Summary
The Drosophila neuromuscular junction (NMJ) has recently provided new insights into the roles of various proteins in neurodegenerative diseases including Amyotrophic Lateral Sclerosis (ALS), Spinal Muscular Atrophy (SMA), Multiple Sclerosis (MS) Hereditary Spastic Paraplegia (HSP), and Huntington’s Disease (HD). Several developmental signaling pathways including WNT, MAPK and BMP/TGF-β signaling play important roles in the formation and growth of the Drosophila NMJ. Studies of the fly homologues of genes that cause neurodegenerative disease at the NMJ have resulted in a better understanding of the roles of these proteins in vivo. These studies may shed light on the pathological mechanisms of these diseases, with implications for reduced BMP/TGF-β signaling in ALS, SMA and HD and increased signaling in HSP and MS.
Introduction
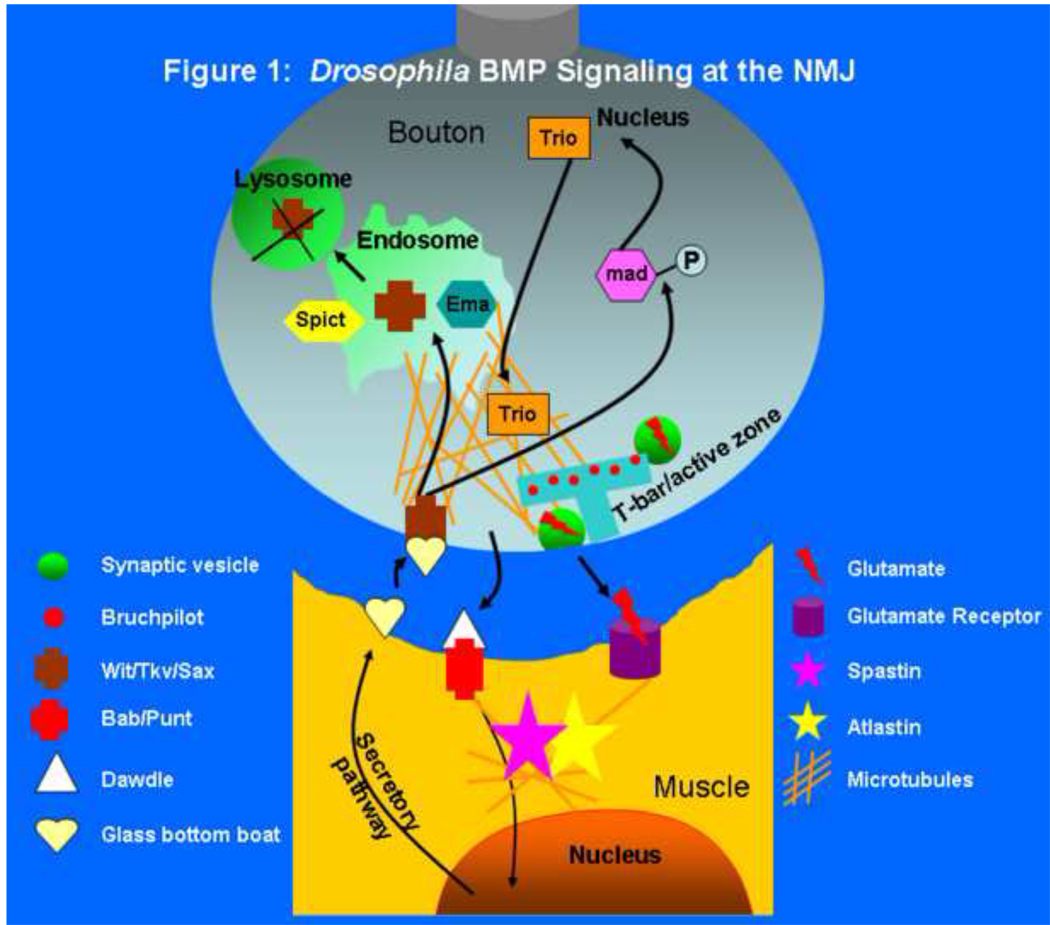
Neurodegenerative diseases are among the most common diseases and yet probing the pathological mechanisms has remained a challenge. The diseases typically come in both sporadic and hereditary forms, and mutations in numerous genes in familial cases have been identified. To obtain insights into how these genes and their mutations are involved in specific diseases, one of our best strategies is to study these genes and the corresponding mutations in model organisms like Drosophila and mice. The Drosophila larval neuromuscular junction (NMJ) is an especially amenable system to study the role of these genes in invertebrates[1]. As shown in Figure 1, the motor neuron’s terminals form close spherical connections with the muscle, called boutons[1]. At each bouton, many synapses, the presynaptic regions of which are known as active zones, form between neuron and muscle. These zones have a T-shaped structure known as a T-Bar where synaptic vesicles (SVs, green) cluster, fuse and release glutamate (red lightning) into the extracellular space. The neurotransmitter is then bound by glutamate receptors (purple cylinders) on the muscle, that triggers calcium influx and subsequent muscle contraction[2]. Given that Drosophila permits sophisticated genetic manipulations[3,4], numerous probing experiments can be performed. For example, in a mutant background, proteins can be expressed pre- or post-synaptically to determine where they are required. Electrophysiological experiments on individual nerves targeting specific muscles allow one to derive very valuable information about the ability to release vesicles, endocytose membranes, the function of postsynaptic receptors, etc[5]. FM1–43 dye uptake experiments allow one to establish how much membrane is taken up during endocytosis and how much is released during exocytosis, thus providing real-time analysis of vesicle trafficking[6]. Antibodies against numerous proteins that are present at the NMJ pre- and post-synaptically are available and allow one to assess which proteins may be