Abstract
Yeast Rsp5p and its mammalian homologue, Nedd4, are hect domain ubiquitin-protein ligases (E3s) required for the ubiquitin-dependent endocytosis of plasma membrane proteins. Because ubiquitination is sufficient to induce internalization, E3-mediated ubiquitination is a key regulatory event in plasma membrane protein endocytosis. Rsp5p is an essential, multidomain protein containing an amino-terminal C2 domain, three WW protein-protein interaction domains, and a carboxy-terminal hect domain that carries E3 activity. In this study, we demonstrate that Rsp5p is peripherally associated with membranes and provide evidence that Rsp5p functions as part of a multimeric protein complex. We define the function of Rsp5p and its domains in the ubiquitin-dependent internalization of the yeast α-factor receptor, Ste2p. Temperature-sensitive rsp5 mutants were unable to ubiquitinate or to internalize Ste2p at the nonpermissive temperature. Deletion of the entire C2 domain had no effect on α-factor internalization; however, point mutations in any of the three WW domains impaired both receptor ubiquitination and internalization. These observations indicate that the WW domains play a role in the important regulatory event of selecting phosphorylated proteins as endocytic cargo. In addition, mutations in the C2 and WW1 domains had more severe defects on transport of fluid-phase markers to the vacuole than on receptor internalization, suggesting that Rsp5p functions at multiple steps in the endocytic pathway.
INTRODUCTION
Ubiquitin is a 76-amino acid polypeptide that is highly conserved and expressed in all eukaryotic cells. One role of ubiquitin is to tag proteins for degradation by the cytosolic 26S proteasome (reviewed by Hershko and Ciechanover, 1998). Another is to trigger the internalization of cell surface proteins (reviewed by Bonifacino and Weissman, 1998; Hicke, 1999; and Strous and Govers, 1999). Ubiquitin is linked to substrates by a covalent isopeptide bond between the carboxy-terminal glycine of the ubiquitin molecule and the ε-amino group of lysines within the substrate protein. Protein ubiquitination is an ATP-dependent reaction catalyzed by the sequential activity of a cascade of three enzymes: ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin-protein ligases (E3s). In most ubiquitination reactions, E3s recognize specific substrates. E3s are broadly defined as proteins that bind to a substrate, directly or indirectly, and promote the transfer of ubiquitin from a thiolester intermediate to the protein substrate or a growing polyubiquitin chain on the substrate (Hershko and Ciechanover, 1998). There are two known major classes of E3s. Members of the first class contain a conserved ∼350-amino acid hect (homologous to E6-AP carboxy terminus) catalytic domain that participates directly in catalysis by forming a thiolester bond with ubiquitin during the ubiquitination reaction (Huibregtse et al., 1995). The second class of ubiquitin-protein ligases are characterized by the presence of a zinc-binding RING finger domain that promotes E2-dependent ubiquitination, apparently without the formation of an E3-ubiquitin thiolester intermediate (reviewed by Freemont, 2000).
Both classes of E3s regulate the activity of plasma membrane proteins by endocytosis. The c-Cbl proto-oncoprotein, an E3 of the RING finger domain family, functions as a negative regulator of receptor tyrosine kinases (Lupher et al., 1999). c-Cbl binds to tyrosine-phosphorylated growth factor receptors through a variant SH2 domain and promotes their ligand-induced ubiquitination, a modification that has been shown to regulate the endocytosis of epidermal growth factor receptor and the colony-stimulating factor-1 receptor (Levkowitz et al., 1998; Miyake et al., 1998; Joazeiro et al., 1999; Lee et al., 1999; Levkowitz et al., 1999; Miyake et al., 1999; Waterman et al., 1999). Nedd4, a hect domain E3, promotes the ubiquitin-dependent turnover of the epithelial sodium channel (ENaC; Staub et al., 1997; Goulet et al., 1998). Nedd4 recognizes ENaC via a direct interaction between its WW protein-protein interaction domains and PPXY motifs in the cytosolic domains of ENaC subunits (Staub et al., 1996).
The yeast homologue of Nedd4, Rsp5p, is an essential protein implicated in a number of cellular processes regulated by the ubiquitin system, including endocytosis (reviewed by Harvey and Kumar, 1999 and Rotin et al., 2000). A role for Rsp5p in endocytosis was first demonstrated using the npi1 mutant, a strain carrying a mutation in the RSP5 promoter that causes a reduction in Rsp5p expression. The npi1 mutation impairs the ubiquitination and endocytosis of the uracil and general amino acid permeases that occur in response to changes in nutrient availability and stress (Galan et al., 1996; Springael and André, 1998). Rsp5p is a member of a growing family of hect domain ubiquitin-protein ligases that are characterized by an amino-terminal C2 domain, two to four copies of the WW protein-protein interaction domain, and a carboxy-terminal hect domain. The C2 domain is a motif that mediates Ca2+-dependent and -independent phospholipid binding in a number of proteins (reviewed by Rizo and Südhof, 1998). However, there are also a number of reported C2 domain-protein interactions (Zhang et al., 1994; Morrione et al., 1999; Plant et al., 2000). WW domains are evolutionarily conserved protein-protein interaction modules that recognize proline-rich sequences such as PPXY, PPLP, or PGM/PPR (Bedford et al., 2000; reviewed by Kay et al., 2000) and phosphoserine and phosphothreonine residues (Lu et al., 1999). The Rsp5p hect domain is the site of a number of temperature-sensitive mutations (Zoladek et al., 1997; Fisk and Yaffe, 1999; Wang et al., 1999) and has been shown to form a thiolester bond with ubiquitin through Cys777 (Huibregtse et al., 1995). The mechanism by which Rsp5p recognizes and ubiquitinates plasma membrane proteins is undefined.
As a model for the process of ubiquitin-dependent internalization, we have studied the down-regulation of the Saccharomyces cerevisiae mating pheromone receptor, Ste2p. Ste2p is a G protein-coupled receptor that binds the peptide pheromone α-factor and initiates a signal transduction pathway that is required for mating. Upon ligand binding, α-factor receptors are rapidly internalized and delivered to the vacuole where they are degraded (Singer and Riezman, 1990; Schandel and Jenness, 1994). Ligand binding induces hyperphosphorylation of tail serine residues (Reneke et al., 1988), a modification that positively regulates receptor ubiquitination (Hicke et al., 1998). A monoubiquitin moiety is sufficient to direct receptor internalization, and ubiquitin carries internalization information in its three-dimensional structure (Terrell et al., 1998; Shih et al., 2000).
In this study, we show that Rsp5p is multimeric and is localized to cellular membranes. We demonstrate that Rsp5p is essential for Ste2p ubiquitination and internalization, and we analyze the contribution of each of the domains of Rsp5p to receptor endocytosis. Additionally, we show that mutants in the noncatalytic domains of Rsp5p have differential effects on receptor internalization and lucifer yellow (LY) localization to the vacuole. Our data indicate that the Rsp5p WW domains are required for recognition of phosphorylated endocytic substrates and suggest that Rsp5p may also function at a step downstream of receptor ubiquitination in the endocytic pathway.
MATERIALS AND METHODS
Strains, Media, and Reagents
All strains were propagated in synthetic minimal (SD) medium (Sherman, 1991), rich (YPUAD) medium (2% bacto peptone, 1% yeast extract, 2% glucose supplemented with 20 mg/l adenine, uracil, and tryptophan), or casamino acids-galactose medium (0.67% yeast nitrogen base, 0.5% vitamin assay casamino acids, 2% galactose supplemented with 50 mg/l adenine, histidine, tryptophan, and methionine and with 20 mg/l uracil). Galactose (2%) was substituted for 2% glucose where indicated in the figure legends. The purification of 35S-labeled α-factor and Ste2p antibody was performed as previously described (Singer and Riezman, 1990; Dulic et al., 1991; Hicke and Riezman, 1996). Robert A. Lamb (Northwestern University, Evanston, IL) generously provided hemagglutinin 12CA5 monoclonal antibodies, and hexokinase antibodies were a gift from Gottfried Schatz (Biozentrum, University of Basel, Basel, Switzerland). A plasmid encoding myc-EMP47 was a gift from Howard Riezman (Biozentrum, University of Basel). The MTY300 strain bearing pHA-CNS1 was provided by M. Tesic and R. Gaber (Northwestern University).
Table 1 shows the genotypes of strains used in this study. Strains carrying rsp5-ww domain mutants as the sole source of Rsp5p were constructed as follows: ww mutant plasmids were transformed into LHY1512 (rsp5Δ pGAL-RSP5[URA3]). Two independent transformants were propagated on YNB plates (0.7% yeast nitrogen base, 2% glucose) containing amino acids and supplemented with 0.1% 5-fluoroorotic acid (5-FOA) to select for cells that had lost the pGAL-RSP5[URA3] plasmid. Single 5-FOA–resistant colonies from each of the two independent transformants were tested for growth at various temperatures. In each case, the two transformants exhibited identical growth phenotypes. Loss of the pGAL-RSP5[URA3] plasmid was confirmed by growth on medium lacking uracil. LHY2066 and LHY2232 strains were constructed using the same procedure. LHY1098, LHY1101, and LHY1103 strains were generated by sporulating heterozygous RSP5/rsp5Δ diploids carrying the appropriate RSP5 variant on a TRP1-marked plasmid and selecting rsp5Δ TRP1 haploid progeny.
Table 1.
