Abstract
Endoscopic ultrasonography (EUS) represents the combination of endoscopy and intraluminal ultrasonography. This allows use of a high-frequency transducer (5-20 MHz) that, due to the short distance to the target lesion, provides ultrasonographic images of higher resolution than those obtained from other imaging modalities, including multiple-detector-row-computed tomography, magnetic resonance imaging, and positron emission tomography. EUS is now a widely accepted modality for diagnosing pancreatic diseases. However, the most important limitation of EUS has been the lack of specificity in differentiating between benign and malignant changes. In 1992, EUS-guided fine needle aspiration (FNA) of lesions in the pancreas head was introduced into clinical practice, using a curved linear-array echoendoscope. Since then, EUS has evolved from EUS imaging to EUS-FNA and wider applications. Interventional EUS for pancreatic cancer includes EUS-FNA, EUS-guided fine needle injection, EUS-guided biliary drainage and anastomosis, EUS-guided celiac neurolysis, radiofrequency ablation, brachytherapy, and delivery of a growing number of anti-tumor agents. This review focuses on interventional EUS, including EUS-FNA and therapeutic EUS for pancreatic cancer.
Keywords: Endoscopic ultrasonography-biliary drainage, Endoscopic ultrasonography-choledochoduodenostomy, Endoscopic ultrasonography-fine needle aspiration, Endoscopic ultrasonography-guided biliary drainage, Interventional endoscopic ultrasonography
INTRODUCTION
Endoscopic ultrasonography (EUS) is a combination of endoscopy and intraluminal ultrasonography, using a high-frequency transducer at 5-20 MHz. In addition, the short imaging distance to the target lesion through the endoscopic device allows high-resolution ultrasonographic images. EUS is now a widely accepted modality for the diagnosis of gastrointestinal and pancreatobiliary diseases. In 1992, Vilmann et al[1] published the first case report of EUS-guided fine needle aspiration (FNA) of a lesion in the pancreas head using a curved linear-array echoendoscope. Since then, many researchers have expanded the indications for EUS-FNA to include various types of lesions, and for therapeutic purposes[2]. EUS techniques have evolved from aspiration to EUS-guided fine needle injection (FNI), providing EUS with even wider applications. This advance has resulted in interventional EUS as an important modality for the diagnosis and treatment of pancreatic cancer. Interventional EUS techniques include EUS-FNA, gene analysis from EUS-FNA specimens, drug delivery, biliary drainage, biliary anastomosis, celiac neurolysis, and brachytherapy. This review focuses on the present status of interventional EUS for pancreatic cancer, including the specific roles of EUS-FNA and therapeutic EUS.
EUS-FNA
Indications and contraindications
A fundamental principle of EUS-FNA is that the information obtained should have the potential to affect patient management[3]. In addition, indications for EUS-FNA in patients with pancreatic cancer should be considered based on diagnostic accuracy, cost effectiveness, and patient comfort and safety. In Japan, the current indications for EUS-FNA include[4,5]: (1) differentiating between benign and malignant lesions; (2) staging of cancer; and (3) obtaining histological evidence of malignancy before chemotherapy and/or radiotherapy, or even surgery. Such diagnoses are based on one or more of cytological (Diff-Quik and Papanicolaou staining), histological (hematoxylin and eosin staining), immunohistochemical or genetic analyses.
Contraindications for EUS-FNA include situations in which FNA results would not affect management, inability to clearly visualize a lesion, tumor mass or vessel interposed in the path between the needle and target, bleeding diathesis, and high risk of tumor seeding[3,6]. In Japan, use of EUS-FNA for cystic tumor is considered to carry a high risk of tumor seeding, particularly for intraductal papillary mucinous neoplasm (IPMN) and mucinous cyst neoplasm. However, only one case of tumor seeding by EUS-FNA for IPMN has been reported to date[7].
Equipment for EUS-FNA
A curved linear-array echoendoscope (convex echoendoscope) is usually available for EUS-FNA. This instrument generates longitudinal sector images parallel to the axis of the endoscope and is equipped with color Doppler functioning[8]. A forward-viewing curved linear-array echoendoscope is sometimes useful in therapeutic EUS, especially for the purpose of drainage. At present, the most important function of the echoendoscope is as a large instrument channel to allow not only histological biopsies to be taken, but also therapeutic applications.
