Abstract
The concept of modularity provides a useful tool for exploring the relationship between genotype and phenotype. Here, we use quantitative genetics to identify modularity within the mammalian dentition, connecting the genetics of organogenesis to the genetics of population-level variation for a phenotype well represented in the fossil record.
We estimated the correlations between dental traits due to the shared additive effects of genes (pleiotropy) and compared the pleiotropic relationships among homologous traits in two evolutionary distant taxa – mice and baboons. We find that in both mice and baboons, who shared a common ancestor >60 Ma, incisor size variation is genetically independent of molar size variation. Furthermore, baboon premolars show independent genetic variation from incisors, suggesting that a modular architecture separates incisors from these posterior teeth as well. Such genetic independence between modules provides an explanation for the extensive diversity of incisor size variation seen throughout mammalian evolution--variation uncorrelated with equivalent levels of postcanine tooth size variation. The modularity identified here is supported by the odontogenic homeobox code proposed for the patterning of the rodent dentition. The baboon postcanine pattern of incomplete pleiotropy is also consistent with predictions from the morphogenetic field model.
Introduction
Developmental genetics can provide insights for how the information stored within the genome may be translated into the phenotype during early ontogeny. Evolutionary biologists have incorporated some of these insights into paleontology, with tremendous success at higher taxonomic levels, such as the origins of body plans (e.g., Raff. ‘96) and the fin-limb transition (e.g., Davis et al, 2007). However, given that much of evolution is characterized by smaller scale variation, it is logical to consider whether those genes involved in making an organ are the same that influence minor variation in the ultimate phenotype (Hlusko, 2004). Selection typically operates at this population level. Therefore, making a connection between developmental genetic mechanisms and normal population-level variation is essential for bringing an “evo-devo” approach to most of vertebrate evolution.
Quantitative genetic analyses can be used to make this phenotype-genotype connection, as they enable the investigation of the genetics of normal adult phenotypic variation, working back towards the genome. Our goal is to link these two approaches—quantitative and developmental genetics—together to form a more complete understanding of the relationship between genotype and phenotype, and ultimately, to incorporate this knowledge into our understanding of phenotypic evolution as evidenced in the fossil record (Hlusko, 2004).
Since antiquity biologists have recognized the importance of the size and shape of an animal’s teeth (e.g., Aristotle’s On the Generation of Animals, Book V, Chapter 8). Given the dentition’s fundamental role in procuring and processing food and in social interactions with conspecifics, the dentition has evolved to be one of the most informative parts of the skeleton for inferring evolutionary relationships and adaptations. Because teeth are largely inorganic, they also survive well in the fossil record—for many extinct vertebrates, all we know of them is what their teeth looked like. Considerable advances have also been made in identification and functional analyses of the genes necessary to make and pattern the dentition (Jernvall and Thesleff, 2000; Tucker and Sharpe, 2004). Consequently, the dentition is an important organ system for developmental biologists, neontologists, and paleontologists alike, making it an ideal system for an integrated developmental, genetic, and paleontological approach (Jernvall and Jung, 2000; Hlusko, 2004). Here, we report on the first comparative quantitative genetic analysis of dental variation in two mammalian taxa: mouse and baboon.
Background
In 1939 Butler proposed the morphogenetic field theory which became the foundation for most morphologists’ understanding of dental variation. In this model, primordial teeth are pluripotent, and tooth type is determined by extrinsic factors (“morphogens”). An alternative was later proposed, the clone model (Osborn, ‘78) in which tooth type is intrinsically determined. Neither of these hypotheses relied on actual knowledge of genetics, but rather posed speculative hypotheses that were difficult to test, but tested nonetheless via adult phenotypic variation yielding inconclusive results (e.g., Dahlberg, ‘45; Van Valen, ‘61; Henderson and Greene, ‘75; Lombardi, ‘75).
Advances in developmental genetics over the last 20 years have dramatically improved our understanding of tooth organogenesis and patterning (Tucker and Sharpe, 2004). This research has primarily focused on the mouse model, as has much of mammalian developmental genetics. We now know that the dentition is patterned quite early during development, by mouse embryonic day 11. At this stage, the oral cavity has started to form in a layer of epithelial cells oral to neural crest derived mesenchyme. Patterning information for the dental arcade appears to be regulated by this epithelial layer, called the dental lamina. Once the epithelium invaginates into the mesenchyme at mouse embryonic day 13, control of tooth type shifts to the surrounding mesenchymal cells. By mouse embryonic day 14 the primary signaling for continued tooth formation has returned to the epithelium, but now is centered within a mass of non-proliferating cells that form the enamel knot, a known signaling center (Jernvall et al, ‘94).
The genetic mechanism formally proposed for how genes determine tooth type during the dental lamina stage is the odontogenic homeobox code (Thomas and Sharpe, ‘98). This model suggests that bone morphogenetic proteins (BMP) and fibroblast growth factor (FGF) proteins in the epithelium induce and inhibit expression of 8 homeobox genes in various permutations, with specific combinations resulting in a particular tooth type. For example, Msx1, Msx2, Lhx6, and Lhx7 are expressed in presumptive incisor tissue and Dlx1, Dlx2, Barx1, Lhx6, and Lhx7 are expressed in presumptive molar tissue. The molecular evidence for this derives from experiments on mice, and as such, the odontogenic code is only proposed for determining the reduced dentition (incisors and molars) of rodents (but see McCollum and Sharpe, 2001). Since a recent study by Munne et al (2010) suggests that this odeontogenic homeobox code may be based on a misinterpretation of gene knock-out morphology, the genetic patterning mechanism for the dental arcade remains speculative.
Developmental genetics more generally shows that organisms have morphological and developmental modularity that results from modules at the genomic level, such as gene families, and from modules in embryogenesis (Raff, ‘96; Carroll et al, 2005). This modularity is critical since it enables an organism to be “evolvable” (Wagner and Altenberg, ‘96; Scholsser and Wagner, 2004; Draghi and Wagner, 2009). This modularity has been defined more specifically as “a genotype-phenotypic map in which there are a few pleiotropic effects among characters serving different functions, with pleiotropic effects falling mainly among characters that are part of a single functional complex” (Wagner and Altenberg, ‘96: 967). Considerable research has demonstrated modularity within the vertebrate limb (Wagner and Vargas, 2008, Reno et al, 2008; Shubin et al, ‘97; Shubin, 2002; Davis et al, 2007) and the skull (Richtsmeier et al, ‘84; Kohn et al, ‘93; Cheverud, ‘96; Ackermann and Cheverud, 2002; Marroig et al, 2004; Roseman, 2004; Marroig and Cheverud, 2005; Wolf et al, 2005; Ackermann, 2007; Hallgrimsson et al, 2007; Mitteroecker and Bookstein, 2008; Sherwood et al, 2008), for example.
Although the dentition is in a sense its own module, given the hierarchical nature of its development (Bateson, 1894; Stock, 2001), in this paper we focus on modularity within the dentition. This is the level of modularity often thought to be represented by characters in paleontological analyses, especially those at the sub-family level or below (Hlusko, 2004).
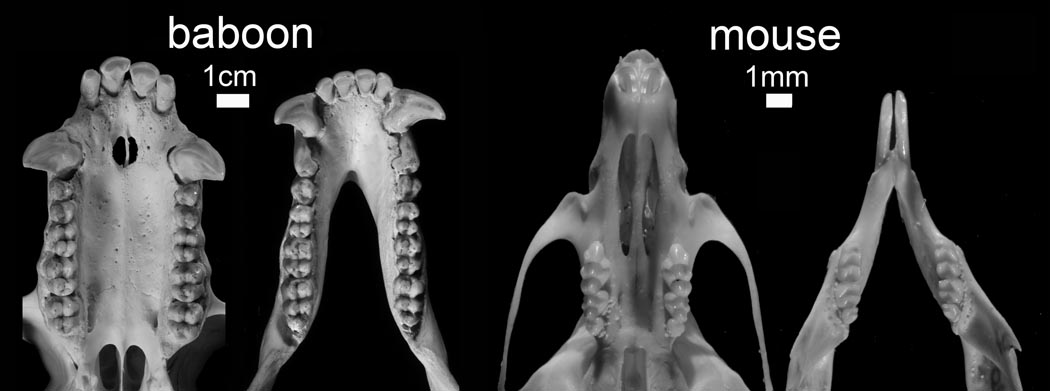
The modularity reported here is defined by the genetic architecture of mammalian population-level dental variation. We employ two animal models (Fig. 1). The first is the baboon because this primate has a relatively generalized mammalian dental pattern in that it is dyphyodont with incisors, canines, premolars and molars. The second is the mouse, as this taxon provided the source for most of the developmental genetics research to date despite its highly derived and reduced dentition (mice are monophyodont with only incisors and molars).
FIGURE 1.
Photograph of mouse and baboon maxillae and mandibles. Baboons have a more evolutionarily primitive dental formula (diphyodont: 2 incisors, 1 canine, 2 premolars, 3 molars) compared to the highly derived and reduced mouse dentition (monophyodont: 1 incisor, 3 molars).
Using quantitative genetic analyses in pedigreed populations, we detected and estimated additive genetic correlations between linear measurements of tooth size for teeth along the maxillary and mandibular dental arcades of these two taxa. These additive genetic correlations were compiled into matrices, each matrix characterizing the contribution of pleiotropy to the genetic architecture underlying observed patterns of covariation in tooth size measurements. Our results demonstrate significant similarity between mouse and baboon dental genetic architectures, a common pattern of modularity that may result from a conserved mammalian genetic patterning mechanism.
Materials and Methods
BABOON POPULATION
For 630 baboons we measured mesiodistal length and buccolingual widths of all incisors, premolars, and molars (maxillary and mandibular). These animals are part of a captive, pedigreed breeding colony of Papio hamadryas (as defined in Jolly, ‘93) housed at the Southwest National Primate Research Center (SNPRC) in San Antonio, Texas. The colony is maintained in pedigrees with all mating opportunities controlled. Age and sex (as well as other life history and health data) are known for all individuals.
Genetic management of the colony was started over 30 years ago and allows for data collection from non-inbred animals. All non-founder animals in this study resulted from matings that were random with respect to dental, skeletal, and developmental phenotype. The female-to-male sex ratio is approximately 2:1. The animals from which data were collected are distributed across eleven extended pedigrees that are 3–5 generations deep. The mean number of animals with data per pedigree was 44, and these individuals typically occupied the lower two or three generations of each pedigree. All pedigree data management and preparation was facilitated through use of the computer package PEDSYS (Dyke, ‘96).
The Institutional Animal Care and Use Committee, in accordance with the established guidelines (National Research Council, '96), approved all procedures related to the treatment of the baboons during the conduct of this study.
MOUSE POPULATION
We measured mesiodistal length and buccolingual width of all teeth (1 incisor and 3 molars for each dental quadrant, maxillary and mandibular) of 207 mice that are part of a large pedigreed colony made by R.D.S. between 1977 and 1992, currently housed at the University of California at Berkeley’s Museum of Vertebrate Zoology.
The colony was established in 1977 with either mice wild-caught by R.D.S., or outbred mice from another lab that established their colony with wild-caught animals. For example, Mus cervicolor popaeus founders were from the pedigreed breeding colony established and maintained by the National Cancer Institute (Escot et al, ‘86). We restrict our analyses to animals that are first-generation from these founders in order to minimize the chances of inbreeding. As such, all mice used in this study are from litters born between 1977 and 1981. Pedigrees were reconstructed from breeding records, enabling ascertainment of age at death and sex, as well as familial relationships. Seven taxa are represented (Table 1). No in-bred laboratory strains were used in this study.
Table 1.
Taxonomic composition and pedigree structure of mouse population
| Taxon | Mating pairs | Litters | Offspring | Total |
|---|---|---|---|---|
| Mus caroli | 5 | 8 | 31 | 41 |
| M. cervicolor cervicolor | 6 | 13 | 42 | 54 |
| M. c. popaeus | 3 | 8 | 35 | 41 |
| M. cookii | 4 | 11 | 40 | 48 |
| M. musculus | 2 | 3 | 17 | 21 |
| M. domesticus brevirostris | 2 | 3 | 5 | 9 |
| M. d. praetextus | 1 | 1 | 1 | 3 |
| M. pahari | 2 | 5 | 11 | 15 |
| M. spretus | 1 | 1 | 1 | 3 |
| Mus total | 235 |
Two of four subgenera within Mus (Nowak, ‘91) are represented: 15 are Coelomys (shrew mice: M. pahari), and 220 are Mus (house and rice field mice: M. caroli, M. cervicolor cervicolor, M. c. popaeus, M. cookii, M. musculus, M. domesticus brevirostris, M. d. praetextes, M. spretus). Our taxonomy follows Sage et al. (’93) and Prager et al. (’93). Each taxon has 1–6 pure mating pairs and 1–13 litters from these pairs (Table 1). There are no hybrids included in the analysis, only offspring from conspecific (or con-subspecific) matings. Although our sample represents non-inbred populations, the taxonomic structure makes it less than ideal for these analyses. By including parent/offspring sets from multiple taxa we artificially inflate the degree of correlation, as interspecific differences will increase the appearance of intra-familial resemblance. Therefore, analyses of this population are prone to overestimate correlations. Our results need to be interpreted with this caveat in mind.
In total, pedigree data for 235 mice were used to reconstruct the pedigrees, 207 with phenotype data. The female to male ratio is approximately 1:1. Mice were maintained and sacrificed under protocols approved by the Office of Laboratory Animal Care, University of California Berkeley.
PHENOTYPIC DATA
All dental measurements from the baboons were collected from casts, as described in detail elsewhere (Hlusko et al, 2002). Linear measurements for the baboons were collected with calipers for the incisors and premolars, and from digital photographs for the molars, following a protocol described elsewhere (Hlusko et al, 2002). Measurements were taken from photographs for the molars because of the need for a protocol that avoided the problem of the gumline obscuring the maximum buccolingual width of the crown – maximum width was standardized as 1 mm below the maximum depth of the occlusal surface. The shape of the other teeth makes caliper measurements more reliable than the two-dimensional representations of photographs. All dental data from the mice were collected from digital photographs using the software program Image Pro Plus©. Because mouse teeth are very small, they are more easily measured with digital photographs that can be magnified. Definitions of length and widths follow standard odontological methods (e.g., Hillson, ‘86).
Abbreviations: I = incisor, P = premolar, M = molar; number following first letter indicates tooth position; ll = labiolingual width of the incisor; md = mesiodistal length of the incisor; l = mesiodistal length (the longest mesiodistal axis of the premolar or molar), w = maximum buccolingual width of the premolar (not necessarily perpendicular to the mesiodistal length); mw = maximum buccolingual width of the molar through the mesial-most pair of cusps (not necessarily perpendicular to the mesiodistal length); dw = maximum buccolingual width of the molar through the distal cusp pair on baboons, and the second cusp pair for mice (not necessarily perpendicular to the mesiodistal length).
ANALYTICAL METHODS
Quantitative genetic analyses test the hypothesis that environmental, or rather non-genetic factors alone can account for the phenotypic similarities seen among family members. A significant heritability estimate and significant genetic correlation indicate that environmental effects by themselves cannot account for, respectively, the pattern of phenotypic variation and covariation between phenotypes seen in a population of related individuals; that is, the degree of interrelatedness – and, hence, genetic similarity – contributes to observed phenotypic similarities.
Our statistical genetic analyses were performed using a maximum likelihood based variance decomposition approach implemented in the computer package SOLAR (Almasy and Blangero, ‘98). Accordingly, the phenotypic covariance for each trait within a pedigree in this study is modeled as , where Φ is a matrix of kinship coefficients for all relative pairs in a pedigree, is the additive genetic variance, I is an identity matrix (composed of ones along the diagonal and zeros for all off diagonal elements), and is the environmental variance. Because the components of the phenotypic variance are additive, such that σ2P = σ2G + σ2E, we estimated heritability, or the proportion of the phenotypic variance attributable to additive genetic effects, as h2 = σ2G / σ2P. Identifying such additive genetic effects are essential to evolutionary theory, as only phenotypic variation that is inherited will respond to selective pressure. Phenotypic variance attributable to non-genetic factors is estimated as e2 = 1 – h2. The mean effects of sex and age were included in the analyses when they had a significant influence on the phenotypic variance (age serves as a proxy for wear in these analyses).
Using extensions to univariate genetic analysis that encompass the multivariate state (Hopper and Mathews, ‘82; Lange and Boehnke, ‘83; Boehnke et al, ‘87), we follow an approach described in detail elsewhere (Mahaney et al, ‘95) to model the multivariate phenotype of an individual as a linear function of the measurements on the individual's traits, the means of these traits in the population, the covariates and their regression coefficients, plus the additive genetic values and random environmental deviations. From this model, we obtained the phenotypic variance-covariance matrix from which we partitioned the additive genetic and random environmental variance-covariance matrices, given the relationships (kinship coefficients) observed in the pedigree. From these two variance-covariance matrices, we estimated the additive genetic correlation, ρG, and the environmental correlation, ρE, between trait pairs. Respectively, these correlations are estimates of the additive effects of shared genes (i.e., pleiotropy) and shared environmental (i.e., unmeasured and nongenetic) factors on the variance in a trait.
