Abstract
Recent data suggests that megakaryocytes (MKs) play a role in skeletal homeostasis. In vitro and in vivo data show that MKs stimulate osteoblast (OB) proliferation and inhibit osteoclast (OC) formation, thus favoring net bone deposition. There are several mouse models with dysregulated megakaryopoiesis and resultant high bone mass phenotypes. One such model that our group has extensively studied is GATA-1 deficient mice. GATA-1 is a transcription factor required for normal megakaryopoiesis, and mice deficient in GATA-1 have increases in immature MK number and a striking increase in bone mass. While the increased bone mass could simply be a result of increased MK number, here we take a more in depth look at the MKs of these mice to see if there is a unique factor inherent to GATA-1 deficient MKs that favors increased bone deposition. We show that increased MK number does correspond with increased OB proliferation and decreased OC proliferation, that stage of maturation does not alter the effect of MKs on bone cell lineages beyond the megakaryoblast stage, and that GATA-1 deficient MKs survive longer than wild-type controls. So while increased MK number in GATA-1 deficient mice likely contributes to the high bone mass phenotype, we propose that the increased longevity of this lineage also plays a role. Since GATA-1 deficient MKs live longer they are able to exert both more proliferative influence on OBs and more inhibitory influence on OCs.
Keywords: Megakaryocytes, Osteoblasts, Osteoclasts, Maturation, Longevity
Cells of the hematopoietic and mesenchymal lineage both reside in the bone marrow cavity and are thus intimately related in space, and recent research shows that these two systems originally studied in isolation are in fact functionally connected. MKs are platelet progenitor cells, and their reciprocal relationship with bone cells is one of the burgeoning areas of research just described. Multiple studies have now provided in vitro and in vivo evidence showing that MKs stimulate OB proliferation and development, and inhibit OC development.
In vitro studies show that MKs can influence bone mass by the secretion of bone matrix proteins and growth factors, by directly increasing osteoblastogenesis, and by affecting OB differentiation. MKs or their platelet products secrete multiple bone matrix proteins and growth factors crucial for bone remodeling (Thiede et al., 1994; Kelm et al., 1992; Breton-Gorius et al., 1992; Sipe et al., 2004; Vannucchi et al., 2002; Wickenhauser et al., 1995). Therefore MKs could directly impact bone formation and bone remodeling, potentially in the setting of increased high local concentrations. MKs have a direct affect on osteoblastogenesis mediated by direct cell-to-cell contact, with co-cultures of MKs and OBs showing a 3–6 fold increase in OB proliferation (Kacena et al., 2004; Miao et al., 2004). There is also in vitro evidence that MKs alter OB differentiation. Bord et al., (Bord et al., 2005) demonstrated that when MKs are co-cultured with OBs for 1–2 days, OB expression of collagen (COL1 A1) and OPG was increased. We recently reported that in long-term co-cultures (14 days), MKs inhibited all measured markers of OB differentiation studied, including expression of type I collagen, osteocalcin, and alkaline phosphatase, as well as functional measures of alkaline phosphatase activity and mineralization (Ciovacco et al., 2009). Overall, the data clearly shows that MKs alter OB proliferation and differentiation.
MKs also have the ability to directly and indirectly affect osteoclastogenesis. Directly, in vitro OC formation is significantly inhibited, up to 10-fold, by MKs or MK conditioned medium (CM) (Kacena et al., 2006; Beeton et al., 2006). The latter suggests a soluble factor(s) is responsible for inhibiting osteoclastogenesis (Kacena et al., 2006). MKs also indirectly influence osteoclastogenesis by stimulating OB and fibroblast proliferation (Kacena et al., 2004; Miao et al., 2004; Schmitz et al., 1995; Schmitz et al., 1999; Udagawa et al., 1999; Nakano et al., 2004; Mitani et al., 2005; Wei et al., 2005; Gao et al., 1998). Fibroblasts and OBs could contribute to osteoclastogenesis as they both secrete osteoprotegerin (OPG), a known inhibitor of osteoclastogenesis. In addition to increasing OB and fibroblast proliferation, MKs also indirectly inhibit OC development by increasing OB expression of OPG (Bord et al., 2005; Kacena et al., 2006).
Thus there is growing body of evidence that MKs cultured from wild-type mice influence bone lineage cells in vitro. Additional in vivo evidence of MK influence on skeletal homeostasis comes from the study of mouse models with dysregulated megakaryopoiesis and a resultant high bone mass phenotype. At least 4 mouse models have been described in which MK number is significantly elevated with a concomitant increase in bone mass. Mice overexpressing thrombopoietin (TPO), the main MK growth factor, have approximately a 4-fold increase in MK number and an osteosclerotic bone phenotype (Frey et al., 1998a; Frey et al., 1998b; Yan et al., 1996; Yan et al., 1995; Villeval et al., 1997). We previously demonstrated that mice deficient in transcription factors required for the development of mature MKs (GATA-1 or NF-E2) have elevated numbers of abnormally immature MKs and exhibit a striking increase in bone mass (Kacena et al., 2004; Shivdasani et al., 1995; Shivdasani et al., 1997; Kacena et al., 2005). More recently, a mouse model of platelet-type von Willebrand disease, a gain-of-function mutation, has also been shown to result in increased numbers of MKs and a high bone mass phenotype (Suva et al., 2008).
Overall, these findings demonstrate the complex regulatory interactions at play between MKs and bone cells. The in vivo data shows that the in vitro observations are not just an artifact of an artificial environment forcing cell lineages to interact, but that MKs, OBs, and OCs may affect each other in their natural environment, as dysregulation of the MK lineage corresponds with bone pathology both in mice and in humans (Thiele et al., 1999; Lennert et al., 1975; Chagraoui et al., 2006b; Chagraoui et al., 2006a). But until now, most theories explaining the high bone mass phenotype in the aforementioned mouse models focused on the increase in MK number, with the logical assumption that if MKs favor bone deposition, more MKs equates to a higher bone mass. However, here we probe further into one of these models, and examine MKs cultured from GATA-1 deficient mice to see if there is an inherent quality about the cell lineage itself, such as stage of maturation or increased viability, that favors net bone formation more than wild-type MKs.
Materials and Methods
Mice
For these studies GATA-1 deficient and C57BL/6 mice were used. Generation and breeding of mutant mice with selective loss of GATA-1 was described previously (Shivdasani et al., 1997; McDevitt et al., 1997). In brief, a DNAse I-hypersensitive region (HS) was identified upstream of the GATA-1 promoter and was subsequently knocked-out by insertion of a neomycin-resistant cassette. This resulted in mice with reduced levels of GATA-1 mRNA and protein (3–5 fold reduction in protein), a functional knock-down (Shivdasani et al., 1997; McDevitt et al., 1997). GATA-1 deficient mice are maintained on the C57BL/6 background.
