Abstract
Brain arteriovenous malformations (BAVMs) can cause lethal hemorrhagic stroke and have no effective treatment. The cellular and molecular basis for this disease is largely unknown. We have previously shown that expression of constitutively-active Notch4 receptor in the endothelium elicits and maintains the hallmarks of BAVM in mice, thus establishing a mouse model of the disease. Our work suggested that Notch pathway could be a critical molecular mediator of BAVM pathogenesis. Here, we investigated the hypothesis that upregulated Notch activation contributes to the pathogenesis of human BAVM. We examined expression of the canonical Notch downstream target Hes1 in the endothelium of human BAVMs by immunofluorescence, and showed increased levels relative to either autopsy or surgical biopsy controls. We then analyzed receptor activity using an antibody to the activated form of the Notch1 receptor, and found increased levels of activity. These findings suggest that Notch activation may promote the development and even maintenance of BAVM. We also detected increases in Hes1 and activated Notch1 expression in our mouse model of BAVM induced by constitutively-active Notch4, demonstrating molecular similarity between the mouse model and the human disease. Our work suggests that activation of Notch signaling is an important molecular candidate in BAVM pathogenesis and further validates that our animal model provides a platform to study the progression as well as the regression of the disease.
Keywords: angiogenesis, arteriovenous malformation, arterial-venous differentiation, cell signaling, endothelial cell, Notch signaling, stroke
Brain arteriovenous malformations (BAVM)s are characterized by a nidus of coiled and tortuous and enlarged vascular lesions that shunt blood directly from feeding arteries to veins1. They often rupture, resulting in hemorrhagic stroke in young people, most commonly between 20 – 40 years of age1. BAVMs contribute to half of the hemorrhagic stroke in children2, and 2% of all stroke1. Currently, surgical resection is the primary treatment, but the efficacy is questionable3. Most BAVMs are sporadic, making it difficult to identify the molecular cause by genetic association1. To date, the cellular and molecular basis for BAVM pathogenesis remains largely unknown. This limited knowledge of BAVM etiology has impeded the rational design of molecular interventions.
Fundamentally, AVMs are a disruption of normal arteriovenous (AV) hierarchy, which was historically thought to be governed by hemodynamic forces4. The discovery of genes with arterial or venous specific expression in the developing mouse embryo has catalyzed advances in our understanding of the genetic control of AV specification and the establishment of AV hierarchy5. Notch, a transmembrane receptor first identified in fruit-fly, and involved in cell fate determination and tissue patterning throughout metazoans, has emerged as a critical mediator of AV differentiation6. Studies in zebrafish and mouse development demonstrated that Notch signaling was necessary and sufficient for the expression of arterial-specific genes6, 7. Furthermore, we have demonstrated that endothelial Notch signaling regulates the luminal size of developing mouse arteries by promoting of arterial specification, and increasing the arterial allocation of endothelial cells8. Abnormal Notch signaling induced enlarged AV connections and shunting in both mouse and zebrafish embryo, suggesting a link between AV specification and arteriovenous malformations (AVMs)6, 7.
Among the four mammalian Notch receptors and five ligands, Notch receptors 1 and 4 and their ligands Dll1, Dll4, and Jag1 are preferentially expressed in the arterial and not venous endothelium9. Cell-cell mediated activation of the Notch receptor, by ligand binding to the extracellular domain, results in sequential cleavage events and release of an active intracellular domain (ICD)10. Once cleaved, the ICD translocates to the nucleus, where it must form a complex with the sequence-specific DNA binding protein Rbpj to promote the transcription of downstream genes10. Transcription factors of the Hairy/Enhancer of Split and Hes-related families of proteins, such as Hes1, are canonical target genes, and mediate many of Notch’s downstream functions10. Therefore, Notch-ICD is a constitutively-active mutant. Likewise, the Notch4 mutant that lacks the extracellular domain is constitutively cleaved and constitutively-activated (Notch4*)11. Thus, nuclear localization of cleaved Notch-ICD and expression of Hes1 are features of active Notch signaling.