implicated in the process or pathology that is being studied[7]. These data, combined with Transmission Electron Microscopy and Immuno-EM studies provide very valuable information about the number, distribution and size of SV, and the number and size of active zones[7,8]. Finally, genetic interactions and epistasis with a vast number of mutants as well as the ability to overexpress specific proteins allow for a detailed analysis of genetic pathways[9]. In summary, no synapses are currently more amenable to a diverse set of manipulations in vivo than the Drosophila NMJ, and by combining the information obtained from different experimental datasets, very valuable biological information can be derived.
Figure 1.
The development and growth of the Drosophila NMJ requires an anterograde as well as a retrograde input from the muscle[2]. The anterograde signaling is primarily mediated by the Wnt ligand Wingless (Wg)[10], whereas retrograde signaling occurs mostly by a Bone Morphogenetic Protein (BMP) ligand named Glass bottom boat (Gbb) that is released from the muscle and binds to its tetrameric presynaptic BMP receptors containing Wishful Thinking (Wit), Thickveins (Tkv) and Saxophone (Sax) on the neuronal cell membrane (Figure 1)[11]. Binding of Gbb to its receptor has two parallel effects. The first involves the activation of the Williams Syndrome-associated kinase LIMK1 to act in the stabilization of the synapse[12]. In the absence of BMP signaling or LIMK1, one sees many ‘synaptic footprint’ or ‘retraction’ sites at the NMJ, in which one still sees clustered postsynaptic proteins like Discs Large (Dlg) but no longer any presynaptic molecules[12]. The other involves receptor-mediated phosphorylation of the Smad family transcription factor Mad (Mothers against decapentaplegic), signaling the neuron to expand the number of synapses[11]. In the absence of key components of the pathway, including Wit and Gbb, small NMJs with reduced neurotransmission develop, and in the absence of negative pathway components such as Daughters against decapentaplegic (Dad), the NMJs overgrow, with characteristic ‘satellite boutons’ that appear disconnected from the other boutons[13]. In addition to the BMP and WNT pathways, a MAP Kinase pathway regulated by Highwire, a putative RING finger E3 ubiquitin ligase, also controls NMJ growth and branching[2].
Here we will review some of the salient recent work related to several genes that cause familial forms of neurodegenerative diseases whose culprits are conserved in flies and have been studied at the Drosophila NMJ. Some of the discussed work was based on forward genetics and only later was it realized that these genes caused or were related to neurological diseases. Other genes were studied specifically because they are known to be mutated in such diseases and the NMJ offers a highly relevant toolkit to study the pathogenesis of neurodegenerative diseases.