Yeast strains
| Strain | Genotypea |
|---|---|
| LHY1 | ura3 leu2 his4 lys2 bar1 |
| LHY10 | ste2Δ∷LEU2 ura3 leu2 his3 trp1 bar1 |
| LHY23 | rsp5-1 ura3 leu2 trp1 bar1 GAL |
| LHY291 | his3 trp1 lys2 ura3 leu2 bar1 |
| LHY433 | rsp5Δ∷HIS3 leu2∷rsp5-2∷LEU2 bar1Δ∷HIS3 his3 lys2 trp1 ura3 |
| LHY434 | rsp5Δ∷HIS3 leu2∷RSP5∷LEU2 bar1Δ∷HIS3 his3 lys2 trp1 ura3 |
| LHY501 | end4-1 ura3 leu2 his4 bar1 |
| LHY776 | trp1 leu2 his3 ade2 lys2 ura3 gal4 gal80 cyhr2 LYS2∷GAL1UAS-HIS3TATA-HIS3 URA3∷GAL1UAS-GAL1TATA-LACZ gal |
| LHY837 | rsp5Δ∷HIS3 leu2∷rsp5-2∷LEU2 bar1Δ∷HIS3 his3 lys2 trp1 ura3 pRSP5[TRP1] |
| LHY855 | rsp5Δ∷HIS3 leu2∷rsp5-2∷LEU2 bar1Δ∷HIS3 his3 lys2 trp1 ura3 pHA-RSP5[TRP1] |
| LHY896 | his3 trp1 lys2 ura3 bar1 leu2∷myc-EMP47∷LEU2 pHA-RSP5[TRP1] |
| LHY1098 | rsp5Δ∷HIS3 his3 trp1 ura3 lys2 leu2 pHA-RSP5 [TRP1] |
| LHY1101 | rsp5Δ∷HIS3 trp1 leu2 ura3 lys2 ade2 bar1 or bar1Δ∷HIS3 pHA-C2ΔRSP5[TRP1] |
| LHY1103 | rsp5Δ∷HIS3 his3 trp1 ura3 lys2 leu2 bar1Δ∷LYS2 pHA-RSP5[TRP1] |
| LHY1394 | ubc1Δ∷HIS3 ubc4Δ∷HIS3 ura3 leu2 trp1 lys2 his3 bar1 |
| LHY1654 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pRSP5[TRP1] |
| LHY1655 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww1AXXA[TRP1] |
| LHY1692 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww2FXXA[TRP1] |
| LHY1693 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww3FXXA[TRP1] |
| LHY1729 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww1AXXP[TRP1] |
| LHY1734 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww2AXXP[TRP1] |
| LHY1735 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww3AXXP[TRP1] |
| LHY1776 | ste2Δ∷LEU2 rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pRSP5[TRP1] |
| LHY1856 | vat2Δ∷LEU2 his3 ade2 lys2 ura3 leu2 bar1 MATα |
| LHY1916 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww2,3AXXP[TRP1] |
| LHY1921 | pGAL-RSP5[URA3], same as LHY1729 |
| LHY1922 | pGAL-RSP5[URA3], same as LHY1734 |
| LHY1923 | pGAL-RSP5[URA3], same as LHY1735 |
| LHY2066 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pHA-RSP5[TRP1] |
| LHY2067 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pHA-rsp5-ww1AXXP[TRP1] |
| LHY2068 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pHA-rsp5-ww2AXXP[TRP1] |
| LHY2069 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pHA-rsp5-ww3AXXP[TRP1] |
| LHY2096 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww1,2AXXP[TRP1] |
| LHY2097 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww1,3AXXP[TRP1] |
| LHY2098 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL prsp5-ww1,2,3AXXP[TRP1] |
| LHY2161 | ste2Δ∷LEU2 pGAL-RSP5[URA3], same as LHY1654 |
| LHY2162 | ste2Δ∷LEU2 pGAL-RSP5[URA3], same as LHY1729 |
| LHY2163 | ste2Δ∷LEU2 pGAL-RSP5[URA3], same as LHY1734 |
| LHY2164 | ste2Δ∷LEU2 pGAL-RSP5[URA3], same as LHY1735 |
| LHY2168 | ste2Δ∷LEU2 pGAL-RSP5[URA3], same as LHY2098 |
| LHY2169 | ste2Δ∷LEU2 pGAL-RSP5[URA3], same as LHY23 |
| LHY2232 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL pHA-C2ΔRSP5[TRP1] |
| LHY2248 | rsp5Δ∷HIS3 leu2 ura3 his3 trp1 lys2 bar1 GAL p6xHis-myc-RSP5[TRP1] |
| LHY2254 | vat2Δ∷LEU2, same as LHY1921 |
| LHY2255 | vat2Δ∷LEU2, same as LHY1922 |
| LHY2256 | vat2Δ∷LEU2, same as LHY1923 |
| MTY300 | cns1Δ∷HIS3 leu2 ura3 his3 trp1 ade2 GAL pHA-CNS1[URA3] |
All strains are MATa unless otherwise indicated.
RSP5 plasmids were based on the yeast-Escherichia coli shuttle vector pRS414. To generate epitope-tagged versions of Rsp5p, a NotI site was introduced at the amino terminus of a genomic RSP5 clone (a gift from Jon Huibregtse, Rutgers University, New Brunswick, NJ) by site-directed mutagenesis using the two-step PCR procedure (Higuchi et al., 1988). Addition of the NotI site introduced three codons (Arg Gly Arg) immediately after the start codon of RSP5 in pNotI-RSP5 (LHP477). A sequence encoding a triple hemagluttinin (HA) epitope flanked by NotI sites was inserted into pNotI-RSP5 to generate pHA-RSP5 (LHP478). NotI- and HA-tagged RSP5 plasmids were able to fully complement rsp5 phenotypes. The C777A mutation was generated by PCR (Higuchi et al., 1988) and was introduced by multiple subcloning steps into pHA-RSP5 to generate LHP590. A precise deletion of the C2 domain (amino acids 2–140) was made by amplifying a fragment of RSP5 by PCR with a NotI site-containing primer that annealed at codon 141. The resulting product was ligated into LHP477 to generate prsp5-C2Δ (LHP510). A triple HA epitope flanked by NotI sites was inserted into prsp5-C2Δ to generate prsp5-C2Δ-HA (LHP511). PCR-derived sequences were verified by automated or manual DNA sequencing.
Plasmids encoding WW domain mutants were created by Quikchange site-directed mutagenesis with Pfu Turbo polymerase (Stratagene, La Jolla, CA). Oligonucleotides encoding a single Trp to Ala mutation in each WW domain were used to amplify a pRS414-based RSP5 wild-type plasmid (LHP472), generating LHP730 (ww1-AXXP), LHP845 (ww2-AXXP), and LHP846 (ww3-AXXP). F/AXXA mutants (LHP691, ww1-AXXA; LHP692, ww2-FXXA; LHP693, ww3-FXXA) were generated using the same procedure. WW domain double and triple mutants were generated by sequential mutation of each respective WW domain using the same Quikchange site-directed mutagenesis procedure. All mutations were confirmed by automated DNA sequencing. To generate epitope-tagged versions of WW domain mutants, an RSP5 BstEII fragment encoding all three WW domains was subcloned from plasmids encoding the WW domain mutations into BstEII-digested pHA-RSP5 (LHP478).
α- Factor Internalization Assays
α-Factor internalization assays were performed essentially as described by Dulic et al. (1991) and Terrell et al. (1998). Growth and specific assay conditions are indicated in the figure legends. Cells assayed by the continuous presence protocol were grown to early to mid log phase growth at 24°C, washed in YPUAD medium, and suspended in YPUAD medium at 5 × 108 cells/ml. Cells were shifted to 37°C for 15 min, and 35S-labeled α-factor was added to initiate internalization. Cells assayed by the pulse-chase protocol were treated in the same way with the following modifications. 35S-labeled α-factor was bound to cells on ice for 45–60 min. Unbound radioactivity was removed by centrifugation at 4°C, and internalization was initiated by the addition of media warmed to 30°C. Percentage internalization is expressed as the ratio of internalized to total cell-associated radioactivity. Curves represent the average of at least three independent assays, and error bars indicate the SD at each time point.
Cell Lysates and Immunoblots
Lysates for Ste2p immunoblotting were prepared as previously described (Hicke and Riezman, 1996) with minor modifications. Cells were grown in SD medium to early logarithmic phase, harvested by centrifugation, and transferred to YPUAD or YPUA galactose medium. Cells were incubated for 15 min at 37°C before the addition of 1 μM α-factor. Cells were not treated with cycloheximide. After lysis by mechanical agitation with glass beads, lysates were incubated 10–20 min at 37°C in urea/SDS buffer and clarified by centrifugation at 4°C. β-Mercaptoethanol and bromophenol blue were added to lysates to 2% and 0.002%, respectively. Samples were incubated at 37°C for 10–15 min before loading.
To test the stability of Rsp5p mutant proteins, cells were harvested, resuspended in 5 ml of YPUAD medium, split into two equal aliquots, and incubated at 30 or 37°C for 15 min. Two milliliters of each culture were removed to ice and metabolically inhibited with 20 mM NaN3 and 20 mM NaF. Lysate preparation was the same as described above.
Proteins were resolved on SDS-PAGE gels and were transferred to nitrocellulose membranes at 50–60 V for 1–2 h. Membranes were blocked with 5% dry milk in Tris-buffered saline, 0.1% Nonidet P-40 for 1–2 h or overnight at 4°C. Blots were incubated 1–4 h with 12CA5 anti-HA antibodies (1:1000–1:2500), anti-Ste2p antibodies, anti-c-myc antibodies (1:1000), or anti-hexokinase antibodies (1:5000). Blots were washed four times and incubated with 1:5000 horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G or 1:5000 horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G antibodies (Sigma, Saint Louis, MO). After several washes, blots were developed with SuperSignal reagents (Pierce, Rockford, IL).
β-Galactosidase Assays
RSP5 was cloned into the pAS2–1 and pACT2 GAL4 yeast two-hybrid fusion vectors (CLONTECH Laboratories, Palo Alto, CA) by standard cloning procedures. Expression of Rsp5p-Gal4p fusion proteins of the expected molecular weights was confirmed by immunoblotting. RSP5 fusion plasmids and control vectors were transformed into LHY776 (Y190 strain, CLONTECH), and multiple transformants were assayed for β-galactosidase activity. Three independent cultures of representative transformants were assayed in parallel according to the protocol recommended in the CLONTECH Yeast Protocols Handbook.
