Abstract
Recent reports have shown the involvement of tumor burden as well as GM-CSF in supporting myeloid-derived suppressor cells (MDSC). However, it is not known what progenitor cells may differentiate into MDSC in the presence of GM-CSF, and whether FVBN202 transgenic mouse model of spontaneous breast carcinoma may exhibit distinct subset distribution of CD11b+Gr1+ cells. In addition, it is not known why CD11b+Gr1+ cells derived from tumor-free and tumor-bearing animals exhibit different functions. In this study, we determined that GM-CSF was one of the tumor-derived soluble factors that induced differentiation of CD11b−Gr1− progenitor cells from within monocytic/granulocytic bone marrow cells into CD11b+Gr1+ cells. We also showed that CD11b+ Gr1+ cells in FVBN202 mice consisted of CD11b+Ly6G−Ly6C+ suppressive and CD11b+Ly6G+Ly6C+ non-suppressive subsets. Previously reported variations between tumor-free and tumor-bearing animals in the function of their CD11b+Gr1+ cells were found to be due to the variations in the proportion of these two subsets. Therefore, increasing ratios of CD11b+Gr1+ cells derived from tumor-free animals revealed their suppressive activity on T cells, in vitro. Importantly, GM-CSF supported the generation of CD11b+Ly6G−Ly6C+ suppressor subsets that inhibited proliferation as well as anti-tumor function of neu-specific T cells. These findings suggest revisiting the use of GM-CSF for the expansion of dendritic cells, ex vivo, for cell-based immunotherapy or as an adjuvant for vaccines for patients with cancer in whom MDSC play a major role in the suppression of anti-tumor immune responses.
Keywords: Myeloid-derived suppressor cells (MDSC), GM-CSF, Breast cancer, Dendritic cells, HER-2/neu
Introduction
Myeloid-derived suppressor cells (MDSC) have been observed to accumulate in many cancer models, including Lewis lung carcinoma (3LL), murine colon carcinoma (CT26), the highly metastatic breast carcinoma 4T1, the neu+ breast carcinoma MMC, and B16 melanoma [1–4]. Patients with renal cell carcinoma, melanoma, head and neck cancer, and breast cancer had increased MDSC [5–9]. Importantly, increases in the circulating levels of MDSC correlate with clinical cancer stage and metastatic burden of breast cancer [9]. In pre-clinical studies, it has been documented that MDSC can cause downregulation of the T cell receptor (TCR) zeta chain, and may explain reduced TCR zeta chain expression in patients with breast cancer, melanoma, and gastric cancer [10–13].
A correlation between tumor burden and increased MDSC suggests that tumor-derived factors may cause accumulation of MDSC in cancer patients. Pan et al. found that abrogating the secretion of stem-cell factor, or blocking its receptor, c-Kit, reduced MDSC expansion in mice bearing MCA26 colon carcinomas [14]. In a transgenic model of breast carcinoma which expresses the activated form of the HER-2/neu oncogene, VEGF serum levels were found to correlate with tumor multiplicity and progression, as well as accumulation of MDSC [15]. GM-CSF has also been linked to the accumulation of MDSC in humans, where a correlation was made between the levels of circulating CD34+ myeloid cells in patients with head and neck carcinoma and the ability of the tumor cells to secrete GM-CSF [16]. However, bone marrow progenitor cells which are differentiated into MDSC by GM-CSF remain elusive. Interestingly, a review compiling the results from multiple clinical trials using GM-CSF has found that the dosage of GM-CSF was paramount, with lower doses (40–80 μg) eliciting an immune response, whereas higher doses showed no advantage [17]. For example, melanoma patients receiving peptide vaccination along with either 100 or 500 μg doses of GM-CSF for 6 days showed a decrease in the induction of specific T cell responses [18] which was associated with increased levels of MDSC [19]. In a mouse model of melanoma, increasing the concentration of GM-CSF to the therapeutic dose of 1,500 ng/106 cells/24 h resulted in decreased survival and increased CD11b+Gr1+ cells in multiple organs [20]. Ex vivo culture of bone marrow with GM-CSF has also been shown to generate MDSC, which suppressed allogeneic as well as OVA-specific CD4+ and CD8+ T cell responses [21]. However, there is no direct evidence identifying the progenitor MDCS in the bone marrow or the ability of GM-CSF as one of the major tumor-derived soluble factors responsible for differentiating CD11b−Gr1− bone marrow progenitor cells into CD11b+Gr1+ MDSC that exhibit suppressor activity against tumor-specific T cells. There are also controversial reports on the CD11b+Gr1+ subsets [2, 21–23] in different tumor models.
Materials and methods
Mouse model
Parental FVB (Jackson Laboratories) and FVBN202 transgenic female mice (Charles River Laboratories) were used between 6 and 10 weeks of age throughout these studies [24, 25]. These studies have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University.
Tumor cell line
The MMC cell line was established from a spontaneous tumor harvested from an FVBN202 transgenic mouse as previously described [26]. MMC cells were maintained in RPMI1640 supplemented with 10% fetal bovine serum (FBS).