Hereditary Spastic Paraplegia
The Hereditary Spastic Paraplegias (HSPs) are a diverse set of diseases that share the primary feature of progressive, severe, lower extremity spasticity, with many causative genes identified so far[14]. Autopsies have revealed, among many other findings, myelin pallor and axonal loss in the corticospinal tracts, and in the brain, a high density of irregular tau-positive neurofibrillary tangles[15]. Mutations, frequently nonsense, in the gene spastin are responsible for almost half of all cases. Some of the earliest studies of spastin function in vivo were performed at the Drosophila NMJ. Spastin was found to localize to the NMJ and to be present both in neuronal and muscle cytoplasm. Loss of Spastin causes an increase in the number of boutons that are smaller than normal and clustered together, in a pattern reminiscent of the ‘satellite boutons’ seen in some endocytic mutants (Tables 1, 2)[16]. spastin mutants also display a thickened microtubule (MT) network, while tissue-specific overexpression results in a loss of the MT network. This work, along with other data and in vitro experiments, provided further evidence that Spastin is a microtubule-severing protein[17,18]. Another HSP protein encoded by atlastin (atl), responsible for 10% of HSP cases, was found to localize to punctae throughout the muscle cytoplasm. In mutant atl larvae synaptic boutons are clustered and satellite boutons are prominent, similar to spastin mutants (Tables 1, 2), and similar to overexpression of BMP pathway components. Furthermore, the Subsynaptic Reticulum (SSR), composed of postsynaptic infoldings of the muscle surrounding the boutons, is greatly reduced. In addition, muscle-specific but not neuron-specific expression of the gene in mutant larvae can rescue the phenotype, indicating that its muscle-specific function is sufficient for NMJ development. This is in contrast to spastin mutants, which can be rescued by presynaptic Spastin expression. However, Atlastin binds to Spastin in GST-pulldown assays, and forms a functional complex[19]. Similarities between the spastin and atlastin mutant and overexpression phenotypes are summarized in Tables 1 and 2. This work supports the idea that these two disease proteins function together to regulate the microtubule network.
Table 1.
Phenotypic Comparisons between Spastin, Atlastin, and Spichthyin
| Null allele | Bouton clustering | Satellite boutons |
Bouton number | Temperature- sensitivity |
Microtubules | Escaper Sterility |
Escaper movement |
|---|---|---|---|---|---|---|---|
| Atlastin | more clustered | Yes | increased by 17% | Yes | dense network | yes | Highly impaired |
| Spastin | more clustered | Yes | increased by 60% | Yes | dense network | yes | Highly impaired |
| Spichthyin | more clustered | No* | increased by 100% | Not tested* | dense network | fertile | Normal* |
| Overexpression | Muscle attachment | Acetylated Tubulin | Microtubules | ||||
| Atlastin | partial/totally detached | significantly less | faint and sparse | ||||
| Spastin | partial/totally detached | significantly less | faint and sparse | ||||
| Spichthyin | Not discussed | significantly less | faint and sparse |
Personal communication (Cahir O’Kane, 9 July 2010)
Table 2.
| Selected diseases discussed | OMIM # |
Drosophila genes discussed |
Loss of function NMJ phenotypes |
|---|---|---|---|
| Hereditary Spastic Paraplegia 3 | #182600 | Atlastin | more boutons, bouton clustering, satellite boutons, dense MT network, impaired movement |
| Hereditary Spastic Paraplegia 4 | #182601 | Spastin | more boutons, bouton clustering, satellite boutons, dense MT network, impaired movement |
| Hereditary Spastic Paraplegia 6 | #600363 | Spichthyin | more boutons, bouton clustering, dense MT network |
| Multiple Sclerosis | #126200 | Ema | more boutons, increased synaptic area, enlarged endosomes |
| Huntington's Disease | #143100 | CIP4 | more boutons, satellite boutons, increased synaptic area |
| Amyotrophic Lateral Sclerosis 8 | #608627 | Vap-33-1 | fewer and larger boutons, severely disorganized and fragmented MT network |
| Spinal Muscular Atrophy I, II, III | #253300, #253550, #253400 | Smn | fewer and larger boutons |
A possible connection between HSP proteins and BMP signaling was revealed by the study of spichthyin (spict), the fly ortholog of another HSP gene, Nonimprinted in Prader-Willi/Angelman (NIPA1). Drosophila Spict is widely expressed and colocalizes with an early endosomal marker Rab5. Unlike in the case of atlastin, however, loss of spict is rescued by neuronal-specific driven expression only, indicating that its function is primarily presynaptic. spict mutants have twice as many synaptic boutons as normal (Tables 1, 2) and Spict overexpression produces a loss of boutons reminiscent of the loss of gbb, sax, and tkv suggesting that it may inhibit the BMP pathway. Indeed, levels of pMad were 4-fold higher in spict mutants and mutations in tkv, sax, wit, gbb, and the co-Smad medea suppressed the spict overgrowth phenotype[20]. Another group, using a frog oocyte expression system, found that NIPA1 encodes a Magnesium transporter that depending upon the Magnesium concentration localizes either to the endosomes or the cell’s plasma membrane[21]. Magnesium is an essential cofactor for Rab5 and Rab7 GTPases[22] necessary for endosomal formation and maturation, suggesting the possibility that an endosomal maturation defect may impair the degradation and processing of BMP receptors in spict mutants.