Glutathione S-Transferase (GST)-Fusion Protein Precipitations
BL21 CodonPlus (Stratagene, La Jolla, CA) E. coli cultures were induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside to stimulate synthesis of GST (pGEX-4T-3, Pharmacia, Piscataway, NJ) or GST-Rsp5p (RSP5 in pGEX-6P-1, a gift from Jon Huibregtse, University of Texas at Austin). Induction of GST-Rsp5p was performed at 18–20°C to optimize fusion protein solubility. Induction of GST was performed at 37°C. Cells were harvested, lysed by sonication in phosphate-buffered saline (PBS) with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, and 1 mM EDTA), and incubated with glutathione-Sepharose beads (Pharmacia) for 1–2 h at 4°C. The beads were washed four times with PBS and stored in PBS with protease inhibitors and 10 mM NaN3.
For yeast lysate preparation, cells were grown in YPUAD medium at 30°C to early to mid logarithmic phase, harvested, and washed in 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5. Cell pellets were lysed by agitation with glass beads in 100 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 0.5 mM MgCl2, 1 mM EGTA, 0.2 mM dithiothreitol, 10 mM NaN3 (Wendland and Emr, 1998), and protease inhibitors (0.1 μg/ml chymostatin, 1 μg/ml leupeptin, 2.5 μg/ml antipain, 1 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride). Lysates were subsequently extracted in the same buffer with 0.25% Triton X-100 and 2 mg/ml dodecyl β-d-maltoside for 1 h on ice. Lysates were clarified by centrifugation at 15,000 × g at 4°C. The protein concentration of lysates was ∼10 mg/ml. A fraction of the total lysate was reserved for immunoblot analysis. Lysates (∼5 mg of protein) were incubated with GST or GST-Rsp5p beads for ∼6 h at 4°C with agitation. After the sample was washed four times in the same buffer, bound proteins were eluted by boiling in Laemmli sample buffer (Laemmli, 1970) containing protease inhibitors. Samples were analyzed by immunoblotting as described above.
Subcellular Fractionation
Subcellular fractionation experiments were performed as described by Pryer et al. (1993) with minor modifications. Enzymatic digestion of the yeast cell wall was with β-1,3-glucanase either prepared according to the protocol of Shen et al. (1991) or purchased from ICN Pharmaceuticals (Costa Mesa, CA). To regenerate metabolic activity after digestion of the cell wall, we incubated cells in osmotically supported rich media for 15–30 min. For analysis, cell extract equivalents of each fraction were resolved by SDS-PAGE. Fractions shown in Figure 3B were dissolved in twofold diluted urea buffer/2% SDS. All other samples were in 1× Laemmli sample buffer. Specific variations in the growth conditions and treatment of lysates are indicated in the figure legends. Resolved samples were transferred to nitrocellulose and analyzed by immunoblotting as described above.
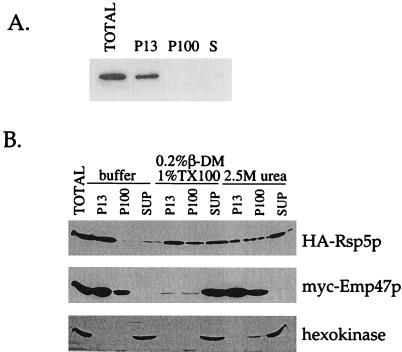
Figure 3.
Rsp5p associates with the membrane fraction of a cell lysate. (A) Differential centrifugation of a lysate prepared from cells expressing HA-Rsp5p (LHY2066). LHY2066 cells were propagated at 24°C in YPUA galactose medium; 13,000 × g (P13), 100,000 × g (P100), and soluble (S) cell fractions were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with HA antibodies. (B) Differential centrifugation after biochemical treatments. LHY896 cells were grown in YPUAD medium at 30°C. The cell lysate was mock (buffer) treated, treated with 2.5 M urea, or treated with 1.0% (vol/vol) Triton X-100 (TX100) and 0.2% (wt/vol) dodecyl β-d-maltoside (β-DM) for 1 h on ice before fractionation. Fractions were analyzed as in A by immunoblotting with HA, c-myc, or hexokinase antibodies to detect HA-Rsp5p, myc-Emp47p, and hexokinase, respectively. An equivalent amount of the total cell extract is shown for comparison. SUP, supernatant.
LY Endocytosis Assays
LY endocytosis assays were performed essentially as described by Dulic et al. (1991). Cells were grown to early to mid logarithmic phase in YPUAD medium. Cells (1–2 × 107) were harvested by centrifugation and suspended in 90 μl of YPUAD. As indicated in the figure legends, cells were shifted to 30°C for 15 min before the addition of 10 μl of 40 mg/ml LY carbohydrazide (Fluka Chemika-Biochemika, Buchs, Switzerland) or LY was immediately added at 30°C. After the sample was incubated for 60–75 min, 1 ml of ice-cold phosphate/stop buffer (50 mM sodium phosphate, pH 7.5, 10 mM NaN3, 10 mM NaF) was added to the cells. Cells were washed in the same buffer three to four times and mounted on a slide by embedding in 1.7% low-melt agarose. Cells were viewed using an LSM410 confocal microscope (Zeiss, Thornwood, NY) equipped with fluorescein isothiocyanate filter sets. Images were taken under identical parameter conditions.
RESULTS
Rsp5p Is Required for Ste2p Ubiquitination and Internalization
Upon binding α-factor, Ste2p undergoes sequential hyperphosphorylation and ubiquitination (Reneke et al., 1988; Hicke et al., 1998). Receptor ubiquitination requires E2s of the Ubc1p/Ubc4p/Ubc5p family (Hicke and Riezman, 1996). Rsp5p binds preferentially to E2s of this family (Nuber et al., 1996; Kumar et al., 1997) and is required for the endocytosis of amino acid permeases (Galan et al., 1996; Springael and André, 1998). To investigate the role of Rsp5p in Ste2p down-regulation, we used two rsp5 temperature-sensitive alleles. The rsp5–1 allele was isolated by F. Winston and colleagues (Harvard Medical School, Cambridge, MA) and was characterized by J. Huibregtse and colleagues (University of Texas at Austin). The rsp5-1 mutation results in a single amino acid change in the hect catalytic domain, and the mutant form of the protein is deficient in the formation of ubiquitin-thiolester intermediates (Wang et al., 1999). The rsp5-2 allele was isolated by B. Seraphin (European Molecular Biology Laboratory, Heidelberg, Germany) and S. Jentsch (Max Planck Institute for Biochemistry, Martinsreid, Germany) and has not been mapped (S. Jentsch, personal communication). Both mutations confer a temperature-sensitive growth defect at 37°C.
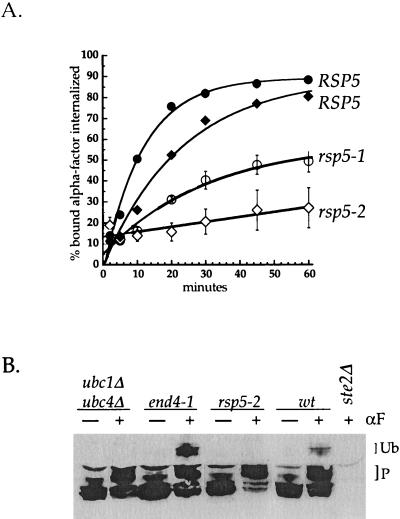
To test whether Rsp5p plays a role in Ste2p internalization, we used an assay that measures the internalization of 35S-labeled α-factor. At 37°C, wild-type cells internalized α-factor rapidly, although the half-time of internalization varied somewhat with strain background. By contrast, rsp5-1 and rsp5-2 cells internalized α-factor very slowly (Figure 1A). To investigate whether Rsp5p is required for Ste2p ubiquitination, we examined the modification of Ste2p in rsp5-2 cells before and after α-factor treatment. We compared the levels of receptor ubiquitination in the rsp5-2 mutant, in the ubc1Δ ubc4Δ mutant lacking E2s that are required for Ste2p ubiquitination, in another endocytosis mutant, end4-1, and in wild-type cells (Figure 1B). In wild-type cells, α-factor induced the appearance of hyperphosphorylated and monoubiquitinated forms of the receptor. Ubiquitinated species accumulated in the endocytosis mutant, end4-1. Similar to ubc1Δ ubc4Δ cells, rsp5-2 cells were able to phosphorylate receptors normally but were unable to ubiquitinate activated receptors. These data demonstrate that Rsp5p is required for the ligand-stimulated ubiquitination and internalization of Ste2p.
Figure 1.
Rsp5p is required for Ste2p ubiquitination and internalization. (A) α-Factor internalization assays performed by the continuous presence protocol at 37°C (see MATERIALS AND METHODS). Cells were propagated in SD medium at 24°C and shifted to 37°C for 15 min before the addition of radiolabeled α-factor. RSP5 (LHY434, ♦), rsp5-2 (LHY433, ⋄), RSP5 (LHY291, ●), rsp5–1 (LHY23, ○). (B) Ste2p modification before and after α-factor (αF) treatment. ubc1Δ ubc4Δ (LHY1394), end4-1 (LHY501), rsp5-2 (LHY433), wild-type (LHY434), and ste2Δ (LHY10) cells were treated (+) or not (−) with 1 μM α-factor for 8 min at 37°C. Total cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with anti-Ste2p antibodies. Phosphorylated (P) and monoubiquitinated (Ub) receptor species are indicated by the labeled brackets.
Because Ste2p undergoes requisite ubiquitination before internalization, the catalytic activity of Rsp5p might be essential for receptor endocytosis. Consistent with this idea, a catalytically inactive Rsp5p mutant with cys777 mutated to alanine failed to rescue the α-factor internalization defect in rsp5-1 cells (Dunn and Hicke, unpublished results). This observation confirms that the catalytic function of Rsp5p is required for α-factor receptor internalization.
