Abstract
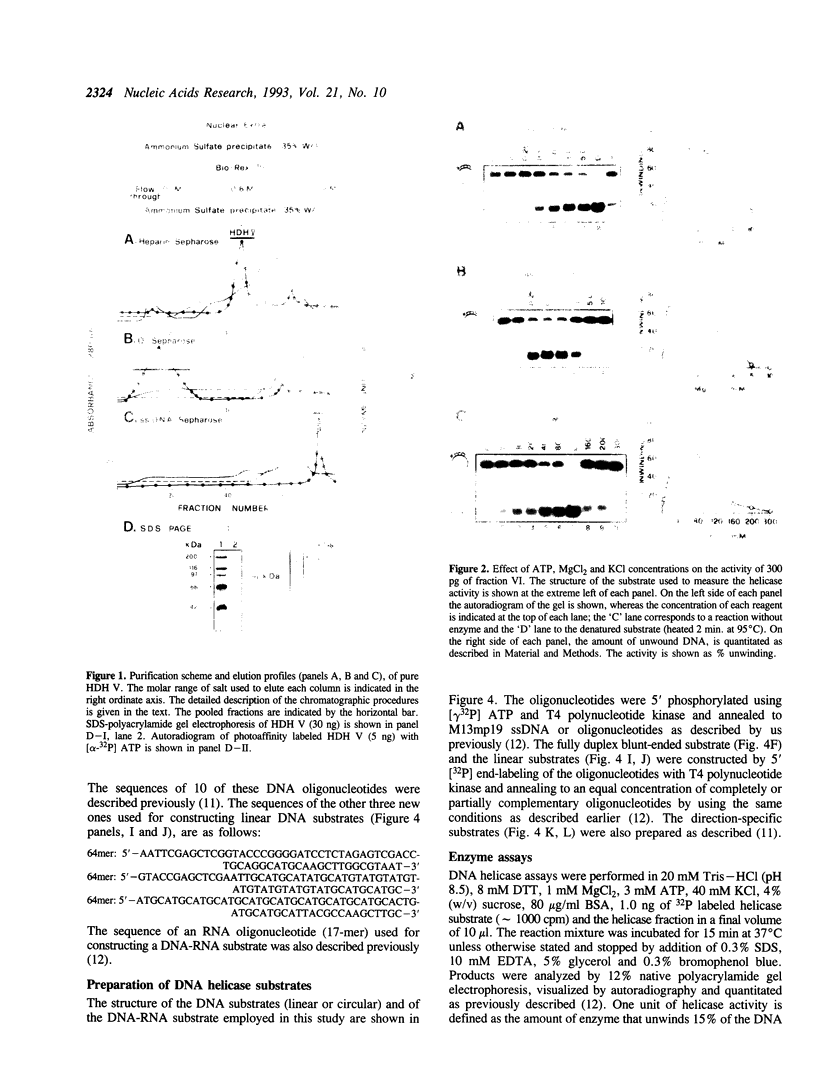
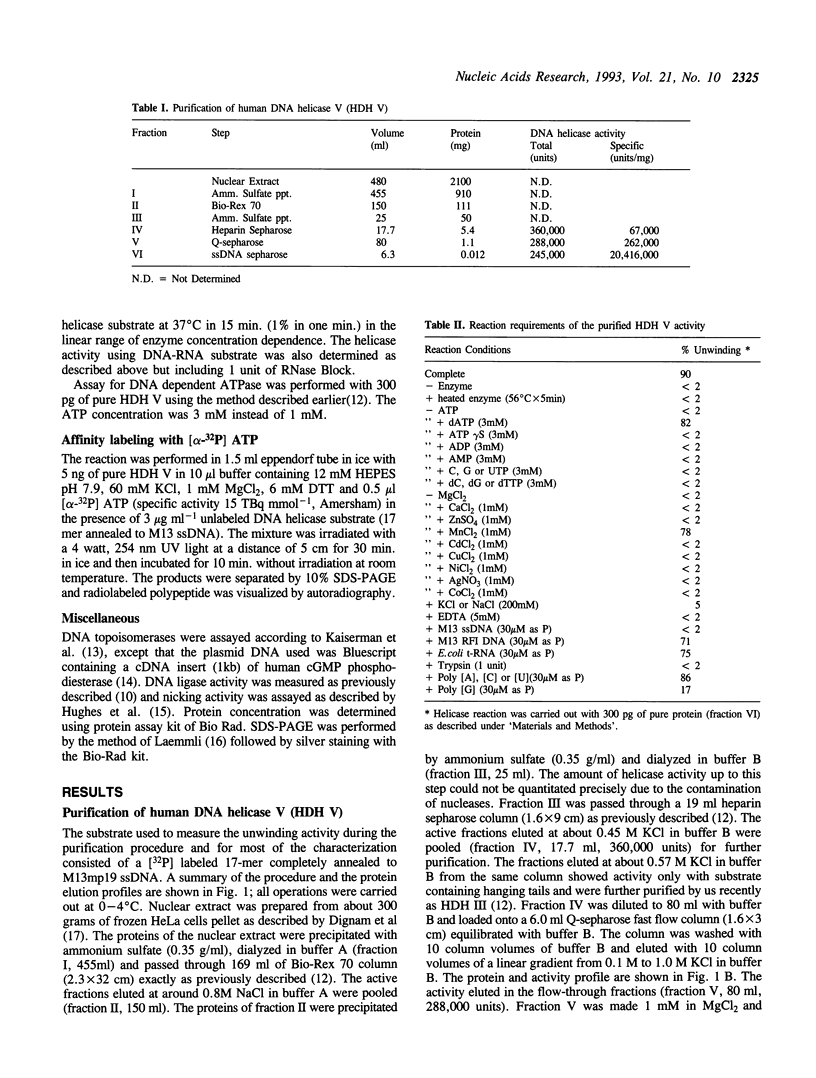
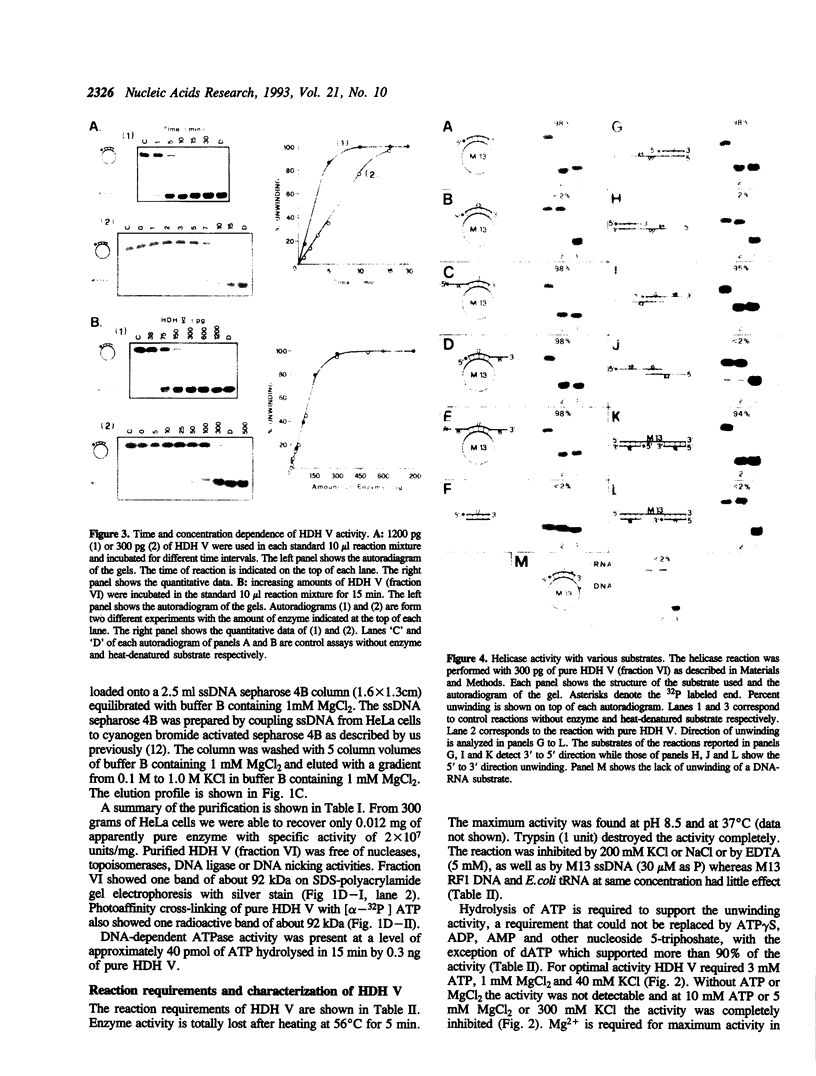
Using a strand-displacement assay with 32P labeled oligonucleotide annealed to M13 ssDNA we have purified to apparent homogeneity and characterized a novel DNA unwinding enzyme from HeLa cell nuclei, human DNA helicase V (HDH V). This is present in extremely low abundance in the cells and has the highest turnover rate among other human helicases. From 300 grams of cultured cells only 0.012 mg of pure protein was isolated which was free of DNA topoisomerase, ligase, nicking and nuclease activities. The enzyme also shows ATPase activity dependent on single-stranded DNA and has an apparent molecular weight of 92 kDa by SDS-polyacrylamide gel electrophoresis. Only ATP or dATP hydrolysis supports the unwinding activity. The helicase requires a divalent cation (Mg2+ > Mn2+) at an optimum concentration of 1.0 mM for activity; it unwinds DNA duplexes less than 25 bp long and having a ssDNA stretch as short as 49 nucleotides. A replication fork-like structure is not required to perform DNA unwinding. HDH V cannot unwind either blunt-ended duplex DNA or DNA-RNA hybrids; it unwinds DNA unidirectionally by moving in the 3' to 5' direction along the bound strand, a polarity similar to the previously described human DNA helicases I and III (Tuteja et al. Nucleic Acids Res. 18, 6785-6792, 1990; Tuteja et al. Nucleic Acid Res. 20, 5329-5337, 1992) and opposite to that of human DNA helicase IV (Tuteja et al. Nucleic Acid Res. 19, 3613-3618, 1991).