Preparation of Fetal Liver Derived MKs
Murine MKs were prepared as previously described (Kacena et al., 2004; Kacena et al., 2006). In brief, fetuses were dissected from pregnant C57BL/6 mice at E13-15. The livers were removed and single cell suspensions made by forcing cells thru sequentially smaller gauge needles (18G, 20G, 23G). Cells were washed 2x with DMEM + 10% FCS and then seeded (5 fetal livers/100 mm dish) in 100 ml culture dishes, in DMEM + 10% FCS + 1% murine TPO (Villeval et al., 1997). After 3–5 days, MK were obtained by separating them from the lymphocytes and other cells using a one-step albumin gradient to obtain a 90–95% pure MK population (Drachman et al., 1997). The bottom layer was 3% albumin in PBS (Bovine Albumin, protease free, fatty acid poor, Serologicals Proteins Inc., Kankakee, IL), the middle layer was 1.5% albumin in PBS, and the top layer was serum free media containing the cells to be separated. All of the cells sedimented through the layers at 1g for approximately 40 minutes at room temperature. The MK fraction was collected from the bottom of the tube.
Preparation of MK CM
To generate MK CM, 1×106 MKs/ml were cultured in α-media containing no serum for 3 days. After 3 days CM was collected, sterile filtered, and stored at −80° C until use. For the studies here CM was used at 3%, 10%, and 30% (vol:vol).
Preparation of neonatal calvarial cells (OB)
Murine calvarial cells were prepared as previously described (Horowitz et al., 1994). Our technique was a modification of the basic method described by Wong and Cohn (Wong and Cohn, 1975). Briefly, calvaria from C57BL/6 mice less than 48 hours old were pretreated with EDTA in PBS for 30 min. The calvaria were then subjected to sequential collagenase digestions. Cells were collected following incubation in collagenase. Fractions 3–5 were used as the starting population. These cells were > 95% OB or OB precursors by a variety of criteria (Horowitz et al., 1994; Simmons et al., 1982; Jilka and Cohn, 1981). Freshly prepared OBs were used for all studies.
Proliferation Analysis
To examine the proliferative capacity of OBs (2500 OBs/well, optimal, pretested) cultured with and without MKs (2500 or 5000 MKs/well, optimal, pretested), cells were seeded in triplicate into 96-well tissue culture plates and incubated for up to 6 days at 37°C in α-MEM supplemented with 10% FCS. Proliferation was measured every 2–3 days by the incorporation of 3H-thymidine (1 µCi/well; 5–8 Ci/mmol) added during the last 16 hours of culture (Centrella et al., 1991). To assess OB proliferation alone, MK were removed from wells (4 washes) prior to measuring incorporation of 3H-thymidine (Kacena et al., 2004).
In Vitro Osteoclast-like Cell Formation Models
OC-like cells were generated by three previously described methods (Udagawa et al., 1989; Yasuda et al., 1998; Horowitz et al., 2004; Kim et al., 2005). First, co-cultures containing 2×106 BM cells/ml and 20,000 primary calvarial OB/ml were grown in α-MEM supplemented with 10% FCS and 10−8 M 1,25(OH)2D3. The media was changed every other day for 6–8 days. Second, 2×106 BM cells/ml were cultured in α-MEM supplemented with 10% FCS and 30 ng/ml of recombinant murine M-CSF (Research Diagnostics Inc., Flanders, NJ) and 50 ng/ml of recombinant human RANKL (Research Diagnostics Inc.). Media was changed every third day for 6–9 days (until OC were visible). Third, a new OC generation model, using a Pax5−/− spleen cell line (SCL) as the source of osteoclast precursors, was used as has been previously described (Horowitz et al., 2004). In brief, the Pax5−/− SCL is highly enriched in OC precursors and when cultured with M-CSF and RANKL, OC develop in a shorter time (3–4 days) than C57BL/6 BM cell cultures (6–8 days). As detailed by Horowitz et al. (Horowitz et al., 2004) the Pax5−/− SCL is 97% CD11b+ (Mac-1), 96% CD16/32+ (Fcγ R), and >90% CD115+ (c-fms) as demonstrated by FACS analysis. In addition, the Pax5−/− SCL does not express CD19, CD45R (B220), CD117 (c-kit), Ly-6A/E, Ly-6C, Ly-6G (Gr-1), NK1.1, or TCRαβ (TCR). These data indicate that the Pax5−/− SCL expresses a monocyte/macrophage-like phenotype with no T or B cells present (Horowitz et al., 2004). For experiments using the Pax5−/− SCL, 100,000 cells/ml were cultured with M-CSF and RANKL (as above) and if necessary the media was changed on the third day. Cells were usually fixed at day 4 or 5, stained, and counted as described below.
Flow Cytometry
Fetal liver cells were removed from dishes prior to BSA gradient separation as described above and washed with PBS containing 2% FCS. Staining was performed in PBS with 2% serum. Anti-CD41, CD61, CD49b, and CD49d, were purchased from PharMingen. Anti- CD42d was purchased from Research Diagnostics Inc. Light scatter and fluorescence of individual cells was measured by a Facstar Plus flow cytometer, and cells were sorted base on their antigen expression as described here. Cells were sorted into 3 separate subpopulations (in order of increasing maturity): megakaryoblast, MK immature, and MK mature. Megakaryoblasts are CD61+ CD41−cells (Vainchenker et al., 1994; Bojko et al., 1998). Immature MKs are CD41+ CD49d+ cells (Mossuz et al., 1997). Mature MKs are CD41+ CD49b+ (Mossuz et al., 1997) or CD41+ CD42d+ (Sato et al., 2000) cells. These 3 subpopulations of cells were then analyzed for their ability to induce OB proliferation or inhibit OC formation as described above.
Longevity Studies
C57BL/6 and GATA-1 deficient MKs (4000 cells/ml) were cultured in the presence or absence of TPO (100 ng/ml) and with or without OBs (20,000 cells/ml). Thus, there were 4 groups examined for each type of MK: 1) MK alone; 2) MK+TPO; 3) MK+OB; and 4) MK+OB+TPO. Cells were cultured in 24-well culture plates and the media used in these studies was α-MEM supplemented with 10% FCS. As MKs can be removed with normal feedings, media was replenished 1/week (if counts were performed, feeding followed counting). Specifically, for the first feeding, an additional 1 ml of medium was added to cultures. On the second feeding 1 ml of medium was carefully removed from the top on the well using a pipet and an additional 1 ml of fresh medium was added to the cultures. All subsequent feedings were done as described for the second feeding. Fresh TPO was added to the cultures indicated (100 ng). Number of MKs present in these cultures was recorded.