Rsp5p Interacts with Itself
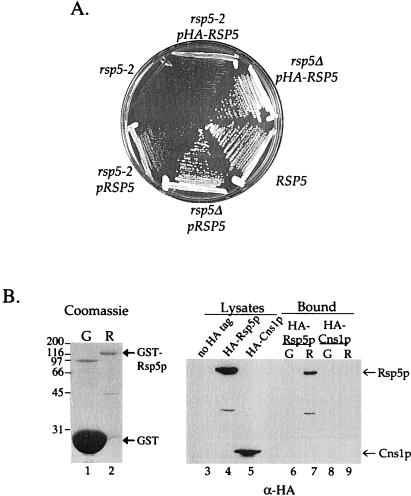
Several experiments with the rsp5-2 mutant suggested that this mutation was semidominant. To test this idea we introduced RSP5 on a centromere-based plasmid into rsp5-2 cells. Figure 2A shows that a plasmid bearing wild-type RSP5 or HA-epitope-tagged RSP5 was able to fully rescue the growth defect of an rsp5Δ null mutation. However, the growth of rsp5-2 cells carrying either of these plasmids was severely impaired compared with wild-type cells, indicating that this mutation is semidominant.
Figure 2.
Rsp5p self-interaction. (A) Semidominance of the rsp5-2 mutation. Cells of the indicated genotype were streaked onto YPUAD medium and grown for 2 d at 37°C. (B) GST-fusion protein precipitations. Left, Coomassie blue staining of the GST (G, lane 1) and GST-Rsp5p (R, lane 2) proteins used for each binding experiment. Bold arrows indicate the migration of GST and GST-Rsp5p. Molecular mass standards in kilodaltons are indicated at the left. Right, immunoblot analysis of GST pull-down experiment. Lysates prepared from LHY2066 (HA-RSP5) and MTY300 (HA-CNS1) cells were incubated with glutathione beads prebound to GST or to GST-Rsp5p. Bound proteins were eluted with Laemmli sample buffer. Eluted proteins and a fraction of the total cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with HA antibodies. Lanes 3–5, lysates of LHY2248 (no HA tag, lane 3), LHY2066 (HA-RSP5, lane 4), and MTY300 (HA-CNS1, lane 5) cells. Lanes 6–7, proteins from the LHY2066 lysate that bound to GST (lane 6) or GST-Rsp5p (lane 7). Lanes 8–9, proteins from the MTY300 lysate that bound to GST (lane 8) or GST-Rsp5p (lane 9). Arrows indicate the migration of HA-Rsp5p and HA-Cns1p.
One possible explanation for the semidominance of the rsp5-2 mutation is that Rsp5p functions as a multimer. In this case, Rsp5–2p and Rsp5wtp might form nonfunctional heteromers. To investigate the possibility of Rsp5p homo-interaction, we took two independent approaches. First, we tested whether Rsp5p could interact with itself in the yeast two-hybrid system. Cells carrying Rsp5p fused to the Gal4p DNA-binding domain and Rsp5p fused to Gal4p activation domain showed a 320-fold increase in β-galactosidase activity relative to control cells (Table 2). We also performed experiments to test whether a GST-Rsp5p fusion protein purified from E. coli could interact with HA-Rsp5p in a yeast cell lysate. HA-Rsp5p bound to GST-Rsp5p but not to GST alone (Figure 2B), indicating that Rsp5p can interact with itself in vitro. A fraction of the total HA-Rsp5p bound to GST-Rsp5p; however, this may be because much of the HA-Rsp5p in the lysate already existed in a stable Rsp5p-Rsp5p multimer. To confirm the specificity of the interaction, we tested whether GST-Rsp5p could interact with an unrelated HA-tagged protein involved in Hsp90 chaperone activity, HA-Cns1p (Marsh et al., 1998). HA-Cns1p was not precipitated by either GST or by GST-Rsp5p (Figure 2B). Thus, Rsp5p interacts with itself, directly or indirectly, in vitro and in vivo.
Table 2.
Rsp5p-Rsp5p yeast two-hybrid interaction.
| DNA-binding domain plasmid | Activation domain plasmid | β-Galactosidase activity (Miller units)a |
|---|---|---|
| GAL4 DBD-RSP5 | GAL4-ACT | 0.7 ± 0.2 |
| GAL 4 DBD | GAL4-ACT-RSP5 | 0.04 ± 0.04 |
| GAL4 DBD-RSP5 | GAL4-ACT-RSP5 | 224 ± 17 |
β-galactosidase activity is represented as the mean activity and the SD for three independent cultures.
Rsp5p Is a Peripheral Membrane Protein
Rsp5p is required for the ubiquitination of a number of membrane proteins (Rotin et al., 2000). To determine whether Rsp5p is associated with membranes, we performed subcellular fractionation. In these experiments, yeast cells were lysed after removal of the cell wall and subjected to differential centrifugation to separate the insoluble and soluble components of the cell. The actin cytoskeleton and large cellular membranes, such as plasma membrane and vacuoles, sediment after centrifugation at 13,000 × g; lighter membrane compartments such as endoplasmic reticulum, Golgi, and endosomes sediment after centrifugation at 100,000 × g (Bénédetti et al., 1994). Soluble proteins remain in the 100,000 × g supernatant. To detect Rsp5p, we used cells expressing a fully functional HA-tagged Rsp5p (see Figure 2). Rsp5p from lysed and fractionated cells was associated almost completely with the 13,000 × g pellet (Figure 3A).
To determine the nature of this particulate association, lysates were treated with the nonionic detergents Triton X-100 and dodecyl-β-d-maltoside or with 2.5 M urea. The addition of dodecyl-β-d-maltoside facilitates solubilization of the Triton X-100-resistant yeast plasma membrane (S. Pacheco, S. Shih, and L. Hicke, unpublished results). A cytosolic protein, hexokinase, was found in the soluble fraction in each case. The Golgi resident integral membrane protein, c-myc-tagged Emp47p, sedimented with the 13,000 and 100,000 × g pellets. As expected, the behavior of Emp47p did not change upon treatment with urea, but it was almost completely solubilized by the detergents. A significant fraction of Rsp5p was solubilized by detergent, and an even greater fraction was solubilized by urea, a protein-protein interaction–disrupting reagent (Figure 3B). The efficiency of Rsp5p solubilization by detergents and by urea varied from experiment to experiment, ranging from the partial solubilization shown here to nearly complete solubilization. Rsp5p was also solubilized by 0.1 M sodium carbonate, pH 11.5, a treatment that strips peripherally associated proteins from membranes (Fujiki et al., 1982), and by 0.5 M NaCl (Dunn and Hicke, unpublished results). These data indicate that Rsp5p is a peripheral membrane protein and suggest that the peripheral association is mediated by protein-protein interactions. The shift of Rsp5p from the P13 to the P100 fraction after treatment with either urea or with detergent suggests that Rsp5p may be associated with a large, sedimentable protein complex that is linked to cellular membranes in the P13 and sediments in the P100 after release from membranes. Alternatively, Rsp5p may be associated with multiple distinct subcellular structures that are affected differently by treatment with urea and detergent. Treatment with latrunculin A, an actin-disrupting drug, over a broad range of concentrations had no effect on Rsp5p sedimentation, and pellet association was insensitive to mutations in genes encoding actin and the actin-associated proteins Pan1p and Vrp1p (Dunn and Hicke, unpublished results). These observations strongly suggest that Rsp5p fractionation with the particulate fraction of a cell lysate is independent of the actin cytoskeleton.
Rsp5p Domains Required for Receptor-mediated Endocytosis
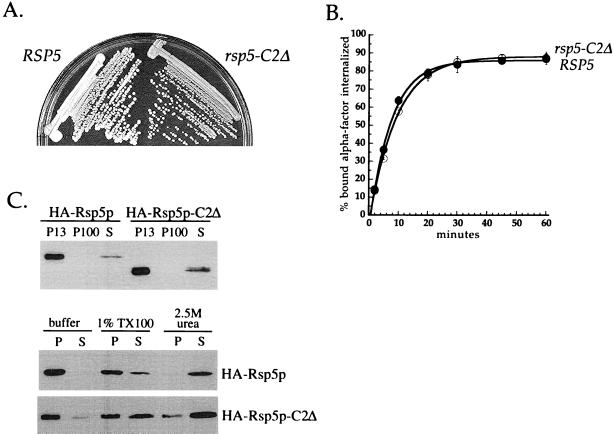
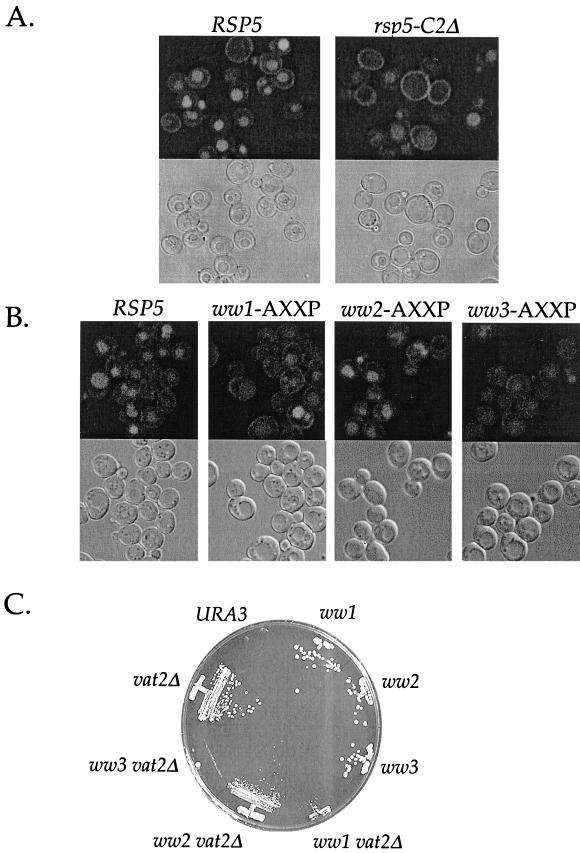
To determine which domains of Rsp5p are required for its role in endocytosis, we made mutations in each of the Rsp5p C2 and WW domains. It has been shown previously that the Rsp5p C2 domain is not essential (Wang et al., 1999). Springael et al. showed that a mutant Rsp5p lacking the C2 domain could ubiquitinate but could not internalize Gap1p, the yeast general amino acid permease (Springael et al., 1999a). We constructed an HA-tagged Rsp5p with a precise deletion of the C2 domain and provided this mutant as the sole source of Rsp5p in the cell. This strain grew normally at 16, 30, and 37°C (Figure 4A; and Dunn and Hicke, unpublished results), consistent with previous reports. We then compared the ability of cells expressing HA-Rsp5p-C2Δ or HA-Rsp5p to internalize α-factor. The rsp5-C2Δ mutant was able to internalize α-factor as efficiently as cells expressing wild-type Rsp5p (Figure 4B). These data indicate that the C2 domain plays no role in α-factor receptor internalization.
