right flanks of Rag1−/− (unfilled) or TCRα−/− (filled) mice on day 0. The error bars represent SEM of three pooled independent experiments. Local expression of scFv-NKG2D significantly (P<0.05 at days 5-19) suppressed the MC-38 tumor growth in TCRα−/− but not Rag1−/− mice. (B) Either WT (◆) (n=10) or scFv-NKG2D-expressing (■) (n=17) B16F10 cells were injected s.c. (5×105) into right flanks of B6 mice on day 0. The tumor areas are represented as Mean + SEM. The error bars represent SEM. Local expression of scFv-NKG2D significantly (P<0.01, at days 5–17) suppressed B16F10 tumor growth.
γδ T cells constitute a major T-cell subset in skin and intestine and can be harnessed for cancer immunotherapy (19). Therefore, scFv-NKG2D may mobilize and activate γδ T cells against subcutaneous tumors, resulting in tumor reduction. The role of γδ T cells was investigated by inoculating either WT MC-38 or scFv-mNKG2D-expressing MC-38/scFv-NKG2D cells into TCRα-deficient mice, in which αβ T cells are deficient and γδ T cells remain intact (20). Compared to WT tumors, MC-38 cells that express scFv-NKG2D show markedly slower growth (Fig. 5A). However, no tumor-free mice were obtained. This result suggests that γδ T cells play a role in scFv-NKG2D-mediated therapeutic efficacy against subcutaneous tumors, but αβ T cells are also required for efficient anti-tumor effects.
Tumor-associated host cells play roles in scFv-NKG2D-mediated anti-tumor efficacy
Several studies have shown that some immune cells, such as regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs) express NKG2D ligands (10). Nausch et al. demonstrated that Gr-1+CD11b+F4/80+ MDSCs isolated from tumor-bearing mice but not myeloid cells from naïve mice, expressed a NKG2D ligand Rae1. Interestingly, these tumor-associated MDSCs can stimulate NK cells to produce IFN-γ in a manner that is partially dependent on the interaction between NKG2D and its ligands (10). In fact, NKG2D can be used to target Tregs at the tumor site in ovarian cancer (11). To determine whether tumor-associated host cells are involved in scFv-NKG2D-mediated therapeutic efficacy, we tested the effects of local expression of scFv-NKG2D on the growth of B16F10 tumors, which do not express NKG2D ligands in vitro or in vivo (Supplemental Fig. 3). If B16F10 tumor-associated host cells (such as MDSCs and Tregs) are targeted by scFv-NKG2D, it would be expected that B16F10 tumor growth may be reduced by the local expression of scFv-NKG2D. As shown in Fig. 5B, local expression of scFv-NKG2D significantly reduced B16F10 tumor growth, suggesting that scFv-NKG2D may target ligand+ tumor-associated host cells. There was no difference between B16F10 and B16F10/scFv-NKG2D in terms of in vitro growth (Supplemental Fig. 4).