Statistics
Unless otherwise stated, all data are presented as the Mean ± SD. For studies where type of megakaryocyte population alone was studied (Figures 3 and 4), one-way ANOVA was used to determine significant differences (p<0.05). Two-way factorial analyses of variances were used to compare groups, with megakaryocyte cell number and type (e.g. GATA-1 deficient or C57BL/6) being the independent variables. In the event of a significant interaction, pair-wise Bonferroni comparisons were made to explore individual group differences while controlling for the elevated family-wise error associated with performing multiple comparisons. All analyses were performed with the Statistical Package for Social Sciences (SPSS 6.1.1; Norusis/SPSS Inc., Chicago, IL) software and were two-tailed with a level of significance set at 0.05. Experiments are always repeated, in some cases multiple times. Within individual experiments, data points are based on a minimum of triplicate samples.
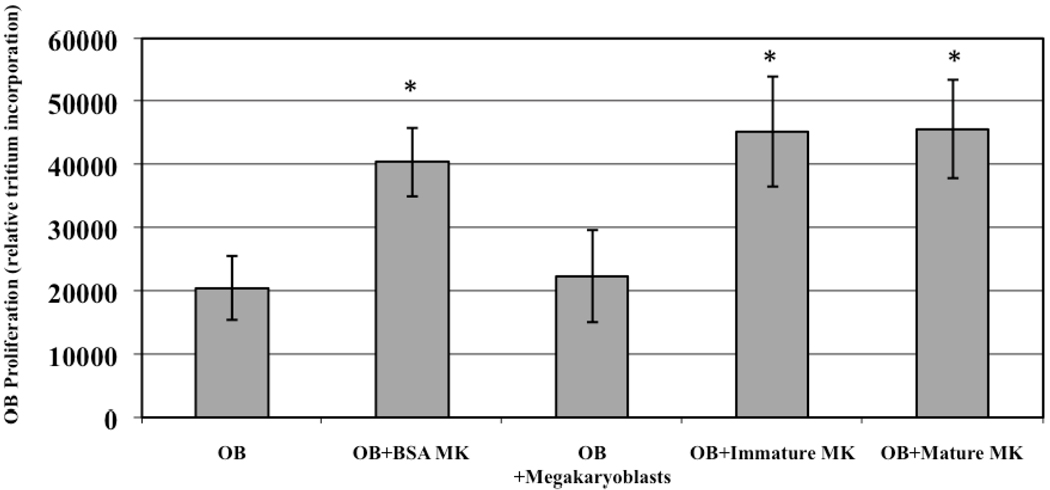
Figure 3.
Affect of MK maturation stage on OB proliferation. 2500 BSA separated C57BL/6 MKs or flow cytometry separated immature and mature MKs all similarly enhanced OB proliferation, while 2500 megarkaryoblasts did not alter OB proliferation. * Denotes a significant difference in proliferation compared to the OB control group (p<0.05).
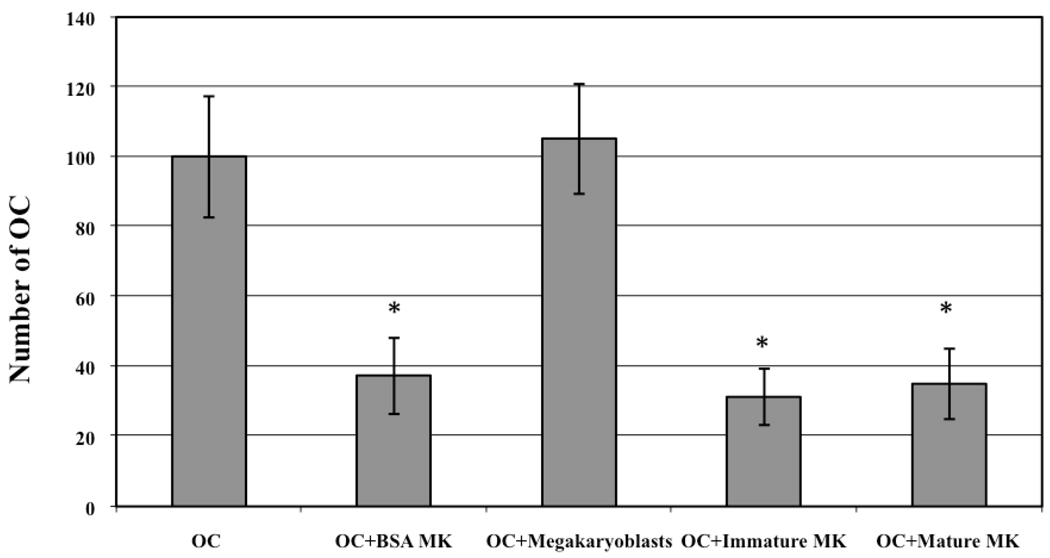
Figure 4.
Affect of MK maturation stage on OC inhibition. 3% C57BL/6 MK CM from BSA separated or flow cytometry separated immature and mature MKs similarly inhibited OC development, while 3% CM from megakaryoblasts did not inhibit OC development. * Denotes a significant difference in OC number compared to OC control group (p<0.05).