Figure 4.
Phenotypic analysis of rsp5-C2Δ cells. (A) Growth of RSP5 and rsp5-C2Δ cells. LHY1103 (RSP5) and LHY1101 (rsp5-C2Δ) cells were streaked onto YPUAD medium and grown for 2 d at 30°C. (B) α-Factor internalization assays performed by the pulse-chase protocol at 30°C (see MATERIALS AND METHODS). Cells were propagated in SD medium at 30°C. RSP5 (LHY1103, ●), rsp5-C2Δ (LHY1101, ○). (C) Fractionation of lysates prepared from cells expressing HA-Rsp5p or HA-Rsp5p-C2Δ. Top, differential centrifugation of LHY2066 (HA-Rsp5p) and LHY2232 (HA-Rsp5p-C2Δ) lysates. Cells were propagated in casamino acids-galactose medium at 24°C. Fractionation and immunoblot analysis were performed as described for Figure 3A. Bottom, fractionation of LHY1098 (HA-Rsp5p) and LHY1101 (HA-Rsp5p-C2Δ) cell lysates. Cells were propagated in YPUAD medium at 30°C. Lysates were fractionated into 100,000 × g pellet and supernatant fractions after biochemical treatment with 1.0% Triton X-100 (TX100), 2.5 M urea, or buffer as described for Figure 3.
Because the C2 domain has been shown to mediate lipid interactions in a number of proteins, this domain was a candidate for the determinant that mediates Rsp5p membrane association. However, we observed that the majority of Rsp5p-C2Δ was still associated with the pellet fraction of a yeast lysate (Figure 4C). Furthermore, the fractionation behavior of the C2Δ mutant after various biochemical treatments was similar to wild-type Rsp5p (Figure 4C). These data indicate that Rsp5p can associate with cellular membranes in the absence of its C2 domain.
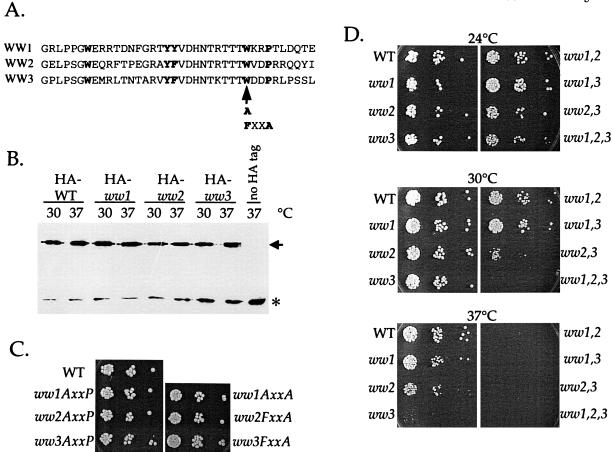
To analyze the function of Rsp5p WW domains in endocytosis, we engineered mutations in conserved residues in the ligand-binding pocket of the WW domain. First, we made mutations in each of the WW domains to convert the conserved WXXP sequence to FXXA or to AXXA (Figure 5A). Each of these point mutations individually abolishes binding of WW domains to proline-rich peptides (Chen et al., 1997), and rsp5-ww-FXXA mutants were used previously to show that WW2 and WW3 are essential domains (Wang et al., 1999). Second, we mutated the second conserved tryptophan of the WW domain to alanine, converting the conserved WXXP to AXXP (Figure 5A). This mutation abolishes the binding of Pin1p WW domains to phosphoserine/threonine in vitro (Lu et al., 1999), and the WXXP tryptophan was shown to be unimportant for WW domain stability (Macias et al., 2000). Thus, the ww-AXXP mutants are likely to be deficient specifically in ligand binding and not in WW domain structure. The ww-AXXP and ww-FXXA mutant proteins were expressed normally and were stable at 37°C (Figure 5B; Dunn and Hicke, unpublished results). Unexpectedly, we found that none of these mutations were lethal. ww-FXXA or ww-AXXP mutants in any of the three WW domains could complement the growth of a strain with a chromosomal deletion of RSP5 at 30°C (Figure 5C). To define the residues of the WW domains that are essential for in vivo function, we made several other mutations and analyzed their effect on growth. We mutated residues that are predicted to be important for WW domain function based on multiple criteria: conservation of the residue among WW domains, the crystallographic structure of a WW domain bound to its proline-rich ligand, and biochemical experiments identifying residues important for ligand binding (Macias et al., 1996; Chen et al., 1997; Lu et al., 1999). None of the mutations were lethal, but most mutations that block the ligand-binding function of WW domains in vitro resulted in a temperature-sensitive growth defect (Table 3). However, mutation of the second conserved tryptophan of WW3 to phenylalanine resulted in completely normal cell growth, even though this mutation blocked ligand binding in the YAP (Yes-associated protein) WW domain (Chen et al., 1997).
Figure 5.
Growth phenotypes of rsp5-ww domain mutants. (A) Sequence alignment of Rsp5p WW domains. WW1, amino acids 229–266. WW2, amino acids 331–368. WW3, amino acids 387–424. The highly conserved residues of the WW domain are in bold type. The arrow indicates the position of AXXP and FXXA mutations. (B) Expression of HA epitope-tagged ww-AXXP mutant proteins in cells expressing the mutant protein as the sole source of Rsp5p. Cells of the indicated genotypes were grown to early logarithmic phase in SD medium at 24°C. Cells were transferred to YPUAD medium, and aliquots of the cultures were shifted to 30 or 37°C for 15 min. Extracts prepared from these cells were prepared and analyzed by immunoblotting with HA antibodies. The asterisk indicates an unrelated cross-reacting band that serves as a loading control. WT, wild type. (C) ww-AXXP and ww-FXXA mutants are viable. Equal numbers of rsp5Δ cells carrying the indicated RSP5 plasmids were plated onto YPUAD medium (three serial 1:10 dilutions) and grown 3 d at 30°C. (D) Growth of single, double, and triple ww-AXXP mutants. Cells of the indicated genotype were serially diluted as in C and were grown on YPUAD medium at 24°C for 3 d, 30°C for 2 d, or 37°C for 2 d.
Table 3.
Effects of Rsp5p WW domain mutations on cell growth
| Domain | Mutation | Growth at 37°C |
|---|---|---|
| (wild type) | none | + |
| ww1 | W1→F | + |
| ww1 | YY→AY | t.s. |
| ww1 | YYV→YYA | + |
| ww1 | WXXP→AXXP | t.s. |
| ww1 | WXXP→AXXA | t.s. |
| ww2 | YF→FF | t.s. |
| ww2 | WXXP→AXXP | t.s. |
| ww2 | WXXP→FXXA | t.s. |
| ww3 | YF→AF | t.s. |
| ww3 | WXXP→FXXP | + |
| ww3 | WXXP→AXXP | t.s. |
| ww3 | WXXP→FXXA | t.s. |
rsp5Δ cells carrying the indicated RSP5 plasmids were grown on YPUAD plates at 37°C. t.s. indicates temperature-sensitive slow growth. Resides in bold are those indicated in bold in Figure 5A. W1 indicates the first conserved tryptophan of the WW domain.
Because none of the individual ww mutations were lethal, we made combinations of double mutants in the WW domains (ww1,2, ww1,3, ww2,3) and a triple mutant (ww1,2,3). The growth of single, double, and triple ww-AXXP mutants was analyzed at 24, 30, and 37°C (Figure 5D). The single ww-AXXP mutants grew at a rate indistinguishable from that of wild-type at 24 or 30°C but exhibited distinct growth defects at 37°C (Figure 5D). The growth defect of the rsp5-ww1 mutant was modest, whereas the rsp5-ww2 and rsp5-ww3 mutants exhibited a severe temperature-sensitive growth defect. The ww2,3 and ww1,2,3 mutants exhibited marked growth defects at 30°C, and the double and triple mutant combinations were unable to grow at 37°C. A similar growth analysis of single, double, and triple ww-F/AXXA mutants gave the same results (Dunn and Hicke, unpublished results). These data suggest that the WW domains have partially overlapping functions in vivo.