Flow cytometry
Flow cytometry analysis was performed as previously described by our group [3, 26]. Briefly, non-specific binding to Fc receptors was blocked with anti-CD16/CD32 antibody (Biolegend) for 20 min on ice. Cells were stained sequentially with antibody toward Ly6G (FITC, clone IA8), washed and then antibodies toward CD11b (PE, clone M1/70 Biolegend) and Gr1 (PE/Cy5, clone RB6-8C5 from Biolegend) were added. Cells were washed twice and fixed with 1% paraformaldehyde or were washed again in 1× Annexin V buffer (BD Pharmingen) and the Annexin V staining protocol was followed. Staining of whole blood was done by retro-orbital bleed and aliquoting of 50 × 106 whole blood cells per tube followed by blocking and staining as described above, and subsequent red blood cell lysis. We also used mouse anti-neu (Ab-4) Ab (Calbiochem, San Diego, CA) and PE anti-mouse Ig (Biolegend, San Diego, CA). Samples were run on a Beckman Coulter FC 500 and analyzed using Expo 32 software.
MMC-derived supernatants
MMC-derived conditioned medium (CM) was generated by culturing 10 × 106 MMC cells in 10 ml of medium for 24 h in RPMI1640 supplemented with 10% FBS. Supernatant was then collected and concentrated to a volume of 600 μl using 10 kDa molecular weight cut-off columns (Vivaspin). Concentrated supernatant was then injected intradermally into FVB mice (n = 3, 200 μl per mouse). Control medium with 10% FBS was prepared and concentrated in the same way in the absence of tumor cells. Injections were repeated once per day on 3 consecutive days; mice were then killed on day 4.
Multiplex cytokine array of MMC supernatants
MMC cells were cultured at a concentration of 106 cells/ml for 24 h. Supernatants were collected and sent to Allied Biotech, Inc. for multiplex array analysis in a blinded fashion.
Cell sorting
Bone marrow cells were harvested from naïve FVBN202 mice and stained for surface expression of CD11b and Gr1 as described. Cells were sorted on a Cytomation MoFlo by gating on the monocyte/granulocyte region (Fig. S1) and then sorting CD11b+Gr1+ and CD11b−Gr1− cells. The CD11b+Gr1+ cells were sorted into two major populations from the spleens of tumor-bearing mice by first staining with anti-Ly6G antibody, followed by anti-CD11b, and anti-Gr1 antibodies. Cells were gated on CD11b+ cells, and this population was then sorted based on expression of Ly6G and Ly6C. Purity of sorted cells was consistently greater than 96%.
Culture of sorted bone marrow cells
Sorted bone marrow populations (CD11b+Gr1+ and CD11b−Gr1−) were cultured in six-well plates (1–2 × 105 cells/well) for a total of 6 days. GM-CSF (100 ng/ml), VEGF (50 ng/ml), or MCP-1 (50 ng/ml) were added directly to the culture medium on day 0 (all cytokines/chemokines from Peprotech) or sorted cells were cultured in the bottom of a six-well dish with a 24 mm Transwell insert with a 0.4 μm pore size on top (Corning Life Sciences). To the Transwell insert, 0.1 × 105 MMC cells were added on day 0. On day 3, cells were split and fresh cytokine was added. Transwell inserts were discarded on day 3 and a fresh insert with 0.1 × 105 MMC cells was added.
In vitro T cell proliferation and BrdU labeling
Plates were coated with 1 μg/ml of anti-CD3 antibody (BD Pharmingen) and were washed twice with PBS after 24 h to remove any unbound antibody. Soluble anti-CD28 antibody (BD Pharmingen) was prepared in the culture medium at 1 μg/ml. Splenocytes (106 cells/ml in complete medium) were labeled by adding BrdU (5-bromo-2-deoxyuridine, BD Pharmingen) to a final concentration of 10 μM directly to the culture medium. Cells were plated at 2 × 105 cells/well and were allowed to proliferate for 72 h at 37 °C, 5% CO2. Staining for BrdU was done using the FITC-conjugated anti-BrdU Flow Kit. Where indicated, sorted subsets of MDSC were added directly to the splenocytes (1 × 105 MDSC per well).
Cytotoxicity assay
Cytotoxicity assays were performed as previously described by our group [26, 27]. Briefly, neu-specific effector lymphocytes derived from MMC-sensitized FVB mice [26] were cultured with MMC at 10:1 E:T ratios in complete medium (RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS) and 20 U/ml recombinant IL-2 (Preprotech) in six-well culture dishes. After 48 h, cells were harvested and stained for neu (anti-neu), Annexin V and PI according to the manufacturer's protocol (BD Pharmingen).
Statistical analysis
Graphical data are presented as averages with standard errors. Statistical comparisons between groups were made using the Student's t test with P < 0.05 being statistically significant.