Several needles have been developed. Recent models for EUS-FNA can be lure-locked in a fixed position on the echoendoscope. The endoscopist can then advance the needle into the lesion independently under ultrasonographic guidance. With regard to needle technology, the shape of the tip and diameter of the needle have been continuously developed and improved. Needles range in size from 19 to 25 gauge. Sufficient specimens can be obtained to diagnose pancreatic cancer utilizing immunohistochemical and gene analysis, even with a 25-gauge needle[9].
Diagnostic accuracy and complications
High rates of adequate tissue sampling and diagnostic accuracy have been reported for EUS-FNA. In a study of nearly 1700 patients, DeWitt[10] reported 88% accuracy, 85% sensitivity, and 98% specificity of EUS-FNA for pancreatic tumors. EUS-FNA of solid pancreatic masses provided 95% sensitivity, 92% specificity, 98% positive predictive value, and 80% negative predictive value. The overall accuracy of EUS-FNA was 94.1%[11]. The overall complication rate of EUS-FNA appears to be 1%-2%. The major complications reported with EUS-FNA are infections, bleeding, pancreatitis, and duodenal perforation. Cystic pancreatic lesions appear to be associated with a greater risk of infective complications and bleeding than solid pancreatic masses. Two deaths have been reported with EUS-FNA. One patient developed fulminant cholangitis following EUS-FNA of a liver metastasis, while the other developed uncontrolled bleeding from a pseudoaneurysm after EUS-FNA of the pancreas[12]. We have encountered severe EUS-FNA-related complications, including rupture of a pancreatic pseudoaneurysm followed by massive gastrointestinal bleeding, and acute portal vein obstruction[13]. Both of these cases might have been caused by acute focal pancreatitis. The risk of acute pancreatitis after EUS-FNA of pancreatic masses has been estimated in 19 centers, with reported frequencies of 0.29% in a retrospective analysis and 0.64% in prospective study[14].
EUS-GUIDED DRAINAGE AND ANASTOMOSIS
EUS-guided pancreatic pseudocyst drainage
Pancreatic cancer is sometimes associated with pancreatitis and pseudocyst. Pancreatic pseudocyst in association with infection or symptoms should be aspirated or drained. A single puncture and aspiration of the cystic fluid with a 19-gauge needle is a simple method to drain the cyst cavity without any complicated procedures. However, cysts tend to recur with this method alone. In most cases, persistent drainage is indispensable for complete reduction of the cyst cavity.
In 1992, Grimm et al[15] provided the first report of EUS-guided cyst drainage with linear-array echoendoscope in a patient with chronic pancreatitis associated with pancreatic tail pseudocyst. EUS-guided pseudocyst drainage has some advantages for identifying pseudocysts that show no overt protrusion into the gastrointestinal lumen, and also for selecting the shortest puncture pathway under real-time scan, compared to endoscopy-guided drainage. Color Doppler images also help to avoid inadvertent puncture of blood vessels near the cyst puncture route. According to recent reports, EUS-guided pseudocyst drainage is feasible in more than 90% of patients, with a complication rate below 5%[16]. EUS-guided pancreatic pseudocyst drainage can now be considered a very effective treatment for patients with pancreatic pseudocyst.
EUS-guided biliary drainage
Wiersema et al[17] first described EUS-guided cholangiopancreatography in 1996 as a diagnostic alternative for two patients following failed ERCP. Recent reports have demonstrated the feasibility of EUS-guided cholangiography with biliary stent placement in patients with failed cannulation at ERCP. EUS-guided biliary drainage includes two methods, a rendezvous technique and a direct access technique, and two main approach routes, a transgastric approach and a transduodenal approach.
