Abstract
Background
Fibrosing Mediastinitis (FM) is a rare complication of infection with Histoplasma capsulatum, which can lead to obstruction of pulmonary and systemic vasculature and large airways, often resulting in significant morbidity and mortality. Medical therapy is ineffective and surgical intervention is often not feasible. Stent implantation offers a potential treatment for vascular obstruction due to FM, but this has not been well studied.
Methods and Results
We conducted a retrospective review of all patients undergoing cardiac catheterization for FM. Anatomic site of stenosis and hemodynamic information before and after intervention, as well as clinical presentation and follow-up data were recorded. From 1996 to 2008, 58 patients underwent cardiac catheterization for FM, with intervention performed in 40 (69%). A total of 77 stents were used to relieve 59 lesions (pulmonary artery = 26, pulmonary vein = 21, superior vena cava = 12). Significant reduction in pressure gradients (p<0.001) and increase in vessel caliber (p<0.001) was seen at all locations. Symptomatic recurrent stenosis requiring further intervention occurred in 11 (28%) patients. Median time to recurrence was 115 months. Thirty-two (87%) of 37 patients for whom follow-up was available reported symptomatic improvement following stent placement. Procedure-related complications occurred in 14 patients (24%). Overall mortality was 19%, with the majority of deaths in patients with bilateral disease. Among patients with bilateral disease, intervention was associated with improved survival at 5 years.
Conclusions
Percutaneous vascular stent implantation is an effective therapy for central vascular obstruction due to FM, providing significant relief of anatomic obstruction and sustained clinical improvement.
Keywords: stents, stenosis, pulmonary heart disease, *fibrosing mediastinitis, *pulmonary vascular obstruction
BACKGROUND
Fibrosing Mediastinitis (FM) is a rare, debilitating, and often life-threatening complication of infection with the fungus Histoplasma capsulatum, for which there is no known effective medical or surgical therapy. In susceptible individuals, uncontrolled fibrosis around previously infected mediastinal lymph nodes leads to invasion and obstruction of surrounding structures including central pulmonary vasculature, intrathoracic systemic veins, and mainstem bronchi 1–5. Patients present years to decades after primary infection with symptoms associated with the sites of mediastinal involvement: most commonly cough, dyspnea, hemoptysis, chest pain, and recurrent pulmonary infections 3. Although vascular or airway involvement leading to loss of function of a single lung can usually be tolerated, bilateral disease carries a much poorer prognosis and is often fatal 3, 6.
Medical and surgical therapies for FM remain largely ineffective. Antifungal and anti-inflammatory agents have been used with varying results, but there are no studies demonstrating long-term benefit 2, 3, 7–11. Furthermore, the extensive fibrotic invasion caused by FM leads to the destruction of normal tissue planes, which complicates and often limits surgical intervention 3, 7, 11, 12.
The use of percutaneously placed vascular stents has been well described as an effective therapy for relief of central pulmonary or systemic vascular obstruction in other diseases 13–15. Reports on the safety and efficacy of central vascular stent implantation in cases of FM, however, have been limited to individual or small case series 16–23, and there have been no studies of long-term outcomes.
In 2001, we reported our early experience with stent relief of pulmonary vascular obstruction in 4 patients with FM, all of whom demonstrated immediate hemodynamic and clinical improvement based on short-term follow-up 17. This initial success encouraged our continued use of stent implantation in selected patients with FM. Here we review our interim experience with more than 50 patients – to our knowledge, the largest series of its kind – which suggests that stent placement can provide not only acute relief of anatomic obstruction, but also sustained clinical improvement in this cohort of patients for whom few treatment options exist.
METHODS
Data Collection
Approval was obtained from the Vanderbilt University Institutional Review Board. A retrospective review of medical records and cardiac catheterization data was conducted to identify all patients undergoing cardiac catheterization for FM. Clinical presentation and diagnostic evaluation prompting referral for catheterization, as well as anatomic site of stenosis, type of intervention, and hemodynamic information before and after intervention were recorded. Follow-up was through review of records and/or phone interview where available. Clinical improvement following stent placement was assessed by subjective patient report as documented in the medical record or obtained through phone interview.
Catheterization Procedure
Patients presented for cardiac catheterization based on clinical, radiographic, and echocardiographic findings consistent with central vascular obstruction due to FM. The definitive diagnostic test for FM was CT imaging, and patients were referred for intervention when bilateral disease was evident or suspected, clinical condition appeared worse than expected for unilateral disease, or there was symptomatic superior vena cava obstruction. Informed consent was obtained for all procedures. Venous access was obtained from the femoral, jugular, or subclavian veins as needed, and atrial transseptal puncture was performed if there was evidence or suspicion of pulmonary vein involvement. A hemodynamic right and left heart catheterization was performed, followed by site specific angiography to assess stenosis. Transesophageal echocardiogram (TEE) was performed routinely during the catheterization to aid in the assessment of pulmonary vein involvement.
The decision to proceed with stent placement was based on assessment of the patient’s clinical condition, extent of vascular obstruction, and anticipated benefit from obstruction relief. Stent implantation was deferred in patients for whom re-establishment of blood flow to a nonfunctional segment of lung was not expected to provide clinical benefit, such as in cases of long-standing complete occlusion to a lung segment or significant ipsilateral airway obstruction. In patients with both arterial and venous obstruction to the same lung segments, pulmonary venous obstruction was addressed first in order to prevent the development of acute pulmonary edema, which can occur if pulmonary arterial relief is effective but pulmonary venous relief is ineffective. The stent implantation technique has been previously described 17. Pre-dilation of the stenosis was not routinely performed, as vascular tissue in cases of FM is friable and susceptible to intimal damage or rupture. Stent lengths were chosen to cover the length of the obstruction and balloon diameters for stent deployment were chosen to achieve a diameter equal to adjacent, noninvolved vessels. All stents were bare metal, balloon-expandable stents that were either pre-mounted or hand crimped onto the delivery balloons; stent types included the Palmaz and Genesis series (Johnson and Johnson Interventional Systems, Warren, NJ) and the DoubleStrut LD and Mega LD open-cell design series (IntraTherapeutics, St. Paul, MN). Overlapping stents were used when indicated in cases of long-segment stenosis or occlusion. High pressure (20 atmospheres) dilation was often needed to achieve maximal improvement.
Following stent placement, hemodynamic measurements and angiography were repeated to assess the extent to which pressure gradients and anatomic obstruction had been relieved. Additional stents were placed as indicated to achieve optimal results.