Results
MMC-derived soluble factors increases CD11b+Gr1+ MDSC in tumor-free FVB mice
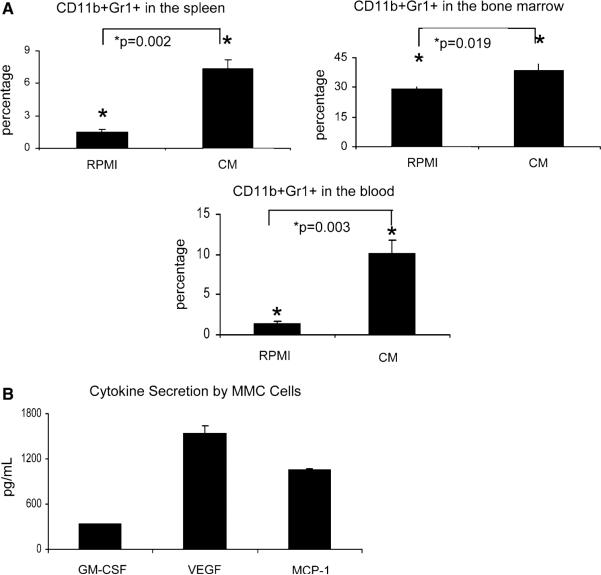
Inoculation of FVBN202 mice with MMC tumors increases MDSC in these animals [2, 3]. To determine if soluble factors released by MMC cells are entirely responsible for the increase in MDSC, MMC cells (10 × 106cells/10 ml) were cultured for 24 h in RPMI1640 supplemented with 10% FBS. FVB mice (n = 3) were then injected intradermally with 200 μl of the concentrated supernatant. FVB control mice (n = 3) were injected intradermally with 200 μl of control medium alone. Figure 1a shows a significant increase in the proportion of CD11b+Gr1+ MDSC in the spleens of mice injected with MMC-derived conditioned medium (CM) versus mice injected with medium alone (1.5% MDSC in medium-treated mice versus 7.3% MDSC in CM-treated mice, P = 0.002). A significant increase in MDSC was also seen in the blood, where control mice had 1.5% MDSC and CM-treated mice had 10% MDSC (P = 0.003), as well as in the bone marrow (29.3% MDSC in control mice versus 38.7% MDSC in CM-treated mice, P = 0.019). Animals did not show splenomegaly.
Fig. 1.
MMC-derived soluble factors cause the accumulation of CD11b+Gr1+ cells in FVB mice. FVB mice (3 per group) were injected intradermally with MMC-conditioned medium (CM) or with control medium (RPMI) for 3 consecutive days and killed on the fourth day. a The total percentage of CD11b+Gr1+ cells in the spleens, bone marrow, and blood of medium-treated (RPMI) or CM-treated (CM) mice as measure by flow cytometry. b Multiplex array analysis of MMC-derived supernatant after a 24-h culture shows substantial levels of GM-CSF, VEGF, and MCP-1. Data are averages of duplicate samples
A multiplex array detecting IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IFN-γ, TNF-α, GM-CSF, MCP-1, and VEGF was performed on MMC-conditioned medium after a 24 h culture. We found that high levels of GM-CSF, VEGF, and MCP-1 were secreted by MMC (Fig. 1b). Other cytokines were below detectable levels (data not shown).
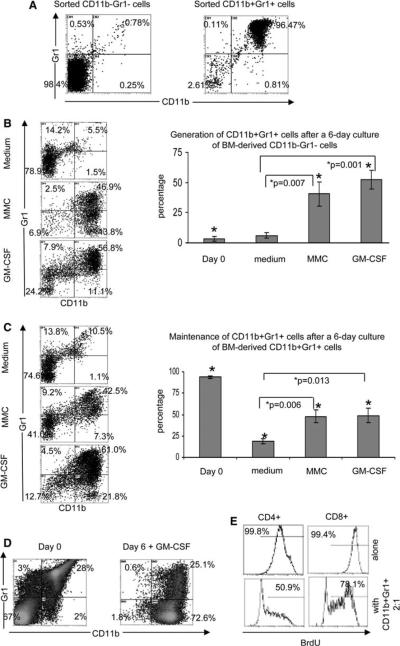
GM-CSF is one of the major cytokines produced by MMC that induces hematopoietic cells to generate CD11b+Gr1+ MDSC and supports their maintenance
Bone marrow cells from naïve FVBN202 mice (6–8 weeks old) were sorted into two populations: CD11b+Gr1+ cells and CD11b−Gr1− cells from within the gated monocyte/granulocyte regions (Fig. S1). The purity of the sorted cells was >96% (Fig. 2a). Sorted cells were cultured separately in the presence of GM-CSF (100 ng/ml), VEGF (50 ng/ml), MCP-1 (50 ng/ml), or MMC cells on top of a Transwell insert. We chose the concentration of GM-CSF based on the fact that such concentration is being used for the generation of mouse or human DC. Control wells were added with medium only. Figure 2b shows that the MMC-derived soluble factors or GM-CSF caused generation of CD11b+Gr1+ from hematopoietic CD11b−Gr1− cells. Blocking of GM-CSF in the tumor-derived supernatant by means of antibody did slightly inhibit but did not completely abrogate generation of MDSC (data not shown). At the end of a 6-day culture 46.9 and 56.8% of cells were MDSC in the presence of MMC (P = 0.007 as compared to medium alone) and GM-CSF (P = 0.001 as compared to medium alone), respectively. VEGF or MCP-1 did not induce differentiation of CD11b+Gr1+ cells from CD11b−Gr1− progenitor cells, and all cells died within a few days of culture (data not shown). Figure 2c shows that both GM-CSF (61%) and MMC (42.5%), supported the maintenance of CD11b+Gr1+ cells when compared with medium alone (10.5%) (P = 0.013 and P = 0.006, respectively). Each cytokine/chemokine was also tested for the induction of proliferation in sorted CD11b+Gr1+ cells using a BrdU kit, and it was determined that none could induce proliferation of MDSC exceeding the baseline level (data not shown).
Fig 2.