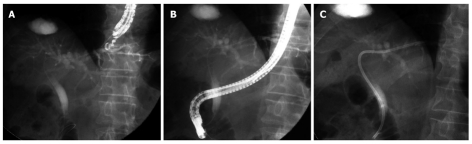
EUS-biliary drainage using the rendezvous technique: EUS-guided bile duct drainage with the rendezvous technique (Figure 1) was first described by Mallery et al[18] in 2004. Other researchers have since reported EUS-biliary drainage (BD) using the rendezvous technique. Six reports[18-23] on EUS-guided rendezvous technique have been published, with a total of 45 patients. Both 19-gauge and 22-gauge EUS-FNA needles have been used. Site of puncture included the duodenum in 19 cases, stomach in 18 cases, and esophagus in 1 case. The overall success rate was 80% (36/45) and the complication rate was 4% (2/45), including 1 case each of pneumoperitoneum and bile leakage. Kim et al[23] reported moderately severe pancreatitis and bacteremia in each case as a complication, but unrelated to the EUS-guided rendezvous technique. According to the largest case series, reported by Maranki et al[24], the overall success rate of trans-papillary stenting was 65% (32/49) among the 49 patients who underwent intra- and extrahepatic approaches using only the EUS-guided rendezvous technique.
Figure 1.
Endoscopic ultrasonography biliary drainage using the rendezvous technique. A: Intrahepatic approach: fluoroscopic image of the wire crossing the hilar stricture and advancing into the duodenum; B, C: Subsequent endoscopic retrograde cholangiopancreatography with bile duct access and plastic stent placement.
The rendezvous technique is feasible only when the endoscope can be advanced to the papillary orifice or site of surgical anastomosis for retrieval of the guide wire. EUS-rendezvous is used solely to puncture the obstructed bile duct and pass a guide wire in an antegrade manner through the native papilla to allow subsequent ERCP[23]. However, the guide wire cannot be advanced across the obstruction in some cases. Potential advantages of EUS-rendezvous access are seen for patients with large amounts of ascites.
EUS-guided choledochoduodenostomy
EUS-choledochoduodenostomy (CDS) (Figure 2) was first reported by Giovannini et al[25] in 2001. Recently, some studies have evaluated the role and technique of EUS-CDS[24-30]. A needle knife or fistulotome and/or 19- or 22-gauge EUS-FNA needles were used to access the extrahepatic bile duct. Although the procedure was unsuccessful in a few patients, transduodenal stents (including a metal stent] were successfully inserted in almost all patients (> 95%). The rate of treatment success was almost 100% among patients with successful EUS-CDS access.
Figure 2.
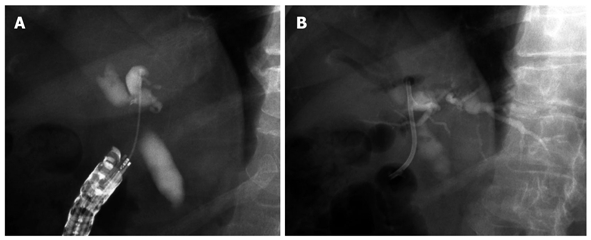
Endoscopic ultrasonography-guided choledochoduodenostomy. A: Cholangiography after puncture of extrahepatic bile duct by needle knife; B: Plastic stent was inserted from duodenum into extrahepatic bile duct.
The advantage of the EUS-CDS technique is that the puncture site is very close to the extrahepatic bile duct and away from the obstructing tumor[28]. Compared with approaches from the stomach, the duodenum is easily punctured to reach the bile duct and dilation of the route is simple. To prevent dislocation of the guide wire and dilator, an appropriate puncture site should be selected aiming at the extrahepatic bile duct between the upper margin of the pancreas and the hepatic hilum. Comparatively high complication rates (15%) have been reported, including 2 cases of small focal bile peritonitis[29,31] and 3 cases of pneumoperitoneum[13,28,32]. The one-step method with direct puncture of the extrahepatic bile duct may reduce the risk of complications.
