Abstract
Background
Salivary biomarkers of periodontitis were assessed longitudinally to determine response to therapy.
Methods
A 6-month case-controlled study of adults with chronic periodontitis was performed, with 33 participants receiving oral hygiene instructions (OHI) alone and 35 with scaling and root planing (SRP) combined with OHI. Saliva samples collected at week 0, 16 and 28 were analyzed for interleukin-1β (IL-1β), IL-8, macrophage inflammatory protein (MIP)-1α, matrix metalloproteinase-8 (MMP-8), osteoprotegerin (OPG) and tumor necrosis factor-α (TNF)-α. Clinical measures of periodontal disease were recorded at each visit.
Results
All parameters of periodontal health improved significantly in both groups by week 16 (p<0.0001) with the SRP group demonstrating greater benefit at week 16 and 28. Baseline OPG and TNF-α levels changed significantly at both follow-up visits (p<0.03), regardless of treatment group. IL-1β and MMP-8 levels decreased significantly from baseline (p≤0.04) in the SRP group only. OPG, MMP-8 and MIP-1α were significantly reduced in responders compared with non-responders (p=0.04, 0.01, 0.05 respectively). In receiver operating characteristic analyses, MMP-8 produced the highest area under the curve (≥ 0.7; p=0.01).
Conclusion
Salivary levels of IL-1β, MMP-8, OPG and MIP-1α reflected disease severity and response to therapy suggesting their potential utility for monitoring periodontal disease status.
Keywords: Interleukin-1β, macrophage inflammatory protein (MIP)-1α, matrix metalloproteinase (MMP), osteoprotegerin (OPG), salivary biomarkers, biological markers, periodontal disease, therapy
Periodontal disease is a chronic microbial and inflammatory process characterized by the presence of sulcular pathogenic bacteria, impaired host immune response, and destruction of the connective tissue attachment. In affected tissues, biochemical signaling involving three biological phases (inflammation, connective tissue degradation, and alveolar bone turnover) contributes to the clinical morbidity observed. Circulating molecules in these biological phases have been detected at elevated levels in the gingival crevicular fluid and whole saliva of patients who have periodontal disease making them putative biomarkers of the disease (Sorsa et al. 1994, Lamster 1997, Faizuddin et al. 2003, Lamster et al. 2003, Miller et al. 2006, Goncalves Lda et al. 2010). Specifically, interleukin (IL)-1β, C-reactive protein, human macrophage inflammatory protein (MIP)-1α, matrix metalloproteinase (MMP)-8 and -9, osteoprotegerin (OPG), tumor necrosis factor (TNF)-α, receptor activator of nuclear factor-kappa B ligand (RANKL), and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) (Bertolini et al. 1986, Pederson et al. 1995, Gorska and Nedzi- Christodoulides et al. 2005, Gora 2006, Miller et al. 2006, Christodoulides et al. 2007, Ng et al. 2007, Scannapieco et al. 2007, Frodge et al. 2008, Tobon-Arroyave et al. 2008, Gursoy et al. 2009, Miller et al. 2010) show promise for aiding in the recognition of some aspects of periodontitis. In addition, data are accumulating in children and young adult populations (Ulker et al. 2008, Fine et al. 2009, Thomas et al. 2009, Heikkinen et al. 2010), as well as in patients with co-morbid systemic inflammatory disease (Costa et al. 2010, Mirrielees et al. 2010) as to the utility of these biomarkers for monitoring periodontal disease.
Saliva is appealing for use as a diagnostic fluid for point-of-care analysis for oral related disease because it is rapid, easy and non-invasive to collect, and generally readily abundant (Lamster 1990, Malamud et al. 2005, Christodoulides et al. 2007, Herr et al. 2007, Miller et al. 2010). However, few studies have longitudinally monitored salivary biomarker profiles in patients with respect to periodontal status (Henskens et al. 1996, Thomas et al. 2009) or determined if salivary biomarkers accurately represent periodontal disease status over time. If salivary biomarkers are to demonstrate clinical utility, then analyte concentrations should diminish in response to periodontal therapy. Thus, this investigation sought to test the hypothesis that salivary biomarkers reflect periodontal status over time in participants who received localized periodontal therapy. A secondary hypothesis was that salivary biomarkers reflect response to therapy.