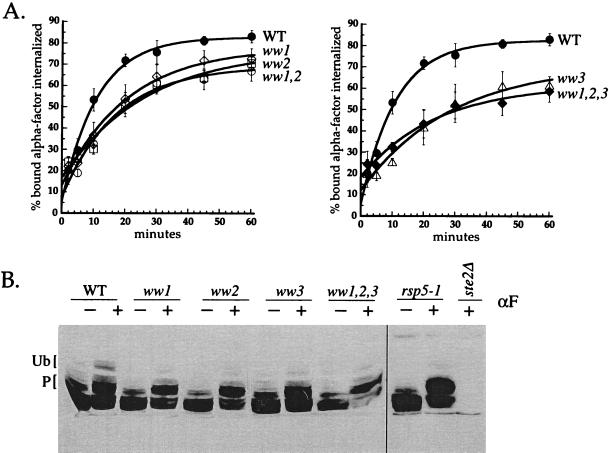
To analyze the function of the WW domains in receptor internalization, we performed α-factor internalization assays on cells expressing rsp5-ww domain mutants in an rsp5Δ background. All three single ww-AXXP mutants exhibited a defect in the rate of α-factor internalization (Figure 6A). Calculation of the half-times of internalization indicated that the ww1, ww2, and ww3 mutants exhibited 1.9-, 2.4-, and 2.6-fold defects, respectively. We also assayed α-factor internalization in cells carrying double and triple rsp5-ww mutations. The defect of a ww1,2 double mutant was not more severe than the single ww2 mutant, and the defect of a ww1,2,3 mutant was not significantly more severe than the single ww3 mutant (Figure 6A). Similarly, the defect in ww1,3 and ww2,3 mutants was similar to that of the ww3 single mutant (Dunn and Hicke, unpublished results). Although the effects of the FXXA and AXXP mutations on cell growth were identical, we observed that ww-FXXA mutants displayed only a minor α-factor internalization defect compared with the ww-AXXP mutants (Dunn and Hicke, unpublished results).
Figure 6.
Rsp5p WW domains are required for receptor ubiquitination and internalization. (A) Continuous presence α-factor internalization assays. Cells were grown at 24°C to early logarithmic phase in SD medium and shifted to 37°C for 15 min before the addition of 35S-labeled α-factor. Wild-type (WT) (LHY1654, ●), rsp5-ww1 (LHY1729, ⋄), rsp5-ww2 (LHY1734, □), rsp5-ww1,2 (LHY2096, ○), rsp5-ww3 (LHY1735, ▵), rsp5-ww1,2,3 (LHY2098, ♦). (B) Ste2p modification before and after α-factor treatment. Wild-type (LHY2161), ww1 (LHY2162), ww2 (LHY2163), ww3 (LHY2164), ww1,2,3 (LHY2168), and rsp5-1 cells (LHY2169) expressing Ste2p from a galactose-inducible promoter were grown to early logarithmic phase in SD medium containing 2% galactose at 24°C. Cells were shifted to 37°C for 15 min and then treated (+) or not (−) with 1 μM α-factor for 8.5 min. Lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with anti-Ste2p antibodies. Labeled brackets indicate the migration of hyperphosphorylated (P) and ubiquitinated (Ub) species.
One possible explanation for the α-factor internalization defect in ww mutants is that Rsp5p WW domains are important for the recognition of activated α-factor receptors. To test whether Ste2p is ubiquitinated in the ww mutants, we examined the ligand-induced modifications of Ste2p in cells expressing wild-type and ww-AXXP mutant Rsp5p (Figure 6B). For comparison, we also analyzed receptor ubiquitination in rsp5-1 cells deficient in the catalytic activity of Rsp5p. In wild-type cells, receptors were efficiently ubiquitinated after α-factor stimulation, whereas ubiquitinated forms of Ste2p were reduced in each single ww mutant. In a ww1,2,3 mutant, receptor ubiquitination occurred at a level comparable to that of single ww mutants, indicating that the defects in receptor ubiquitination in single ww mutants were not additive, paralleling the results of internalization assays. Receptor ubiquitination was not completely blocked in the ww mutants, and the residual ubiquitination may account for the slow receptor internalization that still occurs in these mutants. These data indicate that Rsp5p WW domains are required for efficient recognition of phosphorylated receptors. The results also suggest that the three WW domains do not have redundant functions in promoting receptor ubiquitination and internalization, but instead, all are uniquely required for these functions.
Fluid-Phase Endocytosis Defects in Rsp5 Domain Mutants
The previous experiments tested the function of Rsp5p domains in the internalization step of receptor-mediated endocytosis. To analyze the function of Rsp5p in fluid-phase endocytosis, we used an assay that monitors the transport of LY to the vacuole. LY is a soluble fluorescent molecule that is internalized by fluid-phase endocytosis and delivered to the vacuole where it accumulates over time (Dulic et al., 1991). Mutants that block endocytosis at the internalization step or at later transport steps cannot localize LY to the vacuole (Dulic et al., 1991; Wiederkehr et al., 2000). It was shown previously that an allele of RSP5 carrying a mutation in the hect domain, mdp1-1, is defective in LY accumulation in the vacuole (Zoladek et al., 1997). We further investigated the role of Rsp5p in fluid-phase endocytosis by analyzing the function of each its domains in this process. We observed that rsp5-1 cells were unable to efficiently transport LY at the nonpermissive temperature (Dunn and Hicke, unpublished results), consistent with previous reports (Zoladek et al., 1997). Compared with a congenic wild-type strain, an rsp5-C2Δ mutant was also defective in its ability to accumulate LY in the vacuole (Figure 7A). Cells carrying rsp5-ww1 and rsp5-ww3 cells were strongly impaired in their ability to transport LY to the vacuole at 30°C, whereas the rsp5-ww2 mutation had a small, if any, effect on LY endocytosis (Figure 7B). These strains were not assayed at 37°C because LY transport to the vacuole in the congenic wild-type strain was not efficient at this temperature. These data suggest that a subset of the amino-terminal domains of Rsp5p, in addition to the catalytic domain, is required for efficient fluid-phase endocytosis.
Figure 7.
Fluid-phase endocytosis in rsp5 domain mutants. (A) LY uptake in RSP5 (LHY2066) and rsp5-C2Δ (LHY2232) cells. Cells were grown to early logarithmic phase at 30°C in YPUAD medium and then incubated with LY for 1 h. (B) LY uptake in ww-AXXP mutants. RSP5 (LHY1654), ww1 (LHY1729), ww2 (LHY1734), and ww3 (LHY1735) cells were grown to early logarithmic phase in YPUAD at 24°C, shifted to 30°C for 15 min, and then incubated for 75 min with LY at 30°C. Top panels are fluorescein isothiocyanate images. Bottom panels are differential interference contrast images of the same field of cells. (C) Synthetic lethality of ww1-AXXP and ww3-AXXP mutants with vat2Δ. ww-AXXP and ww-AXXP vat2Δ mutant cells carrying pRSP5(WT)[URA3] were grown on YNB medium containing 5-FOA. The growth of URA3 cells and cells carrying a disruption in VAT2 alone (vat2Δ) are shown for comparison.
As an independent assay to assess the function of the Rsp5p WW domains in fluid-phase endocytosis, we performed a genetic analysis of rsp5-ww mutants in combination with the vat2Δ mutation. VAT2 encodes an integral membrane subunit of a H+-ATPase that is required for vacuole acidification (Yamashiro et al., 1990). Deficiency in both Vat2p function and endocytosis results in cell lethality, presumably because multiple pathways for vacuole acidification are blocked (Munn and Riezman, 1994). To investigate whether the WW domain mutations interact genetically with VAT2, we analyzed the growth of cells carrying both a disruption of VAT2 and ww-AXXP mutations. vat2Δ ww-AXXP cells carrying wild-type RSP5 on a URA3-marked plasmid were grown on medium containing 5-FOA to select for loss of the wild-type RSP5 URA3 plasmid (Figure 7C). A URA3 strain was unable to grow on this medium, indicating that growth was dependent on the loss of wild-type RSP5. Compared with cells carrying a single vat2Δ or ww-AXXP mutation, vat2Δ ww-AXXP double mutants exhibited pronounced synthetic growth defects. The synthetic growth defect of vat2Δ ww-AXXP mutants mirrored the defect in LY localization in the ww-AXXP mutants. ww1-AXXP vat2Δ and ww3-AXXP vat2Δ exhibited a strong defect, and ww2-AXXP vat2Δ exhibited a very weak synthetic growth defect compared with vat2Δ alone. These results support the conclusion that WW domains 1 and 3 are required for fluid-phase endocytosis.
DISCUSSION
In this study, we demonstrate that the Rsp5 ubiquitin-protein ligase and its WW domains are required for down-regulation of the yeast α-factor receptor. Cells carrying a temperature-sensitive rsp5 mutation were unable to internalize Ste2p at the nonpermissive temperature. rsp5 mutants were also defective in Ste2p ubiquitination, consistent with a direct role for Rsp5p in endocytic cargo modification. This study expands the current list of Rsp5p/Nedd4 plasma membrane targets from amino acid permeases, nutrient transporters, and ion channels to include signal-transducing receptors. A recent study indicated that Rsp5p is required for normal expression of OLE1, a gene required for synthesis of oleic acid, and it was suggested that many rsp5 phenotypes could be explained by an indirect effect of deficient fatty acid metabolism (Hoppe et al., 2000). However, the rsp5-2 endocytic defect cannot be rescued by the addition of oleic acid to the growth medium, although the rsp5-2 growth defect is substantially rescued in the same medium (Dunn and Hicke, unpublished results).
Using the yeast two-hybrid assay and biochemical experiments with differently tagged Rsp5p proteins, we showed that Rsp5p interacts with itself. The strong yeast two-hybrid interaction suggests that the Rsp5p-Rsp5p interaction is direct, but we cannot rule out the possibility that the interaction occurs through an intermediate protein(s). For instance, hect domain ubiquitin-protein ligases may multimerize by a stable interaction with E2-E2 multimers (Gwozd et al., 1995). Alternatively, Rsp5p may be part of a multimeric protein complex that contains more than one molecule of Rsp5p, a proposal supported by the observation that Rsp5p sediments in a large protein complex on sucrose gradients (Yashiroda et al., 1996). The rsp5-2 mutation is semidominant, because wild-type RSP5 was unable to fully rescue the growth defect of rsp5-2 cells. Nonfunctional Rsp5p mutant proteins may retain the ability to interact with wild-type Rsp5p, and mixed protein complexes may have reduced Rsp5p function.