Subsequent mammalian cell culture experiments have confirmed that NIPA1 is an inhibitor of BMP signaling. Although the mechanism is still unclear, in that same report, knockdown of Spastin and Spartin resulted in an almost identical increase in phosphorylated Smad levels as observed for NIPA1, suggesting that a common mechanism may be involved[23]. In Drosophila, then, it is possible that when Atlastin and Spastin are reduced, secretion of the postsynaptic BMP ligand is increased, whereas in spict mutants, the BMP signal is somewhat constitutive because of an endosomal trafficking defect.
Interestingly, pMad has recently been shown to bind to the promoter of the trio gene, which encodes an activator of the Rac GTPase, essential for MT bundle formation and bouton growth. In the absence of retrograde BMP signaling, very little Trio is transcribed and little Rac is activated[24], which may underlie the phenotype associated with spict presynaptic loss but does not provide a rationale for the observations associated with the loss of Atlastin post-synaptically.
An important new insight into the BMP pathway has recently been uncovered that might explain how a microtubule-severing protein in the muscle may affect BMP signaling in the neuron. The activin family ligand Dawdle plays an important role at the fly NMJ too, with mutants in this activin and its target Type 1 receptor Baboon having strikingly similar small NMJ phenotypes to mutants in the BMP signaling pathway like mad. This activin signaling pathway from the neuron to the muscle was found to lie upstream of the BMP signaling pathway that operates in the opposite direction, thus creating a signaling loop between muscle and neuron (Figure 1)[25]. Smads specifically bind to MT and in response to phosphorylation become dissociated from MT and relocate to the nucleus[26]. Hence, the MT impairment in atlastin-mutant muscles may result in higher levels of activin signaling and hence Gbb production, a hypothesis that should be tested. Finally, considering that activins play a neuroprotective role and that activin inhibition results in glutamatergic transmission and synaptic plasticity defects in mice, it would be useful to determine if secretion of BMP ligands by postsynaptic neurons in response to Activins occurs in mammals[27].
Amyotrophic Lateral Sclerosis
Amyotrophic Lateral Sclerosis is a devastating motor neuron disease characterized by late-onset progressive upper and lower motor neuron degeneration. About 90% of the cases are sporadic, but mutations in 7 genes have been identified in familial cases, including VapB[14]. Histologic and ultrastructural studies of ALS patients’ NMJs have provided strong evidence of frequent denervation and reinnervation of the muscle endplates. In addition, synaptic terminals are on average smaller than normal but they are frequently unusually large [28]. Prior to its identification as an ALS locus, loss of VapB in Drosophila was found to reduce bouton number as well as increase their average size (Table 2). Overexpression of wild-type VapB results in the opposite phenotype: an expanded NMJ with many more boutons. Furthermore, loss of VapB causes a depolymerization of the MT in synaptic boutons, while overexpression results in denser, thickened MT[29]. Subsequent studies confirmed this disorganized microtubule phenotype as well as a ‘floating T-Bar’ phenotype observed in mutants with impaired BMP signaling[30]. Ratnaparkhi et al. (2008) showed that VapB overexpression results in higher levels of pMad while loss of VapB results in lower levels of pMad compared to controls[31]. How VapB affects BMP signaling is still unclear however. The VapB N-terminal domain is cleaved and secreted by neurons, and binds to Eph receptors as well as other receptors present on muscles (paracrine) and probably neurons (autocrine). The latter pathway may affect the MT network in VapB mutants via Rac signaling via an unknown receptor, as Eph loss does not affect NMJ morphology[32,33]. Overexpression of the VapB protein with the mutation found in ALS patients, P58S, also results in aggregates in the Endoplasmic Reticulum with a corresponding upregulated Unfolded Protein Response (UPR)[33]. Interestingly, there is increasing evidence from pathology specimens to support the idea of impaired BMP/TGF-β signaling in ALS, with motor neurons from human Sporadic ALS spinal cords possessing severely reduced nuclear pSmad levels and aggregated pSmad in their cytoplasmic Round Hyaline Inclusions (RHIs)[34]. Enhancing BMP/TGF- β signaling could therefore be a promising therapeutic strategy.