Material and Methods
Participants
Sixty eight patients were enrolled in this longitudinal, case-controlled, clinical study. Participants were recruited from the general clinic population of the College of Dentistry as well as the surrounding counties by advertisement and had the diagnosis of chronic periodontitis based on the criteria defined by the American Academy of Periodontology (Armitage 1999, Armitage 2004). Inclusion criteria included ≥18 years of age who were in good general health, (excluding the case definition) and had ≥18 erupted teeth. Participants had to have five qualifying sites in two quadrants with a minimum of two affected teeth in each quadrant with each site having probing depth ≥ 5 mm, clinical attachment loss (CAL) of ≥ 3 mm, and bleeding upon probing (BOP) score of ≥ 2 (0= one, 1=pinpoint, 2=interdental bleeding, 3=spontaneous/heavy bleeding). Exclusion criteria were periodontal therapy in the past two years, a history of alcoholism; liver, kidney, or salivary gland dysfunction; inflammatory bowel disease; granulomatous diseases; diabetes or were undergoing or had undergone organ transplant or cancer therapy. Pregnancy or lactation, use of antibiotics or immunosuppressant medication within the last 6 months, need for antibiotics for infective endocarditis prophylaxis during dental procedures, symptoms of acute illness (i.e., fever, sore throat, body aches, and diarrhea), orthodontic appliances or presence of an oral mucosal inflammatory condition (e.g., aphthous, lichen planus, leukoplakia, and oral cancer) also were exclusion criteria. The study was performed at the University of Kentucky between August 2005 and August 2009 and was approved by the University Institutional Review Board. All subjects understood the study, provided written informed consent and received incentives (i.e., monetary compensation and a clinical examination) as part of the study protocol.
Clinical Evaluation and Therapy
Complete medical and dental histories were obtained from the patient’s records and confirmed by interview. Periodontal health measures including BOP, probing depth (PD), and clinical attachment loss (CAL) were assessed as previously described (Frodge et al. 2008, Dawson et al. 2009) at baseline, week 16 and week 28. Participants were randomly assigned to receive either oral hygiene instructions (OHI) only or scaling and root planing (SRP) and OHI using a computer randomization schedule constructed prior to study initiation. OHI and SRP were performed by a registered dental hygienist during the first 30 days following the baseline visit. Patients returned to the clinic at week 8 as part of a separate study protocol for dental plaque sampling, and subjects had repeat OHI or SRP by the treatment hygienist following clinical examination at week 16. A single calibrated periodontist blinded to the participant group assignments, performed the clinical evaluations. Scaling and root planing therapy were performed as we have described previously (Preshaw et al. 2008). Response to therapy was defined based on published findings where non-surgical pocket therapy was expected to yield improvements in PD, CAL and BOP in the time frame examined (Cobb 1996, Kaldahl et al. 1996, Fleming 1999). Responders were defined as individuals who demonstrated 20% improvement in all four categories (%BOP sites, %CAL≥2mm sites, %PD≥4mm sites and %PD≥5mm sites) at both follow-up visits. This threshold was expected to underestimate the number of individuals who responded at individual sites at a single follow-up visit (i.e., increased stringency for response to therapy).
Saliva Collection
Unstimulated whole expectorated saliva (5 mL) was collected from each subject between 9 and 11 a.m. according to a modification in the method described by Navazesh (Navazesh 1993). Subjects rinsed their mouth with tap water, then expectorated whole saliva into sterile tubes while seated in an upright position. Collected samples were placed immediately on ice and aliquoted prior to freezing at −80°C. Samples were thawed and analyzed within six months of collection.
Biomarker Analysis
Concentrations of salivary IL-1β, IL-8, MIP-1α and TNF-α were determined in duplicate using Luminex human cytokine/chemokine multiplex kits (Millipore, St. Charles, MO, USA) and salivary levels of OPG and MMP-8 were determined in duplicate for each subject using human quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s directions in the University of Kentucky General Clinical Research Center Core laboratory. Standards were included on all runs and all results are reported within the linearity of the assays.
Statistical Analysis
Demographic variables were compared between the OHI and SRP groups using a Chi square test or Fisher’s exact test for categorical responses and a two sample t-test for interval level responses. Changes from baseline in the periodontal indices were compared between the treatment groups using analysis of covariance (ANCOVA) with adjustments for age, race and tobacco use. In these analyses, BOP was used as dichotomous score as previously described (Lang et al. 1990), with ≥2 being positive and < 2 being negative. Since the biomarker measurements were not normally distributed due to the presence of large values, the ranks of the biomarker levels (or change from baseline) became the dependent variables in the remaining analyses. The overall comparison of biomarker levels relied on linear mixed model for a two factor design: factor one being treatment group (OHI versus SRP) and factor two being visit (baseline, week 16 or week 28). At each follow-up visit, the comparison of biomarker levels relied on rank ANCOVA for a two factor design: factor one being treatment group (OHI versus SRP) and factor two being responder status (yes or no) with age as a covariate. Correlations were based on Spearman’s rank correlation coefficient. All analyses were performed using the PC SAS 9.1 (SAS Institute Inc., Cary, NC, USA) with statistical significance determined at the 0.05 level. Of note, in the presence of multiple comparisons, some of the results reported below could be due to chance alone.