Results
Effect on MK Number on OB Proliferation
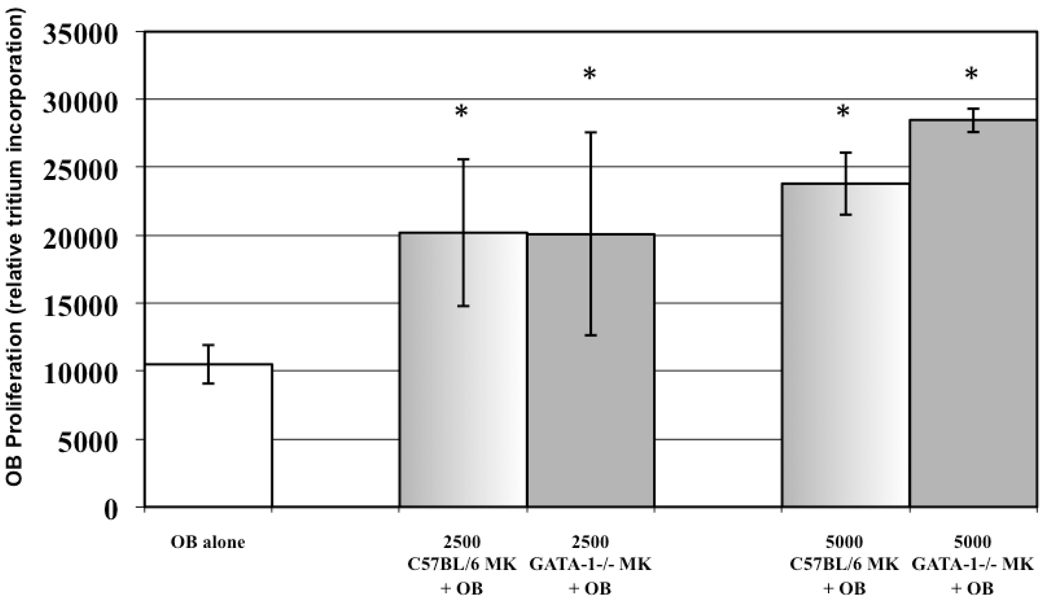
To determine if MK number affected the degree of OB proliferation we cultured increasing numbers of C57BL/6 and GATA-1 deficient MKs (0, 2500 and 5000) with 2500 OBs, and OB proliferation was determined by relative tritium incorporation as outlined above (n=9). The results at day 3 are shown in Figure 1. When OBs were co-cultured with 2500 MKs, derived from either C57BL/6 or GATA-1 deficient mice, OB proliferation was similarly elevated by 92% and 91%, respectively. When OBs were co-cultured with 5000 MKs, derived from either C57BL/6 or GATA-1 deficient mice, OB proliferation was enhanced by 127% and 171%, respectively. It should be noted that OB proliferation was significantly increased when co-cultured with either C57BL/6 or GATA-1 deficient MKs (as compared to OBs cultured alone) at both concentrations. Importantly, although it appears that OB proliferation is further elevated when co-cultured with 5000 GATA-1 deficient MKs as compared to 5000 C57BL/6 MKs, no significant difference was detected (p=0.11). Thus, these results show that as MK number increases, OB proliferation increases regardless of whether C57BL/6 or GATA-1 deficient MKs are used.
Figure 1.
Affect of MK number on OB proliferation. Increasing numbers of GATA-1 deficient and C57BL/6 MKs similarly enhanced OB proliferation. A significant, greater than 90% increase in OB proliferation was observed when OBs were cultured with either 2500 C57BL/6 or GATA-1 deficient MKs. Culturing OBs with 5000 MKs further enhanced OB proliferation. * Denotes a significant difference in proliferation compared to the OB alone control group (p<0.05).
Effect on MK Number on OC Inhibition
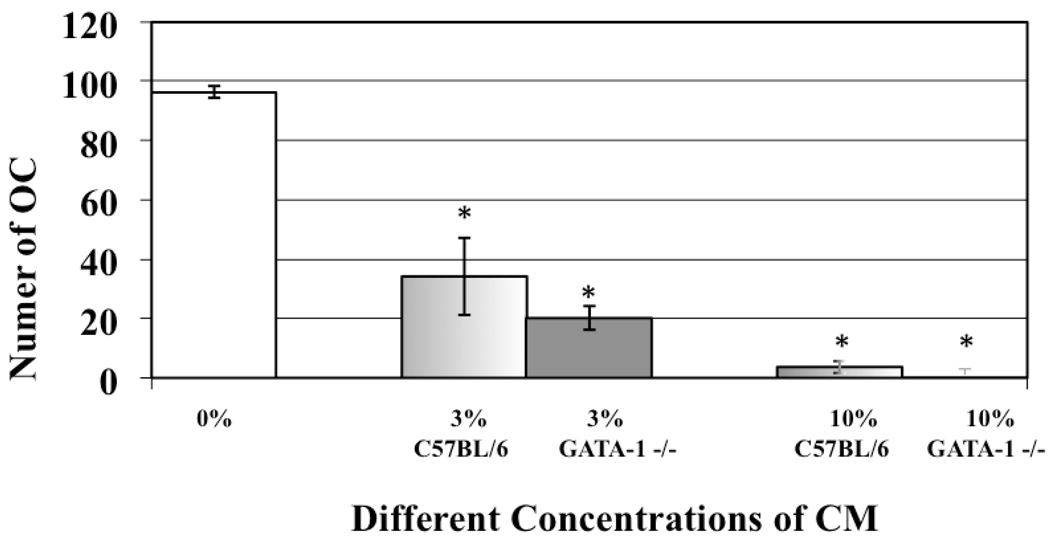
To determine if MK number affected the degree of OC inhibition we cultured 100,000 OC progenitor cells/ml with increasing concentrations of C57BL/6 and GATA-1 deficient MK CM (0%, 3%, 10%, and 30%, vol:vol), and mature multi-nucleated OC number was determined (n=9). The results are shown in Figure 2. Of note, the OC data presented here utilize the Pax5−/− spleen cell line model system as OCs develop in the shortest time with this system and for the flow cytometry, we required a model system where OCs were generated in the shortest amount of time (fewer media changes mean fewer sorted MKs required to generated enough CM). We did however demonstrate that in all the OC generation models described, GATA-1 deficient and C57BL/6 MK CM similarly inhibited OC formation (data not shown and (Kacena et al., 2006)). When Pax5−/− OC progenitors were cultured with 3% C57BL/6 MK CM or 3% GATA-1 deficient MK CM, there was a 66% and 80% reduction, respectively, in OC formation as compared to OC progenitors cultured without CM. Like with OB proliferation, no significant difference was detected between the ability of 3% GATA-1 deficient MK CM and 3% C57BL/6 CM to inhibit OC formation (p=0.14). With 30% MK CM no OCs were detectable in cultures containing C57BL/6 or GATA-1 CM. These results show that just as with OB proliferation, MK number plays a critical role in the inhibition of OC formation.
Figure 2.
Affect of MK CM concentration on osteoclastogenesis. Increasing concentrations of GATA-1 deficient and C57BL/6 MK CM similarly inhibited OC development. A significant, greater than 65% reduction in the number of OCs formed was observed with just 3% CM. With 30% CM from either C57BL/6 or GATA-1 deficient MKs, complete inhibition in OC formation was seen. * Denotes a significant difference (p<0.05) in OC number compared to control groups cultured without CM (0%).
Effect on MK Maturation Stage on OB Proliferation
To determine whether the stage of MK maturation influenced OB proliferation, C57BL/6 MKs were sorted into 3 subpopulations (megakaryoblasts, immature MKs, and mature MKs) based on antibody binding to cell surface markers characteristic of each subpopulation (flow cytometry). 2500 MKs from each individual population, as well as 2500 MKs from a mixed MK population (~90–95% pure, BSA separated MK population harvested from fetal livers as outlined above (Drachman et al., 1997)), were added to cultures containing 2500 OBs, and OB proliferation was determined (n=6). The results are shown in Figure 3. Like before, we examined OB proliferation on day 3. Interestingly, the megakaryoblasts had no significant effect on OB proliferation. However, the BSA separated MK population, the immature MK population, and the mature MK population all had a similar effect on OB proliferation (no significant differences were detected between these groups). Importantly these results show that while megarkaryoblasts have no effect on OB proliferation, immature and mature MKs have virtually the same proliferative effect on OBs.