All patients received antibiotic prophylaxis with Ancef (one dose prior to and 2 doses after stent placement). All patients were placed on anticoagulation with aspirin or Coumadin (in the case of pulmonary vein stent placement) for a minimum of 6 months following stent implantation.
Statistical Analysis
Data are presented as mean ± standard deviation unless otherwise noted. Standard graphing and screening techniques were used to detect outliers and ensure data accuracy. Interventions were grouped for comparison based on anatomic site: pulmonary arteries (PA), pulmonary veins (PV), and SVC. Pressure gradients and vessel diameters before and after stent placement were compared at each anatomic site using the Wilcoxon signed-rank test (for SVC interventions) or a mixed-effects model (for pulmonary artery and vein interventions, because independence could not be assumed when more than one intervention was performed per patient). Kaplan-Meier curves were constructed to graphically illustrate freedom from reintervention and survival data. Because patients with bilateral disease have a distinctly different clinical course and prognosis, the effect of intervention on survival was evaluated separately in patients with bilateral disease and those with unilateral disease. The log-rank test and Cox proportional-hazards model were used for survival analysis. Statistical analysis was performed using SPSS software v.19.0 (SPSS Inc., Chicago, IL), GraphPad Prism software v.5.0 (GraphPad software Inc., San Diego, CA), and R software 2.10.1 (R-project.org).
RESULTS
Patient characteristics
From 1996 to 2008, 58 patients underwent cardiac catheterization for FM. Patients were referred for catheterization based on clinical symptomatology in addition to objective evidence of significant pulmonary vascular or SVC involvement as documented by CT angiography (n = 58, 100%), nuclear medicine perfusion scan (n = 43, 74%), and/or echocardiography (n = 41, 71%). Biopsy of the mass or adjacent mediastinal lymph nodes was performed in 38 patients (66%) to rule-out malignancy.
Patient demographics and clinical characteristics are summarized in Table 1. Patients with significant pulmonary vascular disease were more likely to present with exertional intolerance (p<0.001) and hemoptysis (p=0.054), while patients with SVC involvement presented more often with symptoms typical of SVC syndrome (p<0.001). Thirty-one patients (53%) had bilateral disease on presentation, and an additional 12 patients (21%) had isolated right-sided disease with symptomatic SVC involvement. Twenty-six patients (45%) had documented evidence of pulmonary hypertension based on pre-procedure evaluation including echocardiography or, in some cases, catheterization data from the referring institution.
Table 1.
Patient demographics and clinical characteristics
| Total (n = 58) | |
|---|---|
| Age (years) – Mean ± SD | 36.7 ± 10.5 |
| Gender – n (%) | |
| Male | 20 (34) |
| Female | 38 (66) |
| Race – n (%) | |
| Caucasian | 43 (74) |
| African-American | 13 (22) |
| Other | 2 (4) |
| Weight (kg) – Mean ± SD | 84.8 24.2 |
| Vessel Involvement – n (%) | |
| RPA | 43 (74) |
| LPA | 21 (36) |
| RPV | 23 (40) |
| LPV | 20 (35) |
| SVC | 20 (35) |
| Presenting symptoms – n (%) | |
| Exercise intolerance | 46 (79) |
| Chest pain | 28 (48) |
| Hemoptysis | 14 (24) |
| SVC syndrome | 14 (24) |
| Bilateral disease – n (%) | 31 (53) |