Results
Sixty eight adults with chronic periodontitis ranging in age from 25 to 69 years were evaluated at baseline, week 16 and week 28 (Table 1). There were 33 in the OHI and 35 in the SRP groups. Participants were predominantly Caucasian and Hispanic, and male. The groups had a similar mean number of teeth, however the OHI group was 7 years older (p=0.003). There were no significant differences between groups in gender, race and smoking. Clinical measures of %CAL ≥2 mm, %PD sites ≥4 mm, %PD sites ≥5 mm and %BOP reflected the presence of generalized chronic periodontitis and were similar between the two groups (p>0.05).
Table 1.
Comparison of demographics and clinical characteristics between study groups at baseline.
| OHI (n=33) | SRP (n=35) | P Value | |
|---|---|---|---|
| Age (years; mean +/− SD) | 47.3 +/− 8.8 | 40.3 +/− 10.0 | 0.003* |
| Female (%) | 36.4 | 25.7 | |
| White (%) | 42.4 | 40.0 | |
| Hispanic (%) | 21.2 | 37.1 | |
| African American | 27.3 | 11.4 | |
| Asian (%) | 9.1 | 11.4 | |
| Current tobacco use (%) | 33.3% | 22.9% | |
| # Teeth | 26.1 +/− 6.4 | 26.5 +/− 4.8 | |
| Periodontal indices (% sites; mean +/− SD) | |||
| BOP Sites | 56.1 +/− 22.7 | 63.0 +/− 22.5 | |
| PD Sites ≥4 mm | 26.5 +/− 14.8 | 27.2 +/− 14.7 | |
| PD Sites ≥5 mm | 15.3 +/− 11.3 | 16.6 +/− 11.8 | |
| CAL ≥2 mm | 30.9 +/− 21.4 | 24.3 +/− 18.1 |
determined by t test.
Periodontal indices analyzed by ANCOVA
Clinical Parameters and Response to Therapy
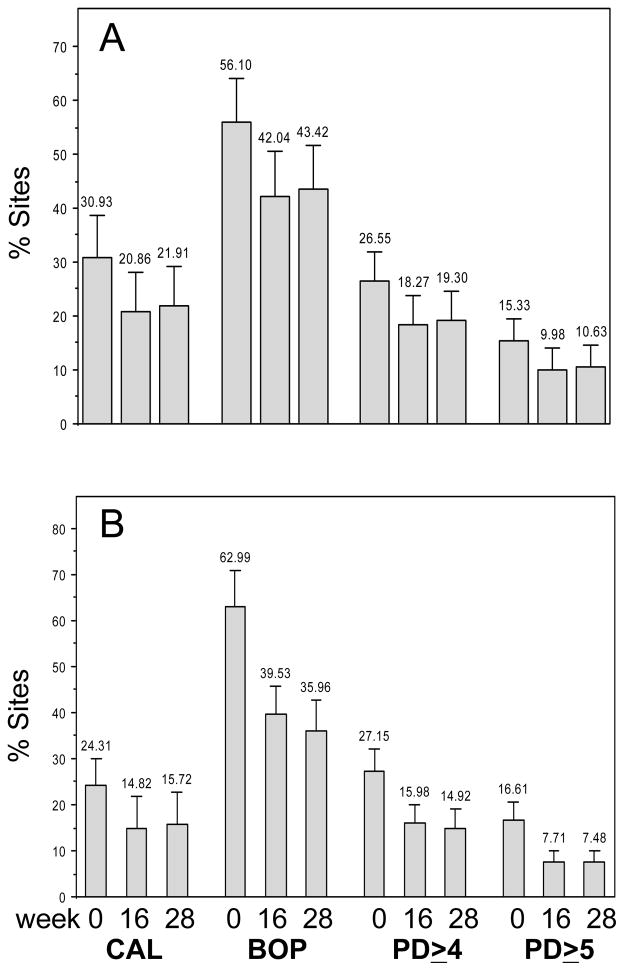
All parameters of periodontal health improved significantly in both groups by week 16 (Figure 1) compared with baseline (p≤0.001). The improved levels persisted in both groups at week 28 (p<0.0001). Overall, SRP was more beneficial (37% to 56% reductions) than OHI (22% to 35% reductions) at both visits. As demonstrated in Figure 1, the percent change in means was significantly greater in the SRP group for %BOP (P≤0.005), %PD sites ≥5 mm (P≤0.002), and %PD sites ≥4 mm (P≤0.04) than the OHI group at both follow-up visits.
Figure 1.
Clinical periodontal measures by group and visit. A) OHI and B) SRP. Significant reductions (p<0.0001) occurred in each parameter compared to baseline within groups as determined by ANCOVA.