EUS-guided hepaticogastrostomy
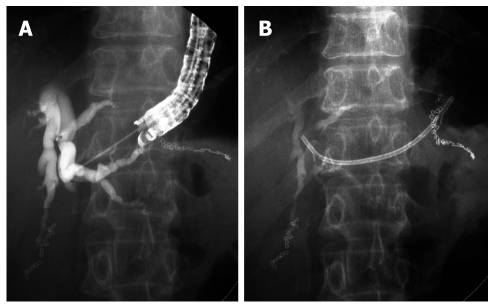
EUS-guided hepaticogastrostomy (Figure 3) was first reported by Burmester et al[29] in 2003. The technique is broadly similar to EUS-CDS. EUS-guided hepaticogastrostomy has been reported in some studies[29,30,33-35]. In all patients, 19- or 22-gauge fine needles, or fine needles followed by a needle knife or cystotome, were used to puncture the intrahepatic bile ducts. The procedure was successful in almost all patients (> 96%)[29,30,33-35]. Various types of stents, including plastic stents, uncovered metallic stent (MS), and covered MS have been used for drainage. The rate of treatment success was over 96% among patients with successful EUS-GHS access[29,30,33-35]. The rate of procedure-related early complications was 14%, with no mortality, and included: 1 case of ileus probably due to the use of morphine during anesthesia, 1 case of bilioma, and 2 cases of cholangitis[33]. Stent migration has been reported as a late complication in 1 case[35].
Figure 3.
Endoscopic ultrasonography-guided hepaticogastrostomy. A: Cholangiography after puncture of intrahepatic bile duct by 19G fine needle. B: Plastic stent was inserted from stomach into intrahepatic bile duct.
Kahaleh et al[30] noted that the advantages of EUS-guided hepaticogastrostomy over percutaneous transhepatic drainage included puncture of the biliary tree with real-time US when using color-Doppler information to limit the possibility of vascular injury, the lack of ascites in the interventional field when present in the peritoneum, and the lack of an external drain. Based on their experience, they also pointed out that the extrahepatic approach has a greater risk of complications compared to the intrahepatic approach. Itoi et al[31] reported the limitations of this technique as follows: (1) certain displacement between the puncture site of the gastric wall and the intrahepatic bile duct, resulting in possible failure to carry out this procedure; (2) risk of mediastinitis with a transesophageal approach; (3) difficulty achieving puncture in cases of liver cirrhosis; (4) risk of injury to the portal vein; and (5) necessity of using small-caliber stents or MS with a small-diameter delivery device.
EUS-FNI THERAPY
EUS-guided celiac plexus neurolysis or block
EUS-guided celiac plexus neurolysis (EUS-CPN) was first reported by Wiersema et al[36] in 1996. Since then, EUS-CPN (Figure 4) has been applied for relief of intractable pain. Patients with significant abdominal pain and unresectable pancreatic cancer may represent suitable candidates for EUS-guided CPN. The technique of EUS-CPN resembles that of EUS-FNA, except for the injection. The procedure begins with identification of the celiac trunk. The celiac plexus is located anterior and lateral to the celiac trunk take-off from the aorta. Bupivacaine (3-10 mL, 0.25%) is injected, followed by 10 mL of (98%) dehydrated ethanol[37,38]. For EUS-guided celiac plexus block (EUS-CPB), a steroid (triamcinolone suspension, 40 mg each side, bilaterally) is used instead of alcohol. In a study of 30 patients, Wiersema et al[36] reported a 79%-88% improvement in pain score at a median follow-up of 10 wk, while Gress et al[39] reported reduced pain score and medication use in 55% patients with chronic pancreatitis treated using EUS-CPB. Levy et al[40] recently reported the utility of EUS-guided direct injection of agents into the celiac ganglia as EUS-guided celiac ganglion neurolysis (EUS-CGN) in patients with pancreatic cancer and chronic pancreatitis. Use of EUS-CPN to provide pain relief from pancreatic cancer has substantially increased initial response rates to over 90%, and no major complications were encountered in that study.
Figure 4.

Endoscopic ultrasonography-guided celiac plexus neurolysis. A: At first, we visualize the celiac trunk by linear array echoendoscope; B: Endoscopic ultrasonography image during ethanol injection.