| Pulmonary hypertension – n (%) | 26 (45) |
| Received Intervention – n (%) | 40 (69) |
n = Number of patients, SD = standard deviation, kg = kilograms, RPA = right pulmonary artery, LPA = left pulmonary artery, RPV = right pulmonary vein, LPV = left pulmonary vein, SVC = superior vena cava
Interventions
Intervention was performed in 40 patients (69%). Of those receiving intervention, 33 (83%) had either bilateral disease and/or symptomatic SVC stenosis.
A total of 77 stents were placed as initial intervention in 59 vessels: 24 stents in 17 right pulmonary arteries (RPA); 11 stents in 9 left pulmonary arteries (LPA); 9 stents in 8 right pulmonary veins (RPV); 15 stents in 13 left pulmonary veins (LPV); and 18 stents in 12 SVC. Balloon angioplasty was performed as the only intervention in one patient with isolated SVC involvement (Table 2).
Table 2.
Anatomic site of stent placement and recurrence
| n Receiving Stents |
Number of Original Stents |
Number of Vessels Stented |
n with Recurrence |
n Receiving Reintervention |
n with Multiple Reintervention |
|
|---|---|---|---|---|---|---|
| RPA | 14 | 24 | 17 | 2 | 0 | 0 |
| LPA | 8 | 11 | 9 | 1 | 1 | 0 |
| RPV | 7 | 9 | 8 | 3 | 1 | 0 |
| LPV | 12 | 15 | 13 | 4 | 3 | 0 |
| SVC | 12 | 18 | 12 | 7 | 7 | 6 |
n = Number of patients, RPA = right pulmonary artery, LPA = left pulmonary artery, RPV = right pulmonary vein, LPV = left pulmonary vein, SVC = superior vena cava
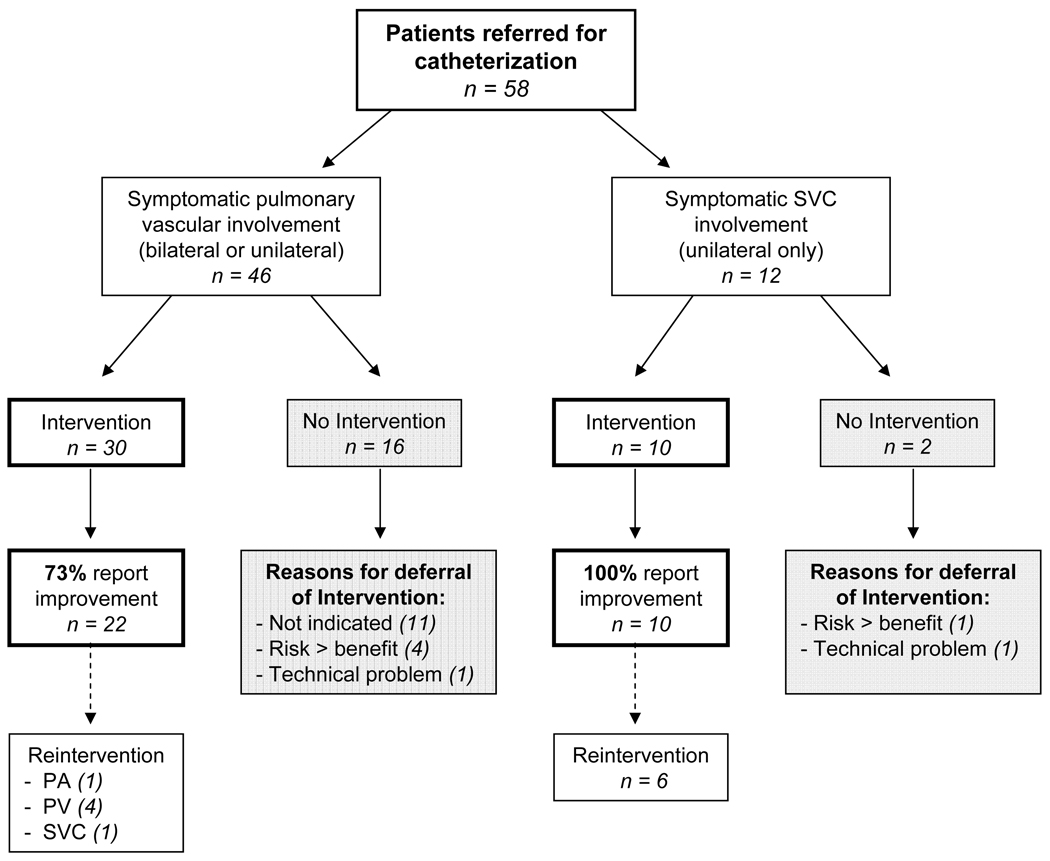
Eighteen patients (31%) did not receive stent implantation. Intervention was not indicated in 11 patients (19%) based on hemodynamic and angiographic data obtained at catheterization. The majority of these patients had unilateral pulmonary disease with an otherwise normal contralateral lung. Intervention was deferred in 5 patients (9%) with either long-standing vessel occlusion and/or extensive bilateral pulmonary vascular disease in whom it was felt that the anticipated benefit from revascularization would not outweigh the significant risk of stent placement. Of the remaining 2 patients, intervention was not performed due to a procedure-related complication in 1, and due to a malfunction of catheterization lab equipment in the other (Figure 1).
Figure 1. Flow diagram illustrating interventions, reasons for deferral of intervention, and outcomes based on disease location.
SVC = superior vena cava, f/u = Follow-up
Pressure Gradients and Vessel Diameters
Pulmonary Arteries
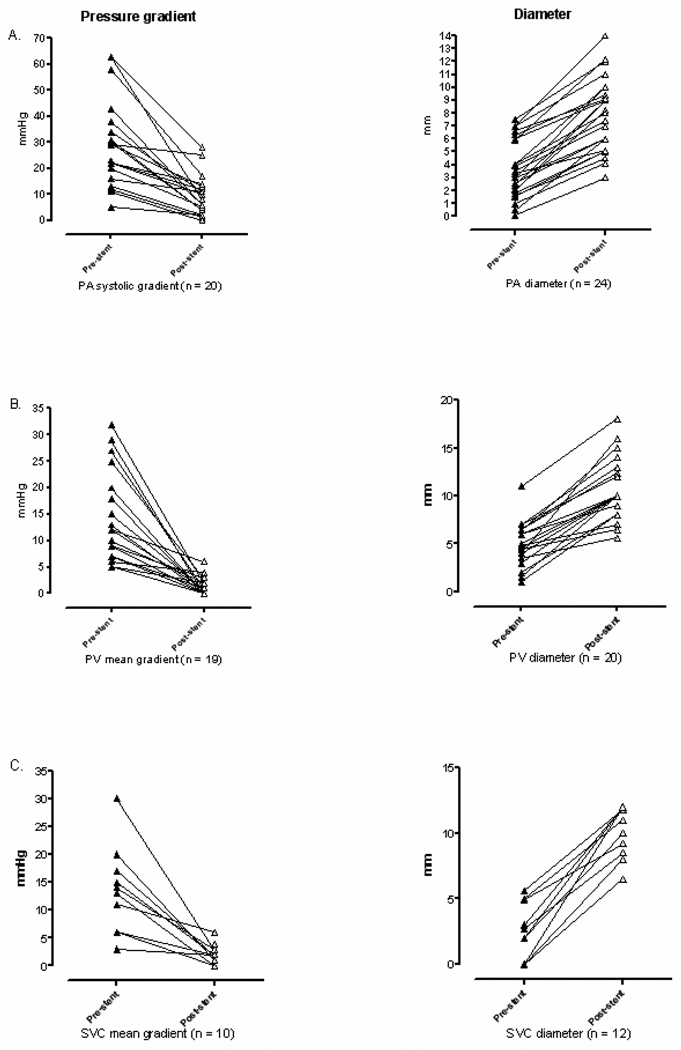
The majority of pulmonary artery stents (85%) were placed in distal lobar or subsegmental branches. Taken together, pulmonary arterial systolic pressure gradients were significantly reduced after stent placement (p<0.001). PA caliber was significantly increased after stent placement (p<0.001). Final measured stent diameter averaged 90% ± 13% (range 60 – 113%) of goal vessel diameter based on adjacent vessel and/or chosen balloon diameter (Figure 2A, Table 3).
Figure 2. Peak systolic or mean pressure gradient (left) and narrowest vessel diameter (right) measured at individual sites of pulmonary artery (PA) stenosis (A), pulmonary venous (PV) stenosis (B), and superior vena cava (SVC) stenosis (C), before and after stent placement.
n = Number of vessels for which hemodynamic or angiographic data were available, mmHg = millimeters of mercury, mm = millimeters
Table 3.