Subcellular fractionation of yeast lysates showed that Rsp5p is localized to membranes. This association appears to be mediated primarily by protein-protein interactions. Consistent with our results, J. Huibregtse and colleagues have observed that an Rsp5p-GPF fusion protein was localized to the plasma membrane and a perivacuolar compartment (Jon Huibregtse, personal communication). Deletion of the C2 domain, a putative lipid-interacting domain, had a minor effect on the sedimentation behavior of Rsp5p. Thus, Rsp5p can associate with cellular membranes in the absence of its C2 domain. The C2 domain of Nedd4 has been shown to mediate Ca2+-dependent localization of Nedd4 to raft lipid microdomains in the apical membrane of polarized cells (Plant et al., 2000). Because our experiments examined crude total cellular membranes, we cannot rule out the possibility that the C2 domain mediates interaction with particular cellular membranes or with certain membrane microdomains. Our unpublished observations indicate that point mutations in the WW domains or catalytic hect domain do not solubilize Rsp5p, suggesting that Rsp5p may contain redundant signals for membrane association or may associate with membranes via an uncharacterized motif. The finding that Rsp5p is localized to membranes is consistent with its function in modifying plasma membrane proteins.
To determine which WW domains of Rsp5p are important for its endocytic function, we generated point mutations in each domain. We chose this approach because a previous report indicated that internal deletions of the WW domains resulted in an unstable protein (Springael et al., 1999). Unexpectedly, none of the point mutations, individually or in combination, resulted in cell inviability in an rsp5Δ null background, even though many of these mutations abolish the ability of WW domains to (detectably) bind ligands in vitro. In contrast, a previous study of the essential domains of Rsp5p indicated that WW2 and WW3 are essential domains, because point mutants in either domain could not rescue the temperature sensitivity of rsp5-1 cells (Wang et al., 1999). A more recent study used truncation experiments to show that WW3 and the hect domain are sufficient to provide the essential Rsp5p functions (Hoppe et al., 2000). In contrast to these studies, we examined the loss of function of individual domains in the context of the full-length protein and in the absence of any other form of Rsp5p. Our results indicate that the WW domains share overlapping functions in vivo, given that mutation of multiple WW domains caused a pronounced synthetic growth defect. We focused on the ww-AXXP mutants for our analysis of WW domain functions in endocytosis because, based on previous structural and biochemical studies, this mutation seemed the most likely to specifically disrupt ligand binding, particularly to phosphorylated substrates, without altering the WW domain fold (Lu et al., 1999; Macias et al., 2000).
α-Factor internalization and Ste2p ubiquitination assays indicated that all three WW domains are required for normal receptor ubiquitination and internalization. A triple ww1,2,3 mutant was still able to associate with the membrane fraction of a lysate (Dunn and Hicke, unpublished results), providing evidence that the effects of ww mutations on endocytosis are direct and are probably not due to mislocalization of Rsp5p. A subset of the WW domains may contribute directly to recognition of receptors, whereas an overlapping or distinct subset may promote receptor ubiquitination by mediating the formation of an E3-containing protein complex that functions at the plasma membrane. It is likely that Rsp5p and its WW domains also perform a function in internalization downstream of receptor ubiquitination because the internalization of a Ste2p-ubiquitin chimeric protein is still dependent on Rsp5p (Dunn and Hicke, unpublished results). WW domains 2 and 3 are important for binding another substrate of Rsp5p, the large subunit of RNA polymerase II (Wang et al., 1999). WW3 also binds to Spt23p, an Rsp5p-regulated transcription factor (Hoppe et al., 2000). Our finding that WW1 is required for endocytosis is the first function ascribed to this domain.
WW domains bind to proline-rich motifs, such as PPXY and PPLP, and to phosphoserine/threonine sites. The cytoplasmic domain of Ste2p does not contain a proline-rich motif that could mediate a WW domain interaction. However, the WW domains of Rsp5p may bind directly to α-factor-induced phosphoserines in the cytoplasmic tail. This scenario would explain how receptor phosphorylation positively regulates ubiquitination by providing binding sites for Rsp5p WW domains (Hicke et al., 1998). Alternatively, Rsp5p WW domains may recognize activated receptors through an unidentified adaptor protein or an as yet unidentified WW-binding motif. One Rsp5p-interacting protein that carries a PPXY motif, Bul1p, is not involved in receptor ubiquitination or internalization, because null mutations in both BUL1 and its homologue, BUL2, have no effect on α-factor internalization (M. Haulberg and Hicke, unpublished results). Our results suggest a model in which Rsp5p WW domains recognize phosphorylated endocytic cargo proteins, directly or indirectly, to promote subsequent hect domain-dependent ubiquitination.
Using a mutant deleted of the entire Rsp5p C2 domain, we show that the C2 domain is not required for α-factor internalization. In contrast, a mutant lacking the Rsp5p C2 domain was deficient in the endocytosis of Gap1p, the general amino acid permease (Springael et al., 1999a). This disparity is not surprising given that the internalization information in the cytoplasmic domains of Ste2p and Gap1p is significantly different. The major internalization signal in Ste2p is the SINNDAKSS sequence, a motif that promotes phosphorylation-dependent ubiquitination of the receptor (Hicke et al., 1998; Shih et al., 2000). In the case of Ste2p, a single ubiquitin moiety is sufficient to drive internalization in the absence of any other cis-acting elements in the receptor cytoplasmic tail (Shih et al., 2000). Internalization of Gap1p depends on ubiquitination and cis-acting elements including a dileucine motif and glutamate residue in a predicted α-helix (Springael and André, 1998). A normal rate of regulated Gap1p internalization requires the formation of di-ubiquitin chains linked through Lys63 of ubiquitin (Springael et al., 1999b). The C2 domain may be involved in these specific aspects of regulated permease endocytosis.
Using an assay that measures transport through several different steps of the endocytic pathway, we show that the domain requirements of Rsp5p in fluid-phase endocytosis are distinct from those for α-factor internalization. The C2 domain was completely dispensable for α-factor internalization but was required for efficient LY transport to the vacuole. Furthermore, LY localization assays indicated that WW1 and WW3, but not WW2, are required for fluid-phase transport to the vacuole. In support of this conclusion, mutants in WW domains 1 and 3 exhibited a strong synthetic growth defect with the vat2Δ mutation, a phenotype observed in mutants that block the uptake of extracellular fluid (Munn and Riezman, 1994). These results suggest that Rsp5p has multiple functions in endocytosis that are mediated by distinct amino-terminal (noncatalytic) domains of the protein. Because the C2Δ and ww1-AXXP mutants exhibited a more severe defect in fluid-phase transport to the vacuole than in receptor internalization, we suggest that Rsp5p functions at a step downstream of internalization in the endocytic pathway.
Evidence from other studies is consistent with a role for ubiquitination in sorting and transport in the late secretory and endocytic pathways (reviewed by Lemmon and Traub, 2000). Upon starvation, the tryptophan permease Tat2p undergoes vacuolar degradation that is dependent on ubiquitination sites in the permease cytoplasmic domain (Beck et al., 1999). Although most of Tat2p is localized intracellularly and appears to be transported directly to the vacuole without endocytosis from the plasma membrane, Tat2p degradation is still dependent on Rsp5p (Beck et al., 1999). Furthermore, proteins that function in sorting into multivesicular bodies, specifically the yeast protein Vps23p and its mammalian homologue TSG101, contain a domain related to E2s (Li et al., 1999; Babst et al., 2000). Another recent study has identified Rcy1p, an F-box protein, as a protein component required for endosomal sorting in yeast (Wiederkehr et al., 2000). F-box proteins mediate phosphorylation-dependent substrate recognition by SKp1-cullin-F-box protein ubiquitin ligase complexes, suggesting that Rcy1p function in endocytosis may involve ubiquitination. These observations suggest that Rsp5p and ubiquitination play a role in sorting to the vacuole after the convergence of the endocytic and secretory pathways.
Ubiquitin-protein ligases are emerging as key regulators of the activity of plasma membrane proteins. The RING-finger E3 c-Cbl recognizes tyrosine-phosphorylated growth factor receptors and promotes receptor ubiquitination (Joazeiro et al., 1999; Levkowitz et al., 1999). It will be interesting to determine whether Rsp5p/Nedd4 proteins share this mechanism, directly recognizing serine/threonine-phosphorylated plasma membrane proteins. Nedd4 WW domains can bind to phosphorylated peptides but bind with ∼10-fold lower affinity than the WW domains of the yeast peptidyl-prolyl isomerase Pin1p (Lu et al., 1999). The binding of Nedd4 WW domains to the epithelial Na+ channel occurs through an interaction with PY motifs. Nedd4 may also recognize phosphorylated plasma membrane proteins, or another C2-WW-hect protein in mammalian cells, such as mouse Itch or human WWP2, may perform this function. Further characterization of the role of Rsp5p in endocytosis in yeast will facilitate the understanding of how members of the Rsp5p/Nedd4 family of ubiquitin-protein ligases function as negative regulators of plasma membrane proteins.
ACKNOWLEDGMENTS
We gratefully acknowledge the gift of plasmids, strains, and antibody reagents from Guangli Wang and Jon Huibregtse (University of Texas at Austin, Austin, TX), Anita Hopper (Pennsylvania State University College of Medicine, University Park, PA), Stefan Jenstch (Max Planck Institute, Martinsreid, Germany), Gottfried Schatz (Biozentrum, University of Basel, Basel, Switzerland), Howard Riezman (Biozentrum, University of Basel), Fred Winston (Harvard Medical School, Cambridge, MA), and Mike Yaffe (University of California, San Diego, San Diego, CA). We thank Robert Lamb (Northwestern University, Evanston, IL) for his generosity in providing HA antibodies and for the use of his confocal microscope facility. We are indebted to Abby Fleisch for constructing the ww domain mutants. We thank Magda Houlberg for her technical assistance in many aspects of this project. We also particularly thank Marija Tesic and Rick Gaber (Northwestern University) for providing unpublished reagents. We are grateful to Ben Sehgal and Hilary Godwin (Northwestern University), Jon Huibregtse (Rutgers University), and Anita Hopper (Pennsylvania State University College of Medicine) for the communication of unpublished results. We acknowledge the use of instruments in the Keck Biophysics Facility at Northwestern University. R.D. was supported by National Institutes of Health training grant T32GM08061. Funding from the Burroughs Wellcome Fund, the Searle Scholars Program, and the National Institutes of Health (grant DK 53257) supported this research.