EUS-FNT
Intraoperative detection of very small lesions already detected on preoperative examination is sometimes difficult for surgeons. Endoscopic tattooing is a very useful method to identify such previously detected small lesions at surgery. Gress et al[41] described the tattooing of small pancreatic tumors using the technique of EUS-FNI with Indian ink in 2002. As a number of reports have noted side effects caused by Indian ink, we reported on EUS-FNT with indocyanine green for small pancreatic tumors[42]. We have now safely performed EUS-FNT in five cases of small pancreatic tumors, including four cases of endocrine tumor and one case of multiple serous cystic tumors (unpublished data). EUS-FNT represents a safe and useful method for preoperative marking of small pancreatic tumors. This technique appears to not only reduce operative time, but also the total cost of pre- and intraoperative tumor identification. Further trials are needed to confirm which substance provides the best efficacy and greatest safety for EUS-FNT to identify small pancreatic tumors.
EUS-guided delivery of anti-tumor agent
Injection of materials into pancreatic cancer by EUS-guided delivery appears to represent an attractive treatment strategy. Materials with anti-tumor effects include ethanol and molecules with biological anti-tumor actions. EUS-guided ethanol injection has been used to ablate pancreatic tissue. In animal models, ethanol ablation of normal pancreatic tissue was found to be safe and resulted in well-controlled ablation[43-45].
Some clinical trials of EUS-guided anti-tumor injection therapy for pancreatic cancer have been reported. Anti-tumor agents that have already been reported include allogenic mixed lymphocyte culture (cytoimplants)[46], ONYX-015[47], TNFerade[48], immature dendritic cells, and oncolytic herpes simplex virus carrying the GM-CSF gene (Onco VEX)[49]. EUS-guided delivery of anti-tumor agents seems likely to become popular and many other anti-tumor agents will be developed in the near future.
EUS-GUIDED BRACHYTHERAPY AND RADIOFREQUENCY ABLATION
EUS-guided brachytherapy was first reported by Maier et al[50] in 1999 for head and neck cancers. Brachytherapy is a relatively safe procedure in which radiation seeds are delivered directly to the target gland. Brachytherapy has been widely used for various malignancies, including pancreatic cancer.
Goldberg et al[51] reported EUS-guided radiofrequency ablation (RFA) in the pancreas of a porcine model, using a modified EUS needle and a commercial RF needle. RFA could provide localized tissue ablation of a 1-cm zone from the needle catheter. One of 13 pigs developed pancreatitis.
In 2008, Carrara et al[52] demonstrated the feasibility and efficacy of EUS-guided RFA using a newly developed flexible bipolar ablation probe combining RFA and cryotechnology in 14 pigs. The size of the ablation area was related to the duration of ablation. Complications were less common than for use of conventional RFA needles.
CONCLUSION
EUS is now an indispensable imaging modality for pancreatic diseases. Interventional EUS includes EUS-FNA and therapeutic EUS. The clinical utility of EUS-FNA has been widely accepted. EUS-FNA appears to offer a safe and reliable technique to obtain tissue from pancreatic masses with a low risk of complications. Interventional EUS for pancreatic cancer has also been investigated in experimental studies and clinical trials, and some EUS-guided techniques are now well established. Interventional EUS will play a more important role in the treatment of pancreatic cancer in the near future.