Spinal Muscular Atrophy
Spinal Muscular Atrophy (SMA) is a disease in which the lower motor nerves degenerate, resulting in progressive muscular atrophy[14]. SMA shares some key features with ALS, although SMA affects lower motor neurons, whereas ALS typically affects both upper and lower motor neurons. SMA Type I is a recessive disease often caused by deletions of Survival Motor Neuron 1 (Smn1). Interestingly, abnormal copy numbers (1 or 3) of Smn1 have also been found in 12% of patients with sporadic ALS as compared to 4.5% in the wider population[35]. Histologic studies of human fetuses with homozygous deletions of the Smn1 gene have found the nuclei of motor neurons to be frequently small and unusually shaped [36]. In addition, like in ALS, observations from studies of SMA mutant mice have revealed a large accumulation of neurofilaments in the presynaptic terminals. The Smn1 gene encodes a protein that localizes to both the nucleus and the cytoplasm. It is proposed to play a role in pre-mRNA splicing, but whether or not this is relevant to the NMJ defects found in SMA patients is still unclear. Smn in Drosophila localizes to the nucleus, but it is also found to colocalize with Discs large (Dlg) at the NMJ in the muscle, indicating that it may have a non-nuclear function too. Loss of function mutations result in a severe reduction in the number of synaptic boutons, a phenotype enhanced by loss of BMP signaling components like Mad (Table 2). Levels of pMad in Smn mutants were also observed to be reduced, providing further evidence that the Smn protein may play a critically important and previously unrecognized role in the BMP signaling pathway[37]. Intriguingly, the same P58S mutation in the previously mentioned VapB, besides ALS, has also been documented to cause SMA, and since VapB affects BMP signaling as well, reduced BMP signaling may be a common theme. It is possible therefore that impaired activation and trafficking of BMP pathway molecules like Smad may be a common theme in these diseases [38].
Multiple Sclerosis
Multiple Sclerosis is a neuroinflammatory disorder in which patients develop demyelinated plaques in their CNS with corresponding neurological deficits. At least five independent genetic studies have linked polymorphisms in the Clec16A gene to the disease[39]. In a screen designed to identify new genes that regulate synaptic terminal growth, fly mutants of the homolog of Clec16A, endosomal maturation defective (ema), were observed to possess dramatically overgrown synapses (Table 2)[40]. This is similar to the mutant phenotype seen in a putative lysosomal sugar carrier spinster[41]. ema mutant neurons possess enlarged immature endosomal compartments, with the BMP receptor Tkv present at levels almost double those of wild-type, and a more than 4-fold increase in the levels of pMad[40]. In ema mutants, the endolysosomal pathway is unable to efficiently degrade Tkv and as anticipated, the overgrowth phenotype is suppressed by mutations in Mad.
The fly neuron must limit how much BMP signaling occurs under normal conditions, as excessive signaling results in overgrown NMJs, as in ema mutants. Hence, loss of numerous endocytic genes frequently cause characteristic satellite bouton phenotypes[4,16]. In light of ALS and MS’s potential connections with impaired BMP signaling, it is interesting that children of patients with ALS are almost three times as likely to develop MS than the average person[42]. It is interesting too that very high levels of BMPs 4 and 5 have been observed in MS patient lesions[43] as well as elevated BMPs 4, 6, and 7 levels in a mouse model of the disease[44]. Finally, considering the phenotypic similarities of endosomal maturation defects and increased BMP signaling between ema and spinster mutants[45], it is worth noting that 16p11 and 17p13, the loci containing human Spinster 1 and 2 respectively, have both shown linkage in patients with Multiple Sclerosis[46,47].