The genetic and environmental components of the phenotypic correlation matrix are additive, like those of the corresponding variance-covariance matrix, so we use the maximum likelihood estimates of the additive genetic and environmental correlations to obtain the total phenotypic correlation between two traits, ρP, as
We conducted bivariate quantitative genetic analyses of trait pairs using multivariate extensions to the basic variance decomposition methods implemented in SOLAR (Almasy and Blangero 1998). We used this approach to obtain simultaneous maximum likelihood estimates of the phenotypic means (μ), phenotypic standard deviations (σ), heritabilities (h2), and the mean effects of covariates on all traits, and the genetic and environmental correlations between them.
Significance of the maximum likelihood estimates for heritability and other parameters is assessed by means of likelihood ratio tests. Twice the difference of the maximum likelihoods of a general model (in which all parameters are estimated) and a restricted model (in which the value of a parameter to be tested is held constant at some value, usually zero) are compared. This difference is distributed asymptotically approximately as either a ½:½ mixture of χ2 and a point mass at zero, for tests of parameters like h2 for which a value of zero in a restricted model is at a boundary of the parameter space, or as a χ2 variate for tests of covariates for which zero is not a boundary value (Hopper and Mathews, ‘82). In both cases degrees of freedom is equal to the difference in the number of estimated parameters in the two models (Boehnke et al, ‘87). However, in tests of parameters like h2, whose values may be fixed at a boundary of their parameter space in the null model, the appropriate significance level is obtained by halving the P-value (Boehnke et al, ‘87).
For bivariate models in which genetic correlations are found to be significantly greater than zero, additional tests are performed to compare the likelihood of a model in which the value of the genetic correlation is fixed at 1 or 0 to that of the unrestricted model in which the value of the genetic correlation is estimated. A significant difference between the likelihoods of the restricted and polygenic models suggests incomplete pleiotropy, i.e., not all of the additive genetic variance in the two traits is due to the effects of the same gene or genes.
Genetic correlations between traits can result from either pleiotropy or gametic phase disequilibrium (Lynch and Walsh, ‘98). The degree of gametic phase disequilibrium (or linkage disequilibrium, LD) is a function of a population’s genetic history and demography: e.g., it will be lower in outbred populations with many unrelated founders as recombination exerts its effects each generation, higher in populations undergoing rapid expansion from a small number of founders and those resulting from recent admixture. Given a conducive set of population characteristics, the likelihood of genetic correlation between two traits being due to LD is higher for simple traits, with monogenic (or nearly so) inheritance. However, if variation in a pair of traits is attributable to the effects of multiple alleles at multiple loci, LD is not likely to be a major contributor to the genetic correlation (Lande, ‘80; Lynch and Walsh, ‘98). Therefore, we are cautiously confident that significant additive genetic correlations estimated in our analyses on pairs of complex, multifactorial dental measures from our non-inbred, extended baboon and mouse pedigrees are primarily indicative of pleiotropy rather than LD. Ongoing and planned whole genome screens and LD analyses will help confirm this.
Results
The last 50 years of quantitative genetics have repeatedly shown that dental phenotypes tend to have the highest heritability estimates reported for the skeleton (Rizk et al, 2008), indicating that dental variation is largely influenced by genetic variation, although non-genetic affects can be significant. This is not surprising given that the size and shape of teeth are unaltered after eruption, save for wear and breakage, unlike the rest of the mammalian skeleton that continues to remodel over the animal’s lifespan.
As expected, the tooth size variation reported here is highly heritable for both baboons and mice, and as such, highly susceptible to selective pressures (see Table 2a and b for residual h2 estimates). These tables report the residual heritability (h2) estimate after the affects of the covariates (c2) are removed (i.e., sex and age). The remaining variance is attributed to non-genetic effects (e2) such as measurement error, environmental influences, and/or unaccounted for covariates.
Table 2a.
Polygenic models for individual baboon tooth measurements1
| Trait | Mean | Var | n | Kurtosis | Total h2 |
p-value | Total c2 |
Total e2 |
Residual h2 ± SE |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Baboon Right Maxillary | I1ll | 9.07 | 1.08 | 473 | −0.3274 | 0.49 | <0.0001 | 0.194 | 0.32 | 0.605±0.12 |
| I1md | 9.51 | 0.55 | 480 | 0.4816 | 0.51 | <0.0001 | 0.125 | 0.37 | 0.578±0.11 | |
| I2ll | 7.98 | 1.09 | 463 | 0.5029 | 0.51 | <0.0001 | 0.204 | 0.28 | 0.642±0.11 | |
| I2md | 7.05 | 0.91 | 474 | 0.5982 | 0.52 | <0.0001 | 0.141 | 0.33 | 0.611±0.11 | |
| P3l | 6.71 | 0.31 | 276 | −0.1182 | 0.25 | 0.006 | 0.201 | 0.55 | 0.316±0.15 | |
| P3w | 7.82 | 0.44 | 317 | 0.7641 | 0.43 | <0.0001 | 0.346 | 0.22 | 0.659±0.20 | |
| P4l | 7.63 | 0.27 | 400 | 0.4849 | 0.48 | <0.0001 | 0.295 | 0.23 | 0.680±0.12 | |
| P4w | 8.51 | 0.38 | 430 | 0.0152 | 0.37 | <0.0001 | 0.368 | 0.26 | 0.591±0.12 | |
| M1l | 10.68 | 0.40 | 471 | 0.2626 | 0.44 | <0.0001 | 0.336 | 0.23 | 0.659±0.11 | |
| M1mw | 8.38 | 0.30 | 438 | 0.7627 | 0.55 | <0.0001 | 0.184 | 0.27 | 0.672±0.14 | |
| M1dw | 7.87 | 0.29 | 439 | 0.4530 | 0.62 | <0.0001 | 0.190 | 0.19 | 0.763±0.16 | |
| M2l | 12.47 | 0.69 | 531 | 1.4037 | 0.46 | <0.0001 | 0.425 | 0.12 | 0.798±0.11 | |
| M2mw | 9.88 | 0.47 | 530 | 0.5056 | 0.39 | <0.0001 | 0.291 | 0.32 | 0.544±0.12 | |
| M2dw | 8.85 | 0.40 | 517 | 0.5223 | 0.37 | <0.0001 | 0.305 | 0.32 | 0.533±0.13 | |
| M3l | 12.62 | 0.83 | 243 | 2.2095 | 0.14 | 0.06 | 0.429 | 0.43 | 0.241±0.19 | |
| M3mw | 9.97 | 0.75 | 444 | 0.9430 | 0.35 | <0.0001 | 0.381 | 0.27 | 0.562±0.13 | |
| M3dw | 8.50 | 0.56 | 286 | 0.0408 | 0.22 | 0.021 | 0.345 | 0.44 | 0.331±0.19 | |
| Baboon Right Mandibular | Trait | Mean | Var | n | Kurtosis |
Total h2 |
p-value |
Total c2 |
Total e2 |
Residual h2 ± SE |
| I1ll | 8.82 | 1.46 | 474 | 0.6858 | 0.53 | <0.0001 | 0.099 | 0.37 | 0.589±0.12 | |
| I1md | 6.79 | 0.30 | 465 | 0.6396 | 0.55 | <0.0001 | 0.053 | 0.40 | 0.581±0.11 | |
| I2ll | 8.30 | 1.66 | 468 | 0.4795 | 0.28 | <0.0001 | 0.189 | 0.54 | 0.340±0.11 | |
| I2md | 5.67 | 0.35 | 463 | −0.1269 | 0.26 | <0.0001 | 0.116 | 0.62 | 0.293±0.10 | |
| P3l | 11.46 | 13.08 | 162 | 5.1955 | 0.30 | 0.06 | 0.365 | 0.33 | 0.473±0.41 | |
| P3w | 5.58 | 0.42 | 274 | 0.1837 | 0.22 | 0.0003 | 0.513 | 0.27 | 0.442±0.16 | |
| P4l | 8.45 | 0.46 | 409 | 0.1038 | 0.39 | <0.0001 | 0.413 | 0.19 | 0.672±0.10 | |
| P4w | 6.99 | 0.32 | 368 | 0.2922 | 0.56 | <0.0001 | 0.234 | 0.21 | 0.729±0.14 | |
| M1l | 10.47 | 0.30 | 362 | 0.7650 | 0.59 | <0.0001 | 0.360 | 0.05 | 0.927±0.14 | |
| M1mw | 7.33 | 0.25 | 326 | 0.1957 | 0.56 | <0.0001 | 0.226 | 0.22 | 0.722±0.15 | |
| M1dw | 7.36 | 0.26 | 334 | 0.4025 | 0.67 | <0.0001 | 0.146 | 0.18 | 0.781±0.16 | |
| M2l | 12.36 | 0.62 | 490 | 0.7256 | 0.49 | <0.0001 | 0.444 | 0.06 | 0.886±0.10 | |
| M2mw | 9.22 | 0.45 | 501 | 0.5369 | 0.53 | <0.0001 | 0.305 | 0.17 | 0.760±0.10 | |
| M2dw | 8.61 | 0.38 | 475 | 0.3637 | 0.43 | <0.0001 | 0.309 | 0.26 | 0.622±0.12 | |
| M3l | 15.28 | 1.60 | 232 | 2.2398 | 0.40 | 0.0004 | 0.449 | 0.15 | 0.722±0.22 | |
| M3mw | 9.68 | 0.62 | 483 | 0.0916 | 0.49 | <0.0001 | 0.394 | 0.11 | 0.811±0.11 | |
| M3dw | 8.68 | 0.51 | 463 | 0.2065 | 0.38 | <0.0001 | 0.391 | 0.23 | 0.630±0.11 | |
| Trait | Mean | Var | n | Kurtosis |
Total h2 |
p-value |
Total c2 |
Total e2 |
Residual h2 ± SE |
|
| Baboon Left Maxillary | I1ll | 8.96 | 1.06 | 469 | 0.5843 | 0.37 | <0.0001 | 0.176 | 0.46 | 0.446±0.11 |
| I1md | 9.58 | 0.48 | 471 | 0.0452 | 0.55 | <0.0001 | 0.156 | 0.29 | 0.654±0.10 | |
| I2ll | 7.12 | 0.60 | 481 | 0.3304 | 0.54 | <0.0001 | 0.099 | 0.36 | 0.595±0.12 | |
| I2md | 5.62 | 0.48 | 471 | 0.3270 | 0.36 | <0.0001 | 0.212 | 0.43 | 0.452±0.11 | |
| P3l | 6.69 | 0.34 | 287 | −0.1619 | 0.20 | 0.017 | 0.148 | 0.65 | 0.236±0.14 | |
| P3w | 7.75 | 0.41 | 323 | 0.5493 | 0.18 | 0.004 | 0.388 | 0.43 | 0.292±0.14 | |
| P4l | 7.65 | 0.28 | 418 | 0.5649 | 0.34 | <0.0001 | 0.285 | 0.37 | 0.478±0.10 | |
| P4w | 8.52 | 0.37 | 454 | −0.0675 | 0.42 | <0.0001 | 0.303 | 0.27 | 0.608±0.12 | |
| M1l | 10.66 | 0.37 | 470 | −0.1161 | 0.47 | <0.0001 | 0.379 | 0.15 | 0.751±0.12 | |
| M1mw | 8.38 | 0.30 | 458 | 0.5261 | 0.56 | <0.0001 | 0.221 | 0.22 | 0.722±0.11 | |
| M1dw | 7.89 | 0.27 | 454 | 0.3962 | 0.62 | <0.0001 | 0.206 | 0.17 | 0.786±0.12 | |
| M2l | 12.55 | 0.69 | 539 | 0.6799 | 0.44 | <0.0001 | 0.479 | 0.08 | 0.847±0.10 | |
| M2mw | 9.90 | 0.45 | 539 | 0.7125 | 0.49 | <0.0001 | 0.276 | 0.23 | 0.676±0.11 | |
| M2dw | 8.92 | 0.39 | 530 | 0.2218 | 0.39 | <0.0001 | 0.302 | 0.31 | 0.557±0.11 | |
| M3l | 12.49 | 0.87 | 234 | 0.8855 | 0.13 | 0.07 | 0.432 | 0.44 | 0.231±0.19 | |
| M3mw | 9.98 | 0.61 | 440 | 0.3233 | 0.15 | 0.002 | 0.373 | 0.48 | 0.234±0.11 | |
| M3dw | 8.52 | 0.51 | 271 | 0.0973 | 0.16 | 0.01 | 0.411 | 0.43 | 0.271±0.16 | |
| Baboon Left Mandibular | Trait | Mean | Var | n | Kurtosis |
Total h2 |
p-value |
Total c2 |
Total e2 |
Residual h2 ± SE |
| I1ll | 8.62 | 1.38 | 467 | 0.1537 | 0.49 | <0.0001 | 0.125 | 0.39 | 0.560±0.12 | |
| I1md | 6.80 | 0.27 | 456 | −0.0292 | 0.60 | <0.0001 | 0.095 | 0.30 | 0.668±0.11 | |
| I2ll | 8.16 | 1.40 | 468 | 0.3457 | 0.30 | <0.0001 | 0.224 | 0.48 | 0.386±0.11 | |
| I2md | 5.62 | 0.48 | 457 | 0.3326 | 0.25 | <0.0001 | 0.087 | 0.66 | 0.277±0.10 | |
| P3l | 11.18 | 14.57 | 134 | 10.076 | 0.32 | 0.055 | 0.273 | 0.41 | 0.440±0.32 | |
| P3w | 5.59 | 0.43 | 274 | 0.3597 | 0.21 | 0.0003 | 0.468 | 0.32 | 0.403±0.16 | |
| P4l | 8.49 | 0.44 | 389 | 1.0291 | 0.29 | <0.0001 | 0.435 | 0.28 | 0.511±0.12 | |
| P4w | 7.03 | 0.33 | 366 | 0.2336 | 0.49 | <0.0001 | 0.183 | 0.33 | 0.598±0.14 | |
| M1l | 10.47 | 0.33 | 357 | 0.0992 | 0.61 | <0.0001 | 0.284 | 0.11 | 0.848±0.13 | |
| M1mw | 7.32 | 0.28 | 336 | −0.2091 | 0.42 | <0.0001 | 0.215 | 0.36 | 0.539±0.16 | |
| M1dw | 7.32 | 0.26 | 342 | 1.2749 | 0.24 | 0.053 | 0.186 | 0.57 | 0.289±0.19 | |
| M2l | 12.31 | 0.66 | 485 | 1.2870 | 0.32 | <0.0001 | 0.489 | 0.19 | 0.628±0.11 | |
| M2mw | 9.22 | 0.49 | 480 | 0.8268 | 0.30 | <0.0001 | 0.362 | 0.34 | 0.464±0.11 | |
| M2dw | 8.60 | 0.46 | 490 | 0.3373 | 0.31 | <0.0001 | 0.341 | 0.35 | 0.469±0.12 | |
| M3l | 15.20 | 1.58 | 336 | 0.5854 | 0.25 | 0.0005 | 0.404 | 0.35 | 0.415±0.16 | |
| M3mw | 9.62 | 0.63 | 500 | 0.5294 | 0.27 | <0.0001 | 0.384 | 0.34 | 0.441±0.10 | |
| M3dw | 8.62 | 0.48 | 470 | 0.3896 | 0.26 | <0.0001 | 0.343 | 0.40 | 0.392±0.10 |
Total c2 = amount of phenotypic variance attributable to covariates. Total h2 = (Residual h2)(1-Total c2). Total e2 = [1 – (Total c2 + Total h2)]; All data are presented in mm but were analyzed as multiples of 10 to raise the variance above 1.0.
Table 2b.