Levels of several salivary biomarkers changed in response to therapy
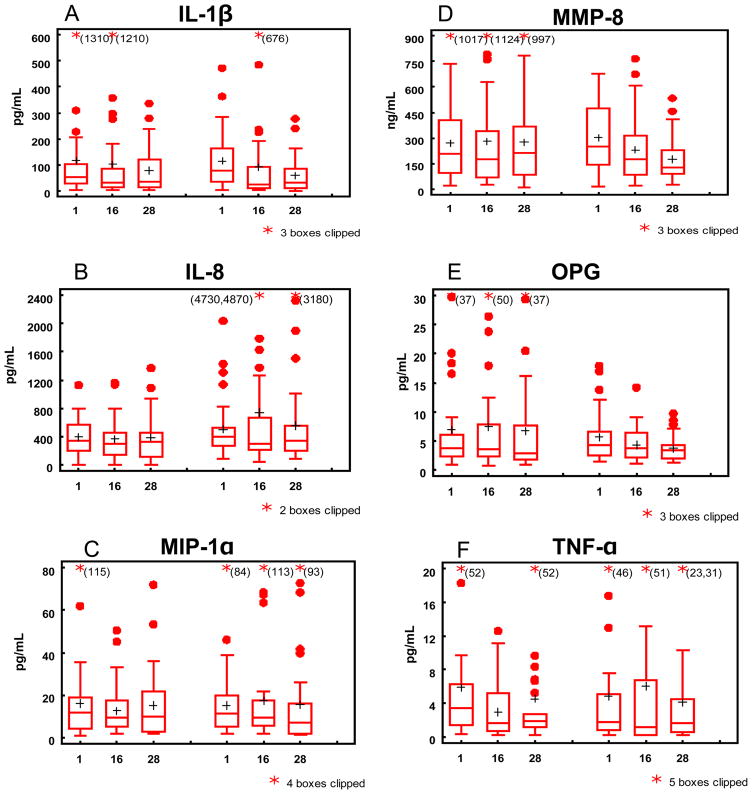
Levels of salivary biomarkers were detected in all samples, had distinct ranges of distribution, and mean levels that were similar between the groups at baseline (Figure 2). The levels of MIP-1α and IL-8 did not vary from baseline at 16 or 28 weeks. The levels of OPG varied significantly from baseline at week 28 (p<0.004), regardless of treatment group. The levels of TNF-α varied significantly from baseline at both visits (p<0.03), regardless of treatment group. Similarly the levels of IL-1β varied significantly from baseline at both visits (p<0.04) in the SRP group, and only at week 28 for the OHI group (p<0.05). Levels of MMP-8 reduced significantly from baseline at both visits in the SRP group only (p<0.0001).
Figure 2.
Boxplots of biomarkers by group and visit. A) IL-1β, B) IL-8, C) MIP-1α, D) MMP-8, E) OPG, and F) TNF-α. Left three boxes correspond to OHI and right three boxes for SRP. Horizontal lines represent the 25th, 50th and 75th percentiles, plus sign represents the mean, and filled circles represent outliers and * indicates outliers clipped from boxplot. X-axis corresponds with weeks.
Correlates between biomarkers and changes in clinical measures of periodontal health
Correlations between each salivary biomarker and changes in clinical measures of periodontal health were calculated for each follow-up visit. In the week 16 analysis for the entire study population, MMP-8 and OPG showed statistically significant correlations with respect to the change in all four clinical measures of periodontal health (r≥0.25, p≤0.04). This relationship persisted for MMP-8 at week 28 for the entire population for PD≥4mm, PD≥5mm and positive BOP (r≥0.27, p≤0.03). OPG correlated with change in CAL≥2mm (r=0.29, p=0.017) at week 28 for the entire population. Also MIP-1α correlated with the change of PD≥4mm and positive BOP (r≥0.26, p≤0.04). In the OHI group alone, MMP-8 correlated with change in PD≥4mm (r=0.40, p=0.025) and positive BOP (r=0.48, p=0.006) at week 28. In the SRP group, MMP-8 showed a significant correlation with respect to the change in all four clinical measures of periodontal health (r≥0.34, p≤0.05) at week 16. MIP-1α correlated with the change of positive BOP (r=0.44, p=0.01) at week 16. MIP-1α also correlated with the change of PD≥5mm (r=0.35, p=0.04), CAL≥2mm (r=0.43, p=0.01) and positive BOP (r=0.41, p=0.01) in the SRP group at week 28.