Pressure gradients and vessel diameters before and after stent placement
| Pre-stent (Mean ± SD) |
Post-stent (Mean ± SD) |
p-value | 95% CI (for the change) |
|
|---|---|---|---|---|
| Pulmonary artery | ||||
| Systolic pressure gradient (mmHg) | 28.1 ± 17.2 | 8.6 ± 7.7 | <0.001 | −12.4 to −26.1 |
| Vessel diameter (mm) | 3.6 ± 2.2 | 7.9 ± 2.8 | <0.001 | 3.6 to 5.0 |
| Percent of goal vessel diameter (%) | 90 ±13 | |||
| Pulmonary vein | ||||
| Mean pressure gradient (mmHg) | 14.5 ± 8.5 | 1.3 ± 1.7 | <0.001 | −8.6 to −17.1 |
| Vessel diameter (mm) | 5.1 ± 2.2 | 11.0 ± 3.6 | <0.001 | 4.7 to 7.0 |
| Percent of goal vessel diameter (%) | 94 ± 9 | |||
| Superior vena cava | ||||
| Mean pressure gradient (mmHg) | 13.5 ± 7.5 | 2.1 ± 1.8 | <0.001 | −6.5 to −16.5 |
| Vessel diameter (mm) | 2.3 ± 2.1 | 10.3 ± 2.2 | <0.001 | 6.6 to 9.6 |
| Percent of goal vessel diameter (%) | 89 ± 14 |
SD = standard deviation; CI = confidence interval; mmHg = millimeters of mercury; mm = millimeters; percent of goal vessel diameter = final measured diameter/goal vessel diameter × 100
Pulmonary Veins
All pulmonary vein stents were placed in central pulmonary veins or a confluence of veins entering the left atrium. Overall, pulmonary venous mean pressure gradients were significantly reduced after stent placement (p<0.001). PV caliber was significantly increased after stent placement (p<0.001). Because there was often significant pre-stenotic PV engorgement noted in cases receiving PV stents, final measured stent diameter was compared to chosen balloon diameter, averaging 94% ± 9% (range 70 – 100%) of goal vessel diameter (Figure 2B, Table 3).
Superior Vena Cavae
SVC pressure gradients were significantly reduced after stent placement (p<0.001). SVC caliber was significantly increased after stent placement (p<0.001). Final measured stent diameter averaged 89% ± 14% (range 65–100%) of goal vessel diameter based on adjacent vessel and/or chosen balloon diameter (Figure 2C, Table 3).
Recurrence
Symptomatic recurrent stenosis requiring further intervention (either additional stent placement or balloon angioplasty of previously placed stents) occurred in 11 (28%) of 40 patients who received intervention. This included 1 (5%) of 19 patients receiving PA intervention, 4 (25%) of 16 patients receiving PV intervention, and 7 (60%) of 13 patients receiving SVC intervention. Recurrent stenosis without associated symptoms was noted by routine follow-up CT angiography in an additional 2 patients receiving PA intervention and 3 patients receiving PV intervention. Recurrent obstruction in these cases was primarily related to instent tissue growth, with or without progression of tissue growth distal to the stent. Recurrent stenosis in the SVC was problematic, with 6 patients requiring more than 1 revision (Table 2). There were no clinical features – including final stent diameter, percent of goal diameter, nor complexity of stent placement (multiple or bifurcating stents) – that were associated with recurrent stenosis (using logistic regression analysis, data not shown).
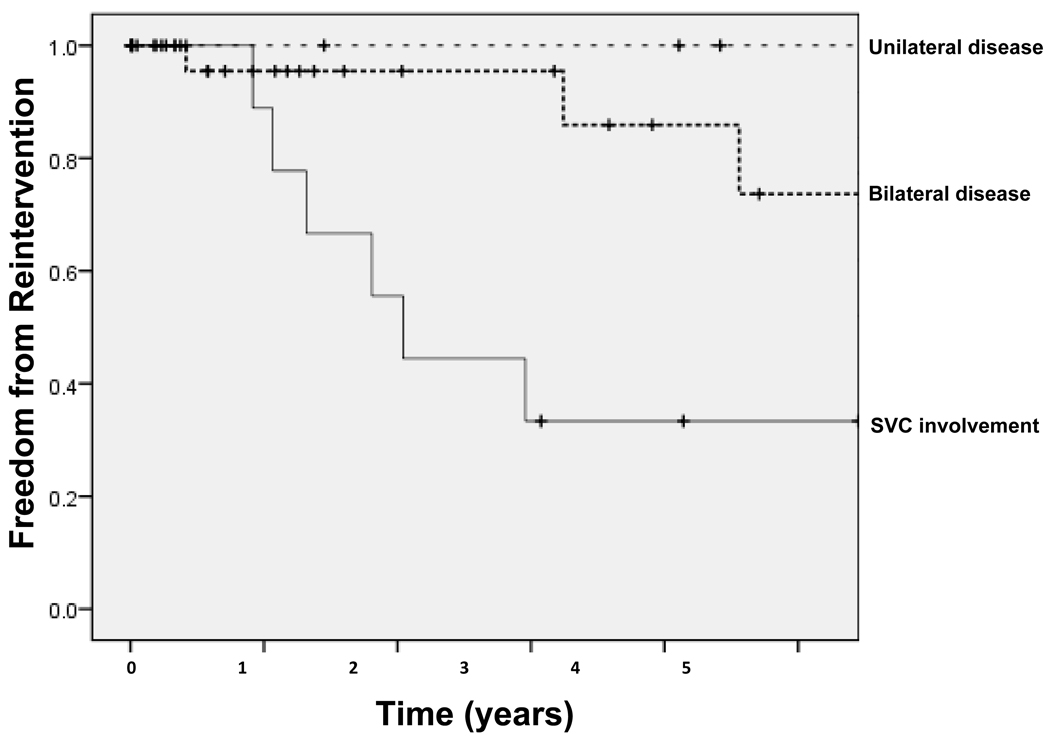
Time to symptomatic presentation of recurrent stenosis ranged from 5 months to 122 months (10.1 years), with an overall median time to recurrence of 115 months. Patients with symptomatic SVC involvement required more frequent stent revisions than patients with bilateral or unilateral pulmonary vascular disease; median time to SVC recurrence was 24.5 months (Figure 3).
Figure 3. Kaplan-Meier curves demonstrating 5-year freedom from reintervention based on site and extent of vascular involvement.
Thin dotted line indicates those with unilateral pulmonary vascular disease, thick dotted line indicates those with bilateral pulmonary vascular disease, and thin solid line indicates those with unilateral disease and SVC involvement. Hash marks (†) indicate censored subjects.
Clinical Improvement
Among the group who received intervention, 37 patients (93%) had documented follow-up at least 1 month following their procedure; 3 were lost to follow-up. Of the 37 patients for whom follow-up was available, 32 patients (87%) reported noticeable clinical improvement immediately following stent placement. Duration of symptomatic clinical improvement in these patients ranged from 2 months to 144 months (12 years).