Huntington’s Disease
Huntington’s Disease is a late-onset progressive disorder characterized by increasingly jerky and uncoordinated movements, rigidity, and neuropsychiatric symptoms[48]. Gradual degeneration of the basal ganglia is a key feature of the disease, which is caused by polyglutamine expansion of the protein Huntingtin. One of the early primary dysfunctions found in Huntington’s Disease is excessive glutamatergic neurotransmission, while later in the disease course, too little glutamatergic transmission becomes evident[49]. The disease is also known to be associated with axonal transport defects, and this has been proposed to be related to the sequestration of Huntingtin-interacting proteins in aggregates[48]. One such Huntingtin-interacting protein is the Arp2/3-interacting F-Bar protein CDC42-interacting protein 4 (CIP4). Patients with HD progressively accumulate high levels of CIP4 in their basal ganglia, and overexpression of CIP4 was shown to be highly neurotoxic[50]. Interestingly, Drosophila CIP4 was identified in a forward genetic screen, with mutant larvae having an overgrown NMJ (Table 2). It was further discovered that CIP4 acts to downregulate the BMP signaling pathway, but, unusually, it downregulates the pathway specifically in the muscle. CIP4 mutants secrete excessive Gbb into the extracellular space, thus activating the pathway[51]. Hence, it is possible that excessive CIP4, as found in the Huntington’s Disease-afflicted brains, causes a downregulation of BMP signaling. Indeed, low blood and neuronal levels of TGF-β1 were recently reported as the earliest known biomarker in asymptomatic Huntington’s Disease patients[52].
Concluding Remarks
In summary, studies at the fly NMJ are rapidly providing a better understanding of the potential pathological mechanisms of fly homologues of human neurodegenerative diseases. The BMP signaling pathway may be playing a central role in many of these diseases. This is important because the BMP signaling pathway is highly conserved in mammals and has been well studied in relation to bone development, angiogenesis and stem cell proliferation. However, there are surprisingly few studies of its role at the vertebrate NMJ so far. Most studies have so far focused primarily on the pathway’s role in eye development, neurogenesis and gliogenesis and not on NMJ synapse formation and growth per say. Interestingly, a recent Xenopus study has drawn a strong parallel. The vertebrate neuromuscular junction is known to require continuous input from terminal Schwann cells to grow in size. In their absence, nerve terminals do not grow and instead retract, perhaps very similar to the constant cycles of motor nerve terminal retraction observed prior to massive neuronal death in ALS and SMA[53]. Schwann cell-conditioned medium (SCTM) allows for this growth to occur. Until recently it was unknown what factor or factors present in this medium were responsible. Based on the work done on the Drosophila NMJ, though, the close BMP relative TGF-β1 was proposed to be a key factor and it was subsequently detected in the medium and shown to be necessary and sufficient to mediate NMJ terminal growth[54]. This suggests that similar signaling pathways operate at the NMJ in vertebrates and may possibly be affecting numerous other synapses as well. In addition, using genetic interactions and drug studies, like the phenotypic suppression by the microtubule-destabilizing drug vinblastine in fly atlastin mutants, may allow us to efficiently identify means of slowing the progression of some of these diseases[19]. Hence, Drosophila studies of the NMJ have provided and will continue to provide new and intriguing insights into potential mechanisms for neurodegenerative disease mechanisms.
Acknowledgements
V. Bayat has received support from the Edward and Josephine Hudson Scholarship Fund and the Developmental Biology Program training grant (T32 HD055200 07/01/2007 - 08/02/2009). We thank Drs. Yao, Giagtzoglou, Tsuda, Jawaid, Yamamoto, Sandoval, DiAntonio and O’Kane for helpful discussions. HJB is an investigator of the HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no competing financial interest.