Abbreviations used:
- E1
ubiquitin-activating enzyme
- E2
ubiquitin-conjugating enzyme
- E3
ubiquitin-protein ligase
- ENaC
epithelial sodium channel
- 5-FOA
5-fluoroorotic acid
- GST
glutathione S-transferase
- HA
hemagluttinin
- LY
lucifer yellow
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- SD medium
synthetic minimal medium
Note added in proof.
The role of Rsp5p WW domains in uracil permease and fluid-phase endocytosis has also been investigated by Gajewska et al. (Gajewska, B., Kaminska, J., Jesionowska, A., Martin, N.C., Hopper, A.K., and Zoladek, T. (2001). WW domains of Rsp5p define different functions: Determination of roles in fluid phase and uracil permease endocytosis in Saccaromyces cerevisiae. Genetics 157, 91–101.)
REFERENCES
- Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- Beck T, Schmidt A, Hall MN. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J Cell Biol. 1999;146:1227–1238. doi: 10.1083/jcb.146.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford MT, Sarbassova D, Xu J, Leder P, Yaffe MB. A novel Pro-Arg motif recognized by WW domains. J Biol Chem. 2000;275:10359–10369. doi: 10.1074/jbc.275.14.10359. [DOI] [PubMed] [Google Scholar]
- Bénédetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Einbond A, Kwak SJ, Linn H, Koepf E, Peterson S, Kelly JW, Sudol M. Characterization of the WW domain of human Yes-associated protein and its polyproline-containing ligands. J Biol Chem. 1997;272:17070–17077. doi: 10.1074/jbc.272.27.17070. [DOI] [PubMed] [Google Scholar]
- Dulic V, Egerton M, Elguindi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Fisk HA, Yaffe MP. A role for ubiquitination in mitochondrial inheritance in Saccharomyces cerevisiae. J Cell Biol. 1999;145:1199–1208. doi: 10.1083/jcb.145.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont PS. RING for destruction? Curr Biol. 2000;10:R84–R87. doi: 10.1016/s0960-9822(00)00287-6. [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JM, Moreau V, André B, Volland C, Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J Biol Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PM. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle's syndrome. J Biol Chem. 1998;273:30012–30017. doi: 10.1074/jbc.273.45.30012. [DOI] [PubMed] [Google Scholar]
- Gwozd CS, Arnason TG, Cook WJ, Chau V, Ellison MJ. The yeast UBC4 ubiquitin conjugating enzyme monoubiquitinates itself in vivo: evidence for an E2–E2 homointeraction. Biochemistry. 1995;34:6296–6302. doi: 10.1021/bi00019a006. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Kumar S. Nedd4-like proteins: an emerging family of ubiquitin-protein ligases implicated in diverse cellular functions. Trends Cell Biol. 1999;9:166–169. doi: 10.1016/s0962-8924(99)01541-x. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hicke L. Gettin' down with ubiquitin: turning off cell surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J Cell Biol. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich HD, Jentsch S. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell. 2000;102:577–586. doi: 10.1016/s0092-8674(00)00080-5. [DOI] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Beaudenon S, Howley PM. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator C-Cb1 as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- Kumar S, Kao WH, Howley PM. Physical interaction between specific E2 and Hect E3 enzymes determines functional cooperativity. J Biol Chem. 1997;272:13548–13554. doi: 10.1074/jbc.272.21.13548. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee PS, Wang Y, Dominguez MG, Yeung YG, Murphy MA, Bowtell DD, Stanley ER. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon SK, Traub LM. Sorting in the endosomal system in yeast and animal cells. Curr Opin Cell Biol. 2000;12:457–466. doi: 10.1016/s0955-0674(00)00117-4. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kane T, Tipper C, Spatrick P, Jenness DD. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol Cell Biol. 1999;19:3588–3599. doi: 10.1128/mcb.19.5.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- Lupher ML, Jr, Rao N, Eck MJ, Band H. The Cbl protooncoprotein: a negative regulator of immune receptor signal transduction. Immunol Today. 1999;20:375–382. doi: 10.1016/s0167-5699(99)01484-x. [DOI] [PubMed] [Google Scholar]
- Macias MJ, Gervais V, Civera C, Oschkinat H. Structural analysis of WW domains and design of a WW prototype. Nat Struct Biol. 2000;7:375–379. doi: 10.1038/75144. [DOI] [PubMed] [Google Scholar]
- Macias MJ, Hyvonen M, Baraldi E, Schultz J, Sudol M, Saraste M, Oschkinat H. Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature. 1996;382:646–649. doi: 10.1038/382646a0. [DOI] [PubMed] [Google Scholar]
- Marsh JA, Kalton HM, Gaber RF. Cns1 is an essential protein associated with the Hsp90 chaperone complex in Saccharomyces cerevisiae that can restore cyclophilin 40-dependent functions in cpr7Δ cells. Mol Cell Biol. 1998;18:7353–7359. doi: 10.1128/mcb.18.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Lupher ML, Jr, Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Mullane-Robinson KP, Lill NL, Douillard P, Band H. Cbl-mediated negative regulation of platelet-derived growth factor receptor-dependent cell proliferation: a critical role for cbl tyrosine kinase-binding domain. J Biol Chem. 1999;274:16619–16628. doi: 10.1074/jbc.274.23.16619. [DOI] [PubMed] [Google Scholar]
- Morrione A, Plant P, Valentinis B, Staub O, Kumar S, Rotin D, Baserga R. mGrb10 interacts with Nedd4. J Biol Chem. 1999;274:24094–24099. doi: 10.1074/jbc.274.34.24094. [DOI] [PubMed] [Google Scholar]
- Munn AL, Riezman H. Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994;127:373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber U, Schwarz S, Kaiser P, Schneider R, Scheffner M. Cloning of human ubiquitin-conjugating enzymes UbcH6 and UbcH7 (E2–F1) and characterization of their interaction with E6-AP and RSP5. J Biol Chem. 1996;271:2795–2800. doi: 10.1074/jbc.271.5.2795. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Lafont F, Lecat S, Verkade P, Simons K, Rotin D. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J Cell Biol. 2000;149:1473–1484. doi: 10.1083/jcb.149.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer NK, Salama NR, Schekman R, Kaiser CA. Cytosolic Sec13p complex is required for vesicle formation from the endoplasmic reticulum in vitro. J Cell Biol. 1993;120:865–875. doi: 10.1083/jcb.120.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneke JE, Blumer KJ, Courchesne WE, Thorner J. The carboxy-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Rizo J, Südhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membr Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- Schandel KA, Jenness DD. Direct evidence for ligand-induced internalization of the yeast α-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S-H, Chretien P, L., Bastein L, Slilaty SN. Primary sequence of the glucanase gene from Oerskovia xanthineolytica. J Biol Chem. 1991;266:1058–1063. [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Shih SC, Sloper-Mold KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 2000;19:187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B, Riezman H. Detection of an intermediate compartment involved in transport of α-factor from the plasma membrane to the vacuole in yeast. J Cell Biol. 1990;110:1911–1922. doi: 10.1083/jcb.110.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springael J-Y, André B. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1253–1263. doi: 10.1091/mbc.9.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springael JY, De Craene JO, André B. The yeast Npi1/Rsp5 ubiquitin ligase lacking its N-terminal C2 domain is competent for ubiquitination but not for subsequent endocytosis of the Gap1 permease. Biochem Biophys Res Commun. 1999a;257:561–566. doi: 10.1006/bbrc.1999.0505. [DOI] [PubMed] [Google Scholar]
- Springael JY, Galan JM, Haguenauer-Tsapis R, André B. NH4+-induced down-regulation of the Saccharomyces cerevisiae Gap1p permease involves its ubiquitination with lysine-63-linked chains. J Cell Sci. 1999b;112:1375–1383. doi: 10.1242/jcs.112.9.1375. [DOI] [PubMed] [Google Scholar]
- Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G, Govers R. The ubiquitin-proteasome system and endocytosis. J Cell Sci. 1999;112:1417–1423. doi: 10.1242/jcs.112.10.1417. [DOI] [PubMed] [Google Scholar]
- Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- Wang G, Yang J, Huibregtse JM. Functional domains of the Rsp5 ubiquitin-protein ligase. Mol Cell Biol. 1999;19:342–352. doi: 10.1128/mcb.19.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- Wendland B, Emr SD. Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J Cell Biol. 1998;141:71–84. doi: 10.1083/jcb.141.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, Avaro S, Prescianotto-Baschong C, Haguenauer-Tsapis R, Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro CT, Kane PM, Wolczyk DF, Preston RA, Stevens TH. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol. 1990;10:3737–3749. doi: 10.1128/mcb.10.7.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiroda H, Oguchi T, Yasua Y, Toh-e A, Kikuchi Y. Bul1, a new protein that binds to the Rsp5 ubiquitin ligase in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3255–3263. doi: 10.1128/mcb.16.7.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Südhof TC, Anderson RGW. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Zoladek T, Tobiasz A, Vaduva G, Boguta M, Martin NC, Hopper AK. MDP1, a Saccharomyces cerevisiae gene involved in mitochondrial/cytoplasmic protein distribution is identical to the ubiquitin-protein ligase gene RSP5. Genetics. 1997;145:595–603. doi: 10.1093/genetics/145.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]