Polygenic models for individual mouse tooth measurements 1
| Trait | Mean | Stdv | n | Kurtosis | Total h2 |
p−value | Total c2 |
Total e2 |
Residual h2 ± SE |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse Right Maxillary | I1ll | 0.16 | 0.02 | 199 | 2.07 | 0.366 | <0.0001 | none | 0.634 | 0.366±0.09 |
| M1l | 2.18 | 0.20 | 207 | −0.58 | 0.765 | <0.0001 | none | 0.235 | 0.765±0.06 | |
| M1mw | 1.16 | 0.09 | 207 | −0.37 | 0.910 | <0.0001 | none | 0.090 | 0.910±0.05 | |
| M1dw | 1.19 | 0.10 | 207 | −0.99 | 0.991 | <0.0001 | none | 0.009 | 0.991±0.03 | |
| M2l | 1.29 | 0.13 | 207 | −0.94 | 0.942 | <0.0001 | none | 0.058 | 0.942±0.03 | |
| M2mw | 0.56 | 0.06 | 207 | 0.18 | 0.758 | <0.0001 | none | 0.242 | 0.758±0.09 | |
| M2dw | 0.98 | 0.09 | 206 | −0.63 | 0.906 | <0.0001 | none | 0.094 | 0.906±0.04 | |
| M3l | 0.70 | 0.08 | 201 | −0.34 | 0.784 | <0.0001 | none | 0.216 | 0.784±0.06 | |
| M3mw | 0.66 | 0.07 | 201 | −0.32 | 0.878 | <0.0001 | none | 0.122 | 0.878±0.06 | |
| Mouse Left Maxillary | M1l | 2.18 | 0.21 | 207 | −0.67 | 0.817 | <0.0001 | none | 0.183 | 0.817±0.06 |
| M1mw | 1.15 | 0.08 | 207 | −0.54 | 0.811 | <0.0001 | none | 0.189 | 0.811±0.06 | |
| M1dw | 1.18 | 0.09 | 207 | −0.99 | 0.939 | <0.0001 | none | 0.061 | 0.939±0.04 | |
| M2l | 1.30 | 0.127 | 207 | −1.00 | 0.952 | <0.0001 | none | 0.048 | 0.952±0.03 | |
| M2mw | 0.56 | 0.06 | 207 | −0.51 | 0.696 | <0.0001 | none | 0.304 | 0.696±0.07 | |
| M2dw | 0.98 | 0.08 | 206 | −0.65 | 0.866 | <0.0001 | none | 0.134 | 0.866±0.05 | |
| M3l | 0.71 | 0.08 | 202 | 12.6 | 0.739 | <0.0001 | none | 0.261 | 0.739±0.07 | |
| M3mw | 0.67 | 0.07 | 202 | 18.8 | 0.731 | <0.0001 | none | 0.269 | 0.731±0.08 | |
| Mouse Right Mandibular | I1ll* | 0.09 | 0.02 | 197 | 92.7 | 0.372 | <0.0001 | none | 0.628 | 0.372±0.09 |
| I1md | 0.05 | 0.01 | 197 | 0.01 | 0.174 | 0.0087 | none | 0.826 | 0.174±0.09 | |
| M1l | 1.62 | 0.13 | 204 | −0.95 | 0.984 | <0.0001 | none | 0.016 | 0.984±0.08 | |
| M1mw | 0.67 | 0.06 | 204 | 0.07 | 0.729 | <0.0001 | 0.009 | 0.262 | 0.736±0.08 | |
| M1dw | 0.91 | 0.07 | 204 | −0.73 | 0.988 | <0.0001 | none | 0.012 | 0.988±0.30 | |
| M2l | 1.01 | 0.09 | 203 | −0.59 | 0.757 | <0.0001 | 0.035 | 0.208 | 0.784±0.04 | |
| M2mw | 0.95 | 0.09 | 203 | −0.55 | 0.886 | <0.0001 | none | 0.114 | 0.886±0.05 | |
| M2dw | 0.89 | 0.10 | 203 | −0.86 | 0.876 | <0.0001 | 0.042 | 0.082 | 0.914±0.04 | |
| M3l | 0.72 | 0.10 | 197 | −0.06 | 0.698 | <0.0001 | none | 0.302 | 0.698±0.08 | |
| Mouse Left Mandibular | M1l | 1.61 | 0.13 | 204 | −0.87 | 0.980 | <0.0001 | none | 0.020 | 0.980±0.03 |
| M1mw | 0.67 | 0.06 | 204 | 0.27 | 0.690 | <0.0001 | none | 0.310 | 0.690±0.10 | |
| M1dw | 0.91 | 0.07 | 204 | −0.91 | 0.892 | <0.0001 | none | 0.108 | 0.892±0.05 | |
| M2l | 1.00 | 0.09 | 203 | −0.81 | 0.744 | <0.0001 | none | 0.256 | 0.744±0.06 | |
| M2mw | 0.95 | 0.09 | 203 | 0.87 | 0.827 | <0.0001 | none | 0.173 | 0.827±0.05 | |
| M2dw | 0.89 | 0.10 | 203 | −0.91 | 0.932 | <0.0001 | none | 0.068 | 0.932±0.04 | |
| M3l | 0.71 | 0.10 | 195 | −0.50 | 0.695 | <0.0001 | none | 0.305 | 0.695±0.08 |
Total c2 = amount of phenotypic variance attributable to covariates. Total h2 = (Residual h2)(1-Total c2). Total e2 = [1 – (Total c2 + Total h2)];
phenotype was also analyzed after being I-normalized to reduce kurtosis; I-normalized h2r estimate was 0.314±0.08 (p < 0.0001; kurtosis −0.23). All data are presented in mm but were multiplied by 100 for the genetic analyses to raise the variance above 1.0.
All but 5 of the 68 baboon tooth measurements yield significant heritabilities (p<0.05), with an average residual heritability of 0.56 and an average total heritability of 0.40. Covariate effects (primarily sex) contribute, on average, 28% to the total phenotypic variance. Non-genetic effects average 32%.
All of the mouse tooth measurements returned significant heritability estimates (p<0.01). The incisor residual heritabilities are lower (average is 0.30) than are those estimated for the molars (average is 0.84). Covariates were found to account for little to no amount of the total phenotypic variance. Non-genetic effects average account for about 16% of the total phenotypic variance.
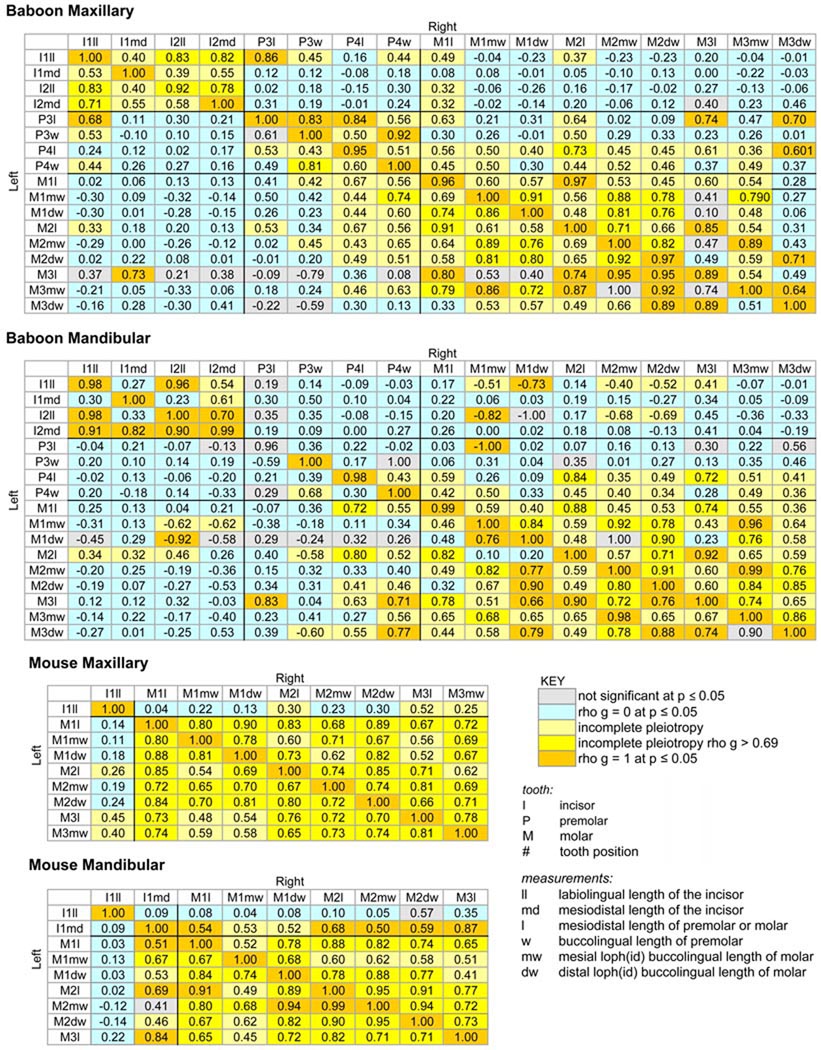
These residual heritability estimates were then used to construct patterns of genetic interrelatedness (correlations), i.e., that aspect of the genetic architecture that is of significance to evolutionary studies (Lande, ‘79; Schluter, 2000), shown as correlation matrices in Figure 2 and reported in detail in Table 3. Genetic correlations were estimated for all possible pair-wise comparisons, even though some of these were based on insignificant heritability estimates. As such, some of the values that populate the matrix, especially those indicated in gray, should be considered tentative at best. The genetic correlation estimates were compared to models in which the correlation was constrained to zero and one. The two far-right columns in Table 3 indicate the probability that the estimated genetic correlation is significantly different from one of these constrained models. Estimates that are significantly different from both one and zero are interpreted to indicate incomplete pleiotropy (see discussion in the Analytical Methods section).
FIGURE 2.
Matrices showing estimated genetic correlations between tooth size measurement pairs for pedigreed baboon and mouse populations described in the main text. All estimates are statistically significant at p ≤ 0.05 unless shaded gray (see key). Specific probabilities and other parameter estimates are reported in Table 3.
Table 3.
Bivariate statistical genetic analyses: Maximum-likelihood estimates of genetic and environmental correlations1
| Baboon Right Maxillary | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v I1md | 473 | 0.397 | 0.206 | 0.016 | <0.0000001 |
| I1ll v I2ll | 444 | 0.831 | 0.340 | <0.0000001 | 0.000016 |
| I1ll v I2md | 455 | 0.820 | −0.065 | <0.0000001 | 0.002 |
| I1ll v P3l | 206 | 0.857 | −0.247 | 0.003 | 0.32 |
| I1ll v P3w | 248 | 0.448 | 0.039 | 0.027 | 0.0004 |
| I1ll v P4l | 309 | 0.156 | 0.344 | 0.31 | <0.0000001 |
| I1ll v P4w | 325 | 0.438 | 0.011 | 0.008 | 0.0000003 |
| I1ll v M1l | 380 | 0.488 | −0.035 | 0.002 | <0.0000001 |
| I1ll v M1mw | 368 | −0.04 | −0.101 | 0.83 | 0.0000003 |
| I1ll v M1dw | 369 | −0.227 | 0.015 | 0.21 | 0.0000003 |
| I1ll v M2l | 429 | 0.366 | 0.161 | 0.018 | <0.0000001 |
| I1ll v M2mw | 427 | −0.233 | 0.052 | 0.19 | 0.0000004 |
| I1ll v M2dw | 418 | −0.232 | 0.078 | 0.20 | 0.0000006 |
| I1ll v M3l | 119 | 0.198 | 0.467 | 0.50 | 0.032 |
| I1ll v M3mw | 353 | −0.035 | 0.162 | 0.85 | 0.0000001 |
| I1ll v M3dw | 201 | 0.011 | −0.018 | 0.97 | 0.020 |
| I1md v I2ll | 452 | 0.391 | −0.233 | 0.017 | 0.0000002 |
| I1md v I2md | 463 | 0.549 | 0.001 | 0.0008 | 0.000006 |
| I1md v P3l | 207 | 0.118 | 0.222 | 0.64 | 0.007 |
| I1md v P3w | 253 | 0.116 | 0.320 | 0.54 | 0.000003 |
| I1md v P4l | 315 | −0.083 | 0.308 | 0.62 | 0.0000005 |
| I1md v P4w | 331 | 0.178 | 0.255 | 0.30 | <0.0000001 |
| I1md v M1l | 384 | 0.080 | 0.361 | 0.63 | <0.0000001 |
| I1md v M1mw | 372 | 0.082 | 0.317 | 0.65 | <0.0000001 |
| I1md v M1dw | 373 | −0.014 | 0.192 | 0.94 | 0.0000004 |
| I1md v M2l | 436 | 0.049 | 0.486 | 0.75 | <0.0000001 |
| I1md v M2mw | 434 | −0.102 | 0.294 | 0.56 | 0.0000001 |
| I1md v M2dw | 425 | 0.128 | 0.116 | 0.47 | <0.0000001 |
| I1md v M3l | 123 | −0.001 | 0.532 | 0.99 | 0.04 |
| I1md v M3mw | 358 | −0.224 | 0.321 | 0.23 | 0.000004 |
| I1md v M3dw | 205 | −0.029 | 0.148 | 0.902 | 0.015 |
| I2ll v I2md | 462 | 0.779 | −0.070 | <0.0000001 | 0.000009 |
| I2ll v P3l | 205 | 0.017 | 0.115 | 0.95 | 0.007 |
| I2ll v P3w | 249 | 0.179 | 0.130 | 0.33 | 0.000001 |
| I2ll v P4l | 310 | −0.152 | 0.698 | 0.30 | <0.0000001 |
| I2ll v P4w | 324 | 0.299 | −0.036 | 0.08 | <0.0000001 |
| I2ll v M1l | 368 | 0.318 | −0.201 | 0.03 | <0.0000001 |
| I2ll v M1mw | 358 | −0.056 | −0.381 | 0.74 | <0.0000001 |
| I2ll v M1dw | 358 | −0.256 | −0.182 | 0.12 | 0.0000002 |
| I2ll v M2l | 421 | 0.162 | 0.357 | 0.28 | <0.0000001 |
| I2ll v M2mw | 418 | −0.166 | −0.026 | 0.33 | 0.0000002 |
| I2ll v M2dw | 409 | −0.019 | −0.095 | 1 | <0.0000001 |
| I2ll v M3l | 121 | 0.266 | 0.110 | 0.31 | 0.043 |
| I2ll v M3mw | 343 | −0.125 | 0.230 | 0.50 | 0.000001 |
| I2ll v M3dw | 203 | −0.060 | −0.014 | 0.81 | 0.020 |
| I2md v P3l | 208 | 0.312 | 0.133 | 0.19 | 0.012 |
| I2md v P3w | 253 | 0.191 | 0.158 | 0.29 | 0.000003 |
| I2md v P4l | 314 | −0.009 | 0.571 | 0.95 | <0.00000001 |
| I2md v P4w | 330 | 0.238 | 0.044 | 0.16 | <0.00000001 |
| I2md v M1l | 377 | 0.322 | 0.062 | 0.03 | <0.00000001 |
| I2md v M1mw | 367 | −0.015 | −0.058 | 0.93 | 0.0000002 |
| I2md v M1dw | 367 | −0.136 | 0.055 | 0.41 | 0.0000001 |
| I2md v M2l | 430 | 0.202 | 0.208 | 0.17 | <0.00000001 |
| I2md v M2mw | 428 | −0.061 | −0.062 | 0.71 | 0.0000001 |
| I2md v M2dw | 419 | 0.119 | −0.125 | 0.49 | 0.0000001 |
| I2md v M3l | 122 | 0.400 | 0.261 | 0.142 | 0.052 |
| I2md v M3mw | 351 | 0.231 | −0.105 | 0.183 | <0.0000001 |
| I2md v M3dw | 204 | 0.460 | −0.185 | 0.046 | 0.033 |
| P3l v P3w | 243 | 0.833 | 0.016 | 0.004 | 0.20 |
| P3l v P4l | 259 | 0.837 | 0.088 | 0.0002 | 0.193 |
| P3l v P4w | 256 | 0.561 | 0.064 | 0.02 | 0.02 |
| P3l v M1l | 212 | 0.631 | 0.093 | 0.014 | 0.038 |
| P3l v M1mw | 198 | 0.213 | 0.437 | 0.47 | 0.003 |
| P3l v M1dw | 198 | 0.309 | 0.233 | 0.305 | 0.013 |
| P3l v M2l | 246 | 0.636 | 0.300 | 0.004 | 0.016 |
| P3l v M2mw | 244 | 0.016 | 0.428 | 0.953 | 0.005 |
| P3l v M2dw | 237 | 0.091 | 0.480 | 0.77 | 0.017 |
| P3l v M3l | 81 | 0.740 | 0.170 | 0.03 | 0.13 |
| P3l v M3mw | 212 | 0.472 | 0.130 | 0.094 | 0.005 |
| P3l v M3dw | 146 | 0.703 | 0.142 | 0.024 | 0.063 |
| P3w v P4l | 311 | 0.495 | 0.213 | 0.003 | 0.0000003 |
| P3w v P4w | 309 | 0.915 | 0.090 | <0.000001 | 0.144 |
| P3w v M1l | 255 | 0.299 | 0.561 | 0.126 | 0.000001 |
| P3w v M1mw | 240 | 0.263 | 0.343 | 0.212 | 0.0000003 |
| P3w v M1dw | 240 | −0.009 | 0.764 | 0.967 | 0.000001 |
| P3w v M2l | 297 | 0.503 | 0.300 | 0.003 | 0.0000008 |
| P3w v M2mw | 293 | 0.293 | 0.320 | 0.136 | 0.0000006 |
| P3w v M2dw | 287 | 0.333 | 0.236 | 0.113 | 0.000003 |
| P3w v M3l | 103 | 0.227 | 0.217 | 0.461 | 0.026 |
| P3w v M3mw | 251 | 0.256 | 0.112 | 0.213 | 0.000006 |
| P3w v M3dw | 166 | 0.010 | 0.319 | 0.97 | 0.02 |
| P4l v P4w | 383 | 0.511 | 0.195 | 0.0006 | <0.00000001 |
| P4l v M1l | 318 | 0.560 | 0.166 | 0.0003 | 0.0000001 |
| P4l v M1mw | 303 | 0.499 | −0.196 | 0.002 | <0.00000001 |
| P4l v M1dw | 304 | 0.402 | 0.114 | 0.024 | 0.0000007 |
| P4l v M2l | 375 | 0.725 | 0.369 | <0.0000001 | <0.00000001 |
| P4l v M2mw | 371 | 0.454 | 0.184 | 0.003 | <0.00000001 |
| P4l v M2dw | 362 | 0.451 | 0.197 | 0.005 | <0.00000001 |
| P4l v M3l | 138 | 0.614 | 0.221 | 0.002 | 0.005 |
| P4l v M3mw | 315 | 0.359 | 0.131 | 0.024 | <0.0000001 |
| P4l v M3dw | 209 | 0.601 | 0.116 | 0.006 | 0.049 |
| P4w v M1l | 332 | 0.448 | 0.247 | 0.007 | <0.00000001 |
| P4w v M1mw | 317 | 0.500 | 0.277 | 0.005 | 0.0000001 |
| P4w v M1dw | 317 | 0.302 | 0.392 | 0.104 | <0.00000001 |
| P4w v M2l | 389 | 0.436 | 0.383 | 0.007 | <0.00000001 |
| P4w v M2mw | 384 | 0.515 | 0.195 | 0.003 | 0.0000001 |
| P4w v M2dw | 375 | 0.460 | 0.106 | 0.013 | 0.0000005 |
| P4w v M3l | 143 | 0.374 | 0.201 | 0.189 | 0.012 |
| P4w v M3mw | 325 | 0.488 | −0.023 | 0.007 | 0.00001 |
| P4w v M3dw | 213 | 0.374 | 0.229 | 0.18 | 0.039 |
| M1l v M1mw | 435 | 0.599 | 0.310 | 0.0003 | <0.0000001 |