MMC-derived soluble factors and GM-CSF can generate CD11b+Gr1+ cells from the CD11b-Gr1-progenitor cells, and can maintain existing CD11b+Gr1+ cells. Bone marrow cells from naïve FVBN202 mice were stained and sorted into two populations: CD11b+Gr1+ and CD11b-Gr1-cells. a Flow cytometry plots of the purity of CD11b+Gr1+ and CD11b-Gr1- populations after sorting. Data are representative of three experiments. b Representative flow cytometry plots showing the percentage of CD11b+Gr1+ cells 6 days after culture of sorted CD11b-Gr1-cells (left) and averages of 3–4 experiments (right). c Representative flow cytometry plots showing the percentage of CD11b+Gr1+ cells remaining on day 6 after culture of sorted CD11b+Gr1+ cells (left) and averaged data from 3–4 experiments (right). (d) Representative flow cytometry staining for CD11b and Gr1 from naïve FVBN202 BM cells on day 0 (left) and after 6 days of culture with 100 ng/ml of GM-CSF. Data are representative of 3–5 separate experiments. e Representative data from duplicate experiments showing BrdU incorporation in gated CD4+ and CD8+ T cells derived from FVB mice and cultured for 72 h in the absence (top panel) or presence of CD11b+Gr1+ cells (bottom panel) generated from culture of unfractionated bone marrow cells (d) with GM-CSF
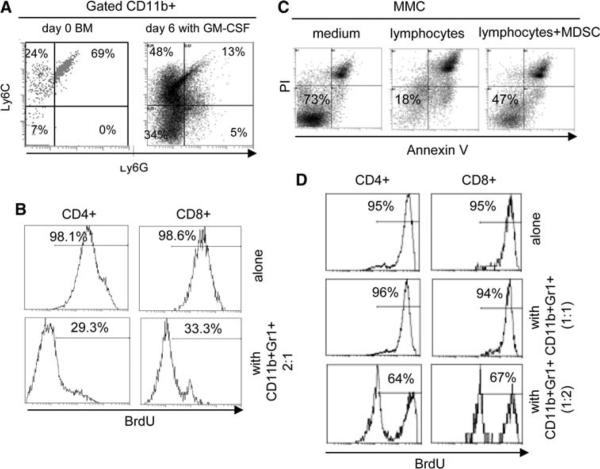
Since GM-CSF is routinely used for the generation of DCs from unfractionated bone marrow cells in vitro, we sought to determine whether GM-CSF might also support MDSC in unfractionated bone marrow cells. We cultured unfractionated bone marrow cells from tumor-free FVBN202 mice in the presence of GM-CSF (100 ng/ml) for 6 days. As shown in Fig. 2d, 28% of total bone marrow cells derived from tumor-free FVBN202 mice were CD11b+Gr1+ and 2% were CD11b+Gr1− cells. Six days after culture with GM-CSF, the majority of cells were CD11b+, of which 25.1% were CD11b+Gr1+. We then performed a suppression assay in vitro and determined that the CD11b+Gr1+ cells from within the monocyte/granulocyte region on day 0 (Fig. S1) did not suppress T cell proliferation when used at 2:1 responder (lymphocytes) : suppressor (bone marrow CD11b+Gr1+) ratios (Fig. S2) whereas a 6-day culture of whole bone marrow cells in the presence of GM-CSF resulted in the acquisition of suppressor activity in the CD11b+Gr1+ cells of naïve mice when co-cultured with T cells at 2:1 responder:suppressor ratios. The CD3/CD28-induced proliferation of CD4+ T cells and CD8+ T cells were reduced from 99.8 to 50.9% and from 99.4 to 78.1%, respectively (Fig. 2e).
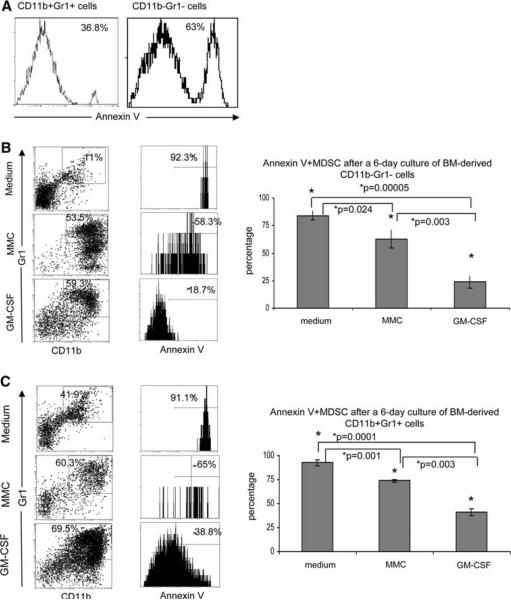
GM-CSF significantly increases the viability of both newly converted and existing CD11b+Gr1+ cells
Bone marrow cells were harvested as before and were sorted into CD11b+Gr1+ and CD11b−Gr1− populations (Fig. S1). Cells were stained with Annexin V after sorting to determine the viability of each population just before culture. The double negative and double positive sorted populations contained 63.0 and 36.8% Annexin V positive apoptotic cells, respectively (Fig. 3a). Cells were then cultured in conditions as described in Fig. 2 and were stained on day 6 with antibodies against CD11b and Gr1, and with Annexin V. As shown in Fig. 3b, GM-CSF significantly reduced the percentage of apoptotic CD11b+Gr1+ cells as compared to the proportion of apoptotic cells in the presence of medium alone (18.7% vs. 92.3%, P = 0.00005). Annexin V levels were also reduced in MDSC cultured with MMC in Transwell as compared to those cultured in medium alone (58.3% vs. 92.3%, P = 0.024). Interestingly, apoptosis was significantly reduced when progenitor CD11b−Gr1− cells were cultured with GM-CSF as compared to those cells cultured with MMC in transwell (18.7% vs. 58.3%, P = 0.003). Figure 3c shows levels of apoptosis in previously generated MDSC after a 6-day culture in vitro. The CD11b+Gr1+ sorted cells exhibited significantly reduced apoptosis when cultured with GM-CSF as compared to medium alone (38.8% vs. 91.1%, P = 0.0001). Annexin V positive MDSC were significantly reduced in the presence of MMC in Transwell as compared to medium alone (65.0% vs. 91.1%, P = 0.001). Apoptosis was also significantly reduced when comparing Annexin V positive CD11b+Gr1+ cells in the presence of GM-CSF compared to those cultured with MMC in Transwell (38.8% vs. 65.0%, P = 0.003). Levels of apoptosis in CD11b+Gr1+ cells on day 0 (Fig. 3a) were similar to those seen on day 6 after culture with GM-CSF (Fig. 3c) (36.8% on day 0 vs. 38.8% on day 6), whereas CD11b−Gr1− cells cultured with GM-CSF showed decreased Annexin V levels on day 6 (Fig. 3b) compared to day 0 (Fig. 3a) (63.0% on day 0 vs. 18.7% on day 6, P = 0.017). Therefore, GM-CSF seems to augment the survival of early stage MDSC as compared to late stage MDSC.