Biomarkers and responders
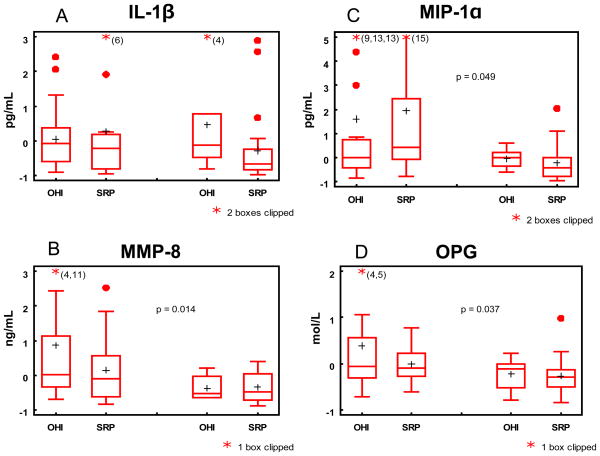
We next investigated whether those who demonstrated a significant response to therapy displayed unique biomarker profiles from those who did not. In contrast to the correlation analyses used above that provided some evidence of salivary response and oral health, a responder in this analysis was defined as an individual who demonstrated 20% improvement in all four clinical categories (frequency of sites with positive BOP, CAL≥2mm, PD≥4mm and PD≥5mm) at both follow-up visits. Of the 68 participants, 51.5% were responders by week 16, with significantly more responders in the SRP (62.9%) than OHI group (27.1%; p=0.0005). OPG, MMP-8 and MIP-1α were significantly reduced in the responders compared with the non-responder (p= 0.008, 0.02, 0.02, respectively) at week 16. At completion of the study, OPG, MMP-8 and MIP-1α demonstrated (Figure 3) significant reductions in the responders compared to the non-responders (p=0.04, 0.01 and 0.05, respectively). Finally, receiver operating characteristic (ROC) analyses were performed to evaluate the change in each biomarker level with respect to response to therapy. As shown in Table 2, MMP-8 consistently produced the highest area under the curve (≥ 0.7; p=0.01) indicating it was the best biomarker for demonstrating response to therapy at weeks 16 and 28. The ROC score for OPG also suggested its usefulness for demonstrating response to therapy.
Figure 3.
Boxplots of change scores of biomarkers (log values) at week 28 by group and responder status. Left two boxes correspond to non-responder and right boxes to responders. Significant p-values for comparing responders to non-responders are indicated on the plots.
Table 2.
Area under the curve (AUC) values for each biomarker at the follow up visits with respect to response to therapy.
| Biomarker | AUC at week 16 | P value at week 16 | AUC at week 28 | P value at week 28 |
|---|---|---|---|---|
| MMP-8 change | 0.70 | 0.01 | 0.72 | 0.01 |
| OPG change | 0.70 | 0.01 | 0.69 | 0.02 |
| MIP-1α change | 0.64 | 0.28 | 0.68 | 0.06 |
| IL-1β change | 0.62 | 0.31 | 0.62 | 0.47 |
| IL-8 change | 0.43 | 0.47 | 0.51 | 0.54 |
| TNF-α change | 0.54 | 0.25 | 0.49 | 0.40 |
Discussion
In dental practice periodontal disease has been historically based on the clinical detection of BOP, PD, CAL, plaque index, and radiographic evidence of bone loss. The diagnostic utility of these clinical measures although reliable, are expensive with respect to time, cost and professional expertise required. Accordingly, the profession has searched for additional chair side measures to aid in early diagnosis and monitoring. Previous studies have shown that constituents present in saliva can provide important complimentary diagnostic information (Malamud 1992, Fox 1993, Kaufman and Lamster 2000, Kaufman and Lamster 2002, Giannobile et al. 2009), that have the potential for point-of-care use by dental professionals and the general public (Malamud et al. 2005, Christodoulides et al. 2007, Miller et al. 2010). However, most previous studies have investigated salivary biomarkers of periodontal disease in cross-sectional studies, and the behavior of various salivary biomarkers in longitudinal studies is limited (Henskens et al. 1996, Van Steijn et al. 2002, Hagewald et al. 2003, Gheren et al. 2008). Thus, a 6-month longitudinal study was designed to follow adults with chronic periodontitis who received either OHI or SRP to better understand the utility of contemporary biomarkers for monitoring periodontal disease and response to different therapies.
Salivary levels of IL-1β, IL-8, MIP-1α, MMP-8, OPG and TNF-α were determined in adults who had chronic periodontitis at baseline, week 16 and week 28 following OHI or SRP therapy. These six proteins are biochemically active for processes integrally involved in inflammation, connective tissue degradation, and osteoclast-modulated alveolar bone turnover (Bertolini et al. 1986, Sorsa et al. 1994, Choi et al. 2000, Engebretson et al. 2002, Silva et al. 2005, Emingil et al. 2005, Roodman 2006, Herr et al. 2007, Miller et al. 2010). All except IL-8 have been previously reported in saliva. IL-8 was included because of its role as a major mediator of the inflammatory response and chemoattractant for neutrophils (Zwahlen et al. 1993). We predicted that significant reductions in levels of these salivary biomarkers would occur in the SRP group compared with baseline and the OHI group. Our data supported the hypothesis that select salivary biomarkers reflected periodontal status over time in participants who received localized mechanical periodontal therapy. Further, the secondary hypothesis that select salivary biomarkers reflect response to therapy was demonstrated.