Footnotes
Peer reviewer: Lei Chen, MD, Associate Professor, Department of Hepatobiliary Surgery, Peking University People’s Hospital, No. 11 Xizhimen South Street, West district, Beijing 10044, China
S- Editor Cheng JX L- Editor Webster JR E- Editor Ma WH
References
- 1.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 2.Bhutani MS. Endoscopic ultrasound guided fine needle aspiration of pancreas. Interventional Endoscopic Ultrasonography. In: Bhutani MS, editor. The Netherlands: Harwood Academic Publishers; 1999. pp. 65–72. [Google Scholar]
- 3.Hawes RH. Indications for EUS-directed FNA. Endoscopy. 1998;30 Suppl 1:A155–A157. doi: 10.1055/s-2007-1001503. [DOI] [PubMed] [Google Scholar]
- 4.Yamao K, Ozawa S, Kida M. Guideline for endoscopic ultrasound guided fine needle aspiration biopsy. In: JSGE , editor. Guidelines for Gastrointestinal Endoscopy. 2nd ed. Tokyo: Igaku-Shoin Ltd; 2002. pp. 327–336. [Google Scholar]
- 5.Kouzu T, Yamao K, Irisawa A. Guideline for Endoscopic ultrasound guided fine needle aspiration biopsy. In: JSGE , editor. Guidelines for Gastrointestinal Endoscopy. 3nd ed. Tokyo: Igaku-Shoin Ltd; 2006. pp. 170–187. [Google Scholar]
- 6.Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013–1023. doi: 10.1007/s00535-005-1717-6. [DOI] [PubMed] [Google Scholar]
- 7.Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323–1324. doi: 10.1046/j.1440-1746.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 8.Mallery S, Van Dam J. Overview of echoendoscope design and mechanics for interventional endosonography. In: Bhutani MS, editor. Interventional Endoscopic Ultrasonography. Harwood Academic Publishers; 1999. pp. 1–7. [Google Scholar]
- 9.Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt J. EUS on pancreatic neoplasms. In: Hawes RH, Fockens P, editors. Interventional Endosonography. London: Elsevier Publishers; 2006. pp. 177–203. [Google Scholar]
- 11.Al-Haddad M, Eloubeidi MA. Interventional EUS for the Diagnosis and Treatment of Locally Advanced Pancreatic Cancer. JOP J Pancreas (Online) 2010;11:1–7. [PubMed] [Google Scholar]
- 12.Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–279. doi: 10.1016/s0016-5107(04)01529-9. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Yamao K, Ohashi K, Watanabe Y, Sawaki A, Nakamura T, Matsuura A, Suzuki T, Fukutomi A, Baba T, et al. Acute portal vein thrombosis after EUS-guided FNA of pancreatic cancer: case report. Gastrointest Endosc. 2003;57:269–271. doi: 10.1067/mge.2003.79. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622–629. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Grimm H, Binmoeller KF, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc. 1992;38:170–171. doi: 10.1016/s0016-5107(92)70384-8. [DOI] [PubMed] [Google Scholar]
- 16.Varadarajulu S. EUS followed by endoscopic pancreatic pseudocyst drainage or all-in-one procedure: a review of basic techniques (with video) Gastrointest Endosc. 2009;69:S176–S181. doi: 10.1016/j.gie.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102–106. doi: 10.1016/s0016-5107(06)80108-2. [DOI] [PubMed] [Google Scholar]
- 18.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–107. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 19.Lai R, Freeman ML. Endoscopic ultrasound-guided bile duct access for rendezvous ERCP drainage in the setting of intradiverticular papilla. Endoscopy. 2005;37:487–489. doi: 10.1055/s-2005-861250. [DOI] [PubMed] [Google Scholar]
- 20.Kahaleh M, Wang P, Shami VM, Tokar J, Yeaton P. EUS-guided transhepatic cholangiography: report of 6 cases. Gastrointest Endosc. 2005;61:307–313. doi: 10.1016/s0016-5107(04)02585-4. [DOI] [PubMed] [Google Scholar]
- 21.Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292–295. doi: 10.1055/s-2007-966215. [DOI] [PubMed] [Google Scholar]
- 22.Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008;40:336–339. doi: 10.1055/s-2007-995455. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496–502. doi: 10.1055/s-0029-1244082. [DOI] [PubMed] [Google Scholar]
- 24.Maranki J, Hernandez AJ, Arslan B, Jaffan AA, Angle JF, Shami VM, Kahaleh M. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy. 2009;41:532–538. doi: 10.1055/s-0029-1214712. [DOI] [PubMed] [Google Scholar]
- 25.Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 26.Yamao K, Sawaki A, Takahashi K, Imaoka H, Ashida R, Mizuno N. EUS-guided choledochoduodenostomy for palliative biliary drainage in case of papillary obstruction: report of 2 cases. Gastrointest Endosc. 2006;64:663–667. doi: 10.1016/j.gie.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Hara K, Yamao K, Mizuno N, Sawaki A, Takagi T, Bhatia V. Endoscopic ultrasound-guided choledochoduodenostomy. Dig Endosc. 2010;22:147–150. doi: 10.1111/j.1443-1661.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340–342. doi: 10.1055/s-2007-995485. [DOI] [PubMed] [Google Scholar]