Effect of MK Maturation Stage on OC Inhibition
To determine whether the stage of MK maturation influenced OC formation, we again sorted C57BL/6 MKs into 3 subpopulations (megakaryoblasts, immature MKs and mature MKs). These individual subpopulations, as well as the BSA separated MKs, were cultured for 3 days (1×106 cells/ml), their CM was collected, sterile filtered, and 3% CM (vol:vol) was added to OC generating cultures as before (n=6). The results are shown in Figure 4. To better compare to our previous data (Figure 2) all OC results were normalized (control cultures were set to 100). Interestingly, similar to the OB proliferation data, 3% megakaryoblast CM did not alter OC formation. Also like OB proliferation data, 3% CM from BSA separated C57BL/6 MKs, immature MKs, and mature MKs all similarly inhibited OC formation by 63%, 69%, and 65% respectively (no significant differences were detected between these groups). Thus, these results show that similar to the OB data, megakaryoblasts have no effect on OC development, but immature and mature MKs have virtually the same inhibitory effect on OC development.
Distribution of MK Subpopulations in C57BL/6 and GATA-1 Fetal Livers
C57BL/6 and GATA-1 deficient fetal liver cultures stimulated with TPO appear similar in their relative distribution of megakaryoblasts and immature MK if they are collected on day 3, but on day 4 the distribution is starting to change with an increase in mature MK in C57BL/6 cultures. Our flow cytometry data indicated day 4 fetal liver C57BL/6 cultures (total cells prior to BSA gradient separation to enrich for MKs) contained 1.83% of megakaryoblasts, 3.22% of immature MKs, and 4.95% of mature MKs, while GATA-1 deficient cultures contained 3.41% of megakaryoblasts, 4.58% of immature MKs, and 2.01% of mature MKs (based on equivalent cell numbers). As would be expected, this data indicates that the C57BL/6 MKs are more differentiated than GATA-1 MKs.
MK Longevity
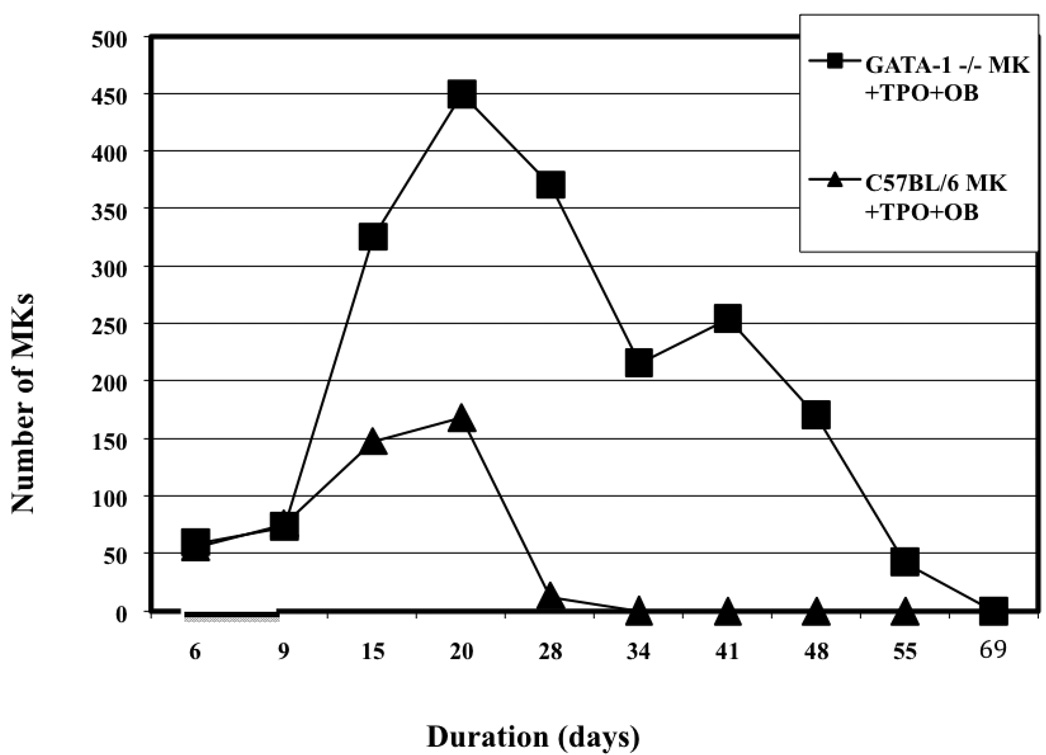
To formally quantify if GATA-1 deficient MKs outlive their C57BL/6 counterparts we cultured C57BL/6 or GATA-1 deficient MKs alone, with TPO, with OBs, or with TPO and OBs. Viable MK number was recorded until no viable MKs were detected (n=4). The data from this study is presented in Figure 5. Virtually all of the C57BL/6 and GATA-1 deficient MKs cultured alone died by day 9 in culture (data not shown). The cultures of MKs with TPO added fared only slightly better, perhaps extending their lifespan by 2+ days (data not shown). Thus without the addition of OBs, viable MKs from both populations were not detected by approximately day 9. Interestingly, when MKs were co- cultured with OBs, MK survival was increased, but by day 15 virtually no viable MKs were detected (data not shown). However, the addition of both TPO and OBs significantly prolonged the lifespan of both C57BL/6and GATA-1 deficient MKs. On day 15 when viable MKs were not detected in the other cultures, viable C57BL/6 MKs and GATA-1 deficient MKs were seen in culture containing both TPO and OBs. Both MK lineages peaked at day 20 with C57BL/6 MKs averaging 169±55 and GATA-1 deficient cultures averaging 450±100. After that, C57BL/6 MK number precipitously declined and by day 34 no viable MKs were detected. However, while the number of viable GATA-1 deficient MKs also declined, they significantly outlived their wild-type counterparts. It was not until day 69 that no viable GATA-1 deficient MKs detected. It is important to remember that in these studies we cultured cells in OB media, not in MK media, which may negatively impact MK survival. That being said, overall these data show that the combination of TPO and OBs enhances MK survival, and that GATA-1 deficient MKs survive longer than C57BL/6 MKs when co-cultured with TPO and OBs.
Figure 5.
Viable MK number over time. C57BL/6 and GATA-1 deficient MKs were cultured alone, with TPO, with OBs, or with TPO and OBs, and viable cell number recorded. The combination of OBs and TPO increased MK survival. GATA-1 deficient MKs survived for longer than did C57BL/6 MKs.