| M1l v M1dw | 437 | 0.565 | 0.061 | 0.0008 | 0.0000004 |
| M1l v M2l | 439 | 0.972 | 0.055 | <0.0000001 | 0.33 |
| M1l v M2mw | 437 | 0.526 | 0.047 | 0.002 | 0.000006 |
| M1l v M2dw | 425 | 0.453 | 0.214 | 0.009 | <0.0000001 |
| M1l v M3l | 137 | 0.601 | 0.418 | 0.021 | 0.043 |
| M1l v M3mw | 362 | 0.544 | 0.079 | 0.002 | 0.00002 |
| M1l v M3dw | 225 | 0.281 | 0.209 | 0.211 | 0.009 |
| M1mw v M1dw | 432 | 0.914 | 0.676 | <0.000001 | 0.0005 |
| M1mw v M2l | 426 | 0.564 | 0.003 | 0.0003 | 0.0000002 |
| M1mw v M2mw | 425 | 0.878 | 0.388 | <0.000001 | 0.004 |
| M1mw v M2dw | 414 | 0.781 | 0.275 | <0.00001 | 0.0006 |
| M1mw v M3l | 133 | 0.414 | 0.151 | 0.19 | 0.06 |
| M1mw v M3mw | 347 | 0.789 | 0.007 | <0.00001 | 0.005 |
| M1mw v M3dw | 213 | 0.269 | 0.383 | 0.316 | 0.014 |
| M1dw v M2l | 426 | 0.482 | −0.065 | 0.002 | 0.0000008 |
| M1dw v M2mw | 426 | 0.806 | 0.218 | <0.00001 | 0.0005 |
| M1dw v M2dw | 414 | 0.763 | 0.278 | <0.00001 | 0.000009 |
| M1dw v M3l | 131 | 0.103 | 0.205 | 0.77 | 0.049 |
| M1dw v M3mw | 349 | 0.482 | 0.168 | 0.015 | 0.00006 |
| M1dw v M3dw | 213 | 0.056 | 0.703 | 0.83 | 0.004 |
| M2l v M2mw | 525 | 0.714 | 0.240 | <0.00001 | 0.000009 |
| M2l v M2dw | 513 | 0.658 | 0.267 | 0.00002 | 0.0000001 |
| M2l v M3l | 167 | 0.854 | 0.548 | 0.003 | 0.28 |
| M2l v M3mw | 424 | 0.538 | 0.219 | 0.002 | 0.00004 |
| M2l v M3dw | 260 | 0.309 | 0.357 | 0.13 | 0.005 |
| M2mw v M2dw | 516 | 0.820 | 0.744 | <0.00001 | <0.0000001 |
| M2mw v M3l | 165 | 0.472 | 0.337 | 0.14 | 0.08 |
| M2mw v M3mw | 422 | 0.891 | 0.273 | <0.000001 | 0.08 |
| M2mw v M3dw | 258 | 0.431 | 0.507 | 0.13 | 0.03 |
| M2dw v M3l | 164 | 0.492 | 0.341 | 0.09 | 0.03 |
| M2dw v M3mw | 418 | 0.589 | 0.367 | 0.002 | 0.000002 |
| M2dw v M3dw | 255 | 0.709 | 0.557 | 0.009 | 0.054 |
| M3l v M3mw | 168 | 0.535 | 0.749 | 0.023 | 0.003 |
| M3l v M3dw | 133 | 0.486 | 0.607 | 0.17 | 0.004 |
| M3mw v M3dw | 275 | 0.643 | 0.699 | 0.04 | 0.047 |
| Baboon Left Maxillary | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v I1md | 469 | 0.529 | 0.178 | 0.0007 | <0.0000001 |
| I1ll v I2ll | 447 | 0.828 | 0.366 | <0.000001 | 0.0015 |
| I1ll v I2md | 456 | 0.711 | −0.069 | 0.0001 | 0.004 |
| I1ll v P3l | 211 | 0.678 | −0.159 | 0.034 | 0.21 |
| I1ll v P3w | 255 | 0.527 | −0.075 | 0.038 | 0.023 |
| I1ll v P4l | 322 | 0.235 | 0.161 | 0.191 | <0.0000001 |
| I1ll v P4w | 339 | 0.441 | −0.133 | 0.018 | 0.00003 |
| I1ll v M1l | 382 | 0.021 | 0.343 | 0.909 | <0.0000001 |
| I1ll v M1mw | 377 | −0.295 | 0.168 | 0.11 | 0.0000002 |
| I1ll v M1dw | 374 | −0.304 | 0.225 | 0.089 | 0.0000002 |
| I1ll v M2l | 428 | 0.330 | 0.126 | 0.027 | <0.0000001 |
| I1ll v M2mw | 429 | −0.292 | 0.183 | 0.121 | 0.000004 |
| I1ll v M2dw | 424 | 0.019 | −0.098 | 0.92 | <0.0000001 |
| I1ll v M3l | 165 | 0.373 | 0.078 | 0.23 | 0.07 |
| I1ll v M3mw | 343 | −0.209 | 0.147 | 0.42 | 0.008 |
| I1ll v M3dw | 193 | −0.159 | 0.255 | 0.56 | 0.012 |
| I1md v I2ll | 448 | 0.401 | −0.131 | 0.011 | <0.0000001 |
| I1md v I2md | 457 | 0.550 | 0.144 | 0.0015 | 0.00006 |
| I1md v P3l | 211 | 0.108 | 0.256 | 0.68 | 0.026 |
| I1md v P3w | 255 | −0.099 | 0.346 | 0.635 | 0.0001 |
| I1md v P4l | 323 | 0.115 | 0.363 | 0.484 | <0.0000001 |
| I1md v P4w | 341 | 0.264 | 0.112 | 0.089 | <0.0000001 |
| I1md v M1l | 384 | 0.063 | 0.531 | 0.669 | <0.0000001 |
| I1md v M1mw | 379 | 0.087 | 0.279 | 0.57 | <0.0000001 |
| I1md v M1dw | 376 | 0.014 | 0.399 | 0.92 | <0.0000001 |
| I1md v M2l | 430 | 0.178 | 0.481 | 0.17 | <0.0000001 |
| I1md v M2mw | 431 | 0.003 | 0.439 | 0.98 | <0.0000001 |
| I1md v M2dw | 426 | 0.224 | 0.163 | 0.144 | <0.0000001 |
| I1md v M3l | 165 | 0.732 | −0.110 | 0.019 | 0.283 |
| I1md v M3mw | 344 | 0.045 | 0.076 | 0.83 | 0.0016 |
| I1md v M3dw | 194 | 0.282 | 0.118 | 0.194 | 0.008 |
| I2ll v I2md | 462 | 0.582 | 0.009 | 0.0015 | 0.0005 |
| I2ll v P3l | 211 | 0.302 | 0.015 | 0.31 | 0.038 |
| I2ll v P3w | 251 | 0.100 | 0.084 | 0.69 | 0.0002 |
| I2ll v P4l | 319 | 0.020 | 0.336 | 0.907 | <0.0000001 |
| I2ll v P4w | 335 | 0.273 | −0.087 | 0.135 | <0.0000001 |
| I2ll v M1l | 374 | 0.125 | −0.015 | 0.484 | <0.0000001 |
| I2ll v M1mw | 369 | −0.323 | 0.234 | 0.075 | <0.0000001 |
| I2ll v M1dw | 366 | −0.277 | 0.313 | 0.114 | <0.0000001 |
| I2ll v M2l | 422 | 0.199 | 0.156 | 0.17 | <0.0000001 |
| I2ll v M2mw | 423 | −0.256 | 0.195 | 0.156 | 0.0000002 |
| I2ll v M2dw | 418 | 0.082 | −0.057 | 0.65 | <0.0000001 |
| I2ll v M3l | 164 | 0.208 | 0.002 | 0.47 | 0.054 |
| I2ll v M3mw | 336 | −0.330 | 0.194 | 0.17 | 0.015 |
| I2ll v M3dw | 191 | −0.296 | 0.426 | 0.23 | 0.012 |
| I2md v P3l | 215 | 0.205 | 0.219 | 0.52 | 0.049 |
| I2md v P3w | 258 | 0.150 | 0.150 | 0.54 | 0.0002 |
| I2md v P4l | 327 | 0.171 | 0.209 | 0.36 | <0.000001 |
| I2md v P4w | 344 | 0.162 | 0.071 | 0.40 | <0.000001 |
| I2md v M1l | 382 | 0.133 | 0.209 | 0.49 | <0.000001 |
| I2md v M1mw | 376 | −0.144 | 0.184 | 0.467 | <0.000001 |
| I2md v M1dw | 373 | −0.145 | 0.163 | 0.45 | <0.000001 |
| I2md v M2l | 430 | 0.130 | 0.405 | 0.413 | <0.000001 |
| I2md v M2mw | 431 | −0.116 | 0.281 | 0.536 | <0.000001 |
| I2md v M2dw | 426 | 0.010 | 0.251 | 0.958 | <0.000001 |
| I2md v M3l | 167 | 0.384 | 0.078 | 0.28 | 0.12 |
| I2md v M3mw | 344 | 0.063 | 0.068 | 0.81 | 0.002 |
| I2md v M3dw | 195 | 0.409 | 0.125 | 0.111 | 0.010 |
| P3l v P3w | 248 | 0.614 | 0.315 | 0.058 | 0.062 |
| P3l v P4l | 263 | 0.530 | 0.254 | 0.036 | 0.0099 |
| P3l v P4w | 262 | 0.493 | 0.261 | 0.052 | 0.017 |
| P3l v M1l | 224 | 0.407 | 0.263 | 0.13 | 0.019 |
| P3l v M1mw | 215 | 0.497 | 0.195 | 0.05 | 0.007 |
| P3l v M1dw | 211 | 0.255 | 0.238 | 0.38 | 0.017 |
| P3l v M2l | 259 | 0.534 | 0.271 | 0.020 | 0.019 |
| P3l v M2mw | 257 | 0.015 | 0.427 | 0.95 | 0.006 |
| P3l v M2dw | 253 | −0.010 | 0.443 | 0.97 | 0.008 |
| P3l v M3l | 133 | −0.094 | 0.570 | 0.84 | 0.09 |
| P3l v M3mw | 214 | 0.181 | 0.310 | 0.62 | 0.005 |
| P3l v M3dw | 143 | −0.222 | 0.394 | 0.71 | 0.18 |
| P3w v P4l | 314 | 0.434 | 0.405 | 0.041 | 0.0001 |
| P3w v P4w | 319 | 0.812 | 0.486 | <0.00001 | 0.009 |
| P3w v M1l | 268 | 0.423 | 0.392 | 0.04 | 0.00004 |
| P3w v M1mw | 262 | 0.423 | 0.466 | 0.06 | 0.0003 |
| P3w v M1dw | 258 | 0.229 | 0.597 | 0.29 | <0.0001 |
| P3w v M2l | 308 | 0.338 | 0.453 | 0.07 | 0.00001 |
| P3w v M2mw | 304 | 0.448 | 0.347 | 0.03 | 0.00001 |
| P3w v M2dw | 301 | 0.198 | 0.420 | 0.35 | 0.00001 |
| P3w v M3l | 146 | −0.789 | 0.553 | 0.058 | 0.345 |
| P3w v M3mw | 252 | 0.237 | 0.291 | 0.44 | 0.002 |
| P3w v M3dw | 153 | −0.584 | 0.483 | 0.16 | 0.19 |
| P4l v P4w | 405 | 0.603 | 0.282 | 0.0001 | <0.0000001 |
| P4l v M1l | 339 | 0.667 | 0.288 | 0.00004 | 0.000005 |
| P4l v M1mw | 328 | 0.437 | 0.257 | 0.013 | <0.0000001 |
| P4l v M1dw | 323 | 0.441 | 0.356 | 0.009 | <0.0000001 |
| P4l v M2l | 396 | 0.668 | 0.450 | <0.000001 | <0.0000001 |
| P4l v M2mw | 392 | 0.431 | 0.134 | 0.011 | <0.0000001 |
| P4l v M2dw | 388 | 0.488 | 0.030 | 0.003 | <0.0000001 |
| P4l v M3l | 189 | 0.355 | 0.442 | 0.184 | 0.031 |
| P4l v M3mw | 321 | 0.462 | 0.107 | 0.028 | 0.0004 |
| P4l v M3dw | 195 | 0.297 | 0.335 | 0.24 | 0.004 |
| P4w v M1l | 358 | 0.558 | 0.086 | 0.0005 | <0.0000001 |
| P4w v M1mw | 348 | 0.736 | 0.057 | <0.000001 | <0.000001 |
| P4w v M1dw | 343 | 0.600 | 0.193 | 0.00009 | <0.0000001 |
| P4w v M2l | 416 | 0.560 | 0.109 | 0.00009 | <0.0000001 |
| P4w v M2mw | 412 | 0.652 | 0.128 | 0.00003 | <0.0000001 |
| P4w v M2dw | 407 | 0.508 | 0.242 | 0.002 | <0.0000001 |
| P4w v M3l | 194 | 0.078 | 0.301 | 0.836 | 0.095 |
| P4w v M3mw | 337 | 0.626 | 0.264 | 0.003 | 0.0007 |
| P4w v M3dw | 203 | 0.126 | 0.426 | 0.69 | 0.009 |
| M1l v M1mw | 454 | 0.692 | 0.066 | <0.00001 | <0.000001 |
| M1l v M1dw | 450 | 0.742 | −0.199 | <0.000001 | 0.00003 |
| M1l v M2l | 455 | 0.913 | 0.072 | <0.000001 | 0.043 |
| M1l v M2mw | 454 | 0.644 | −0.002 | 0.000019 | 0.0000005 |
| M1l v M2dw | 447 | 0.575 | 0.135 | 0.0002 | <0.000001 |
| M1l v M3l | 188 | 0.803 | 0.345 | 0.0004 | 0.053 |
| M1l v M3mw | 368 | 0.794 | 0.140 | 0.00005 | 0.015 |
| M1l v M3dw | 224 | 0.334 | 0.186 | 0.156 | 0.002 |
| M1mw v M1dw | 447 | 0.856 | 0.749 | <0.000001 | <0.000001 |
| M1mw v M2l | 444 | 0.613 | −0.085 | <0.00001 | <0.000001 |
| M1mw v M2mw | 444 | 0.887 | 0.276 | <0.000001 | 0.00012 |
| M1mw v M2dw | 437 | 0.813 | 0.242 | <0.000001 | 0.0002 |
| M1mw v M3l | 182 | 0.528 | 0.216 | 0.082 | 0.092 |
| M1mw v M3mw | 359 | 0.860 | 0.246 | <0.00001 | 0.049 |
| M1mw v M3dw | 216 | 0.528 | 0.093 | 0.024 | 0.019 |
| M1dw v M2l | 440 | 0.576 | −0.203 | 0.00002 | <0.000001 |
| M1dw v M2mw | 440 | 0.758 | 0.296 | <0.000001 | <0.000001 |
| M1dw v M2dw | 434 | 0.803 | 0.406 | <0.000001 | <0.000001 |
| M1dw v M3l | 179 | 0.397 | 0.412 | 0.221 | 0.062 |
| M1dw v M3mw | 354 | 0.721 | 0.281 | 0.0004 | 0.003 |
| M1dw v M3dw | 212 | 0.571 | 0.183 | 0.011 | 0.013 |
| M2l v M2mw | 530 | 0.687 | 0.111 | <0.000001 | <0.000001 |
| M2l v M2dw | 522 | 0.649 | 0.159 | <0.000001 | <0.000001 |
| M2l v M3l | 225 | 0.743 | 0.388 | 0.0007 | 0.085 |
| M2l v M3mw | 427 | 0.868 | 0.055 | <0.000001 | 0.068 |
| M2l v M3dw | 259 | 0.490 | 0.158 | 0.007 | 0.0008 |
| M2mw v M2dw | 528 | 0.916 | 0.532 | <0.000001 | 0.0013 |
| M2mw v M3l | 224 | 0.951 | −0.026 | 0.0006 | 0.42 |
| M2mw v M3mw | 422 | 1.00 | 0.373 | nc | <0.000001 |
| M2mw v M3dw | 257 | 0.663 | 0.210 | 0.0013 | 0.008 |
| M2dw v M3l | 222 | 0.947 | 0.086 | 0.001 | 0.41 |
| M2dw v M3mw | 421 | 0.917 | 0.292 | <0.00001 | 0.12 |
| M2dw v M3dw | 256 | 0.889 | 0.263 | 0.00003 | 0.158 |
| M3l v M3mw | 218 | 0.743 | 0.492 | 0.054 | 0.159 |
| M3l v M3dw | 158 | 0.885 | 0.433 | 0.015 | 0.286 |
| M3mw v M3dw | 268 | 0.511 | 0.702 | 0.224 | 0.012 |
| Baboon Right Mandibular | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v I1md | 456 | 0.273 | 0.310 | 0.116 | <0.000001 |
| I1ll v I2ll | 427 | 0.959 | 0.626 | <0.000001 | 0.19 |
| I1ll v I2md | 435 | 0.539 | 0.129 | 0.033 | 0.019 |
| I1ll v P3l | 120 | 0.186 | 0.623 | 0.66 | 0.34 |
| I1ll v P3w | 203 | 0.140 | −0.148 | 0.62 | 0.009 |
| I1ll v P4l | 302 | −0.093 | 0.292 | 0.59 | <0.0000001 |
| I1ll v P4w | 295 | −0.026 | 0.102 | 0.88 | <0.0000001 |
| I1ll v M1l | 288 | 0.174 | 0.233 | 0.325 | <0.0000001 |
| I1ll v M1mw | 273 | −0.510 | 0.065 | 0.006 | 0.000006 |
| I1ll v M1dw | 282 | −0.730 | 0.480 | 0.0002 | 0.046 |
| I1ll v M2l | 388 | 0.140 | 0.222 | 0.388 | <0.0000001 |
| I1ll v M2mw | 381 | −0.395 | 0.185 | 0.014 | 0.0000001 |
| I1ll v M2dw | 376 | −0.522 | 0.189 | 0.004 | 0.00002 |
| I1ll v M3l | 167 | 0.412 | −0.333 | 0.040 | 0.002 |
| I1ll v M3mw | 372 | −0.070 | −0.134 | 0.67 | <0.0000001 |
| I1ll v M3dw | 354 | −0.006 | −0.046 | 0.97 | <0.0000001 |
| I1md v I2ll | 433 | 0.226 | 0.148 | 0.263 | 0.0001 |
| I1md v I2md | 444 | 0.612 | 0.502 | 0.006 | 0.003 |
| I1md v P3l | 123 | 0.297 | −1.00 | 0.066 | 0.024 |
| I1md v P3w | 208 | 0.497 | −0.298 | 0.045 | 0.026 |
| I1md v P4l | 308 | 0.095 | 0.336 | 0.583 | <0.0000001 |
| I1md v P4w | 302 | 0.043 | 0.343 | 0.795 | <0.0000001 |
| I1md v M1l | 294 | 0.219 | 0.422 | 0.195 | <0.0000001 |
| I1md v M1mw | 279 | 0.057 | 0.150 | 0.764 | 0.0000001 |
| I1md v M1dw | 288 | 0.025 | 0.279 | 0.89 | 0.000004 |
| I1md v M2l | 397 | 0.188 | 0.586 | 0.214 | <0.0000001 |
| I1md v M2mw | 390 | 0.153 | 0.184 | 0.34 | <0.0000001 |
| I1md v M2dw | 384 | −0.273 | 0.440 | 0.138 | 0.00001 |
| I1md v M3l | 170 | 0.338 | −0.364 | 0.066 | 0.0002 |
| I1md v M3mw | 379 | 0.053 | 0.076 | 0.74 | <0.0000001 |
| I1md v M3dw | 360 | −0.092 | 0.288 | 0.60 | <0.0000001 |
| I2ll v I2md | 451 | 0.698 | 0.086 | 0.019 | 0.128 |