- 29.Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246–251. doi: 10.1067/mge.2003.85. [DOI] [PubMed] [Google Scholar]
- 30.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 31.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Moriyasu F, et al. Endoscopic ultrasonography-guided biliary drainage. J Hepatobiliary Pancreat Sci. 2010;17:611–616. doi: 10.1007/s00534-009-0196-1. [DOI] [PubMed] [Google Scholar]
- 32.Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP. 2007;8:438–443. [PubMed] [Google Scholar]
- 33.Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287–291. doi: 10.1055/s-2007-966212. [DOI] [PubMed] [Google Scholar]
- 34.Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, Meyer F. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377–382. doi: 10.1007/s00534-006-1139-8. [DOI] [PubMed] [Google Scholar]
- 35.Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168–2174. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 36.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 37.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 38.Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923–930. doi: 10.1016/s0016-5107(03)70036-4. [DOI] [PubMed] [Google Scholar]
- 39.Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409–416. doi: 10.1111/j.1572-0241.2001.03551.x. [DOI] [PubMed] [Google Scholar]
- 40.Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98–103. doi: 10.1111/j.1572-0241.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 41.Gress FG, Barawi M, Kim D, Grendell JH. Preoperative localization of a neuroendocrine tumor of the pancreas with EUS-guided fine needle tattooing. Gastrointest Endosc. 2002;55:594–597. doi: 10.1067/mge.2002.122580. [DOI] [PubMed] [Google Scholar]
- 42.Ashida R, Yamao K, Okubo K, Sawaki A, Mizuno N, Nakamura T, Tajika M, Kawai H, Shimizu Y. Indocyanine green is an ideal dye for endoscopic ultrasound-guided fine-needle tattooing of pancreatic tumors. Endoscopy. 2006;38:190–192. doi: 10.1055/s-2005-870404. [DOI] [PubMed] [Google Scholar]
- 43.Ashida R, Yamao K, Matsumoto K, Okubo K, Sawaki A, Mizuno N. Experimental study of endoscopic ultra-sound guided ethanol injection in the pancreas; novel strategy for pancreatic lesion. Gatrointest Endosc. 2004;59:A212. [Google Scholar]
- 44.Matsumoto K, Yamao K, Okubo K, Hara K, Sawaki A, Mizuno N, Tajika M, Kawai H, Ashida R. Endoscopic ultrasound-guided ethanol injection in the pancreas in a porcine model: a preliminary study. J Gastroenterol Hepatol. 2008;23:e1–6. doi: 10.1111/j.1440-1746.2007.04918.x. [DOI] [PubMed] [Google Scholar]
- 45.Aslanian H, Salem RR, Marginean C, Robert M, Lee JH, Topazian M. EUS-guided ethanol injection of normal porcine pancreas: a pilot study. Gastrointest Endosc. 2005;62:723–727. doi: 10.1016/j.gie.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 46.Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325–1335. doi: 10.1002/(sici)1097-0142(20000315)88:6<1325::aid-cncr8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 47.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 48.Chang KJ, Senzer N, Chung T, Hecht JR, Vogel S, Rosemurgy A, Nemunaitis J, Gibbs J, Javle M, Reid T, et al. A novel gene transfer therapy against pancreatic cancer (TNFerade) delivered by endoscopic ultrasound (EUS) and percutaneous guided fine needle injection (FNI) Gastrointest Endosc. 2004;59:AB188. [Google Scholar]
- 49.Kasuya H, Takeda S, Nomoto S, Nakao A. The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Ther. 2005;12:725–736. doi: 10.1038/sj.cgt.7700830. [DOI] [PubMed] [Google Scholar]
- 50.Maier W, Henne K, Krebs A, Schipper J. Endoscopic ultrasound-guided brachytherapy of head and neck tumours. A new procedure for controlled application. J Laryngol Otol. 1999;113:41–48. doi: 10.1017/s0022215100143117. [DOI] [PubMed] [Google Scholar]
- 51.Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 52.Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Neugebauer A, Campagnol M, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new internally gas-cooled radiofrequency ablation probe in the liver and spleen of an animal model: a preliminary study. Endoscopy. 2008;40:759–763. doi: 10.1055/s-2008-1077520. [DOI] [PubMed] [Google Scholar]