Fig. 3.
MMC-derived soluble factors and GM-CSF can protect newly derived and existing CD11b+Gr1+ cells from apoptosis. Sorted populations of CD11b+Gr1+ or CD11b-Gr1-cells were cultured for 6 days with medium, MMC, or GM-CSF and stained on day 6 for CD11b, Gr1, and Annexin V. a Representative flow cytometry plots of duplicate experiments showing the expression of Annexin V on sorted CD11b+Gr1+ cells (left) or sorted CD11b-Gr1- cells (right) just after sorting. b Representative flow cytometry plots of CD11b+Gr1+ cells after 6 days of culture of sorted CD11b-Gr1- cells (boxed region) that are Annexin V positive. Bar graph shows the average percentage of CD11b+Gr1+AnnexinV+ cells from 3–4 experiments. c Representative flow cytometry plots of CD11b+Gr1+ after 6 days of culture of sorted CD11b+Gr1+ cells (boxed region) that are Annexin V positive. Bar graph depicts the averages of 3–4 experiments
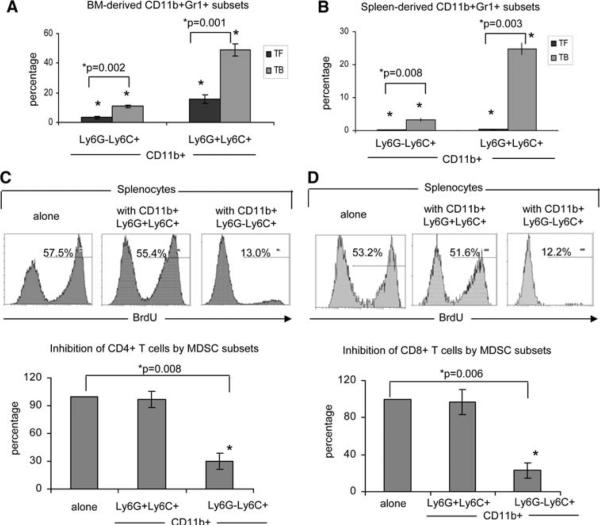
MMC-induced CD11b+Ly6G−Ly6C+ subsets suppress T cell proliferation whereas CD11b+Ly6G+Ly6C+ subsets display no suppressive activity in vitro
Figure 4a shows a significant expansion of both the CD11b+Ly6G−Ly6C+ and CD11b+Ly6G+Ly6C+ fractions (P = 0.002 and P = 0.001, respectively) in the bone marrow of tumor-bearing mice (TB) as compared to tumor-free mice (TF). Both fractions expanded similarly in the bone marrow of tumor-bearing mice (3.2 fold expansion of CD11b+Ly6G−Ly6C+ and 3.1 fold expansion of CD11b+Ly6G+Ly6C+). Expansion of both populations was also seen in the spleens of tumor-bearing mice as compared to tumor-free mice, but at drastically different proportions. Splenocytes from tumor-bearing mice showed as 23.6-fold increase in CD11b+Ly6G−Ly6C+ as compared to splenocytes from tumor-free mice (P = 0.008), whereas CD11b+Ly6G+Ly6C+ showed a drastic 64.2-fold increase in tumor-bearing spleens (P = 0.003) (Fig. 4b). As shown in Fig. 4c–d, the Ly6G−Ly6C+ subsets inhibited T cell proliferation whereas Ly6G+Ly6C+ subsets did not display any suppressive activity when used at a 2:1 responder: suppressor ratio. Representative flow cytometry staining for BrdU incorporation into CD4+ T cells showed 57.5% BrdU+ cells in culture with medium alone, 55.4% when in the presence of sorted CD11b+Ly6G+Ly6C+ cells, and only 13.0% proliferation when cultured with CD11b+Ly6G−Ly6C+ cells (Fig. 4c). Repeated experiments and normalization of data in control splenocytes alone to 100% confirmed these results, with the Ly6G−Ly6C+ fraction causing a 70% inhibition of CD4+ T cell proliferation when compared to the medium alone control (100% BrdU+ in medium alone vs. 30.1% BrdU+ in cultures containing Ly6G−Ly6C+ cells, P = 0.008). In contrast, there was no significant inhibition in cultures containing Ly6G+Ly6C+ cells (96.9% BrdU+; Fig. 4c). Similar results were found in CD8+ T cells, with representative BrdU staining showing 53.2 and 51.6% proliferation in splenocytes cultured with medium alone or with Ly6G+Ly6C+ cells, respectively (Fig. 4d). Splenocytes cultured with sorted Ly6G−Ly6C+ cells showed only 12.2% BrdU+CD8+ T cells. Normalizing the proliferation in control splenocytes alone to 100% shows a 77% inhibition of proliferation in CD8+ T cells cultured with Ly6−GLy6C+ cells from splenocytes alone (P = 0.006), whereas no significant inhibition of proliferation was seen in CD8+ T cells cultured with Ly6G+Ly6C+ cells (Fig. 4d).