| I2ll v P3l | 124 | 0.353 | 0.827 | 0.238 | 0.110 |
| I2ll v P3w | 206 | 0.352 | −0.334 | 0.272 | 0.026 |
| I2ll v P4l | 307 | −0.083 | 0.148 | 0.67 | 0.00004 |
| I2ll v P4w | 296 | −0.146 | 0.268 | 0.457 | 0.00008 |
| I2ll v M1l | 281 | 0.199 | 0.270 | 0.33 | 0.00004 |
| I2ll v M1mw | 267 | −0.824 | 0.197 | 0.0002 | 0.116 |
| I2ll v M1dw | 275 | −1.00 | 0.418 | nc | 0.00002 |
| I2ll v M2l | 385 | 0.167 | 0.297 | 0.373 | 0.00004 |
| I2ll v M2mw | 378 | −0.678 | 0.307 | 0.0008 | 0.033 |
| I2ll v M2dw | 373 | −0.691 | 0.303 | 0.001 | 0.020 |
| I2ll v M3l | 167 | 0.453 | −0.159 | 0.048 | 0.002 |
| I2ll v M3mw | 371 | −0.360 | 0.138 | 0.083 | 0.002 |
| I2ll v M3dw | 357 | −0.326 | 0.150 | 0.136 | 0.0008 |
| I2md v P3l | 126 | 0.186 | 1.00 | 0.588 | 0.011 |
| I2md v P3w | 210 | 0.090 | −0.167 | 0.86 | 0.005 |
| I2md v P4l | 311 | −0.000 | 0.194 | 0.99 | 0.0002 |
| I2md v P4w | 300 | 0.268 | 0.194 | 0.22 | 0.0003 |
| I2md v M1l | 291 | 0.258 | 0.130 | 0.27 | 0.0002 |
| I2md v M1mw | 277 | 0.000 | 0.187 | 1.00 | 0.0003 |
| I2md v M1dw | 285 | 0.021 | 0.204 | 0.94 | 0.0003 |
| I2md v M2l | 395 | 0.175 | 0.503 | 0.395 | 0.0002 |
| I2md v M2mw | 389 | 0.080 | 0.326 | 0.72 | 0.0002 |
| I2md v M2dw | 383 | −0.131 | 0.215 | 0.583 | 0.0006 |
| I2md v M3l | 169 | 0.413 | −0.097 | 0.13 | 0.007 |
| I2md v M3mw | 378 | 0.035 | −0.007 | 0.875 | 0.0002 |
| I2md v M3dw | 362 | −0.187 | 0.214 | 0.439 | 0.0008 |
| P3l v P3w | 167 | −0.364 | 1.00 | 0.26 | 0.007 |
| P3l v P4l | 160 | 0.215 | 1.00 | 0.224 | <0.000001 |
| P3l v P4w | 147 | −0.021 | −1.00 | 0.90 | <0.000001 |
| P3l v M1l | 114 | 0.032 | −1.00 | 0.775 | 0.025 |
| P3l v M1mw | 103 | −1.00 | 0.570 | nc | 0.86 |
| P3l v M1dw | 104 | 0.019 | 1.00 | 0.933 | 0.0003 |
| P3l v M2l | 147 | 0.068 | −1.00 | 0.64 | 0.001 |
| P3l v M2mw | 144 | 0.155 | −1.00 | 0.38 | 0.008 |
| P3l v M2dw | 142 | 0.128 | −1.00 | 0.535 | 0.007 |
| P3l v M3l | 79 | 0.301 | −1.00 | 0.172 | 0.162 |
| P3l v M3mw | 149 | 0.215 | −1.00 | 0.283 | 0.026 |
| P3l v M3dw | 144 | 0.555 | −1.00 | 0.048 | 0.045 |
| P3w v P4l | 255 | 0.171 | 0.175 | 0.520 | 0.031 |
| P3w v P4w | 244 | 1.00 | 0.420 | nc | <0.000001 |
| P3w v M1l | 174 | 0.061 | 0.663 | 0.85 | 0.033 |
| P3w v M1mw | 160 | 0.313 | 0.590 | 0.363 | 0.030 |
| P3w v M1dw | 161 | 0.045 | 0.558 | 0.898 | 0.021 |
| P3w v M2l | 233 | 0.355 | 0.298 | 0.249 | 0.046 |
| P3w v M2mw | 229 | 0.010 | 0.789 | 0.972 | 0.018 |
| P3w v M2dw | 222 | 0.269 | 0.387 | 0.361 | 0.020 |
| P3w v M3l | 108 | 0.127 | −0.120 | 0.656 | 0.026 |
| P3w v M3mw | 238 | 0.350 | 0.318 | 0.230 | 0.025 |
| P3w v M3dw | 225 | 0.458 | 0.133 | 0.102 | 0.029 |
| P4l v P4w | 357 | 0.429 | −0.040 | 0.007 | <0.000001 |
| P4l v M1l | 242 | 0.585 | 0.179 | 0.00009 | <0.000001 |
| P4l v M1mw | 225 | 0.256 | 0.295 | 0.161 | <0.000001 |
| P4l v M1dw | 231 | 0.092 | 0.706 | 0.621 | <0.000001 |
| P4l v M2l | 337 | 0.839 | −0.085 | <0.000001 | 0.0003 |
| P4l v M2mw | 332 | 0.352 | 0.290 | 0.015 | <0.000001 |
| P4l v M2dw | 324 | 0.488 | 0.234 | 0.002 | <0.000001 |
| P4l v M3l | 161 | 0.720 | −0.466 | <0.00001 | 0.009 |
| P4l v M3mw | 334 | 0.508 | −0.008 | 0.0004 | <0.000001 |
| P4l v M3dw | 319 | 0.409 | 0.262 | 0.009 | <0.000001 |
| P4w v M1l | 238 | 0.423 | 0.071 | 0.010 | <0.000001 |
| P4w v M1mw | 217 | 0.496 | 0.246 | 0.004 | <0.000001 |
| P4w v M1dw | 225 | 0.325 | 0.363 | 0.09 | 0.00001 |
| P4w v M2l | 334 | 0.449 | 0.091 | 0.003 | <0.000001 |
| P4w v M2mw | 328 | 0.400 | 0.360 | 0.008 | <0.000001 |
| P4w v M2dw | 323 | 0.340 | 0.350 | 0.037 | <0.000001 |
| P4w v M3l | 163 | 0.283 | −1.00 | 0.162 | 0.0006 |
| P4w v M3mw | 338 | 0.488 | −0.207 | 0.001 | <0.000001 |
| P4w v M3dw | 322 | 0.364 | 0.034 | 0.034 | <0.000001 |
| M1l v M1mw | 321 | 0.586 | −0.195 | 0.0007 | <0.0000001 |
| M1l v M1dw | 330 | 0.403 | 0.471 | 0.03 | 0.000003 |
| M1l v M2l | 342 | 0.876 | −0.365 | <0.0000001 | 0.0003 |
| M1l v M2mw | 336 | 0.445 | −0.078 | 0.002 | <0.0000001 |
| M1l v M2dw | 329 | 0.525 | −0.368 | 0.0009 | <0.0000001 |
| M1l v M3l | 144 | 0.744 | −1.00 | <0.0000001 | 0.02 |
| M1l v M3mw | 309 | 0.547 | −0.240 | 0.0002 | <0.0000001 |
| M1l v M3dw | 296 | 0.358 | 0.196 | 0.03 | <0.0000001 |
| M1mw v M1dw | 314 | 0.842 | 0.750 | 0.00005 | 0.0003 |
| M1mw v M2l | 319 | 0.587 | −0.461 | 0.00004 | <0.000001 |
| M1mw v M2mw | 313 | 0.920 | 0.177 | <0.000001 | 0.039 |
| M1mw v M2dw | 306 | 0.778 | 0.208 | <0.000001 | 0.000001 |
| M1mw v M3l | 132 | 0.434 | −1.00 | 0.004 | 0.00002 |
| M1mw v M3mw | 288 | 0.964 | 0.011 | <0.000001 | 0.211 |
| M1mw v M3dw | 277 | 0.641 | 0.134 | 0.0002 | 0.00006 |
| M1dw v M2l | 327 | 0.478 | −0.155 | 0.001 | <0.0000001 |
| M1dw v M2mw | 321 | 1.00 | 0.124 | nc | <0.000001 |
| M1dw v M2dw | 313 | 0.903 | 0.233 | <0.0000001 | 0.022 |
| M1dw v M3l | 137 | 0.227 | −0.254 | 0.28 | 0.0001 |
| M1dw v M3mw | 295 | 0.755 | 0.194 | 0.00001 | 0.014 |
| M1dw v M3dw | 284 | 0.583 | 0.298 | 0.0005 | 0.00007 |
| M2l v M2mw | 480 | 0.566 | 0.397 | <0.0001 | <0.000001 |
| M2l v M2dw | 474 | 0.706 | −0.098 | <0.000001 | <0.000001 |
| M2l v M3l | 207 | 0.920 | −0.249 | <0.000001 | 0.109 |
| M2l v M3mw | 437 | 0.650 | −0.049 | <0.000001 | <0.000001 |
| M2l v M3dw | 417 | 0.590 | 0.036 | 0.00002 | <0.000001 |
| M2mw v M2dw | 466 | 0.905 | 0.536 | <0.000001 | <0.000001 |
| M2mw v M3l | 200 | 0.595 | −1.00 | <0.00001 | 0.00007 |
| M2mw v M3mw | 427 | 0.985 | 0.016 | <0.000001 | 0.203 |
| M2mw v M3dw | 408 | 0.762 | 0.153 | <0.00001 | 0.0001 |
| M2mw v M3l | 198 | 0.601 | −1.00 | <0.00001 | 0.0004 |
| M2dw v M3mw | 424 | 0.844 | 0.160 | <0.000001 | <0.000001 |
| M2dw v M3dw | 405 | 0.850 | 0.264 | <0.000001 | 0.0008 |
| M3l v M3mw | 227 | 0.739 | −1.00 | <0.000001 | 0.0001 |
| M3l v M3dw | 226 | 0.654 | −1.00 | <0.000001 | 0.00001 |
| M3mw v M3dw | 459 | 0.859 | 0.534 | <0.000001 | 0.00004 |
| Baboon Left Mandibular | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v I1md | 449 | 0.301 | 0.225 | 0.077 | 0.0000001 |
| I1ll v I2ll | 424 | 0.979 | 0.438 | <0.000001 | 0.239 |
| I1ll v I2md | 430 | 0.907 | 0.025 | <0.00001 | 0.159 |
| I1ll v P3l | 109 | −0.044 | 1.00 | 0.884 | 0.0196 |
| I1ll v P3w | 196 | 0.199 | −0.068 | 0.494 | 0.013 |
| I1ll v P4l | 280 | −0.016 | 0.059 | 0.935 | <0.000001 |
| I1ll v P4w | 278 | 0.202 | −0.106 | 0.326 | 0.00004 |
| I1ll v M1l | 288 | 0.251 | 0.073 | 0.142 | <0.000001 |
| I1ll v M1mw | 280 | −0.309 | −0.032 | 0.133 | 0.0001 |
| I1ll v M1dw | 276 | −0.453 | 0.034 | 0.123 | 0.116 |
| I1ll v M2l | 375 | 0.336 | −0.318 | 0.032 | <0.000001 |
| I1ll v M2mw | 375 | −0.201 | −0.075 | 0.259 | <0.000001 |
| I1ll v M2dw | 367 | 0.192 | −0.047 | 0.127 | 0.0000068 |
| I1ll v M3l | 248 | 0.117 | 0.002 | 0.583 | 0.0016 |
| I1ll v M3mw | 373 | −0.137 | −0.016 | 0.494 | 0.0000058 |
| I1ll v M3dw | 350 | −0.266 | 0.030 | 0.177 | 0.00001 |
| I1md v I2ll | 426 | 0.333 | 0.155 | 0.071 | 0.000006 |
| I1md v I2md | 434 | 0.824 | 0.321 | 0.00028 | 0.081 |
| I1md v P3l | 111 | 0.207 | 1.00 | 0.505 | <0.000001 |
| I1md v P3w | 199 | 0.102 | 0.253 | 0.705 | 0.005 |
| I1md v P4l | 284 | 0.128 | 0.167 | 0.514 | <0.000001 |
| I1md v P4w | 281 | −0.175 | 0.251 | 0.443 | 0.003 |
| I1md v M1l | 289 | 0.127 | 0.734 | 0.443 | <0.000001 |
| I1md v M1mw | 281 | 0.130 | 0.260 | 0.522 | 0.00006 |
| I1md v M1dw | 277 | 0.289 | 0.0695 | 0.269 | 0.035 |
| I1md v M2l | 378 | 0.324 | 0.285 | 0.039 | <0.000001 |
| I1md v M2mw | 377 | 0.252 | 0.276 | 0.154 | <0.000001 |
| I1md v M2dw | 370 | 0.068 | 0.263 | 0.732 | <0.000001 |
| I1md v M3l | 249 | 0.122 | 0.020 | 0.547 | 0.00069 |
| I1md v M3mw | 376 | 0.2199 | 0.088 | 0.297 | 0.0000053 |
| I1md v M3dw | 353 | 0.010 | 0.152 | 0.96 | 0.0000046 |
| I2ll v I2md | 448 | 0.897 | 0.067 | 0.00003 | 0.178 |
| I2ll v P3l | 108 | −0.069 | 1.00 | 0.796 | 0.021 |
| I2ll v P3w | 197 | 0.144 | 0.122 | 0.659 | 0.018 |
| I2ll v P4l | 284 | −0.061 | 0.173 | 0.773 | 0.0000017 |
| I2ll v P4w | 282 | 0.144 | 0.153 | 0.518 | 0.000008 |
| I2ll v M1l | 284 | 0.045 | 0.527 | 0.806 | 0.0000001 |
| I2ll v M1mw | 276 | −0.616 | 0.080 | 0.013 | 0.023 |
| I2ll v M1dw | 271 | −0.921 | 0.141 | 0.0099 | 0.422 |
| I2ll v M2l | 373 | 0.464 | −0.147 | 0.004 | 0.0000004 |
| I2ll v M2mw | 372 | −0.187 | −0.087 | 0.358 | 0.0000186 |
| I2ll v M2dw | 365 | −0.266 | −0.055 | 0.231 | 0.0002 |
| I2ll v M3l | 248 | 0.317 | −0.081 | 0.164 | 0.0017 |
| I2ll v M3mw | 374 | −0.165 | 0.069 | 0.469 | 0.00035 |
| I2ll v M3dw | 350 | −0.246 | 0.017 | 0.274 | 0.0003 |
| I2md v P3l | 110 | −0.134 | 1.00 | 0.756 | 0.052 |
| I2md v P3w | 200 | 0.185 | 0.121 | 0.612 | 0.008 |
| I2md v P4l | 289 | −0.203 | 0.207 | 0.466 | 0.002 |
| I2md v P4w | 286 | −0.332 | 0.163 | 0.264 | 0.006 |
| I2md v M1l | 290 | 0.207 | 0.231 | 0.432 | 0.0015 |
| I2md v M1mw | 282 | −0.616 | 0.135 | 0.032 | 0.033 |
| I2md v M1dw | 277 | −0.579 | 0.056 | 0.119 | 0.130 |
| I2md v M2l | 378 | 0.260 | 0.097 | 0.275 | 0.0007 |
| I2md v M2mw | 377 | −0.356 | 0.010 | 0.191 | 0.007 |
| I2md v M2dw | 370 | −0.527 | 0.037 | 0.086 | 0.039 |
| I2md v M3l | 251 | −0.034 | 0.073 | 0.911 | 0.0003 |
| I2md v M3mw | 379 | −0.398 | −0.010 | 0.207 | 0.011 |
| I2md v M3dw | 355 | 0.528 | −0.099 | 0.532 | 0.0162 |
| P3l v P3w | 135 | −0.593 | −1.00 | 0.368 | 0.007 |
| P3l v P4l | 135 | 0.206 | 1.00 | 0.425 | 0.0000001 |
| P3l v P4w | 127 | 0.292 | −1.00 | 0.363 | 0.205 |
| P3l v M1l | 91 | −0.068 | −1.00 | 0.751 | <0.0000001 |
| P3l v M1mw | 90 | −0.378 | −1.00 | 0.27 | 0.00005 |
| P3l v M1dw | 88 | 0.285 | −0.900 | 0.449 | 0.125 |
| P3l v M2l | 125 | 0.396 | 1.00 | 0.097 | <0.0000001 |
| P3l v M2mw | 123 | 0.151 | −1.00 | 0.475 | 0.004 |
| P3l v M2dw | 119 | 0.341 | −1.00 | 0.169 | 0.020 |
| P3l v M3l | 80 | 0.829 | −1.00 | 0.013 | 0.237 |
| P3l v M3mw | 126 | 0.231 | 1.00 | 0.380 | 0.00006 |
| P3l v M3dw | 118 | 0.391 | −1.00 | 0.259 | 0.013 |
| P3w v P4l | 246 | 0.392 | 0.045 | 0.177 | 0.013 |
| P3w v P4w | 233 | 0.676 | 0.581 | 0.015 | 0.007 |
| P3w v M1l | 164 | 0.361 | 0.519 | 0.211 | 0.019 |
| P3w v M1mw | 163 | −0.181 | −0.428 | 0.564 | 0.005 |
| P3w v M1dw | 159 | −0.237 | 0.364 | 0.581 | 0.109 |
| P3w v M2l | 229 | −0.581 | −0.091 | 0.016 | 0.008 |
| P3w v M2mw | 225 | 0.320 | 0.362 | 0.220 | 0.004 |
| P3w v M2dw | 220 | 0.308 | 0.323 | 0.252 | 0.002 |
| P3w v M3l | 153 | 0.044 | −0.126 | 0.902 | 0.003 |
| P3w v M3mw | 230 | 0.414 | 0.186 | 0.141 | 0.002 |
| P3w v M3dw | 213 | −0.599 | 0.083 | 0.022 | 0.008 |
| P4l v P4w | 328 | 0.303 | 0.314 | 0.191 | 0.000076 |
| P4l v M1l | 222 | 0.724 | 0.123 | 0.00005 | 0.0002 |
| P4l v M1mw | 217 | 0.114 | 0.312 | 0.629 | 0.00003 |
| P4l v M1dw | 210 | 0.321 | 0.324 | 0.315 | 0.065 |
| P4l v M2l | 313 | 0.801 | −0.027 | <0.000001 | 0.0005 |
| P4l v M2mw | 309 | 0.334 | 0.454 | 0.104 | <0.000001 |
| P4l v M2dw | 303 | 0.408 | 0.325 | 0.044 | 0.0000001 |
| P4l v M3l | 219 | 0.626 | −0.024 | 0.005 | 0.001 |
| P4l v M3mw | 322 | 0.267 | 0.270 | 0.215 | 0.0000003 |
| P4l v M3dw | 301 | 0.554 | 0.052 | 0.004 | 0.00001 |
| P4w v M1l | 216 | 0.553 | 0.264 | 0.005 | 0.0008 |
| P4w v M1mw | 214 | 0.339 | 0.397 | 0.168 | 0.00004 |
| P4w v M1dw | 205 | 0.264 | 0.356 | 0.470 | 0.087 |
| P4w v M2l | 304 | 0.519 | 0.062 | 0.005 | 0.00003 |
| P4w v M2mw | 300 | 0.395 | 0.257 | 0.056 | 0.00001 |
| P4w v M2dw | 294 | 0.456 | 0.003 | 0.041 | 0.0007 |
| P4w v M3l | 218 | 0.706 | −0.462 | 0.006 | 0.056 |
| P4w v M3mw | 317 | 0.557 | 0.126 | 0.012 | <0.0001 |
| P4w v M3dw | 299 | 0.771 | −0.315 | 0.0003 | 0.060 |
| M1l v M1mw | 330 | 0.463 | 0.574 | 0.023 | 0.00007 |
| M1l v M1dw | 323 | 0.484 | 0.593 | 0.071 | 0.034 |