References
(those of exceptional interest are starred)
- 1.Ruiz-Canada VBaC., editor. The Fly Neuromuscular Junction: Structure and Function. Academic Press; 2006. [Google Scholar]
- 2.Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Venken KJ, Bellen HJ. Transgenesis upgrades for Drosophila melanogaster. Development. 2007;134:3571–3584. doi: 10.1242/dev.005686. [DOI] [PubMed] [Google Scholar]
- 4.Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imlach W, McCabe BD. Electrophysiological methods for recording synaptic potentials from the NMJ of Drosophila larvae. J Vis Exp. 2009 doi: 10.3791/1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstreken P, Ohyama T, Bellen HJ. FM 1–43 labeling of synaptic vesicle pools at the Drosophila neuromuscular junction. Methods Mol Biol. 2008;440:349–369. doi: 10.1007/978-1-59745-178-9_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellen HJ, Budnik V. The neuromuscular junction. In: Sullivan W, Hawley RS, Ashburner M, editors. Drosophila protocols. Cold Spring Harbor Laboratory; 2000. pp. 175–199. [Google Scholar]
- 8.Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 10.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- 12.Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Keshishian H, Kim YS. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 2004;27:143–147. doi: 10.1016/j.tins.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009;10:769–782. doi: 10.1038/nrg2680. [DOI] [PubMed] [Google Scholar]
- 15.White KD, Ince PG, Lusher M, Lindsey J, Cookson M, Bashir R, Shaw PJ, Bushby KM. Clinical and pathologic findings in hereditary spastic paraparesis with spastin mutation. Neurology. 2000;55:89–94. doi: 10.1212/wnl.55.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004;2:e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Paik SK, Lee MJ, Kim YJ, Kim S, Nahm M, Oh SJ, Kim HM, Yim J, Lee CJ, et al. Drosophila Atlastin regulates the stability of muscle microtubules and is required for synapse development. Dev Biol. 2009;330:250–262. doi: 10.1016/j.ydbio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Shaw WR, Tsang HT, Reid E, O'Kane CJ. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat Neurosci. 2007;10:177–185. doi: 10.1038/nn1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goytain A, Hines RM, El-Husseini A, Quamme GA. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J Biol Chem. 2007;282:8060–8068. doi: 10.1074/jbc.M610314200. [DOI] [PubMed] [Google Scholar]
- 22.Simon I, Zerial M, Goody RS. Kinetics of interaction of Rab5 and Rab7 with nucleotides and magnesium ions. J Biol Chem. 1996;271:20470–20478. doi: 10.1074/jbc.271.34.20470. [DOI] [PubMed] [Google Scholar]
- 23.Tsang HT, Edwards TL, Wang X, Connell JW, Davies RJ, Durrington HJ, O'Kane CJ, Luzio JP, Reid E. The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum Mol Genet. 2009;18:3805–3821. doi: 10.1093/hmg/ddp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 66:536–549. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Ellis JE, Parker L, Cho J, Arora K. Activin signaling functions upstream of Gbb to regulate synaptic growth at the Drosophila neuromuscular junction. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGF beta activity. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- 27.Muller MR, Zheng F, Werner S, Alzheimer C. Transgenic mice expressing dominant-negative activin receptor IB in forebrain neurons reveal novel functions of activin at glutamatergic synapses. J Biol Chem. 2006;281:29076–29084. doi: 10.1074/jbc.M604959200. [DOI] [PubMed] [Google Scholar]
- 28.Tsujihata M, Hazama R, Yoshimura T, Satoh A, Mori M, Nagataki S. The motor end-plate fine structure and ultrastructural localization of acetylcholine receptors in amyotrophic lateral sclerosis. Muscle & Nerve. 1984;7:243–249. doi: 10.1002/mus.880070310. [DOI] [PubMed] [Google Scholar]
- 29.Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 30.Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 31.Ratnaparkhi A, Lawless GM, Schweizer FE, Golshani P, Jackson GR. A Drosophila model of ALS: human ALS-associated mutation in VAP33A suggests a dominant negative mechanism. PLoS One. 2008;3:e2334. doi: 10.1371/journal.pone.0002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura M, Ito H, Wate R, Nakano S, Hirano A, Kusaka H. Phosphorylated Smad2/3 immunoreactivity in sporadic and familial amyotrophic lateral sclerosis and its mouse model. Acta Neuropathol. 2008;115:327–334. doi: 10.1007/s00401-007-0337-z. [DOI] [PubMed] [Google Scholar]
- 35.Corcia P, Camu W, Halimi JM, Vourc'h P, Antar C, Vedrine S, Giraudeau B, de Toffol B, Andres CR. SMN1 gene, but not SMN2, is a risk factor for sporadic ALS. Neurology. 2006;67:1147–1150. doi: 10.1212/01.wnl.0000233830.85206.1e. [DOI] [PubMed] [Google Scholar]
- 36.Fidzianska A, Rafalowska J. Motoneuron death in normal and spinal muscular atrophy-affected human fetuses. Acta Neuropathologica. 2002;104:363–368. doi: 10.1007/s00401-002-0566-0. [DOI] [PubMed] [Google Scholar]
- 37. Chang HC, Dimlich DN, Yokokura T, Mukherjee A, Kankel MW, Sen A, Sridhar V, Fulga TA, Hart AC, Van Vactor D, et al. Modeling spinal muscular atrophy in Drosophila. PLoS One. 2008;3:e3209. doi: 10.1371/journal.pone.0003209. This paper provides key insights into the localization of the SMN protein at the NMJ, the Drosophila mutant SMN phenotype, its similarities to impaired BMP signaling mutants and its genetic interactions with the BMP pathway. Thus it provides a valuable new testable pathological mechanism for Spinal Muscular Atrophy.