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgers P. M., Bambara R. A., Campbell J. L., Chang L. M., Downey K. M., Hübscher U., Lee M. Y., Linn S. M., So A. G., Spadari S. Revised nomenclature for eukaryotic DNA polymerases. Eur J Biochem. 1990 Aug 17;191(3):617–618. doi: 10.1111/j.1432-1033.1990.tb19165.x. [DOI] [PubMed] [Google Scholar]
- Dailey L., Caddle M. S., Heintz N., Heintz N. H. Purification of RIP60 and RIP100, mammalian proteins with origin-specific DNA-binding and ATP-dependent DNA helicase activities. Mol Cell Biol. 1990 Dec;10(12):6225–6235. doi: 10.1128/mcb.10.12.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz G. S., Dean F. B., Hurwitz J., Matson S. W. The unwinding of duplex regions in DNA by the simian virus 40 large tumor antigen-associated DNA helicase activity. J Biol Chem. 1988 Jan 5;263(1):383–392. [PubMed] [Google Scholar]
- Harosh I., Naumovski L., Friedberg E. C. Purification and characterization of Rad3 ATPase/DNA helicase from Saccharomyces cerevisiae. J Biol Chem. 1989 Dec 5;264(34):20532–20539. [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Seki M., Enomoto T., Hanaoka F., Yamada M. DNA-dependent adenosinetriphosphatase B from mouse FM3A cells has DNA helicase activity. Biochemistry. 