| M1l v M2l | 327 | 0.818 | 0.073 | <0.0000001 | 0.0005 |
| M1l v M2mw | 326 | 0.487 | 0.268 | 0.004 | <0.0000001 |
| M1l v M2dw | 321 | 0.319 | 0.553 | 0.091 | 0.0000001 |
| M1l v M3l | 206 | 0.777 | −1.00 | <0.0000001 | 0.035 |
| M1l v M3mw | 309 | 0.649 | −0.216 | 0.0001 | 0.00002 |
| M1l v M3dw | 291 | 0.436 | 0.161 | 0.020 | 0.00004 |
| M1mw v M1dw | 320 | 0.760 | 0.902 | 0.008 | 0.099 |
| M1mw v M2l | 321 | 0.102 | 0.516 | 0.58 | 0.00001 |
| M1mw v M2mw | 320 | 0.820 | 0.442 | <0.00001 | 0.024 |
| M1mw v M2dw | 316 | 0.673 | 0.455 | 0.0005 | 0.0007 |
| M1mw v M3l | 199 | 0.506 | 0.062 | 0.04 | 0.007 |
| M1mw v M3mw | 303 | 0.684 | 0.334 | 0.0007 | 0.002 |
| M1mw v M3dw | 286 | 0.575 | 0.277 | 0.007 | 0.0015 |
| M1dw v M2l | 314 | 0.199 | 0.438 | 0.44 | 0.029 |
| M1dw v M2mw | 313 | 0.770 | 0.468 | 0.0008 | 0.061 |
| M1dw v M2dw | 308 | 0.899 | 0.456 | 0.00005 | 0.193 |
| M1dw v M3l | 195 | 0.663 | 0.153 | 0.040 | 0.056 |
| M1dw v M3mw | 294 | 0.652 | 0.274 | 0.014 | 0.031 |
| M1dw v M3dw | 279 | 0.790 | 0.225 | 0.002 | 0.13 |
| M2l v M2mw | 479 | 0.587 | 0.544 | 0.0002 | <0.0000001 |
| M2l v M2dw | 471 | 0.491 | 0.575 | 0.004 | <0.0000001 |
| M2l v M3l | 291 | 0.902 | 0.034 | <0.00001 | 0.157 |
| M2l v M3mw | 440 | 0.651 | 0.020 | 0.0001 | 0.00001 |
| M2l v M3dw | 415 | 0.494 | 0.198 | 0.009 | 0.00003 |
| M2mw v M2dw | 468 | 0.798 | 0.761 | <0.00001 | 0.0000002 |
| M2mw v M3l | 289 | 0.715 | −0.190 | 0.0007 | 0.016 |
| M2mw v M3mw | 436 | 0.976 | 0.230 | <0.0000001 | 0.329 |
| M2mw v M3dw | 412 | 0.780 | 0.224 | 0.00005 | 0.011 |
| M2dw v M3l | 287 | 0.761 | −0.190 | 0.002 | 0.059 |
| M2dw v M3mw | 429 | 0.654 | 0.379 | 0.0019 | 0.0004 |
| M2dw v M3dw | 407 | 0.876 | 0.327 | 0.00001 | 0.054 |
| M3l v M3mw | 320 | 0.674 | 0.294 | 0.013 | 0.029 |
| M3l v M3dw | 314 | 0.738 | 0.235 | 0.006 | 0.049 |
| M3mw v M3dw | 451 | 0.897 | 0.847 | nc | nc |
| Mouse Right Maxillary | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v M1l | 207 | 0.043 | 0.590 | 0.768 | <0.0001 |
| I1ll v M1mw | 207 | 0.215 | −0.193 | 0.15 | <0.0001 |
| I1ll v M1dw | 207 | 0.127 | −0.161 | 0.33 | <0.0001 |
| I1ll v M2l | 207 | 0.296 | −0.842 | 0.019 | <0.0001 |
| I1ll v M2mw | 207 | 0.227 | −0.005 | 0.206 | <0.0001 |
| I1ll v M2dw | 207 | 0.304 | −0.576 | 0.03 | <0.0001 |
| I1ll v M3l | 207 | 0.518 | −0.535 | <0.001 | <0.0001 |
| I1ll v M3mw | 207 | 0.252 | −0.279 | <0.01 | <0.05 |
| M1l v M1mw | 207 | 0.797 | −0.291 | <0.0001 | <0.0001 |
| M1l v M1dw | 207 | 0.895 | −0.572 | <0.0001 | <0.0001 |
| M1l v M2l | 207 | 0.834 | −0.798 | <0.0001 | <0.0001 |
| M1l v M2mw | 207 | 0.683 | −0.220 | <0.0001 | <0.0001 |
| M1l v M2dw | 207 | 0.886 | −1.000 | <0.0001 | <0.01 |
| M1l v M3l | 207 | 0.667 | −0.850 | <0.0001 | <0.0001 |
| M1l v M3mw | 207 | 0.716 | −0.563 | <0.0001 | <0.0001 |
| M1mw v M1dw | 207 | 0.783 | 0.438 | <0.0001 | <0.0001 |
| M1mw v M2l | 207 | 0.603 | −0.148 | <0.0001 | <0.0001 |
| M1mw v M2mw | 207 | 0.707 | −0.270 | <0.0001 | <0.0001 |
| M1mw v M2dw | 207 | 0.666 | −0.252 | <0.0001 | <0.0001 |
| M1mw v M3l | 207 | 0.563 | −0.343 | <0.0001 | <0.0001 |
| M1mw v M3mw | 207 | 0.690 | −0.721 | <0.0001 | <0.0001 |
| M1dw v M2l | 207 | 0.732 | −1.000 | <0.0001 | <0.0001 |
| M1dw v M2mw | 207 | 0.624 | −0.278 | <0.0001 | <0.0001 |
| M1dw v M2dw | 207 | 0.821 | −0.938 | <0.0001 | <0.0001 |
| M1dw v M3l | 207 | 0.524 | −0.911 | <0.0001 | <0.0001 |
| M1dw v M3mw | 207 | 0.671 | −1.000 | <0.0001 | <0.0001 |
| M2l v M2mw | 207 | 0.735 | −0.621 | <0.0001 | <0.0001 |
| M2l v M2dw | 207 | 0.854 | 0.207 | <0.0001 | <0.0001 |
| M2l v M3l | 207 | 0.711 | 0.221 | <0.0001 | <0.0001 |
| M2l v M3mw | 207 | 0.619 | 0.119 | <0.0001 | <0.0001 |
| M2mw v M2dw | 207 | 0.742 | 0.054 | <0.0001 | <0.0001 |
| M2mw v M3l | 207 | 0.806 | −0.325 | <0.0001 | <0.001 |
| M2mw v M3mw | 207 | 0.692 | −0.456 | <0.0001 | <0.0001 |
| M2dw v M3l | 206 | 0.660 | 0.147 | <0.0001 | <0.0001 |
| M2dw v M3mw | 206 | 0.711 | −0.166 | <0.0001 | <0.0001 |
| M3l v M3mw | 201 | 0.782 | 0.515 | <0.0001 | <0.0001 |
| Mouse Left Maxillary | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v M1l | 207 | 0.137 | 0.427 | 0.346 | <0.0001 |
| I1ll v M1mw | 207 | 0.110 | 0.053 | 0.488 | <0.0001 |
| I1ll v M1dw | 207 | 0.176 | −0.135 | 0.199 | <0.0001 |
| I1ll v M2l | 207 | 0.262 | −0.880 | 0.044 | <0.0001 |
| I1ll v M2mw | 207 | 0.187 | 0.145 | 0.257 | <0.0001 |
| I1ll v M2dw | 207 | 0.238 | −0.261 | 0.097 | <0.0001 |
| I1ll v M3l | 207 | 0.446 | −0.472 | 0.003 | <0.0001 |
| I1ll v M3mw | 207 | 0.404 | −0.416 | 0.009 | <0.0001 |
| M1l v M1mw | 207 | 0.801 | −0.183 | <0.0001 | <0.0001 |
| M1l v M1dw | 207 | 0.878 | −0.438 | <0.0001 | <0.0001 |
| M1l v M2l | 207 | 0.852 | −0.961 | <0.0001 | <0.0001 |
| M1l v M2mw | 207 | 0.716 | −0.302 | <0.0001 | <0.0001 |
| M1l v M2dw | 207 | 0.839 | −0.500 | <0.0001 | <0.001 |
| M1l v M3l | 207 | 0.729 | −0.410 | <0.0001 | <0.0001 |
| M1l v M3mw | 207 | 0.739 | −0.398 | <0.0001 | <0.01 |
| M1mw v M1dw | 207 | 0.808 | 0.363 | <0.0001 | <0.0001 |
| M1mw v M2l | 207 | 0.538 | −0.137 | <0.0001 | <0.0001 |
| M1mw v M2mw | 207 | 0.652 | −0.199 | <0.0001 | <0.0001 |
| M1mw v M2dw | 207 | 0.700 | −0.414 | <0.0001 | <0.0001 |
| M1mw v M3l | 207 | 0.484 | −0.063 | <0.001 | <0.0001 |
| M1mw v M3mw | 207 | 0.588 | −0.089 | <0.0001 | <0.0001 |
| M1dw v M2l | 207 | 0.692 | −0.451 | <0.0001 | <0.0001 |
| M1dw v M2mw | 207 | 0.704 | −0.525 | <0.0001 | <0.0001 |
| M1dw v M2dw | 207 | 0.808 | −0.477 | <0.0001 | <0.0001 |
| M1dw v M3l | 207 | 0.537 | −0.046 | <0.0001 | <0.0001 |
| M1dw v M3mw | 207 | 0.578 | −0.072 | <0.0001 | <0.0001 |
| M2l v M2mw | 207 | 0.671 | −0.159 | <0.0001 | <0.0001 |
| M2l v M2dw | 207 | 0.795 | 0.268 | <0.0001 | <0.0001 |
| M2l v M3l | 207 | 0.762 | 0.256 | <0.0001 | <0.0001 |
| M2l v M3mw | 207 | 0.651 | 0.373 | <0.0001 | <0.0001 |
| M2mw v M2dw | 207 | 0.720 | 0.141 | <0.0001 | <0.0001 |
| M2mw v M3l | 207 | 0.721 | −0.009 | <0.0001 | <0.0001 |
| M2mw v M3mw | 207 | 0.728 | −0.094 | <0.0001 | <0.0001 |
| M2dw v M3l | 206 | 0.695 | 0.075 | <0.0001 | <0.0001 |
| M2dw v M3mw | 206 | 0.741 | 0.192 | <0.0001 | <0.0001 |
| M3l v M3mw | 202 | 0.808 | 0.689 | <0.0001 | <0.0001 |
| Mouse Right Mandible | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v I1md | 197 | 0.088 | 0.886 | 0.707 | <0.0001 |
| I1ll v M1l | 204 | 0.079 | 0.228 | 0.571 | <0.0001 |
| I1ll v M1mw | 204 | 0.038 | 0.125 | 0.851 | <0.0001 |
| I1ll v M1dw | 204 | 0.079 | −1.000 | 0.564 | <0.0001 |
| I1ll v M2l | 204 | 0.095 | −0.522 | 0.505 | <0.0001 |
| I1ll v M2mw | 204 | 0.048 | −0.368 | 0.742 | <0.0001 |
| I1ll v M2dw | 204 | 0.574 | 0.522 | nc | nc |
| I1ll v M3l | 204 | 0.349 | −0.554 | 0.030 | <0.0001 |
| I1md v M1l | 204 | 0.538 | 0.325 | 0.003 | 0.074 |
| I1md v M1mw | 204 | 0.533 | 0.112 | 0.016 | 0.023 |
| I1md v M1dw | 204 | 0.521 | −1.000 | 0.0012 | 0.008 |
| I1md v M2l | 204 | 0.675 | −0.523 | <0.001 | 0.072 |
| I1md v M2mw | 204 | 0.503 | −0.183 | 0.008 | 0.042 |
| I1md v M2dw | 204 | 0.593 | −0.408 | 0.001 | 0.075 |
| I1md v M3l | 204 | 0.870 | −0.519 | <0.0001 | 0.218 |
| M1l v M1mw | 204 | 0.524 | 0.441 | <0.0001 | <0.0001 |
| M1l v M1dw | 204 | 0.778 | −1.000 | <0.0001 | <0.0001 |
| M1l v M2l | 204 | 0.876 | −0.732 | <0.0001 | <0.01 |
| M1l v M2mw | 204 | 0.819 | −0.554 | <0.0001 | <0.0001 |
| M1l v M2dw | 204 | 0.741 | −1.00 | <0.0001 | <0.0001 |
| M1l v M3l | 204 | 0.645 | −1.00 | <0.0001 | <0.0001 |
| M1mw v M1dw | 204 | 0.675 | 0.528 | <0.0001 | <0.0001 |
| M1mw v M2l | 204 | 0.599 | −0.074 | <0.0001 | <0.0001 |
| M1mw v M2mw | 204 | 0.623 | 0.115 | <0.0001 | <0.0001 |
| M1mw v M2dw | 204 | 0.582 | −0.017 | <0.0001 | <0.0001 |
| M1mw v M3l | 204 | 0.509 | −0.404 | <0.001 | <0.0001 |
| M1dw v M2l | 204 | 0.782 | −0.128 | <0.0001 | <0.0001 |
| M1dw v M2mw | 204 | 0.884 | −0.522 | <0.0001 | <0.0001 |
| M1dw v M2dw | 204 | 0.767 | −0.431 | <0.0001 | <0.0001 |
| M1dw v M3l | 204 | 0.409 | 1.000 | <0.001 | <0.0001 |
| M2l v M2mw | 203 | 0.947 | 0.036 | <0.0001 | <0.01 |
| M2l v M2dw | 203 | 0.907 | −0.058 | <0.0001 | <0.01 |
| M2l v M3l | 203 | 0.768 | 0.188 | <0.0001 | <0.01 |
| M2mw v M2dw | 203 | 0.942 | −0.008 | <0.0001 | <0.01 |
| M2mw v M3l | 203 | 0.719 | −0.026 | <0.0001 | <0.0001 |
| M2dw v M3l | 203 | 0.729 | 0.268 | <0.0001 | <0.0001 |
| Mouse Left Mandible | |||||
|---|---|---|---|---|---|
| Correlations (MLEs) |
Significance of Correlations P(Hypothesis) |
||||
| Phenotype pair | N | ρG | ρE | ρG =0 | | ρG |=1 |
| I1ll v M1l | 204 | 0.027 | 1.000 | 0.844 | <0.0001 |
| I1ll v M1mw | 204 | 0.126 | −0.138 | 0.548 | <0.0001 |
| I1ll v M1dw | 204 | 0.034 | −0.271 | 0.817 | <0.0001 |
| I1ll v M2l | 204 | 0.018 | −0.491 | 0.906 | <0.0001 |
| I1ll v M2mw | 204 | −0.123 | −0.150 | 0.416 | <0.0001 |
| I1ll v M2dw | 204 | −0.140 | 0.063 | 0.350 | <0.0001 |
| I1ll v M3l | 204 | 0.220 | −0.421 | 0.215 | <0.0001 |
| I1md v M1l | 204 | 0.510 | 1.000 | 0.004 | 0.050 |
| I1md v M1mw | 204 | 0.665 | −0.179 | 0.001 | 0.009 |
| I1md v M1dw | 204 | 0.534 | −0.094 | 0.003 | 0.03 |
| I1md v M2l | 204 | 0.693 | −0.531 | 0.0003 | 0.09 |
| I1md v M2mw | 204 | 0.405 | −0.005 | 0.07 | 0.10 |
| I1md v M2dw | 204 | 0.455 | −0.124 | 0.017 | 0.05 |
| I1md v M3l | 204 | 0.840 | −0.541 | <0.0001 | 0.12 |
| M1l v M1mw | 204 | 0.671 | −0.608 | <0.0001 | <0.0001 |
| M1l v M1dw | 204 | 0.843 | −0.560 | <0.0001 | <0.0001 |
| M1l v M2l | 204 | 0.905 | −1.000 | <0.0001 | 0.036 |
| M1l v M2mw | 204 | 0.803 | −0.388 | <0.0001 | <0.0001 |
| M1l v M2dw | 204 | 0.671 | −1.000 | <0.0001 | <0.0001 |
| M1l v M3l | 204 | 0.647 | −1.000 | <0.0001 | <0.001 |
| M1mw v M1dw | 204 | 0.735 | 0.525 | <0.0001 | <0.0001 |
| M1mw v M2l | 204 | 0.492 | 0.398 | <0.001 | <0.0001 |
| M1mw v M2mw | 204 | 0.678 | −0.140 | <0.0001 | <0.0001 |
| M1mw v M2dw | 204 | 0.621 | −0.139 | <0.0001 | <0.0001 |
| M1mw v M3l | 204 | 0.447 | −0.008 | 0.005 | <0.0001 |
| M1dw v M2l | 204 | 0.886 | −0.091 | <0.0001 | <0.01 |
| M1dw v M2mw | 204 | 0.937 | −0.050 | <0.0001 | 0.011 |
| M1dw v M2dw | 204 | 0.824 | −0.434 | <0.0001 | <0.0001 |
| M1dw v M3l | 204 | 0.715 | −0.306 | <0.0001 | <0.001 |
| M2l v M2mw | 203 | 0.989 | −0.145 | <0.0001 | 0.34 |
| M2l v M2dw | 203 | 0.897 | −0.372 | <0.0001 | <0.01 |
| M2l v M3l | 203 | 0.823 | 0.099 | <0.0001 | <0.01 |
| M2mw v M2dw | 203 | 0.950 | −0.264 | <0.0001 | <0.01 |
| M2mw v M3l | 203 | 0.710 | 0.004 | <0.0001 | <0.0001 |
| M2dw v M3l | 203 | 0.710 | 0.100 | <0.0001 | <0.0001 |
MLE: maximum likelihood estimate; P(Hypothesis): probability of the hypothesis (indicated in columns below) being true given the available pedigreed data; nc = not computable.
For the baboon population, of the 208 incisor:post-canine analyses (maxillary and mandibular), only 26 return significant (p≤0.05) genetic correlations (12 in the maxilla and 14 in the mandible).
In contrast, all of the maxillary incisor:incisor comparisons yield significant genetic correlations, 14 of 16 maxillary premolar:premolar analyses returned significant genetic correlations, as did 65 of 81 maxillary molar:molar comparisons. Approximately half of the 72 maxillary premolar:molar analyses returned significant genetic correlations.