Complications
Complications related to the catheterization procedure or stent placement occurred in 15 (26%) of 58 patients. Minor complications requiring no additional intervention—including bleeding from the catheterization site, mild hemoptysis, and minor vessel injury—occurred in 8 patients. Major complications requiring immediate intervention occurred in 6 (10%) of 58 patients. These included significant vessel injury in 2 patients (including RPA dissection requiring mechanical extracorporeal circulatory support in one patient), reperfusion pulmonary edema in 2 patients, and thrombosis requiring anticoagulation in 2 patients; stent malposition followed by successful retrieval or stabilization also occurred in 2 of these patients. No cases of stent fracture were seen in this cohort. One patient with severe bilateral disease (New York Heart Association [NYHA] class IV) died 19 days following catheterization with pulmonary vein stent placement after suffering a cerebrovascular accident while still in the intensive care unit following the procedure.
Mortality
Of the 40 patients receiving intervention, there were 6 deaths (15%) reported during follow-up. There was one procedure-related death as described above. Five patients died late, ranging from 11 months to 9.5 years following their initial procedure. Four (75%) of 6 patients who died had bilateral disease at presentation; death in 2 patients with isolated SVC involvement was unrelated to their disease. Among the group not receiving intervention, 5 (29%) died during follow-up. Of these, 4 (80%) had bilateral disease.
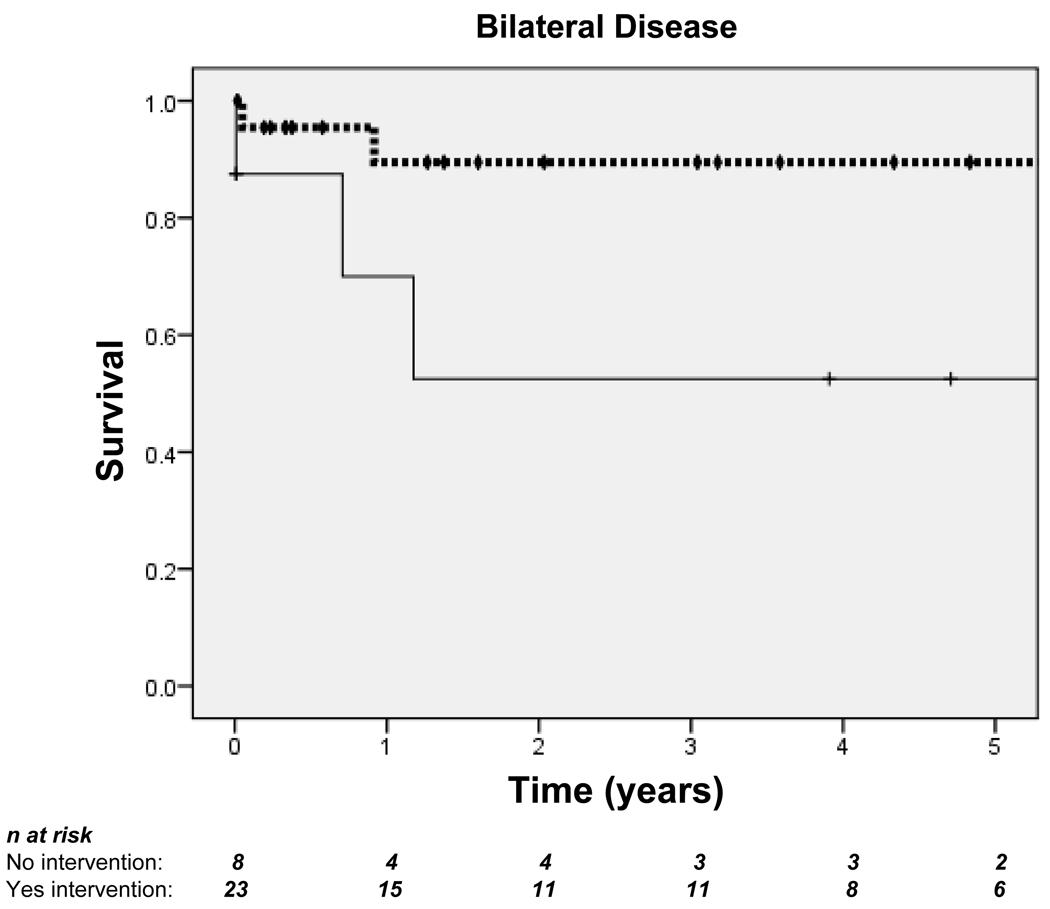
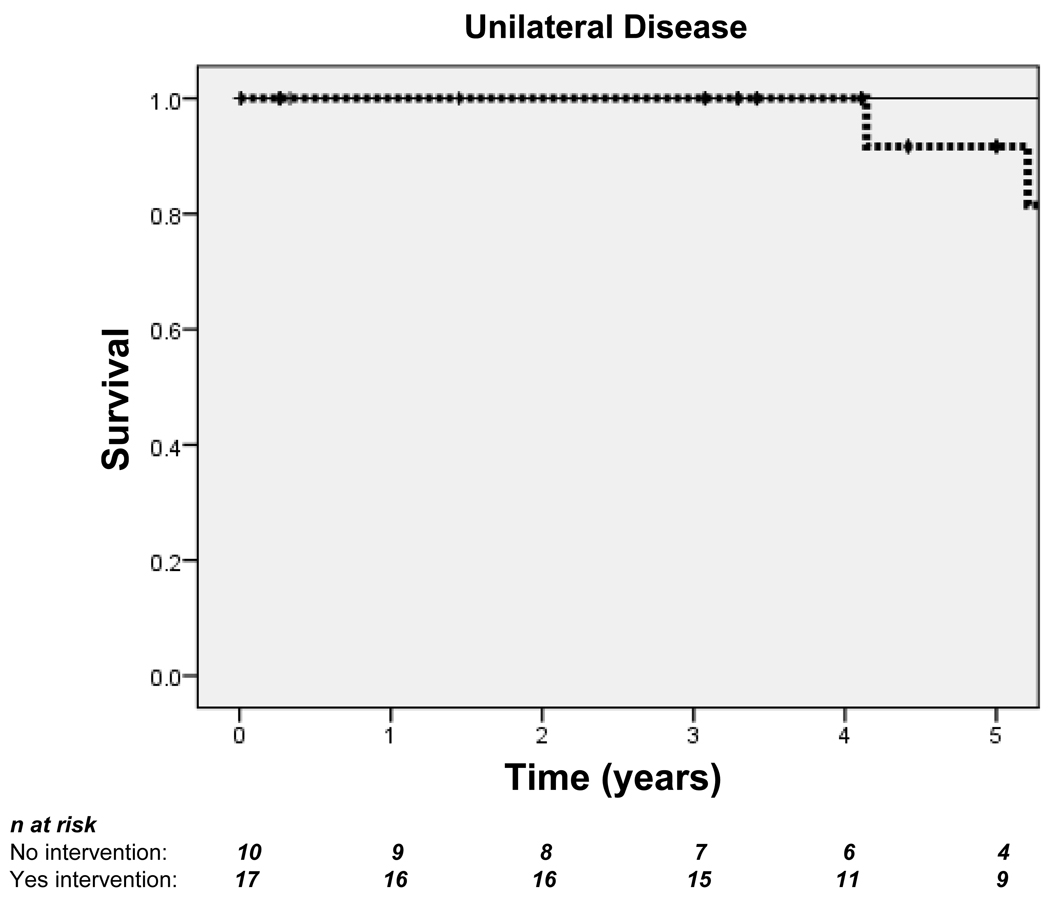
Among patients with bilateral disease, those who received intervention had improved survival at 5 years (89.5% vs. 52.5%) (Figure 4A). There were fewer deaths in patients with unilateral disease, and intervention was not associated with improved survival for this group at any point during follow-up (Figure 4B).