Fig. 4.
The CD11b+Ly6G−Ly6C+ MDSC cause inhibition of T cell proliferation. Bone marrow (BM) cells (a) or splenocytes (b) were isolated from tumor-free (TF) or tumor-bearing (TB) FVBN202 mice (n = 3 per group) and stained for CD11b, Ly6G, and Ly6C and analyzed by flow cytometry. Results are the percentage total cells. (c–d) Sorted CD11b+Ly6G−Ly6C+ and CD11b+Ly6G+Ly6C+ cells from tumor-bearing FVBN202 spleens were cultured at a 1:2 ratio with BrdU-pulsed splenocytes from FVB mice with CD3/CD28 stimulation and stained on day 3 for BrdU incorporation and CD4 and CD8 expression. Representative BrdU plots are gated either on CD4+ (c) or CD8+ (d) lymphocytes. Bar graphs are the averages of triplicate experiments, with the percent suppression of wells containing MDSC derived by: (percent BrdU+ cells in experimental wells/percent BrdU+ cells in splenocyte alone wells) × 100. Each control well was set at 100% and each experimental well was divided by its own corresponding control well and these numbers averaged and presented ±SEM
GM-CSF preferentially supports the generation of CD11b+Ly6G−Ly6C+ subsets that convey suppressor activity
As shown in Fig. 5a, CD11b+Gr1+ cells in the bone marrow on day 0 were comprised of 24% CD11b+Ly6G-Ly6C+, correlating with a suppressive phenotype and 69% CD11b+Ly6G+Ly6C+ subsets, correlating with non-suppressive phenotype. Interestingly, culture of the progenitor cells in the presence of GM-CSF resulted in a significant increase in the suppressive CD11b+Ly6G-Ly6C+ subsets (24% vs. 48%, P = 0.025) and decrease in the non-suppressive CD11b+Ly6G+Ly6C+ subset (69% vs. 13%, P = 0.011). Similar results were obtained in the presence of MMC (Fig. S3). We then performed suppression and cytotoxicity assays in vitro. As shown in Fig. 5b, presence of GM-CSF-induced CD11b+Gr1+ cells resulted in the suppression of T cell proliferation. The CD3/CD28 stimulation induced proliferation of CD4+ and CD8+ T cells up to 98%, as determined by BrdU incorporation. Addition of MDSC at 2:1 responder:suppressor ratios reduced proliferation of CD4+ and CD8+ T cells to 29.3 and 33.3%, respectively. We also used neu-specific lymphocytes derived from MMC-sensitized FVB mice and performed cytotoxicity assay against neu-positive MMC tumor cells in the presence or absence of CD11b+Gr1+ cells that were generated by GM-CSF. As shown in Fig. 5c, while viability of MMC was reduced from 73 to 18% because of the presence of the neu-specific T cells, addition of MDSC to the culture protected MMC from apoptosis such that 47% of MMC remained viable (Annexin V-/PI-) despite the presence of neu-specific T cells.
Fig. 5.
MMC-derived supernatant and GM-CSF cause the generation of suppressive CD11b+Ly6G−Ly6C+ MDSC from CD11b−Gr1− precursor cells. a Sorted CD11b+Gr1+ cells from bone marrow (BM) of tumor-free FVBN202 mice (n = 3) were analyzed on day 0 for the expression of Ly6G and Ly6C by flow cytometry. CD11b−Gr1− cells sorted from these mice and cultured with GM-CSF were analyzed on day 6 for the expression of Ly6G and Ly6C by flow cytometry. b Representative data from duplicate experiments showing BrdU incorporation in gated CD4+ and CD8+ T cells 72 h after culture in the absence (top panel) or presence of cells (bottom panel) that were generated from a 6-day culture of CD11b−Gr1− progenitor cells with GM-CSF. c Gated neu positive MMC were analyzed for Annexin V and PI after a 48 h culture with neu-specific T cells. d Representative data from duplicate experiments showing BrdU incorporation in gated CD4+ and CD8+ T cells 72 h after culture in the absence (top panel) or presence of CD11b+Gr1+ cells derived from tumor-free mice and used at 1:1 (middle panel) or 1:2 (bottom panel) rations
Due to the presence of CD11b+Ly6G-Ly6C+ suppressor subsets in the bone marrow of tumor-free animals at lower ratios compared to these subsets in MDSC generated by GM-CSF (24% vs. 48%; Fig. 5a), we hypothesized that CD11b+Gr1+ cells of tumor-free animals are not intrinsically non-suppressive and their lower proportion masks their suppressor function. Therefore, using these CD11b+Gr1+ cells at a higher ratio, in vitro, may reveal their suppressor function. We showed that the CD11b+ Gr1+ cells from tumor-free mice did not suppress T cell proliferation when used at 2:1 responder:suppressor ratios (Fig. S2). Here, we also detected no suppressive activity even at a 1:1 ratio; however, increasing the number of CD11b+Gr1+ cells over lymphocytes to a 1:2 ratio revealed their suppressor function. As shown in Fig. 5d, presence of the CD11b+Gr1+ at 1:2 ratio inhibited proliferation of CD4+ and CD8+ T cells from 95 to 64 and 67%, respectively.