For the mandible, the mesiodistal breadth of the central incisor is not significantly correlated with the labiolingual breadth of the lateral incisor, although all other mandibular incisor:incisor correlations are insignificantly different from one. The premolar:premolar analyses return fewer positive genetic correlations than found for the maxilla, although we note that the mandibular premolar sample sizes are much smaller (e.g., 150 versus 250). Twenty-nine of the premolar:molar correlations are significantly greater than zero, approximately 40% compared to the approximately 50% for the maxilla. Seventy-four of the 81 molar:molar analyses returned significant genetic correlations, even more than were seen for the maxilla.
The handful of genetic correlations noted between the mandibular incisors and molars suggest that the genetic relationship is inverse, as they returned negative correlations. This would indicate that when the incisors are smaller, the first and second molars are larger. Given that these results are not identical on the right and left sides, and are interspersed with insignificant analyses, this possible pattern needs to be explored in more detail as the evolutionary implications could be quite interesting and important.
In the mouse population, the labiolingual diameter of the mandibular incisors yields no significant genetic correlation with the molars while the mesiodistal diameter of the mandibular incisor is significantly correlated with the molars. For the maxillary incisors, the labiolingual diameter has no genetic correlation with the first molars (as seen in the mandible), and incomplete pleiotropy with the mesiodistal length of the second molars and both length and width of the third molars.
The mouse molar:molar analyses yield a more consistent pattern of high genetic correlations compared to the baboons, but we are hesitant to place emphasis on this distinction given that the mouse pedigree structure will tend to overestimate genetic correlations. While mouse molars do develop almost simultaneously, in contrast to the sequential formation of baboon molars (and this might result in a higher degree of integration), we do not feel that our analyses are robust enough to indicate that this difference is biologically significant at this point in time.
Discussion
In recent years, quantitative genetic methods have been most commonly employed to identify genomic loci that significantly influence phenotypic variation, and often within a medical framework (e.g., lipoprotein metabolism in baboons: Rainwater et al, 2009; MC4R influence on energy expenditure and appetite in children: Cole et al, 2010), but sometimes include other phenotypes (e.g., dog coat color variation: Cadieu et al, 2009; an adaptive allele for deer mouse coloration: Linnen et al, 2009). Quantitative genetics has also been recruited to explore phenotypic response to natural selection (e.g., Boag, ’83), or lack thereof (e.g., Kruuk et al, 2002), sexual selection (e.g., Lande and Kirkpatrick, ’88), selection in the laboratory (e.g., Beldade et al, 2002), adaptive radiations (Schluter, 2000), and complex fitness surfaces (e.g., Blows et al, 2003).
In contrast to these foci, our research uses quantitative genetics to understand how genes influence morphological variation with the specific goal of improving our ability to interpret evolutionary processes from the fossil record. As such, we use a quantitative genetic approach to recast skeletal variation in terms of the underlying pattern of genetic correlations between traits. Our objective has been to detect and exploit genetic correlations -- indicative of additive genetic pleiotropy or shared additive genetic effects between trait pairs. We then use these genetic correlations to infer patterns of morphological integration (similar to Hallgrimsson et al, 2007; for example see Hlusko et al, 2004a, b; Hlusko and Mahaney, 2009; Koh et al, 2010) rather than for identifying specific genomic loci or quantifying selection, as is more typically done.
Our results are the first quantitative genetic evidence for modularity within the mammalian dental arcade and the first evidence of a shared dental genetic architecture across mammals broadly. Developmental studies have shown that mammalian tooth organogenesis relies on many of the same genes, for example Shh expression in mouse (Vaahtokari et al, ‘96), vole (Keränen et al, ‘98), shrew (Yamanaka et al, 2007), ferret (Järvinen et al, 2009), and opossum (Moustakas et al, 2009). However, little is known about how these developmental genes are expressed similarly or differentially across the dental arcade in various mammals (but see Moustakas et al, 2009, for recent results in opossum compared to mouse).
Mice and monkeys last shared a genetic common ancestor ~ 69 million years ago (Eizirik et al, 2001) and extant mice have highly derived dentitions compared to those early mammals, in part by having large continuously growing incisors. As such, genetic independence of the incisors may be expected in extant mice. It is therefore intriguing that incisor size variation is genetically independent from the size variation in the postcanine dentition in both mice and baboons. This similarity predicts that dental variation is controlled in a similar way in other mammalian orders.
Other biologists have shown that the genetic architecture has a significant influence on how, and how quickly a species responds to selective pressure (Lande, ‘79; Schluter, 2000; Beldade et al, 2002). If similar genes or sets of genes influence size variation across the entire dental arcade, we would expect to see more concomitant change in incisor and molar size, as selection for or against change in one region of the arcade would simultaneously affect the other region. However, modularity (Wagner and Altenberg, ‘96; Schlosser and Wagner, 2004) in the dentition, or rather, a level of genetic independence between various regions along the tooth row as we have identified here, would facilitate evolvability in size disparity because each module could respond independently to different selective pressures.
For example, in mammals independent anterior and posterior dental modules would facilitate responses to the different selective pressures that act on the incisors in contrast to the molars (e.g., grooming or food procurement versus food mastication, respectively). This genetic modularity may either have facilitated or been the result of the very different selective pressures and functional constraints experienced by the anterior and posterior dentitions.
A survey of mammalian dental evolution provides strong morphological evidence for the pervasiveness of such a modular genetic architecture. Repeatedly, and in numerous lineages, incisors have undergone tremendous diversification in both size and shape (Wortman 1886), although we focus on size here. In some lineages, incisors have reduced in size tremendously (i.e., felids; manatees – only males have one incisors; and robust Australopithecus hominid species), or have been lost completely (i.e., all extant xenarthrans – armadillos, tree sloths, and anteaters; cervid and bovid maxillae). In other lineages, they have developed highly specialized functions, such as the elongated mandibular tooth combs used by lemurs for grooming (coupled with extreme size reduction in the maxillary incisors), the long spear-like incisors of “shrew” opossums (Caenolestidae, Marsupialia), the continuously growing incisors used for gnawing in several lineages (e.g., aye-ayes within the Primates, mice to porcupines in the Rodentia), the tusks/incisors of hippopotamus and dugongs, the very large tusks/incisors of elephants used for rooting and uprooting trees, and perhaps the most extreme case, the ~3 m long spiraled tusk/incisor in the Arctic Ocean narwhal (Monodon monoceros) thought to be used for breaking ice, weaponry, or possibly even echolocation (Nowak, ‘91). The eutherian mammalian fossil record yields even more variation than is seen in the extant taxa noted above (Rose, 2006). In virtually all of these taxa, the postcanine dentition may vary significantly in shape, but the size variance is not as extreme as in the incisors.
This pattern of dental size diversity is even seen in the earliest mammals in the late Cretaceous. For example, Zalambdalestes had long procumbent mandibular incisors in contrast to the shorter peg-like incisors of Malestes and even shorter Asioryctes, all of which sit outside the placental clade (Wible et al, 2007). Therefore, based on phenotypic data from extant and fossil mammals, and the results from our quantitative genetic analyses of dental variation in mice and baboons, we hypothesize that a genetic independence between incisor and postcanine size variation is symplesiomorphic to eutherian mammals, and perhaps to mammals more generally.
One clear difference between mouse and baboon genetic correlation matrices is the significant genetic correlation between the mesiodistal width of the mouse mandibular incisor and molar size, where baboons have no genetic correlation. Although one might propose multiple explanatory scenarios consistent with these two data points, they are inadequate for identifying an evolutionary trend, much less confirming one. Additional populations need to be studied to identify the evolutionary polarity of this pattern.
We also note that the baboon results suggest that there may be an inverse genetic relationship (genetic correlation) between the mandibular incisors and the mandibular first and/or second molars. While these results are not consistent, and are interspersed with some insignificant results, if further analyses bolster this pattern the evolutionary implications are interesting. Several primate lineages show a simultaneous reduction in the incisor region and expansion of the molar region (for example, in the robust Australopithecus species of hominids and the Theropithecus brumpti lineage of cercopithecoids).
From a developmental perspective, the odontogenic code (Thomas and Sharpe, ‘98) is clearly compatible with these results, as it could be interpreted to predict a certain degree of independence between the incisor and molar regions. However, for the post-canine pattern seen in baboons, this pattern of incomplete pleiotropy may better fit with a morphogenetic gradient, or reaction-diffusion mechanism (Jernvall, 2000; Kangas et al, 2004).
While the field (Butler, ’39) and clone (Osborn, ’78) models for tooth development have received a significant amount of attention historically, it is important to keep in mind that these models were developed primarily on phenotypic data and that hypothesis testing was rarely conclusive (see references cited previously). Our current understanding of tooth development suggests that neither is likely to be entirely right or wrong (as also suggested by the hybrid Cooperative Genetic Integration model proposed by Mitsiadis and Smith, 2006). As we continue to improve our understanding of tooth organogenesis from developmental studies and patterns of genetic correlation from quantitative genetic analyses, we are better off reconstructing tooth patterning mechanisms without these speculative models constraining our interpretation of the actual genetic data.
Here we demonstrated that quantitative genetic analyses provide a useful tool for linking developmental genetics of tooth organogenesis with studies of morphological variation in the adult dentition by employing the concept of modularity. As more pedigreed populations are developed for other taxa, this may prove to be a powerful and common approach through which we can bridge the gap between genotype and phenotype and better understand how this relationship has evolved through time as documented in the fossil record.