Figure 4. Kaplan-Meier curves demonstrating 5-year survival for patients with bilateral disease (A) and unilateral disease (B).
Dotted lines indicate those who received intervention; solid lines indicate those who did not. Hash marks (†) indicate censored subjects. n = Number of patients. In patients with bilateral disease: log-rank p=0.282; overall Hazard ratio (HR)=0.44, 95% CI 0.09–2.04. In patients with unilateral disease: log-rank p=0.253; overall HR=1.06, 95% CI 0.09–11.72.
DISCUSSION
This report represents the largest published experience with stent relief of vascular obstruction secondary to FM. We are encouraged by our results which show that stent placement can provide not only significant relief of anatomic obstruction as measured by reduction in pressure gradients and improvement in vessel caliber at areas of stenosis, but also sustained symptomatic relief in the majority of patients. We found recurrent stenosis to be problematic, particularly with SVC involvement, but this has been responsive to repeated intervention.
Despite widespread use, there is limited data on long-term outcomes for stent implantation as treatment for large vessel obstruction. In adults, the largest body of literature focuses on stent placement as treatment for benign or malignant SVC stenosis, with reported recurrence rates of 16–40%. In these series, most cases are amenable to stent redilation or additional stent implantation 24–27. Literature regarding pulmonary arterial or pulmonary venous stent implantation is limited primarily to children with congenital heart disease (CHD) and adults with pulmonary vein stenosis after catheter ablation procedures. Direct comparisons with these populations are difficult, as indications for stent implantation and reintervention are different. Most reports suggest low rates of neointimal proliferation following pulmonary artery stent placement 28, 29. Restenosis after pulmonary venous stent placement in pediatric and adult patients, however, appears to be more common 30, 31.
Indeed, while percutaneous stent implantation is now preferred as a non-invasive therapy to relieve vascular obstruction in many diseases, our findings are particularly important in cases of FM where stent placement may offer not only an alternative, but rather the only therapeutic option. Despite advances in our understanding of the disease process and improvement in surgical techniques, medical therapies have not yet been shown to alter the course of this disease and surgical intervention remains technically difficult due to the destruction of normal tissue planes. One center recently reported a series of 5 patients with pulmonary artery occlusion due to FM who were successfully treated using artificial conduit material to reconstruct or bypass the obstructed pulmonary artery 32; however, the fact that this type of intervention was performed in only 5 eligible candidates over 28 years at one of the country’s largest referral centers certainly highlights the complexity and limited application of a surgical approach for treatment of FM.
The decision to proceed with catheterization and stent implantation in patients with FM should be made only after a thorough pre-procedure evaluation. Specifically, the presence of bilateral vascular involvement should strongly influence referral for intervention, as patients in this cohort are likely to receive the most clinical benefit from such revascularization procedures. Non-invasive evaluation for bilateral disease should include echocardiography (to detect evidence of pulmonary hypertension) as well as nuclear medicine perfusion scans which can not only reveal bilateral perfusion defects, but can also localize lesions to specific lung segments and their supplying vessels. CT angiography and combined ventilation/perfusion studies are also helpful in assessing both vascular and bronchial involvement, which is particularly important in cases of FM involving the tracheobronchial tree in which the reestablishment of blood flow to an unventilated portion of lung would potentially worsen V/Q mismatch. In our series, CT angiography was the definitive diagnostic test for all patients, with additional testing performed as indicated or as provided by the referring institution.
When referral for catheterization and stent placement is being considered, particularly in cases of presumed pulmonary venous involvement, interventional specialists who have experience specific to the unique aspects of pulmonary vein assessment and intervention should be sought. Furthermore, proceduralists must be aware that well known complications of intravascular stent placement (such as vessel injury and stent malposition), while considered rare in the general population, may occur more frequently in patients with FM. We suspect this is due to the unique vascular pathology and clinical instability inherent to the underlying disease. Surgical and pathology reports of FM specimens commonly describe an unusually dense, often calcified mass – the texture of which has been compared to that of concrete – which causes severe distortion of normal surrounding tissue architecture 3, 12. In our experience, the exceedingly firm nature of the compressing mass often makes stent positioning and deployment more challenging than typical vascular stent placement due to the tendency of the stent to migrate toward uninvolved areas as the balloon is expanded if it has not been well-centered. In addition, the high pressure needed to expand the stent within the fibrotic mass can lead to over-flaring of the terminal portions of the stent which typically extend into adjacent normal vessel. Such exposure of nearby structures to the over-expanded distal tines increases the risk for vessel injury, and may account for one report of late aortic laceration after SVC stent placement in a patient with SVC obstruction due to FM 21. Furthermore, we suspect the potential for disruption of vascular integrity by the invading mass increases the risk for vessel injury even during non-interventional portions of the catheterization procedure.
In our series, the decision to proceed with stent placement was based on the patient’s clinical status in conjunction with hemodynamic and angiographic assessment at the time of catheterization. Anatomic features of the lesions, such as vessel occlusion or length of stenosis, did not necessarily dictate the decision to proceed with stent placement, as we were able to consistently and safely recannulate even completely occluded vessels in the appropriate clinical situation, and we found that all lesions improved to some degree with stent implantation. We also found, however, that although long standing pulmonary arterial occlusions could in some circumstances be crossed and stented, distal vessel disease appeared to prevent long term patency. For this reason, we now defer intervention in cases where the occlusion is known to be long standing.