Discussion
Here we compare characteristics of GATA-1 deficient MKs to wild-type C57BL/6 MKs in an attempt to isolate the characteristics of these mutant cells that contribute to the high bone mass phenotype seen in GATA-1 deficient mice (Kacena et al., 2004). As much attention has been given to the fact that GATA-1 deficient mice have increased MK number, we first cultured increasing number of MKs, or increasing concentrations of MK CM, with OBs and OC precursors respectively, to study the effect of increased MK number or increased MK CM concentration on bone cells. We then looked at the effect of MK stage of maturation on the same populations, and finally looked at MK life span.
As Figure 1 demonstrates, 2500 MKs cause an approximate doubling of the OB population, with MKs cultured from GATA-1 deficient and wild-type MK having almost the exact same effect. When the number of MKs in co-culture was doubled (5000 MKs), OB proliferation increased further, and no significant differences were detected between the ability of C57BL/6 or GATA-1 deficient MKs to enhance OB proliferation. Similarly, as Figure 2 shows, both C57BL/6 and GATA-1 deficient MK CM were equally able to inhibit OC formation. These data, on first glance, appear to support the idea that MK number alone can elicit a response in bone cells.
The next parameter we studied was MK maturation stage. In our previous studies of MK effect on OBs and OCs, no attempt was made to separate out MKs based on stage of maturity, yet 2 of the 4 aforementioned mouse models with high bone mass phenotypes have documented defects in MK maturation. Mice deficient in NF-E2 and GATA-1 transcription factors have a developmental arrest in MK differentiation, resulting in the accumulation of immature MKs (Kacena et al., 2004). Further, immature megakaryocytes from NF-E2 deficient mice have significantly reduced numbers of granules (Shivdasani et al., 1995), while the megakaryocytes from GATA-1 deficient mice are so immature as to have few if any specific granules, inhibiting if not precluding their ability to hold and later secrete proteins like wild-type megakaryocytes (Shivdasani et al., 1997). However, in the TPO overexpressing mice, there are increased numbers of MKs at all stages of differentiation, and these mice have increased bone mass (Yan et al., 1996; Yan et al., 1995; Villeval et al., 1997). As the former mouse models imply that increases in the number of immature MKs may be a requisite for increases in bone mass; perhaps the TPO overexpressing mice have sufficient numbers of immature MKs to produce this high bone mass phenotype. MK maturation stage is also an important parameter to consider because when we culture fetal livers in vitro for MK isolation, C57BL/6 cultures resemble cultures from GATA-1 deficient fetal livers. Our FACS analysis of these cultures in vitro demonstrated that at day 3 they were virtually identical (data not shown), and at day 4 some slight differences were seen. Therefore if our in vitro C57BL/6 and GATA-1 deficient MK cultures are virtually identical in subpopulation distribution, it would not be unreasonable for these MKs to elicit a similar response. Thus like our prior assumption with GATA-1 deficient and NF-E2 deficient mice, perhaps the reason C57BL/6 MKs induce OB proliferation in vitro is because they are a more immature cell population. Here we directly address this issue by sorting MKs from C57BL/6 wild-type animals into populations based on stage of differentiation, and co-culture these subpopulations with OBs and OCs to study the effect of MK maturation on these separated cells. For these studies we examined BSA separated MKs, as well as flow cytometry separated megakaryoblasts, immature MKs, and mature MKs. Figures 3 and 4, respectively show that the degree of OB proliferation induced, or OC formation inhibited, was not significantly different if immature MKs, mature MKs, or BSA separated MKs were used. Megakaryoblasts, however, had no proliferative effect on OBs nor inhibitory effect on OC formation. It should be noted that for these maturation studies we chose to analyze only one time point for osteoblast proliferation (day 3), and used only the lower cell number (2500). Similarly, we only used the lower concentration of MK CM (3% vol:vol). This was done because recovery of sorted subpopulations of MKs was low. In general it is difficult to sort large cells such as MKs, and sorting is done more slowly. Added to this we only obtained approximately 1×106 BSA separated MKs per pregnant mouse. These BSA separated MKs are approximately 90–95% immature and mature MKs and the contaminating cells are megakaryoblasts and other fetal liver cells (Drachman et al., 1997). Thus, many mice and long sorting times are required to obtain sufficient numbers of cells in each subpopulation. To further enhance our ability to obtain sufficient numbers of cells in each subpopulation we did the following. We extended our fetal liver culture duration (stimulated with TPO) from 3–4 days to 5 days to enhance the number of mature MKs obtained. On the other hand, to enhance megakaryoblasts we cultured fetal liver cells for 3 days without TPO. This is important, as both C57BL/6 and GATA-1 deficient fetal liver cultures stimulated with TPO appear similar in their relative distribution of megakaryoblasts and immature MK if they are collected on day 3, but on day 4 the distribution is starting to change with an increase in mature MK in C57BL/6 cultures (data not shown). These technical difficulties aside, it appears that in vitro mature and immature MKs equivalently influence OBs and OCs while megakaryoblasts do not exert an influence.
We now turn to our data on MK longevity. Of note, we chose to culture MKs alone, with TPO, with OBs, and with TPO and OBs (Figure 5). We thought it vital to co-culture MKs with OBs, for just as MKs have a positive proliferative effect on OBs, OBs similarly stimulate megakaryopoiesis (Kacena et al., 2004; Miao et al., 2004). Since our focus is on the interaction of the hematopoietic and bone cell systems, we wanted to create an in vitro environment that more closely resembled the natural in vivo bone marrow environment. To our knowledge this is the first formal attempt to quantify MK lifespan in vitro when cultured with TPO and OBs. In reference to longevity the most striking data was seen when MKs were cultured in the presence of OBs and TPO. In the presence of both OBs and TPO, viable C57BL/6 MK number peaked at day 20 and declined precipitously after that, with only a few viable MKs present at day 28, and none detected at day 34. This result is broadly consistent with the work done by one group who studied MK lifespan in vitro, and found when cultured with TPO the number of mature MKs peaked at days 12– 15, with the majority of MKs beginning to show markers of apoptosis at days 18–21 (ZAULI G et al., 1997). We found similar results when MKs were co-cultured with OBs in the presence of TPO, but in the absence of OBs our wild-type MKs did not survive as long as the above group reports. This may be explained by the fact that we cultured our cells in osteogenic media as opposed to MK culture media. The GATA-1 deficient MK population co-cultured with OBs under the influence of TPO also peaks around day 20. Of note, the peak observed in both populations is due to the impurity of the population. Approximately 5–10% of the sample is megakaryoblasts and as they proliferate in response to TPO the populations of both wild-type and GATA-1 deficient MKs initially increases, and only begins to decline after these cells have presumably finished responding. However, at day 34 when no mature C57BL/6 MKs were detected, there were more than 200 viable GATA-1 deficient MKs still present. It was not until day 69 that there were no viable GATA-1 deficient MKs detected. Our results are broadly consistent with previous work done by Vyas et al. (Vyas et al., 1999) showing that GATA-1 deficient MKs outlive wild-type MKs. Of note, the GATA-1 deficient MKs cultured with TPO alone in the study by Vyas et al. (Vyas et al., 1999) lived up to 4-weeks, while our GATA-1 deficient MK population cultured with TPO alone did not survive as long, again likely owing to our use of osteogenic culture media. However, in the presence of OBs and TPO we saw a marked increase in longevity of 103% which was similar to the 100–133% increase in longevity reported by Vyas et al. (Vyas et al., 1999) with TPO alone. Thus GATA-1 deficient MKs live significantly longer than their wild-type counterparts when both are cultured with OBs in the presence of TPO. Interestingly, we show here that culturing MKs with TPO and OBs has a synergistic effect on both GATA-1 deficient and wild-type MK lifespan. For when MKs of either type are cultured with TPO alone, there are no viable MK cells by approximately day 9, and with OBs alone there are no viable MK cells by day 15. However, when C56BL/6 MKs are cultured with TPO and OB they live up to 34 days, and GATA-1 deficient MKs survive up to 69 days, highlighting the significant synergistic effect of TPO and OBs together on MK survivability.