The two randomly assigned groups had similar levels of periodontal disease and similar baseline levels of each of the salivary biomarkers analyzed. The mean levels for OPG, MMP-8, IL-1β and TNF-α reflected the presence of generalized chronic periodontitis when compared with historical values reported for affected individuals (Miller et al. 2006, Ng et al. 2007, Scannapieco et al. 2007, Frodge et al. 2008). In contrast, salivary levels of MIP-1α and IL-8 have not been previously described in adults. Fine et al. reported MIP-1α levels in adolescents that were similar in concentration range as our adults (Fine et al. 2009), however adolescents who developed localized aggressive periodontitis in that study had much higher levels than our adult participants.
Our baseline clinical and biochemical data provided insight into the profile of salivary biomarker levels of adults who have chronic periodontitis, and allowed us to determine if these values can serve as a reference for monitoring response to therapy. The utility of these biomarkers for monitoring response to therapy was assessed in groups receiving either OHI or SRP. Both groups dramatically improved and progressed towards health (Fig. 1), with the SRP group responding significantly better (37% to 56% reductions) than the OHI group (22% to 35% reductions, P≤0.04). Of interest is the better than expected improvement in the OHI group that may have occurred in relation to the Hawthorne effect and the frequent dental visits employed in this study design. Within the picture of improving oral health, OPG and TNF-α declined significantly from baseline in both treatment groups while IL-1β and MMP-8 only showed significant changes from baseline in the SRP group. A plausible explanation for only these two biomarkers (OPG and TNF-α) changing significantly in the OHI group could be that significant amounts of disease remained in the OHI group at the follow-up visits (i.e., BOP ≥42%, PD≥4mm >18%, PD≥5mm 10%, CAL ≥21%) thus limiting the sensitivity of all of the biomarkers to distinguish the response within that group. Another possibility is that smoking, which was documented in 23% of the SRP group and 33% of the OHI group, influenced salivary biomarkers levels differentially (i.e., more for select biomarkers), as has been reported for OPG in a previous study (Buduneli et al. 2008). In any case, mechanical therapy was sufficiently effective in exceeding a biological threshold required to detect the changes in saliva in all four biomarkers (IL-1β, MMP-8, OPG, TNF-α) in the SRP group.
Analysis by responders allowed us to further explore the role of biomarkers and showed that several specific biomarkers have the potential utility for monitoring periodontal disease. OPG, MMP-8 and MIP-1α were early (week 16) salivary biomarkers of the responders. These biomarkers also identified the responders at week 28. Biomarker utility was further validated by performing ROC analyses with respect to change in biomarker level in responders vs. non-responders. Here, AUCs demonstrated that MMP-8 ≥ OPG > MIP-1α >IL-1β ranked as the best biomarkers indicative of response to therapy. The fact that MMP-8 was highly ranked is not a surprise. Salivary MMP-8 has been a consistent indicator of periodontal disease and connective tissue degradation in several studies (Miller et al. 2006, Ng et al. 2007, Scannapieco et al. 2007, Ramseier et al. 2009, Costa et al. 2010). In comparison, OPG and MIP-1α, have shown promise as biomarkers related to periodontal disease (Miller et al. 2006, Fine et al. 2009). However, their levels are predicted to correlate with osteoclast activity and bone turnover aspects of periodontal disease that may or not be present at any given time during the course of periodontal disease, and single time point measures may inadequately capture these events. Also IL-1β, although a marker of periodontal disease, can be elevated in both gingival and periodontal inflammation (Engebretson et al. 2002, Offenbacher et al. 2007), thus its sensitivity to periodontal tissue change may be insufficient to be used alone for monitoring periodontal disease.
Conclusions
In conclusion, this study is one of the first to examine the role of a panel of salivary biomarkers for monitoring periodontal health in a longitudinal study design. Our findings, although limited by study size, some potential statistical limitations and the rigidity of the inclusion criteria, show that MMP-8, OPG, MIP-1α and IL-1β have associations with biological aspects of periodontitis and reflect improved periodontal health as a result of localized therapy over a 6-month time period. Also, the findings suggest that select salivary biomarkers could have utility for monitoring periodontal health and response to therapy. To further advance the field, large validation studies are needed to confirm these data and investigate if salivary biomarkers are confounded by age, gender, race, smoking, presence of oral inflammatory conditions, or co-morbid conditions. We believe that validation studies could lead to their use in point-of-care devices that could have a major impact on periodontal care. One can envision that these devices could be used to assess periodontal health similar to the way a glucometer assesses blood glucose in diabetic subjects. Specific biomarkers (i.e., MMP-8, OPG, MIP-1α, IL-1β) levels above set thresholds might suggest immediate therapy would be beneficial, similar to how insulin is provided when glucose is elevated in a diabetic. In turn, individualized periodontal care based on salivary biomarker levels could be provided in the near future and these analyses could become an integral part of the assessment of periodontal health in dental and non-dental settings.