Study Limitations
This study is limited by its retrospective nature as well as the inconsistency of objective pre- and post-procedure clinical data available for these patients. Specifically, we found it difficult to retrospectively assign objective clinical assessment scores (such as NYHA classifications) before and after stent placement, particularly in patients with isolated SVC involvement whose symptoms do not necessarily correspond to such a scoring system. Instead, we report only subjective clinical improvement as based on documentation in the medical record or phone interview.
Follow-up is further complicated by the fact that, as a referral center for the diagnosis and management of FM, many of the patients in this cohort are referred from distant institutions. Often, patients return to their primary physicians for routine care following the procedure and do not present back to our institution unless they have clinical indications for further intervention. We recognize that inconsistent follow-up may result in underestimation of recurrence rate. Because symptoms of SVC syndrome are more easily recognized by patients and clinicians, there is likely to be increased reporting and evaluation of SVC recurrence, whereas unilateral pulmonary artery or venous restenosis may go unnoticed and not prompt further investigation. In the past, we have relied on patient report of clinical symptoms and non-invasive assessment during follow-up, but one could make the argument that routine repeat invasive evaluation, in combination with more standardized clinical assessment protocols such as regular pre- and post-procedure exercise testing as well as scheduled follow-up CT angiography or lung perfusion testing, may be warranted.
Finally, because FM remains a rare disease, even this relatively large sample size likely provided insufficient power to detect clinically significant effects of intervention, particularly when comparing clinically relevant sub-groups. Specifically, results of our Cox proportional-hazards model suggest that intervention was associated with a 56% reduction in the risk of death in patients with bilateral disease; however, because of small sample size, the result is not statistically significant (Figure 4A.). Statistical analysis was further confounded by a referral bias which favored intervention for patients with either symptomatic SVC syndrome or bilateral pulmonary vascular involvement, as well as a follow-up bias which favored re-evaluation (and re-intervention) for patients with symptomatic recurrence, ultimately limiting our ability to explore the effect of any predictor variables on intervention, recurrence, or outcome. Yet despite these statistical limitations, we believe the clinical significance of this report is compelling.
Conclusions
We conclude that percutaneous vascular stenting is technically feasible and can provide significant hemodynamic improvement in patients with central vascular obstruction due to Fibrosing Mediastinitis. Because our clinical experience has been that patients with unilateral disease generally have favorable long-term outcomes, we suspect that revascularization of one auto-amputated lung does not provide significant clinical benefit in patients with one remaining normal lung, and therefore, we do not routinely encourage intervention in this group (with the exception of patients with symptomatic SVC syndrome who consistently report significant relief of symptoms following stent placement). In patients with bilateral pulmonary vascular involvement, however, our study suggests that the hemodynamic improvement achieved with stent implantation translates to improved clinical outcomes in this cohort of patients for whom limited treatment options exist.
Clinical Summary.
Fibrosing mediastinitis (FM) is a rare but devastating late complication of infection with Histoplasma that manifests as a proliferation of fibrous tissue within the mediastinum, obstructing central vessels and/or airways. In approximately 20% of affected individuals, bilateral pulmonary arteries, pulmonary veins, and/or airways are affected by the dense fibrous material, which portends an especially grave prognosis. Management of FM is particularly challenging to the clinician: to date no effective pharmacologic or surgical therapy has been shown to provide long-term benefit, and while percutaneous interventions using stents to relieve vascular obstruction in FM have been reported in single case reports and small case series, few centers have robust experience with vascular stenting for this disease. This review is the largest published series of percutaneous vascular stenting to ameliorate central vascular obstruction in FM. Our results show that vascular stenting is technically feasible and provides sustained symptomatic relief in the majority of patients. Minor complications including hemoptysis and catheterization site bleeding were the most common adverse events, and major complications and/or death following percutaneous intervention were rare. The decision to perform vascular stenting for FM is complex and should be carefully considered following a comprehensive radiographic and hemodynamic evaluation and expert consultation to properly select candidates with greatest chance of sustained success. In our experience, percutaneous vascular stenting in selected patients with significant bilateral vascular involvement or superior vena cava syndrome from FM is a good option for relief of clinical symptoms, but should be performed at a center experienced in large vessel stent implantation.
ACKNOWLEDGMENTS
The authors would like to thank Daniel Byrne (Vanderbilt University, Department of Biostatistics) for his advice on statistics and for his editorial assistance.
FUNDING SOURCES
This work was supported in part by funding through the Vanderbilt Institute for Clinical and Translational Research (1 UL1 RR024975 from NCRR/NIH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper is submitted with the full knowledge and authorization of all listed authors.
DISCLOSURES
None of the authors have conflicts of interest to report.