Discussion
We identified GM-CSF as one of the major tumor-derived soluble factors responsible for the generation and maintenance of CD11b+Gr1+ cells from the CD11b-Gr1- fractionated monocytic/granulocytic bone marrow progenitor cells. However, soluble factors other than GM-CSF may also contribute to the generation of MDSC, because blocking of GM-CSF in the tumor-derived supernatant did not completely abrogate generation of MDSC (data not shown). In addition, GM-CSF had similar effects on unfractionated bone marrow cells, supporting MDSC that resulted in the inhibition of T cell proliferation, in vitro. We also identified two major subsets within CD11b+Gr1+ cells in FVBN202 transgenic mice bearing neu+ tumors as CD11b+Ly6G-Ly6C+ being suppressive MDSC, and CD11b+Ly6G+Ly6C+ being non-suppressive. These subsets were also present in tumor-free mice at a different proportion, such that suppressor subsets were lower and non-suppressor subsets were higher as compared with those in tumor-bearing FVBN202 mice. Therefore, increasing the ratios of CD11b+Gr1+ cells up to twofold over T cells resulted in the detection of their suppressor function. These data suggest a possible role for MDSC in the maintenance of immunological tolerance [28, 29]. MMC-derived soluble factors or GM-CSF supported the generation of suppressive CD11b+Ly6G-Ly6C+ MDSC that inhibited T cell proliferation and suppressed tumoricidial activity of neu-specific T cells.
GM-CSF-secreting tumors and GM-CSF administration in vivo (5 μg twice daily for 3 days) have been implicated in increased numbers of MDSC [30]. It was determined that only high-dose GM-CSF had such deleterious effects [20], though differential effects of GM-CSF on progenitor cells versus existing MDSC were not determined. Our data indicated that sorted CD11b-Gr1- progenitor cells were more susceptible to the effects of GM-CSF on the generation and survival of MDSC compared to CD11b+Gr1+ bone marrow cells. This may result from high turnover rates in MDSC such that previously generated MDSC are more prone to apoptosis than newly generated MDSC even in the presence of GM-CSF. Interestingly, the protective effects of GM-CSF on CD11b+Gr1+ cells appeared to be dose-dependent, because culture with GM-CSF at 100 ng/ml caused a significant reduction in apoptosis as compared to MMC secreting lower GM-CSF at the concentration of 336 pg/106 cells/ml. It is therefore conceivable that, since GM-CSF has been found to be secreted in 31% of tested human tumor cell lines [30], administering GM-CSF in the clinical setting may have an additive effect with tumor-secreted GM-CSF and therefore support the generation of MDSC from bone marrow progenitor cells, as well as maintaining existing populations of MDSC and driving the production of highly suppressive subsets of MDSC. However, in tumor-free patients or animals, injection of low-dose GM-CSF in vivo may not produce similar results as MMC supernatant because of short half-life of GM-CSF for producing sustained effects on the bone marrow progenitor cells. In addition, other cytokines in the MMC supernatant may have longer half-life and contribute in the generation of MDSC, as blocking of GM-CSF in MMC supernatant by means of antibody did not completely abrogate generation of MDSC from bone marrow progenitor cells. In fact, intradermal injection of GM-CSF with similar regimen as MMC supernatant did not produce similar results (data now shown). A recent review on the opposing effects of GM-CSF as an adjuvant in cancer patients also suggested activation and expansion of MDSC by endogenous tumor-derived or exogenous GM-CSF [17]. However, a study on patients with high-risk melanoma treated with adjuvant GM-CSF showed that GM-CSF mainly expanded DCs rather than MDSC [31]. Different observations may result from use of different markers used for identification of human MDSC. Although markers of MDSC in humans are not as well characterized as in mouse, we made similar observations in mice when using unfractionated bone marrow cells in culture with GM-CSF that supported both DC and MDSC while its effects on DC expansion was more pronounced. However, by performing suppression assays, we detected suppressive activity of these cells after a 6-day culture with GM-CSF. These data suggest revisiting the use of GM-CSF-expanded DCs for cell-based immunotherapy of breast cancer patients. Our findings are consistent with other reports showing that use of GM-CSF even at low doses, ex vivo, supported MDSC [21].
We showed in Figs. 2 and 3 that MMC supernatant and GM-CSF not only generated MDSC from BM progenitor cells but also supported maintenance of CD11b+Gr1+ cells. These data suggest that an increase in MDSC in the spleen was due both to the generation from the BM progenitor cells and maintenance of the splenic MDSC. An increase in circulating MDSC in the blood also reflected the cells from both the BM and the spleen.