Acknowledgments
The authors thank Jenny O’Nan, Donna Mischel, Vanessa Hodges-Reed, study coordinators, Jason Stevens, research analyst, and Malini Bharadwaj, data management specialist, of the Center for Oral Health Research of the University of Kentucky for clinical, laboratory and data management support.
source of funding statement
This study was supported by grants from the National Institute of Health P20 RR020145 and M01-RR02602 and University of Kentucky General Clinical Research Core.
Footnotes
Conflict of interest
The authors report no conflicts of interest related to this study.
References
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Buduneli N, Biyikoğlu B, Sherrabeh S, Lappin DF. Saliva concentrations of RANKL and osteoprotegerin in smoker versus non-smoker chronic periodontitis patients. J Clin Periodontol. 2008;35:846–852. doi: 10.1111/j.1600-051X.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, Alsina M. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- Christodoulides N, Floriano PN, Miller CS, Ebersole JL, Mohanty S, Dharshan P, Griffin M, Lennart A, Ballard KL, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV, McDevitt JT. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, Ali MF, Bernard B, Romanovicz D, Anslyn E, Fox PC, McDevitt JT. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005;5:261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1:443–490. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB, Jr, Taba M., Jr Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010;81:384–391. doi: 10.1902/jop.2009.090510. [DOI] [PubMed] [Google Scholar]
- Dawson DR, III, Wang C, Danaher RJ, Lin Y, Kryscio RJ, Jacob RJ, Miller CS. Oral Diseases. 2009;15:554–559. doi: 10.1111/j.1601-0825.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- Emingil G, Atilla G, Baskesen A, Berdeli A. Gingival crevicular fluid EMAP-II, MIP-1alpha and MIP-1beta levels of patients with periodontal disease. J Clin Periodontol. 2005;32:880–885. doi: 10.1111/j.1600-051X.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL-1beta profiles in periodontal disease. J Clin Periodontol. 2002;29:48–53. doi: 10.1034/j.1600-051x.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- Faizuddin M, Bharathi SH, Rohini NV. Estimation of interleukin-1beta levels in the gingival crevicular fluid in health and in inflammatory periodontal disease. J Periodontal Res. 2003;38:111–114. doi: 10.1034/j.1600-0765.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Donnelly R, Gunsolley J. Macrophage inflammatory protein-1alpha: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J Periodontol. 2009;80:106–113. doi: 10.1902/jop.2009.080296. [DOI] [PubMed] [Google Scholar]
- Fleming TF. Periodontitis. Ann Periodontol. 1999;4:32–37. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- Fox PC. Salivary monitoring in oral diseases. Ann N Y Acad Sci. 1993;694:234–237. doi: 10.1111/j.1749-6632.1993.tb18356.x. [DOI] [PubMed] [Google Scholar]
- Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone remodeling biomarkers of periodontal disease in saliva. J Periodontol. 2008;79:1913–1919. doi: 10.1902/jop.2008.080070. [DOI] [PubMed] [Google Scholar]
- Gheren LW, Cortelli JR, Rodrigues E, Holzhausen M, Saad WA. Periodontal therapy reduces arginase activity in saliva of patients with chronic periodontitis. Clin Oral Investig. 2008;12:67–72. doi: 10.1007/s00784-007-0146-8. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves Lda R, Soares MR, Nogueira FC, Garcia C, Camisasca DR, Domont G, Feitosa AC, Pereira Dde A, Zingali RB, Alves G. Comparative proteomic analysis of whole saliva from chronic periodontitis patients. J Proteomics. 2010;73:1334–1341. doi: 10.1016/j.jprot.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Gorska R, Nedzi-Gora M. The effects of the initial treatment phase and of adjunctive low-dose doxycycline therapy on clinical parameters and MMP-8, MMP-9, and TIMP-1 levels in the saliva and peripheral blood of patients with chronic periodontitis. Arch Immunol Ther Exp (Warsz) 2006;54:419–426. doi: 10.1007/s00005-006-0047-6. [DOI] [PubMed] [Google Scholar]
- Gursoy UK, Kononen E, Uitto VJ, Pussinen PJ, Hyvarinen K, Suominen-Taipale L, Knuuttila M. Salivary interleukin-1beta concentration and the presence of multiple pathogens in periodontitis. J Clin Periodontol. 2009;36:922–927. doi: 10.1111/j.1600-051X.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- Hagewald SJ, Fishel DL, Christan CE, Bernimoulin JP, Kage A. Salivary IgA in response to periodontal treatment. Eur J Oral Sci. 2003;111:203–208. doi: 10.1034/j.1600-0722.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Sorsa T, Pitkaniemi J, Tervahartiala T, Kari K, Broms U, Koskenvuo M, Meurman JH. Smoking Affects Diagnostic Salivary Periodontal Disease Biomarker Levels in Adolescents. J Periodontol. 2010;81:1299–1307. doi: 10.1902/jop.2010.090608. [DOI] [PubMed] [Google Scholar]