REFERENCES
- 1.Goodwin RA, Loyd JE, Des Prez RM. Histoplasmosis in normal hosts. Medicine (Baltimore) 1981;60:231–266. doi: 10.1097/00005792-198107000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Davis AM, Pierson RN, Loyd JE. Mediastinal fibrosis. Semin Respir Infect. 2001;16:119–130. doi: 10.1053/srin.2001.24242. [DOI] [PubMed] [Google Scholar]
- 3.Loyd JE, Tillman BF, Atkinson JB, Des Prez RM. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 1988;67:295–310. doi: 10.1097/00005792-198809000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin RA, Nickell JA, Des Prez RM. Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis. Medicine (Baltimore) 1972;51:227–246. doi: 10.1097/00005792-197205000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Berry DF, Buccigrossi D, Peabody J, Peterson KL, Moser KM. Pulmonary vascular occlusion and fibrosing mediastinitis. Chest. 1986;89:296–301. doi: 10.1378/chest.89.2.296. [DOI] [PubMed] [Google Scholar]
- 6.Martin J, Prudhomme J, Scott T, Loyd J. Features associated with mortality in fibrosing mediastinits [abstract A57] Amer J Resp & Critical Care Med. 2005;2:A204. [Google Scholar]
- 7.Mathisen DJ, Grillo HC. Clinical manifestation of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg. 1992;54:1053–1057. doi: 10.1016/0003-4975(92)90069-g. discussion 1057–1058. [DOI] [PubMed] [Google Scholar]
- 8.Maholtz MS, Dauber JH, Yousem SA. Case report: fluconazole therapy in histoplasma mediastinal granuloma. Am J Med Sci. 1994;307:274–277. doi: 10.1097/00000441-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mocherla S, Wheat LJ. Treatment of histoplasmosis. Semin Respir Infect. 2001;16:141–148. doi: 10.1053/srin.2001.24244. [DOI] [PubMed] [Google Scholar]
- 10.Wheat LJ, Conces D, Allen SD, Blue-Hnidy D, Loyd J. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med. 2004;25:129–144. doi: 10.1055/s-2004-824898. [DOI] [PubMed] [Google Scholar]
- 11.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 12.Garrett HE, Jr, Roper CL. Surgical intervention in histoplasmosis. Ann Thorac Surg. 1986;42:711–722. doi: 10.1016/s0003-4975(10)64619-x. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer KM, Mullins CE, Grifka RG, O'Laughlin MP, McMahon W, Ing FF, Nihill MR. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol. 1998;31:661–667. doi: 10.1016/s0735-1097(97)00535-4. [DOI] [PubMed] [Google Scholar]
- 14.O'Laughlin MP, Slack MC, Grifka RG, Perry SB, Lock JE, Mullins CE. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation. 1993;88:605–614. doi: 10.1161/01.cir.88.2.605. [DOI] [PubMed] [Google Scholar]
- 15.O'Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation. 1991;83:1923–1939. doi: 10.1161/01.cir.83.6.1923. [DOI] [PubMed] [Google Scholar]
- 16.Dodds GA, 3rd, Harrison JK, O'Laughlin MP, Wilson JS, Kisslo KB, Bashore TM. Relief of superior vena cava syndrome due to fibrosing mediastinitis using the Palmaz stent. Chest. 1994;106:315–318. doi: 10.1378/chest.106.1.315. [DOI] [PubMed] [Google Scholar]
- 17.Doyle TP, Loyd JE, Robbins IM. Percutaneous pulmonary artery and vein stenting: a novel treatment for mediastinal fibrosis. Am J Respir Crit Care Med. 2001;164:657–660. doi: 10.1164/ajrccm.164.4.2012132. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine AB, Borsa JJ, Hoffer EK, Bloch RD, So C. Stent placement in the treatment of pulmonary artery stenosis secondary to fibrosing mediastinitis. J Vasc Interv Radiol. 2001;12:1107–1111. doi: 10.1016/s1051-0443(07)61600-5. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero A, Hoffer EK, Hudson L, Schuler P, Karmy-Jones R. Treatment of pulmonary artery compression due to fibrous mediastinitis with endovascular stent placement. Chest. 2001;119:966–968. doi: 10.1378/chest.119.3.966. [DOI] [PubMed] [Google Scholar]
- 20.Kandzari DE, Warner JJ, O'Laughlin MP, Harrison JK. Percutaneous stenting of right pulmonary artery stenosis in fibrosing mediastinitis. Catheter Cardiovasc Interv. 2000;49:321–324. doi: 10.1002/(sici)1522-726x(200003)49:3<321::aid-ccd20>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Recto MR, Bousamra M, Yeh T., Jr Late superior vena cava perforation and aortic laceration after stenting to treat superior vena cava syndrome secondary to fibrosing mediastinitis. J Invasive Cardiol. 2002;14:624–629. [PubMed] [Google Scholar]
- 22.Satpathy R, Aguila V, Mohiuddin SM, Khan IA. Fibrosing mediastinitis presenting as pulmonary stenosis: stenting works. Int J Cardiol. 2007;118:e85–e86. doi: 10.1016/j.ijcard.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Thomas BP, Bream PR, Jr, Milstone AP, Meranze SG. Treatment of SVC syndrome and hemoptysis in a patient with mediastinal fibrosis. Emerg Radiol. 2006;12:240–243. doi: 10.1007/s10140-005-0442-z. [DOI] [PubMed] [Google Scholar]
- 24.Ganeshan A, Hon LQ, Warakaulle DR, Morgan R, Uberoi R. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol. 2009;71:343–349. doi: 10.1016/j.ejrad.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi AZ, Kalra M, Bjarnason H, Bower TC, Schleck C, Gloviczki P. Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg. 2008;47:372–380. doi: 10.1016/j.jvs.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh MA, Fernandez BB, Jr, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv. 2005;65:405–411. doi: 10.1002/ccd.20458. [DOI] [PubMed] [Google Scholar]
- 27.Urruticoechea A, Mesia R, Dominguez J, Falo C, Escalante E, Montes A, Sancho C, Cardenal F, Majem M, Germa JR. Treatment of malignant superior vena cava syndrome by endovascular stent insertion. Experience on 52 patients with lung cancer. Lung Cancer. 2004;43:209–214. doi: 10.1016/s0169-5002(03)00361-1. [DOI] [PubMed] [Google Scholar]
- 28.Law MA, Shamszad P, Nugent AW, Justino H, Breinholt JP, Mullins CE, Ing FF. Pulmonary Artery Stents: Long-Term Follow-Up. Catheter Cardiovasc Interv. 2010;75:757–764. doi: 10.1002/ccd.22356. [DOI] [PubMed] [Google Scholar]
- 29.Stanfill R, Nykanen DG, Osorio S, Whalen R, Burke RP, Zahn EM. Stent implantation is effective treatment of vascular stenosis in young infants with congenital heart disease: acute implantation and long-term follow-up results. Catheter Cardiovasc Interv. 2008;71:831–841. doi: 10.1002/ccd.21526. [DOI] [PubMed] [Google Scholar]
- 30.Neumann T, Kuniss M, Conradi G, Sperzel J, Berkowitsch A, Zaltsberg S, Wojcik M, Erkapic D, Dill T, Hamm CW, Pitschner HF. Pulmonary vein stenting for the treatment of acquired severe pulmonary vein stenosis after pulmonary vein isolation: clinical implications after long-term follow-up of 4 years. J Cardiovasc Electrophysiol. 2009;20:251–257. doi: 10.1111/j.1540-8167.2008.01316.x. [DOI] [PubMed] [Google Scholar]
- 31.Sreeram N, Emmel M, Trieschmann U, Brockmeier K, Bennink G. Pulmonary vein stents in infants and children: is there lasting benefit? Clin Res Cardiol. 2008;97:463–466. doi: 10.1007/s00392-008-0653-x. [DOI] [PubMed] [Google Scholar]
- 32.Brown ML, Cedeno AR, Edell ES, Hagler DJ, Schaff HV. Operative strategies for pulmonary artery occlusion secondary to mediastinal fibrosis. Ann Thorac Surg. 2009;88:233–237. doi: 10.1016/j.athoracsur.2009.04.012. [DOI] [PubMed] [Google Scholar]