It is now imminently clear that the hematopoietic and bone lineages are connected not just by proximity, but by functionality, and that MK induced effects on OBs and OCs are multiple and complex. While the exact mechanisms of inhibition in osteoclastogenesis and induction in osteoblastogenesis still need to be further elucidated, here we make important strides toward that goal. First we show that to an extent, increasing MK number further increases osteoblasts proliferation and inhibits OC formation. This confirms prior in vivo observation of mouse models that increased MK number corresponds to a high bone mass phenotype. However, here we probed further, wondering if in addition to increased number, there was something inherently different about mutant GATA-1 deficient MKs that favored net bone deposition more than wild-type MKs. We looked at stage of maturation and showed that immature and mature MKs have similar effects on osteoblastogenesis and osteoclastogenesis. Finally, we tried to better simulate the in vivo bone marrow environment by co-culturing MKs with OBs in the presence of TPO and demonstrated that GATA-1 deficient MKs significantly outlive C57BL/6 MKs by up 35 days. Thus in addition to increased MK number, we propose that increased viability contributes to the increased influence of GATA-1 deficient MKs on skeletal homeostasis. If viable MKs are around for a longer duration in the bone marrow cavity, there is more opportunity to stimulate OB proliferation and likewise inhibit OC development. Thus the increased viability of GATA-1 deficient MKs may partially explain the high bone mass phenotype seen in GATA-1 deficient mice.
These findings may represent targets for therapeutic intervention, with the possibility of significant clinical impact on debilitating bone diseases such as osteoporosis if we could harness the anabolic effect of MKs. As we more fully detail the complexities behind the hematopoietic-bone cell interplay, pharmacologic and even genetic therapies for important public health problems may be discovered.
Acknowledgments
We would like to thank Dr. David Pflugh for his assistance with the flow cytometry studies. This work was sponsored in part by the Department of Orthopaedic Surgery at Indiana University School of Medicine, by a Yale University School of Medicine Medical Student Research Fellowship (WAC), by NIH grant AR47342 (MCH), by a grant from the Ralph W. and Grace M. Showalter Research Trust Fund (MAK), and by NIH grant AR055269 (MAK).
References
- Beeton CA, Bord S, Ireland D, Compston JE. Osteoclast formation and bone resorption are inhibited by megakaryocytes. Bone. 2006;39:985–990. doi: 10.1016/j.bone.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Bojko P, Hester JP, Durett AG, Maadani F, Korbling M, Champlin RE. Identification of megakaryocyte precursors in peripheral blood stem cell collections from normal donors. J Clin Apher. 1998;13:7–15. doi: 10.1002/(sici)1098-1101(1998)13:1<7::aid-jca2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE. Megakaryocytes modulate osteoblast synthesis of type-l collagen, osteoprotegerin, and RANKL. Bone. 2005;36:812–819. doi: 10.1016/j.bone.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J, Clezardin P, Guichard J, Debili N, Malaval L, Vainchenker W, Cramer EM, Delmas PD. Localization of platelet osteonectin at the internal face of the alpha-granule membranes in platelets and megakaryocytes. Blood. 1992;79:936–941. [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol Cell Biol. 1991;11:4490–4496. doi: 10.1128/mcb.11.9.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagraoui H, Wendling F, Vainchenker W. Pathogenesis of myelofibrosis with myeloid metaplasia: Insight from mouse models. Best Pract Res Clin Haematol. 2006a;19:399–412. doi: 10.1016/j.beha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Chagraoui H, Wendling F, Vainchenker W. Pathogenesis of myelofibrosis with myeloid metaplasia: Insight from mouse models. Best Pract Res Clin Haematol. 2006b;19:399–412. doi: 10.1016/j.beha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, Kacena MA. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44:80–86. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- Frey BM, Rafii S, Crystal RG, Moore MA. Adenovirus long-term expression of thrombopoietin in vivo: a new model for myeloproliferative syndrome and osteomyelofibrosis. Schweiz Med Wochenschr. 1998a;128:1587–1592. [PubMed] [Google Scholar]
- Frey BM, Rafii S, Teterson M, Eaton D, Crystal RG, Moore MA. Adenovector-mediated expression of human thrombopoietin cDNA in immune-compromised mice: insights into the pathophysiology of osteomyelofibrosis. J Immunol. 1998b;160:691–699. [PubMed] [Google Scholar]
- Gao YH, Shinki T, Yuasa T, Kataoka-Enomoto H, Komori T, Suda T, Yamaguchi A. Potential role of cbfa1, an essential transcriptional factor for osteoblast differentiation, in osteoclastogenesis: regulation of mRNA expression of osteoclast differentiation factor (ODF) Biochem Biophys Res Commun. 1998;252:697–702. doi: 10.1006/bbrc.1998.9643. [DOI] [PubMed] [Google Scholar]
- Horowitz MC, Fields A, DeMeo D, Qian HY, Bothwell AL, Trepman E. Expression and regulation of Ly-6 differentiation antigens by murine osteoblasts. Endocrinology. 1994;135:1032–1043. doi: 10.1210/endo.135.3.7520861. [DOI] [PubMed] [Google Scholar]
- Horowitz MC, Xi Y, Pflugh DL, Hesslein DG, Schatz DG, Lorenzo JA, Bothwell AL. Pax5-deficient mice exhibit early onset osteopenia with increased osteoclast progenitors. J Immunol. 2004;173:6583–6591. doi: 10.4049/jimmunol.173.11.6583. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Cohn DV. Role of phosphodiesterase in the parathormone-stimulated adenosine 3',5'-monophosphate response in bone cell populations enriched in osteoclasts and osteoblasts. Endocrinology. 1981;109:743–747. doi: 10.1210/endo-109-3-743. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Gundberg CM, Nelson T, Horowitz MC. Loss of the transcription factor p45 NF-E2 results in a developmental arrest of megakaryocyte differentiation and the onset of a high bone mass phenotype. Bone. 2005;36:215–223. doi: 10.1016/j.bone.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39:991–999. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- Kelm RJ, Jr, Hair GA, Mann KG, Grant BW. Characterization of human osteoblast and megakaryocyte-derived osteonectin (SPARC) Blood. 1992;80:3112–3119. [PubMed] [Google Scholar]
- Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennert K, Nagai K, Schwarze EW. Patho-anatomical features of the bone marrow. Clin Haematol. 1975;4:331–351. [PubMed] [Google Scholar]
- McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci U S A. 1997;94:6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao D, Murant S, Scutt N, Genever P, Scutt A. Megakaryocyte-bone marrow stromal cell aggregates demonstrate increased colony formation and alkaline phosphatase expression in vitro. Tissue Eng. 2004;10:807–817. doi: 10.1089/1076327041348473. [DOI] [PubMed] [Google Scholar]
- Mitani M, Miura Y, Saura R, Kitagawa A, Fukuyama T, Hashiramoto A, Shiozawa S, Kurosaka M, Yoshiya S. Estrogen specifically stimulates expression and production of osteoprotegerin from rheumatoid synovial fibroblasts. Int J Mol Med. 2005;15:827–832. [PubMed] [Google Scholar]
- Mossuz P, Schweitzer A, Molla A, Berthier R. Expression and function of receptors for extracellular matrix molecules in the differentiation of human megakaryocytes in vitro. Br J Haematol. 1997;98:819–827. doi: 10.1046/j.1365-2141.1997.3013118.x. [DOI] [PubMed] [Google Scholar]
- Nakano K, Okada Y, Saito K, Tanaka Y. Induction of RANKL expression and osteoclast maturation by the binding of fibroblast growth factor 2 to heparan sulfate proteoglycan on rheumatoid synovial fibroblasts. Arthritis Rheum. 2004;50:2450–2458. doi: 10.1002/art.20367. [DOI] [PubMed] [Google Scholar]
- Sato N, Kiyokawa N, Takada K, Itagaki M, Saito M, Sekino T, Suzuki T, Taguchi T, Mimori K, Lanza F, Fujimoto J. Characterization of monoclonal antibodies against mouse and rat platelet glycoprotein V (CD42d) Hybridoma. 2000;19:455–461. doi: 10.1089/027245700750053940. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Thiele J, Witte O, Kaufmann R, Wickenhauser C, Fischer R. Influence of cytokines (IL-1 alpha, IL-3, IL-11, GM-CSF) on megakaryocyte-fibroblast interactions in normal human bone marrow. Eur J Haematol. 1995;55:24–32. doi: 10.1111/j.1600-0609.1995.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Wickenhauser C, Thiele J, Frimpong S, Brockbals C, Selbach B, Mueller C, Fischer R. Megakaryocyte induced fibroblast proliferation is enhanced by costimulation with IL-6/IL-3 and dependent on secretory and adhesion events. Leuk Res. 1999;23:723–729. doi: 10.1016/s0145-2126(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- Simmons DJ, Kent GN, Jilka RL, Scott DM, Fallon M, Cohn DV. Formation of bone by isolated, cultured osteoblasts in millipore diffusion chambers. Calcif Tissue Int. 1982;34:291–294. doi: 10.1007/BF02411253. [DOI] [PubMed] [Google Scholar]
- Sipe JB, Zhang J, Waits C, Skikne B, Garimella R, Anderson HC. Localization of bone morphogenetic proteins (BMPs)-2, −4, and −6 within megakaryocytes and platelets. Bone. 2004;35:1316–1322. doi: 10.1016/j.bone.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Suva LJ, Hartman E, Dilley JD, Russell S, Akel NS, Skinner RA, Hogue WR, Budde U, Varughese KI, Kanaji T, Ware J. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am J Pathol. 2008;172:430–439. doi: 10.2353/ajpath.2008.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede MA, Smock SL, Petersen DN, Grasser WA, Thompson DD, Nishimoto SK. Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology. 1994;135:929–937. doi: 10.1210/endo.135.3.8070388. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Fischer R. Histochemistry and morphometry on bone marrow biopsies in chronic myeloproliferative disorders - aids to diagnosis and classification. Ann Hematol. 1999;78:495–506. doi: 10.1007/s002770050546. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, Higashio K, Gillespie MT, Martin TJ, Suda T. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25:517–523. doi: 10.1016/s8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- Vainchenker W, Debili N, Wendling F. Mpl ligand (thrombopoietin) and the regulation of megakaryocytopoiesis. Focus Growth Factors. 1994;5:6–12. [Google Scholar]
- Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Rana RA, Lorenzini R, Migliaccio G, Migliaccio AR. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice) Blood. 2002;100:1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, Cramer EM, Vainchenker W, Wendling F. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90:4369–4383. [PubMed] [Google Scholar]
- Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93:2867–2875. [PubMed] [Google Scholar]
- Wei X, Zhang X, Zuscik MJ, Drissi MH, Schwarz EM, O'Keefe RJ. Fibroblasts express RANKL and support osteoclastogenesis in a COX-2-dependent manner after stimulation with titanium particles. J Bone Miner Res. 2005;20:1136–1148. doi: 10.1359/JBMR.050206. [DOI] [PubMed] [Google Scholar]
- Wickenhauser C, Hillienhof A, Jungheim K, Lorenzen J, Ruskowski H, Hansmann ML, Thiele J, Fischer R. Detection and quantification of transforming growth factor beta (TGF-beta) and platelet-derived growth factor (PDGF) release by normal human megakaryocytes. Leukemia. 1995;9:310–315. [PubMed] [Google Scholar]
- Wong GL, Cohn DV. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975;72:3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Fletcher F, Hartley C, McElroy P, Sun Y, Xia M, Mu S, Saris C, Hill D, Hawley RG, McNiece IK. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995;86:4025–4033. [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, McNiece IK. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–409. [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- Zauli G, Vitale M, Falcieri E, Gibellini D, Bassini A, Celeghini C, Columbaro M, Capitani S. In vitro senescence and apoptotic cell death of human megakaryocytes. 1997;90:2234–2243. [PubMed] [Google Scholar]