- 38.Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marce M, Griffin JW, Rich MM, Sumner CJ. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. Journal of Neuroscience. 2009;29:842–851. doi: 10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffjan S, Akkad DA. The genetics of multiple sclerosis: An update 2010. Mol Cell Probes. 2010 doi: 10.1016/j.mcp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Wairkar YP, Daniels RW, DiAntonio A. The novel endosomal membrane protein Ema interacts with the class C Vps-HOPS complex to promote endosomal maturation. J Cell Biol. 2010;188:717–734. doi: 10.1083/jcb.200911126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dermaut B, Norga KK, Kania A, Verstreken P, Pan H, Zhou Y, Callaerts P, Bellen HJ. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J Cell Biol. 2005;170:127–139. doi: 10.1083/jcb.200412001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemminki K, Li X, Sundquist J, Sundquist K. Familial risks for amyotrophic lateral sclerosis and autoimmune diseases. Neurogenetics. 2009;10:111–116. doi: 10.1007/s10048-008-0164-y. [DOI] [PubMed] [Google Scholar]
- 43.Deininger M, Meyermann R, Schluesener H. Detection of two transforming growth factor-beta-related morphogens, bone morphogenetic proteins-4 and -5, in RNA of multiple sclerosis and Creutzfeldt-Jakob disease lesions. Acta Neuropathol. 1995;90:76–79. doi: 10.1007/BF00294462. [DOI] [PubMed] [Google Scholar]
- 44.Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–135. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- 45.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 46.Ban M, Stewart GJ, Bennetts BH, Heard R, Simmons R, Maranian M, Compston A, Sawcer SJ. A genome screen for linkage in Australian sibling-pairs with multiple sclerosis. Genes Immun. 2002;3:464–469. doi: 10.1038/sj.gene.6363910. [DOI] [PubMed] [Google Scholar]
- 47.Dyment DA, Cader MZ, Datta A, Broxholme SJ, Cherny SS, Willer CJ, Ramagopalan S, Herrera BM, Orton S, Chao M, et al. A first stage genome-wide screen for regions shared identical-by-descent in Hutterite families with multiple sclerosis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:467–472. doi: 10.1002/ajmg.b.30620. [DOI] [PubMed] [Google Scholar]
- 48.De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 49.Andre VM, Cepeda C, Levine MS. Dopamine and glutamate in Huntington's disease: A balancing act. CNS Neurosci Ther. 2010;16:163–178. doi: 10.1111/j.1755-5949.2010.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holbert S, Dedeoglu A, Humbert S, Saudou F, Ferrante RJ, Neri C. Cdc42-interacting protein 4 binds to huntingtin: neuropathologic and biological evidence for a role in Huntington's disease. Proc Natl Acad Sci U S A. 2003;100:2712–2717. doi: 10.1073/pnas.0437967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nahm M, Kim S, Paik SK, Lee M, Lee S, Lee ZH, Kim J, Lee D, Bae YC. dCIP4 (Drosophila Cdc42-interacting protein 4) restrains synaptic growth by inhibiting the secretion of the retrograde Glass bottom boat signal. J Neurosci. 2010;30:8138–8150. doi: 10.1523/JNEUROSCI.0256-10.2010. This work reports the identification of the NMJ-expanded CIP4 mutant in a Drosophila forward genetic screen and links the observed phenotype to a defect resulting in the over-secretion of the BMP Glass bottom boat by the muscle. This provides an interesting pathological mechanism for Huntington’s Disease, in which CIP4 accumulates to high levels in the striatum.
- 52.Battaglia G, Cannella M, Riozzi B, Orobello S, Maat-Schieman ML, Aronica E, Busceti CL, Ciarmiello A, Alberti S, Amico E, et al. Early defect of transforming growth factor beta-1 formation in Huntington's disease. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 54.Feng Z, Ko CP. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J Neurosci. 2008;28:9599–9609. doi: 10.1523/JNEUROSCI.2589-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]