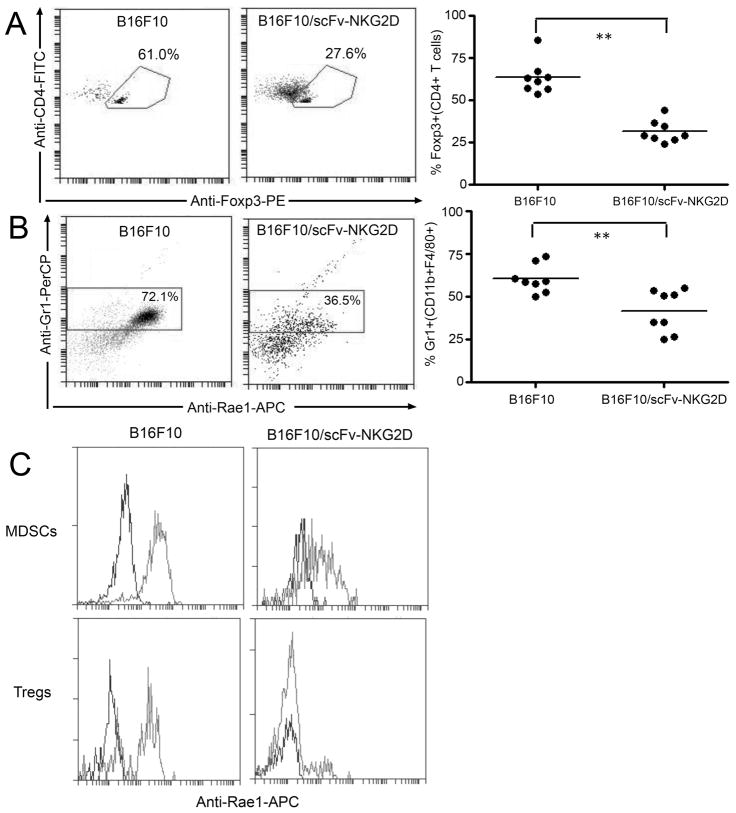
To further understand whether Tregs and MDSCs can be targeted, we first checked an NKG2D ligand Rae1 expression on those cells in both MC-38 and B16F10 models. As shown in supplemental Fig. 5C, Rae1 was expressed on tumor (both MC-38 and B16F10)-derived Tregs (CD4+Foxp3+) and MDSCs (CD11b+F4/80+Gr1+). In contrast, CD4+Foxp3+ and CD11b+F4/80+Gr1+ cells from naïve B6 spleens did not express Rae1, suggesting that tumor microenvironment plays a role in NKG2D ligand expression. Next, it was tested whether local expression of scFv-NKG2D could alter the ratios of effector CD4+/Treg and Gr1-/Gr1+ (CD11b+F4/80+) using the B16F10 melanoma model. If tumor-derived Tregs and MDSCs were targeted, one would expect lower percentages of these cells in tumors. As demonstrated in Fig. 6, local expression of scFv-NKG2D by B16F10 cells significantly (P<0.01) reduced the percentage of both Tregs (Fig. 6A) and MDSCs (Fig. 6B) at the tumor site. In addition, Rae-1 expression by those cells was lower in the presence of scFv-NKG2D (Fig. 6C), consistent with the targeting of tumor-derived Tregs and MDSCs by scFV-NKG2D. Overall, these data support the idea that NKG2D can be used to target both tumor cells and their supportive immunosuppressive cells.
Fig 6. Local expression of scFv-NKG2D in tumors reduces the percentages of Tregs and MDSCs.
The percentages of Tregs (CD4+Foxp3+, A) and MDSCs (CD11b+F4/80+Gr1+, B) in either B16F10 or B16F10/scFv-NKG2D tumors were determined by flow cytometry on excised tumor tissues 15 days post-inoculation. Both representative expression profiles (left panel) and individual values (right panel) are shown. Results are pooled data from two independent experiments. *: p<0.01.(C) Rae1 expression by MDSCs and Tregs is reduced in the presence of scFv-NKG2D. Isotype control: black line; Anti-rae1-APC: grey line.
Local expression of scFv-NKG2D leads to increased recruitment of T cells
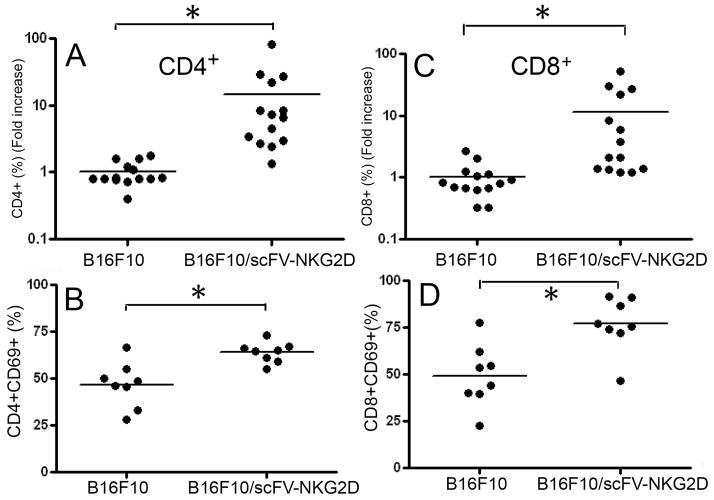
Since local expression of scFv-NKG2D resulted in reduced tumor growth, we hypothesized that more T cells may be recruited to tumor sites and become more activated. To test the hypothesis, WT B16F10 or B16F10/scFv-NKG2D cells (5×105 cells) were injected into the right flanks of B6 mice. The infiltration of T cells (both CD4+ and CD8+) as well as T cell activation, as demonstrated by expression of an activation marker CD69+, were determined by flow cytometry 19 days post-tumor inoculation. As shown in Fig. 7A&C, the percentage of both CD4+ and CD8+ T cells was significantly increased in the presence of scFv-NKG2D. In addition, T cells (especially CD8+ T cells) had a significantly increased expression of CD69, which was consistent with an activated status (Fig. 7B&D).