- Henskens YM, van der Weijden FA, van den Keijbus PA, Veerman EC, Timmerman MF, van der Velden U, Amerongen AV. Effect of periodontal treatment on the protein composition of whole and parotid saliva. J Periodontol. 1996;67:205–212. doi: 10.1902/jop.1996.67.3.205. [DOI] [PubMed] [Google Scholar]
- Herr AE, Hatch AV, Throckmorton DJ, Tran HM, Brennan JS, Giannobile WV, Singh AK. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci U S A. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J Periodontol. 1996;67:93–102. doi: 10.1902/jop.1996.67.2.93. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis--a review. J Clin Periodontol. 2000;27:453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. The diagnostic applications of saliva--a review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- Lamster IB. Evaluation of the host response in crevicular fluid, saliva, and blood: implications and applications for the diagnosis of periodontal disease. Periodontal Case Rep. 1990;12:6–9. [PubMed] [Google Scholar]
- Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2:123–137. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. Beta-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J Periodontol. 2003;74:353–359. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990;17:714–721. doi: 10.1111/j.1600-051x.1990.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Malamud D. Saliva as a diagnostic fluid. BMJ. 1992;305:207–208. doi: 10.1136/bmj.305.6847.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D, Bau H, Niedbala S, Corstjens P. Point detection of pathogens in oral samples. Adv Dent Res. 2005;18:12–16. doi: 10.1177/154407370501800104. [DOI] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campbell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, Ebersole JL, McDevitt JT. Current developments in salivary diagnostics. Biomarkers in Medicine. 2010;4:1–18. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- Mirrielees J, Crofford LJ, Lin Y, Kryscio RJ, Dawson DR, III, Ebersole JL, Miller CS. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol. 2010;37:1068–1074. doi: 10.1111/j.1600-051X.2010.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- Ng PY, Donley M, Hausmann E, Hutson AD, Rossomando EF, Scannapieco FA. Candidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studies. FEMS Immunol Med Microbiol. 2007;49:252–260. doi: 10.1111/j.1574-695X.2006.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78:1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch Oral Biol. 1995;40:1151–1155. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Novak MJ, Mellonig J, Magnusson I, Polson A, Giannobile WV, Rowland RW, Thomas J, Walker C, Dawson DR, Sharkey D, Bradshaw MH. Modified-release subantimicrobial dose doxycycline enhances scaling and root planing in subjects with periodontal disease. J Periodontol. 2008;29:440–452. doi: 10.1902/jop.2008.070375. [DOI] [PubMed] [Google Scholar]
- Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD. Regulation of osteoclast differentiation. Ann N Y Acad Sci. 2006;1068:100–109. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann N Y Acad Sci. 2007;1098:496–497. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva TA, Garlet GP, Lara VS, Martins W, Jr, Silva JS, Cunha FQ. Differential expression of chemokines and chemokine receptors in inflammatory periapical diseases. Oral Microbiol Immunol. 2005;20:310–316. doi: 10.1111/j.1399-302X.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Ding Y, Salo T, Lauhio A, Teronen O, Ingman T, Ohtani H, Andoh N, Takeha S, Konttinen YT. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Ann N Y Acad Sci. 1994;732:112–131. doi: 10.1111/j.1749-6632.1994.tb24729.x. [DOI] [PubMed] [Google Scholar]
- Thomas MV, Branscum A, Miller CS, Ebersole J, Al-Sabbagh M, Schuster JL. Within-subject variability in repeated measures of salivary analytes in healthy adults. J Periodontol. 2009;80:1146–1153. doi: 10.1902/jop.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobon-Arroyave SI, Jaramillo-Gonzalez PE, Isaza-Guzman DM. Correlation between salivary IL-1beta levels and periodontal clinical status. Arch Oral Biol. 2008;53:346–352. doi: 10.1016/j.archoralbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Ulker AE, Tulunoglu O, Ozmeric N, Can M, Demirtas S. The evaluation of cystatin C, IL-1beta, and TNF-alpha levels in total saliva and gingival crevicular fluid from 11- to 16-year-old children. J Periodontol. 2008;79:854–860. doi: 10.1902/jop.2008.070422. [DOI] [PubMed] [Google Scholar]
- Van Steijn GJ, Amerongen AV, Veerman EC, Kasanmoentalib S, Overdijk B. Effect of periodontal treatment on the activity of chitinase in whole saliva of periodontitis patients. J Periodontal Res. 2002;37:245–249. doi: 10.1034/j.1600-0765.2002.00330.x. [DOI] [PubMed] [Google Scholar]
- Zwahlen R, Walz A, Rot A. In vitro and in vivo activity and pathophysiology of human interleukin-8 and related peptides. Int Rev Exp Pathol. 1993;34(Pt B):27–42. doi: 10.1016/b978-0-12-364935-5.50008-0. [DOI] [PubMed] [Google Scholar]