Recent evidence has pointed toward VEGF as an important mediator of myeloid cell accumulation and differentiation through the activation of STAT3 which has been shown to prevent the differentiation of myeloid cells [32]. Likewise, the link between the chemokine MCP-1 (CCL2) and elevated levels of detrimental tumor-associated macrophages in breast cancer patients made it a likely candidate for causing the generation of suppressive macrophage and/or MDSC populations [33, 34]. We have indeed observed a higher percentage of CD11b+Gr1- cells at the tumor site (unpublished observation), an effect that is recapitulated in Fig. 2b with sorted CD11b-Gr1- progenitor cells cultured with MMC in Transwell converting to 43.8% CD11b+Gr1- cells by day 6. However, neither sorted populations of progenitor cells nor MDSC responded to either VEGF or MCP-1 alone. GM-CSF, secreted at an average of 336 pg/106 cells/mL by MMC cells, is therefore one of the main determinant of MDSC generation and maintenance.
It has recently been reported that expression of Ly6G correlates with a granulocytic phenotype, while expression of Ly6C correlates with a monocytic phenotype [2, 22]. We report here that the tumor-bearing FVBN202 transgenic mouse model of HER-2/neu positive mammary carcinoma displays two phenotypes of CD11b+Gr1+ cells. These subsets include CD11b+Ly6G-Ly6C+ suppressive MDSC and CD11b+Ly6G+Ly6C+ non-suppressive cells. These observations on CD11b+Gr1+ subsets are consistent with those reported by Rossner et al. [35] and Lutz et al. [21]. Other reports have differed in regards to which subset exhibits greater expansion and/or suppressive function. Reports by Movahedi et al. using MDSC from mice bearing EG7 thymomas or BW-Sp3 lymphomas and Youn et al. using MDSC from mice with EL-4 thymomas have shown that both subsets of CD11b+Gr1+ cells are suppressive against OT-1 splenocytes stimulated with specific peptide. However, neither group reported suppression against splenocytes stimulated with anti-CD3/CD28 [2, 22]. In contrast, we showed that the CD11b+Ly6G-Ly6C+ MDSC subset from MMC tumor-bearing FVBN202 mice was highly suppressive against anti-CD3/CD28 stimulated splenocytes, while the CD11b+Ly6G+Ly6C+ subset exhibited no suppressive activity. To the best of our knowledge, there is no report thus far on functional analyses of CD11b+Gr1+Ly6C+ subsets, and we identified such non-suppressor subsets within CD11b+Gr1+ cells. These reports suggest that CD11b+Gr1+ subsets and their mechanism of function may vary in different cancers.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 CA104757 grant (M. H. Manjili) and Department of Defense Grant BC083048. We thank Julie Farnsworth for her expertise in cell sorting and immense dedication to furthering the research at our institution. Flow Cytometry supported in part by NIH Grant P30 CA16059. We gratefully acknowledge the support of VCU Massey Cancer Centre and the Commonwealth Foundation for Cancer Research.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-009-0622-8) contains supplementary material, which is available to authorized users.
References
- 1.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res Treat. 2009;114:423–431. doi: 10.1007/s10549-008-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 6.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 7.Valenti R, Huber V, Filipazzi P, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 8.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–252. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezernitchi AV, Vaknin I, Cohen-Daniel L, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 11.Gruber IV, El Yousfi S, Durr-Storzer S, Wallwiener D, Solo-mayer EF, Fehm T. Down-regulation of CD28, TCR-zeta (zeta) and up-regulation of FAS in peripheral cytotoxic T-cells of primary breast cancer patients. Anticancer Res. 2008;28:779–784. [PubMed] [Google Scholar]
- 12.Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding W, Lotze M, Whiteside TL. Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7:947s–957s. [PubMed] [Google Scholar]
- 13.Takahashi A, Kono K, Amemiya H, Iizuka H, Fujii H, Matsumoto Y. Elevated caspase-3 activity in peripheral blood T cells coexists with increased degree of T-cell apoptosis and down-regulation of TCR zeta molecules in patients with gastric cancer. Clin Cancer Res. 2001;7:74–80. [PubMed] [Google Scholar]
- 14.Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 16.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997;73:663–669. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 19.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 20.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 21.Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35(12):3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- 22.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 23.Sawanobori Y, Ueha S, Kurachi M, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 24.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kmieciak M, Morales JK, Morales J, Bolesta E, Grimes M, Manjili MH. Danger signals and nonself entity of tumor antigen are both required for eliciting effective immune responses against HER-2/neu positive mammary carcinoma: implications for vaccine design. Cancer Immunol Immunother. 2008;57:1391–1398. doi: 10.1007/s00262-008-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37:675–685. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58(6):941–953. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180(12):7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 29.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 30.Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 31.Daud AI, Mirza N, Lenox B, et al. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2008;26:3235–3241. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- 32.Nefedova Y, Huang M, Kusmartsev S, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 33.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Yamashiro S, Takeya M, Nishi T, et al. Tumor-derived monocyte chemoattractant protein-1 induces intratumoral infiltration of monocyte-derived macrophage subpopulation in transplanted rat tumors. Am J Pathol. 1994;145:856–867. [PMC free article] [PubMed] [Google Scholar]
- 35.Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.