1987 May 19;26(10):2924–2928. doi: 10.1021/bi00384a038. [DOI] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Thömmes P., Hübscher U. DNA helicase from calf thymus. Purification to apparent homogeneity and biochemical characterization of the enzyme. J Biol Chem. 1990 Aug 25;265(24):14347–14354. [PubMed] [Google Scholar]
- Thömmes P., Hübscher U. Eukaryotic DNA helicases: essential enzymes for DNA transactions. Chromosoma. 1992 Jun;101(8):467–473. doi: 10.1007/BF00352468. [DOI] [PubMed] [Google Scholar]
- Turchi J. J., Siegal G., Bambara R. A. DNA helicase E and DNA polymerase epsilon functionally interact for displacement synthesis. Biochemistry. 1992 Sep 22;31(37):9008–9015. doi: 10.1021/bi00152a043. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Danciger M., Klisak I., Tuteja R., Inana G., Mohandas T., Sparkes R. S., Farber D. B. Isolation and characterization of cDNA encoding the gamma-subunit of cGMP phosphodiesterase in human retina. Gene. 1990 Apr 16;88(2):227–232. doi: 10.1016/0378-1119(90)90035-p. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Tuteja R., Rahman K., Kang L. Y., Falaschi A. A DNA helicase from human cells. Nucleic Acids Res. 1990 Dec 11;18(23):6785–6792. doi: 10.1093/nar/18.23.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. S., Grosse F. Purification and characterization of two DNA helicases from calf thymus nuclei. J Biol Chem. 1991 Oct 25;266(30):20483–20490. [PubMed] [Google Scholar]