Fig 7. scFv-NKG2D treatment significantly increases T cell infiltration in a B16F10 melanoma model.
Infiltration of CD4+ (A) and CD8+ (C) T cells was analyzed by flow cytometry on excised B16F10 tumor tissues 19 days post-inoculation. Results shown are pooled data from two independent experiments and are demonstrated as fold-increase, in which the average % in the WT B16F10-injected group was designated as 1. *: p<0.01. CD69 expression on CD4+ (B) and CD8+ (D) T cells was also analyzed.
DISCUSSION
Immunotherapy has emerged as a promising method to treat cancers due to the ability to selectively eliminate tumors. Both vaccination and adoptive immune cell transfer have been utilized against cancers (3). Dendritic cell (DC)-based vaccines have been tested in both animal models and clinical trials. However, DCs need to be delicately conditioned with toll-like receptor (TLR agonists) and cytokines to ensure delivery of both primary as well as co-stimulatory signals for T cell stimulation (21). Otherwise, T cells may be induced into a tolerant state. Although DC-based immunotherapy has proven clinically safe and efficient to induce tumor-specific immune responses, only a limited number of objective clinical responses have been reported in cancer patients (22). In the second strategy, adoptive transfer of T cells or allogeneic bone marrow cells can be performed (23). However, the traditional approaches for obtaining large numbers of tumor-specific T cells are difficult. Allogeneic bone marrow transplantation can cause severe acute graft-versus-host disease (GVHD) and response rate is less than 15% (23). Compared to these two strategies, scFV-NKG2D no longer relies on generation of specific T cells or a regular antigen presentation by dendritic cells. ScFv-NKG2D stimulates host T cells in a MHC-independent manner. Therefore scFv-NKG2D is unlikely to be affected by well documented tumor-escape strategies, such as “down-regulation” of MHC expression. Unlike most of other bi-specific molecules in which a specific tumor-targeting scFv has to be made for targeting certain types of tumors, scFv-NKG2D is a pan-tumor targeting reagent since NKG2D ligands are expressed on many types of tumor cells (colon, ovarian, breast, prostate, melanoma, leukemia, and lymphoma, among others). In addition, NKG2D ligands have restricted expression in normal tissues (9), but they are also expressed on immunosuppressive cells within the tumor microenvironment.
In this study, scFv-NKG2D treatment reduced or abrogated the growth of NKG2D ligand-positive tumors, suggesting that scFv-NKG2D in vivo can lead to effective T cell activation and elicit host T cell responses to tumor cells. Possible mechanisms for this may be the small size of scFv-NKG2D (~50 KD) and existence of flexible linkers which allow efficient formation of immunological synapses and reduce a steric barrier imposed by large, abundant glycoproteins like CD43 and CD45 (24). In adult humans, about one-half of the T cells are memory cells based on the expression of a memory marker CD45RO and their ability to respond to allogeneic cells (25). Therefore, these memory T cells may be primed by NKG2D ligand-positive tumor cells in the presence of scFv-NKG2D even without the help of co-stimulatory molecules. Full-length anti-CD3 mAb, even in very small quantities, can induce T cell activation and its associated toxicity in vivo (26). Non-specific T cell activation is primarily due to antibody Fc fragment binding to FcR which leads to CD3 cross-linking, since anti-CD3-induced non-specific T cell activation was significantly reduced using enzymatically produced F(ab’) (26, 27). In scFv-NKG2D, the Fc fragment was removed. Therefore, non-specific T cell activation due to Fc-mediated events and related toxicities are expected to be low.