Supplementary Material
Acknowledgments
We thank K.D. Carey, K. Rice and the Veterinary Staff of the Southwest Foundation for Biomedical Research and the Southwest National Primate Research Center; J. Cheverud (Washington University) for access to baboon specimens and project support; D.E. Newman for baboon pedigree data management and processing; University of California Museum of Vertebrate Zoology for mouse curation, assistance, and advice, especially C. Conroy and E. Lacey; Thanks also to D. Duren, L. Havill, T. Grieco, J. Shade, R. Sherwood, E. Simms, O. Rizk, C. Roseman for discussion and methodological advice; Much appreciation goes to the numerous students at the University of Illinois and the University of California Berkeley who helped with data collection: C. Anderson, L. Bates, L. Broughton, L. Buchanan, T. Cannistraro, S. Deldar, N. Do, Z. Fletcher, J. Hernandez, L. Holder, J. Irwin, T. Koh, A. Liberatore, M.L. Maas, C. Page, D. Pillie, N. Reeder, N. Wu, A. Yen, E. Young, and A. Zowghi. Many thanks also to those who provided comments on previous versions of the manuscript: S. Amugongo, T. Grieco, J. Jernvall, J. Moustakas, O. Rizk, D. Su, T.D. White, and two anonymous reviewers. This material is based upon work supported by the National Science Foundation under Grants No. BCS-0500179, BCS-0130277, and BCS-0616308. The University of Illinois, Urbana-Champaign’s Research Board helped fund the baboon research. The University of California, Berkeley’s Committee on Research funded the data collection for the mouse component. NIH/NCRR P51 RR013986 supports the Southwest National Primate Research Center.
Literature Cited
- Ackermann RR. Craniofacial variation and developmental divergence in primate and human evolution. Novartis Found Symp. 2007;284:262–273. doi: 10.1002/9780470319390.ch17. [DOI] [PubMed] [Google Scholar]
- Ackermann RR, Cheverud JM. Discerning evolutionary processes in patterns of tamarin (genus Saguinus) craniofacial variation. Am J Phys Anthropol. 2002;117:260–271. doi: 10.1002/ajpa.10038. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristotle. On the Generation of Animals, Book V. Translated by A. Platt, eBooks@Adelaide. 2007 [Google Scholar]
- Bateson W. Material for the Study of Variation, Treated with Special Regard to Discontinuity in the Origin of Species. London: Macmillan; 1894. [Google Scholar]
- Beldade P, Koops K, Brakefield PM. Developmental constraints versus flexibility in morphological evolution. Nature. 2002;416:844–847. doi: 10.1038/416844a. [DOI] [PubMed] [Google Scholar]
- Blows MW, Brooks R, Kraft PG. Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male guppies. Evolution. 2003;57:1622–1630. doi: 10.1111/j.0014-3820.2003.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Boag PT. The heritability of external morphology in Darwin’s ground finches (Geospiza) on Isla Daphne Major, Galápagos. Evolution. 1983;37:877–894. doi: 10.1111/j.1558-5646.1983.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Boehnke M, Moll PP, Kottke BA, Weidman WH. Partitioning the variability of fasting plasma glucose levels in pedigrees. Am J Epidemiol. 1987;125:679–689. doi: 10.1093/oxfordjournals.aje.a114581. [DOI] [PubMed] [Google Scholar]
- Butler PM. Studies of the mammalian dentition – differentiation of the postcanine dentition. Proc Zool Soc London, Series B. 1939;109:1–36. [Google Scholar]
- Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, Vonholdt BM, Rhue A, Boyko A, Byers A, Wong A, Mosher DS, Elkahloun AG, Spady TC, André C, Lark KG, Cargill M, Bustamante CD, Wayne RK, Ostrander EA. Coat variation in the domestic dog is governed by variants in three genes. Science. 2009;326(5949):150–153. doi: 10.1126/science.1177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity 2nd ed. Malden, MA: Blackwell Publishing; 2005. [Google Scholar]
- Cheverud JM. A comparative analysis of morphological variation patterns in the papionins. Evolution. 1989;43:1737–1747. doi: 10.1111/j.1558-5646.1989.tb02623.x. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Quantitative genetic analysis of cranial morphology in the cotton-top (Saguinus oedipus) and saddle-back (S. fuscicollis) tamarins. J Evol Biol. 1996;9:5–42. [Google Scholar]
- Cole SA, Butte NF, Voruganti VS, Cai G, Haack K, Kent JW, Jr, Blangero J, Comuzzie AG, McPherson JD, Gibbs RA. Evidence that multiple genetic variants of MC4R play a functional role in the regulation of energy expenditure and appetite in Hispanic children. Am J Clin Nutr. 2010;91(1):191–199. doi: 10.3945/ajcn.2009.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg AA. The changing dentition of man. J Am Dent Assoc. 1945;32:676–690. [Google Scholar]
- Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- Dyke B. PEDSYS: A pedigree data management software. San Antonio: Texas, Southwest Foundation for Biomedical Research; 1996. [Google Scholar]
- Draghi J, Wagner GP. The evolutionary dynamics of evolvability in a gene network mode. J Evol Biol. 2009;22:599–611. doi: 10.1111/j.1420-9101.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- Eizirik E, Murphy WJ, O’Brien SJ. Molecular dating and biogeography of the early placental mammal radiation. J Hered. 2001;92:212–219. doi: 10.1093/jhered/92.2.212. [DOI] [PubMed] [Google Scholar]
- Escot C, Hogg E, Callahan R. Mammary tumorigenesis in feral Mus cervicolor popaeus. J Virology. 1986;58:619–625. doi: 10.1128/jvi.58.2.619-625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson B, Lieberman DE, Young NM, Parsons T, Wat S. Evolution of covariance in the mammalian skull. Novartis Found Symp. 2007;284:164–185. doi: 10.1002/9780470319390.ch12. [DOI] [PubMed] [Google Scholar]
- Henderson AM, Greene DL. Dental field theory: an application to primate evolution. J Dent Res. 1975;54:344–350. [PubMed] [Google Scholar]
- Hillson S. Cambridge Manuals in Archaeology. New York: Cambridge University Press; 1986. Teeth. [Google Scholar]
- Hlusko LJ. Integrating the genotype and phenotype in hominid paleontology. Proc Natl Acad Sci U.S.A. 2004;101:2653–2657. doi: 10.1073/pnas.0307678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlusko LJ, Mahaney MC. Quantitative genetics, pleiotropy, and morphological integration in the dentition of Papio hamadryas. Evolutionary Biology. 2009;36(1):5–18. doi: 10.1007/s11692-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlusko LJ, Maas M-L, Mahaney MC. Statistical genetics of molar cusp patterning in pedigreed baboons: implications for primate dental development and evolution. J Exp Zool (Mol Dev Evol) 2004a;302B:268–283. doi: 10.1002/jez.b.21. [DOI] [PubMed] [Google Scholar]
- Hlusko LJ, Suwa G, Kono R, Mahaney MC. Genetics and the evolution of primate enamel thickness: A baboon model. Am J Phys Anthropol. 2004b;124:223–233. doi: 10.1002/ajpa.10353. [DOI] [PubMed] [Google Scholar]
- Hlusko LJ, Mahaney MC, Weiss KM. A statistical genetic comparison of two techniques for assessing molar crown size in pedigreed baboons. Am J Phys Anthropol. 2002;117:182–189. doi: 10.1002/ajpa.10022. [DOI] [PubMed] [Google Scholar]
- Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982;46:373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Järvinen E, Tummers M, Thesleff I. The role of the dental lamina in mammalian tooth replacement. J Exp Zool B Mol Dev Evol. 2009;12B:281–291. doi: 10.1002/jez.b.21275. [DOI] [PubMed] [Google Scholar]
- Jernvall J. Linking development with generation of novelty in mammalian teeth. Proc Natl Acad Sci USA. 2000;97:2641–2645. doi: 10.1073/pnas.050586297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Jung HS. Genotype, phenotype, and developmental biology of molar tooth characters. Am J Phys Anthropol. 2000;31(Suppl):171–190. doi: 10.1002/1096-8644(2000)43:31+<171::aid-ajpa6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463–469. [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Jolly CJ. Species, subspecies, and baboon systematics. In: Kimbel WH, Martin LB, editors. Species, Species Concepts, and Primate Evolution. New York: Plenum Press; 1993. pp. 67–107. [Google Scholar]
- Kangas AT, Evans AR, Thesleff I, Jernvall J. Nonindependence of mammalian dental characters. Nature. 2004;432:211–214. doi: 10.1038/nature02927. [DOI] [PubMed] [Google Scholar]
- Keränen SV, Aberg T, Kettunen P, Thesleff I, Jernvall J. Association of developmental regulatory genes with the development of different molar tooth shapes in two species of rodents. Dev Genes Evol. 1998;208:477–486. doi: 10.1007/s004270050206. [DOI] [PubMed] [Google Scholar]
- Koh C, Bates E, Broughton E, Do NT, Fletcher Z, Mahaney MC, Hlusko LJ. Genetic integration of molar cusp size variation in baboons. Am J Phys Anthropol. 2010;142:246–260. doi: 10.1002/ajpa.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn LA, Leigh SR, Jacobs SC, Cheverud JM. Effects of annular cranial vault modification on the cranial base and face. Am J Phys Anthropol. 1993;90:147–168. doi: 10.1002/ajpa.1330900203. [DOI] [PubMed] [Google Scholar]
- Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness F, Clutton-Brock T. Antler size in red deer: heritability and selection but no evolution. Evolution. 2002;56:1683–1695. doi: 10.1111/j.0014-3820.2002.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Genetic variation and phenotypic evolution during allopatric speciation. Am Nat. 1980;116:463–479. [Google Scholar]
- Lande R, Kirkpatrick M. Ecological speciation by sexual selection. J theor Biol. 1988;133:85–98. doi: 10.1016/s0022-5193(88)80026-2. [DOI] [PubMed] [Google Scholar]
- Lange K, Boehnke M. Extensions to pedigree analysis. IV. Covariance components models for multivariate traits. Am J Med Genet. 1983;14:513–524. doi: 10.1002/ajmg.1320140315. [DOI] [PubMed] [Google Scholar]
- Linnen CR, Kingsley EP, Jensen JD, Hoekstra HE. On the origin and spread of an adaptive allele in deer mice. Science. 2009;325:1095–1098. doi: 10.1126/science.1175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi AV. A factor analysis of morphogenetic fields in the human dentition. Am J Phys Anthrop. 1975;42:99–104. doi: 10.1002/ajpa.1330420114. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Mahaney MC, Blangero J, Comuzzie AG, VandeBerg JL, Stern MP, MacCluer JW. Plasma HDL cholesterol, triglycerides, and adiposity. A quantitative genetic test of the conjoint trait hypothesis in the San Antonio Family Heart Study. Circulation. 1995;92:3240–3248. doi: 10.1161/01.cir.92.11.3240. [DOI] [PubMed] [Google Scholar]
- Marroig G, Cheverud JM. Size as a line of least evolutionary resistance: diet and adaptive morphological radiation in New World monkeys. Evolution. 2005;59:1128–1142. [PubMed] [Google Scholar]
- Marroig G, DeVivo M, Cheverud JM. Cranial evolution in sakis (Pithecia, Platyrrhini) II: evolutionary processes and morphological integration. J Evol Biol. 2004;17:144–155. doi: 10.1046/j.1420-9101.2003.00653.x. [DOI] [PubMed] [Google Scholar]
- McCollum MA, Sharpe PT. Developmental genetics and early hominid craniodental evolution. BioEssays. 2001;23:481–493. doi: 10.1002/bies.1068. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Smith MM. How do genes make teeth to order through development? J Exp Zool (Mol Dev Evol) 2006;306B:177–182. doi: 10.1002/jez.b.21104. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Bookstein F. The evolutionary role of modularity and integration in the hominoid cranium. Evolution. 2008;62:943–958. doi: 10.1111/j.1558-5646.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- Moustakas JE, Smith KK, Hlusko LJ. The Evolution and Development of the Mammalian Dentition: Insights from the Marsupial Monodelphis domestica. Dev Biol. 2009;331:461. doi: 10.1002/dvdy.22502. [DOI] [PubMed] [Google Scholar]
- Munne PM, Felszeghy S, Jussila M, Soumalainen M, Thesleff I, Jernvall J. Splitting placodes: effects of bone morphogenetic protein and Activin on the patterning and identity of mouse incisors. Evol & Dev. 2010;12:383–392. doi: 10.1111/j.1525-142X.2010.00425.x. [DOI] [PubMed] [Google Scholar]
- Nowak RM. Walker’s Mammals of the World 5th ed. Baltimore, MD: The Johns Hopkins University Press; 1991. [Google Scholar]
- Osborn JW. Morphogenetic gradients: fields vs clones. In: Butler PM, Joysey KA, editors. Development, Function and Evolution of Teeth. New York: Academic Press; 1978. pp. 171–213. [Google Scholar]
- Prager EM, Sage RD, Gyllensten U, Thomas WK, Hübne R, Jones CS, Noble L, Searle JB, Wilson AC. Mitochontrial DNA sequence diversity and the colonization of Scandinavia by house mice from East Holstein. Biol J Linn Soc. 1993;50:85–122. [Google Scholar]
- Raff RA. The Shape of Life. Chicago, IL: Univ. of Chicago Press; 1996. [Google Scholar]
- Rainwater DL, Cox LA, Rogers J, VandeBerg JL, Mahaney MC. Localization of multiple pleiotropic genes for lipoprotein metabolism in baboons. J Lipid Res. 2009;50(7):1420–1428. doi: 10.1194/jlr.M800583-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno PL, McCollum MA, Cohn MJ, Meindl RS, Hamrick M, Lovejoy CO. Patterns of correlation and covariation of anthropoid distal forelimb segments correspond to Hoxd expression territories. J Exp Zool B Mol Dev Evol. 2008;310B:240–258. doi: 10.1002/jez.b.21207. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Cheverud JM, Buikstra JE. The relationship between cranial metric and non-metric traits in the rhesus macaques from Cayo Santiago. Am J Phys Anthropol. 1984;64:213–222. doi: 10.1002/ajpa.1330640303. [DOI] [PubMed] [Google Scholar]
- Rizk OT, Amugongo SK, Mahaney MC, Hlusko LJ. The quantitative genetic analysis of primate dental variation: History of the approach and prospects for the future. In: Irish JD, Nelson GC, editors. Technique and Application in Dental Anthropology. New York: Cambridge University Press; 2008. pp. 317–346. [Google Scholar]
- Rose KD. The Beginning of the Age of Mammals. Baltimore, MD: The Johns Hopkins University Press; 2006. [Google Scholar]
- Roseman CC. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc Natl Acad Sci USA. 2004;101:12824–12829. doi: 10.1073/pnas.0402637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RD, Atchley WR, Capanna E. House mice as models in systematic biology. Syst Biol. 1993;42:523–561. [Google Scholar]
- Schlosser G, Wagner GP. Modularity in Development and Evolution. Chicago, IL: Univ. of Chicago Press; 2004. [Google Scholar]
- Schluter D. The Ecology of Adaptive Radiation. New York: Oxford University Press; 2000. [Google Scholar]
- Sherwood RJ, Duren DL, Demerath EW, Czerwinski SA, Siervogel RM, Towne B. Quantitative genetics of modern human cranial variation. J Hum Evol. 2008;54:909–914. doi: 10.1016/j.jhevol.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin N. Origin of evolutionary novelty: examples from limbs. J Morphol. 2002;252:15–28. doi: 10.1002/jmor.10017. [DOI] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Fossils, genes, and the evolution of animal limbs. Nature. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- Stock DW. The genetic basis of modularity in the development and evolution of the vertebrate dentition. Philos Trans R Soc Lond, B, Biol Sci. 2001;356:1633–1653. doi: 10.1098/rstb.2001.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A, Sharpe PT. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Sharpe PT. Patterning of the murine dentition by homeobox genes. Eur J Oral Sci. 1998;106:48–54. doi: 10.1111/j.1600-0722.1998.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Aberg T, Jernvall J, Keränen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996;54:39–43. doi: 10.1016/0925-4773(95)00459-9. [DOI] [PubMed] [Google Scholar]
- Van Valen L. Growth fields in the dentition of Peromyscus. Evolution. 1961;16:272–277. [Google Scholar]
- Wagner GP, Altenberg L. Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Vargas AO. On the nature of thumbs. Genome Biol. 2008;9:213. doi: 10.1186/gb-2008-9-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, Asher RJ. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature. 2007;447:1003–1006. doi: 10.1038/nature05854. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Leamy LJ, Routman EJ, Cheverud JM. Epistatic pleiotropy and the genetic architecture of covariation within early and late-developing skull trait complexes in mice. Genetics. 2005;171:683–694. doi: 10.1534/genetics.104.038885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortman JL. Comparative Anatomy of the Teeth of Vertebrates. The American System of Dentistry. 1886 [Google Scholar]
- Yamanaka A, Yasui K, Sonomura T, Uemura M. Development of heterdont dentition in house shrew (Suncus murinus) Eur J Oral Sci. 2007;115:433–440. doi: 10.1111/j.1600-0722.2007.00499.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.