Immunosuppressive cells within the tumor microenvironment have been shown to be prevent anti-tumor immunity (28, 29). Effective therapies must not only attack the tumor, but they must change the local microenvironment leading to inhibition of tumor growth. Recent studies have shown that myeloid suppressive cells and regulator T cells (Treg) express NKG2D ligands (10, 30). Both cell types make significant contribution in the immunosuppression of tumor microenvironment. The findings that even NKG2D ligand-deficient tumor cells can be affected by scFv-NKG2D treatment suggest that scFv-NKG2D allows T cells to attack host immunosuppressive cells. Nausch et al. showed that adoptive transfer of NK cells reduced numbers of Gr-1+CD11b+F4/80+ MDSC cells but not the percentages of Gr-1+CD11b+F4/80− cells in tumor-bearing mice, suggesting that these MDSCs were targeted in vivo by adoptively transferred NK cells (10). Barber et al. showed that NKG2D can be used as a targeting strategy against Tregs within the tumor microenvironment (11). Our results showed that in both MC-38 and B16F10 models, MDSCs and Tregs expressed an NKG2D ligand Rae1, which is consistent with previous findings. Local expression of scFv-NKG2D in tumors significantly reduced the percentage of these immunosuppressive cells, providing more convincing evidence that MDSCs and Tregs can be targeted by scFv-NKG2D.
In TCRα−/− mice, αβ T cells are lacking, whereas γδ T cells remain normal (20). As shown in Fig. 7, scFv-NKG2D expression by MC-38 cells also reduced tumor growth in TCRα−/− mice, suggesting that host γδ T cells can be redirected against NKG2D ligand-positive tumors by scFv-NKG2D. γδ T cells are abundant in skin and mucosa tissues (31). Many studies have suggested that γδ T cells are important components in anti-tumor immunity (32, 33). Therefore in this model, γδ T cells may play a significant role in scFv-NKG2D-mediated early anti-tumor responses.
Since NKT cells also express CD3, it is possible that scFv-NKG2D may engage NKT cells to tumor cells, leading to anti-tumor responses. It has been shown that administration of α-galactosylceramide (αGalCer, an agonist of NKT cells), αGalCer-loaded DCs, or αGalCer-loaded soluble CD1d molecule (αGalCer/sCD1d) could induce significant IFN-γ producing NKT responses and protect against the development of metastases with B16 melanoma after (34, 35). However, success of α-GalCer treatment in humans has been limited. The partial reason may be that in humans, there exist anti-alpha-linked sugar natural antibodies that do not exist in the mouse (36, 37). Lung and liver contain abundant NKT cells, which may play an important role in keeping tumor metastasis in check (34, 35),(38). Expression of membrane-bound anti-CD3 scFv and CD86 on B16 melanoma cells has been shown to suppress tumor growth in vivo and protect mice from B16 rechallenge. In the subcutaneous B16 model, NKT cells have been shown to play a critical role in in vivo antitumor activity(39). Therefore, it is possible that scFv-NKG2D may also engage both NKT and tumor cells, resulting in vivo anti-tumor activity.
T-cell infiltration into tumors (especially CD8+ T cells) is often correlated with improved survival (40–42). Recent studies have shown that tumor-specific CD4+ T cells could also differentiate in vivo into cytotoxic T cells and induce regression of established melanoma (43, 44). The results that local expression of scFv-NKG2D increased recruitment of T cells and reduced tumor growth are consistent with these findings. The possible mechanisms for enhanced T cell-infiltration after scFv-NKG2D treatment may be that scFv-NKG2D engages NKG2D ligand-positive cells in tumors and resident and/or newly-arrived T cells, resulting in T cell activation and production of IFN-γ leading to IFN-γ-inducible chemokines such as CXCL9 and CXCL10 (the ligands for CXCR3), as well as CCL2 (the ligand for CCR2) and CCL11 (the ligand for CCR2 and CCR5) (44). These chemokines may attract more T cells to tumor sites and help eliminate tumors.
Regulation of NKG2D ligand expression can occur on multiple levels (transcriptional, post-transcriptional and post-translational levels)(9). DNA pathways initiated by ATM (ataxia telangiectasia, mutated) or ATR (ATM- and Rad3-related) protein kinases have been shown to play important roles in up-regulation of NKG2D ligands (45). Many anti-cancer drugs can also up-regulate NKG2D ligands (46), which not only potentially enhances the host NK cell anti-tumor activity but also suggests possible synergistic effects of these drugs with scFv-NKG2D treatment.
In view of the wide expression of NKG2D ligands on many human cancers as well as Treg and myeloid-derived suppressor cells, the strategy described here has a promising potential to treat many human cancers using direct and indirect mechanisms. The ability of scFv-NKG2D to induce long-term immunological memory may be beneficial to cancer patients with minimal residual disease. Collectively, our findings suggest that scFv-NKG2D represents a promising alternative to elicit host immunity for effective immunotherapy against cancer.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the Hitchcock Foundation (250-4032, TZ) at Dartmouth Medical School, the Department of Microbiology and Immunology and Norris Cotton Cancer Center (CLS); National Institutes of Health (CA130911, CLS; T32AR007576, TZ). We thank Dr. William Green (Dartmouth Medical School) for providing TCRα−/− mice and the staff of the Animal Resources Center for assistance with animal care.
Footnotes
This work was supported in part by grants from the Hitchcock Foundation (250-4032), NIH (CA130911 and T32AR007576), and support from the Department of Microbiology & Immunology and Norris Cotton Cancer Center.
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest. Dartmouth College has applied for patent protection for the scFv-NKG2D technology described in this article.
References
- 1.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–7. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 2.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 3.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–5. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 6.Baeuerle PA, Kufer P, Bargou R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 2009;11:22–30. [PubMed] [Google Scholar]
- 7.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 8.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived "suppressor" cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–9. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–47. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shastri N, Gonzalez F. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J Immunol. 1993;150:2724–36. [PubMed] [Google Scholar]
- 13.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–36. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 14.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–8. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Grundy MA, Zhang T, Sentman CL. NK cells rapidly remove B16F10 tumor cells in a perforin and interferon-gamma independent manner in vivo. Cancer Immunol Immunother. 2007;56:1153–61. doi: 10.1007/s00262-006-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–33. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson M, Meadows SK, Basu S, Mselle TF, Wira CR, Sentman CL. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J Immunol. 2006;176:6219–24. doi: 10.4049/jimmunol.176.10.6219. [DOI] [PubMed] [Google Scholar]
- 19.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 20.Philpott KL, Viney JL, Kay G, et al. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–52. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 21.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 22.Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2:57. doi: 10.2217/imt.09.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carnevale-Schianca F, Cignetti A, Capaldi A, et al. Allogeneic nonmyeloablative hematopoietic cell transplantation in metastatic colon cancer: tumor-specific T cells directed to a tumor-associated antigen are generated in vivo during GVHD. Blood. 2006;107:3795–803. doi: 10.1182/blood-2005-10-3945. [DOI] [PubMed] [Google Scholar]
- 24.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 25.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175:4886–96. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 26.Weiner GJ, Kostelny SA, Hillstrom JR, et al. The role of T cell activation in anti-CD3 × antitumor bispecific antibody therapy. J Immunol. 1994;152:2385–92. [PubMed] [Google Scholar]
- 27.Tibben JG, Boerman OC, Claessens RA, et al. Cytokine release in an ovarian carcinoma patient following intravenous administration of bispecific antibody OC/TR F(ab')2. J Natl Cancer Inst. 1993;85:1003–4. doi: 10.1093/jnci/85.12.1003. [DOI] [PubMed] [Google Scholar]
- 28.Davis ID, Desai J. Clinical use of therapies targeting tumor vasculature and stroma. Curr Cancer Drug Targets. 2008;8:498–508. doi: 10.2174/156800908785699388. [DOI] [PubMed] [Google Scholar]
- 29.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2008 doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180:1729–36. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 31.Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human gammadelta T cells. Immunobiology. 2008;213:173–82. doi: 10.1016/j.imbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Todaro M, D'Asaro M, Caccamo N, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–96. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 33.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 34.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 35.Stirnemann K, Romero JF, Baldi L, et al. Sustained activation and tumor targeting of NKT cells using a CD1d-anti-HER2-scFv fusion protein induce antitumor effects in mice. J Clin Invest. 2008;118:994–1005. doi: 10.1172/JCI33249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987;84:1369–73. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cullen R, Germanov E, Shimaoka T, Johnston B. Enhanced tumor metastasis in response to blockade of the chemokine receptor CXCR6 is overcome by NKT cell activation. J Immunol. 2009;183:5807–15. doi: 10.4049/jimmunol.0803520. [DOI] [PubMed] [Google Scholar]
- 39.Lee CH, Chiang YH, Chang SE, Chong CL, Cheng BM, Roffler SR. Tumor-localized ligation of CD3 and CD28 with systemic regulatory T-cell depletion induces potent innate and adaptive antitumor responses. Clin Cancer Res. 2009;15:2756–66. doi: 10.1158/1078-0432.CCR-08-2311. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 41.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 43.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 207:637–50. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y, Akpinarli A, Maris C, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 207:651–67. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diermayr S, Himmelreich H, Durovic B, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111:1428–36. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.