To investigate whether upregulation of endothelial Notch signaling can disrupt AV hierarchy and cause AVMs in adult mice, we used a tetracycline-regulated transgenic system to express Notch4* transgene specifically in the endothelium of adult mice (Notch4*-Tet), and reported AVMs in liver, skin and uterus12. Expression of the transgene in immature Notch4*-Tet mice, during post-natal brain growth, resulted in hallmarks of BAVM in all mice, including enlarged and tortuous AV connections, shunting and hemorrhagic stroke13. In both adult and immature Notch4*-Tet mice, the disease progression was reversed when the Notch4* transgene was turned off, demonstrating that Notch4* is critical to sustain the disease12, 13. The urgent question that arose out of this fundamental research is whether increased Notch signaling underlies the development and maintenance of human BAVM.
Notch loss-of-function mutations in Jag1, Notch3 and Notch1 are known to cause Alagille syndrome14, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)14, and aortic valve anomalies15 respectively, but it is not clear whether Notch signaling is involved in human BAVM pathogenesis. In this study, we test the hypothesis that Notch signaling is upregulated in human BAVMs by examining Notch signaling activity in the endothelium of human BAVM relative to autopsy and surgical biopsy controls. We demonstrate increased levels of the activated-Notch1 receptor and canonical Notch target Hes1 in BAVM tissue. We reveal similar increases in our Notch4*-Tet mouse model of BAVM-like abnormalities. Our work puts forward the hypothesis that Notch activation causes and maintains human BAVM, and provides molecular validation of our Notch4*-Tet model of BAVM as valuable system to dissect the molecular and cellular basis of BAVM pathogenesis.
Materials and Methods
Clinical samples
The UCSF Committee on Human Research approved the use of human tissue samples for this study. BAVM samples and surgical biopsy controls were obtained by surgical resection and prepared by the UCSF hospital pathology lab. Samples were fixed in 10% neutral buffered formalin, paraffin imbedded, and cut at 5 μm. Control sections were either cerebral cortex or cerebellum. The cerebral cortex control sections were histologically normal temporal lobe from seizure resection cases or cerebral cortex from autopsy brains which were histologically normal in patients without evidence of neoplastic disease. Cerebellar control sections were also from autopsy brains in patients with no evidence of neoplastic disease. In addition to 2 autopsy controls from UCSF, 5 autopsy controls were received as formalin fixed sections from the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24-MH 068855. These autopsy samples were also paraffin imbedded and cut at 5 μm. Human small intestine biopsy was formalin fixed, paraffin imbedded, and sectioned at 5 μm by the UCSF hospital pathology lab. Snap frozen human small intestine tissue samples were provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute, and sectioned at 10 μm.
Mice
Brain tissue was harvested from Notch4*-Tet (Tie2-tTA;TRE-Notch4*) mutants and littermate genetic controls (Tie2-tTA) at post-natal day 2013. To suppress gene expression, Tet sucrose solution (0.5 mg/mL Tet, 50 mg/mL sucrose, Sigma) was administered to pregnant mothers from plugging, and withdrawn from pups at birth as we described13. All animals were treated in accordance with the guidelines of the UCSF Institutional Animal Care and Use Committee.
Preparation of mouse tissue
Endovascular labeling of perfused vessels was performed with FITC-lectin (Vector Labs, Burlingame, CA) as described13. Following perfusion with 1% paraformaldehye (PFA) at 100 mmHg, brain tissue was fixed overnight in 4% PFA, and then dehydrated in 70% ethanol in water, and 100% ethanol in water before xylene treatment and paraffin imbedding. Small and large intestine was fixed overnight in 4% PFA, and paraffin imbedded according the methods used for brain tissue.
Immunofluorescent Staining
For the purposes of comparison, BAVM sections were always stained with control sections. Tissue sections were deparaffinized in xylene and rehydrated. Following antigen retrieval by sodium citrate, the samples were blocked with Avidin/Biotin Blocking Kit (Vector Labs) and then 10% goat-serum and 0.2% Triton-X 100 in PBS. Hes1 stained samples were also treated with 500 U/mL DNAse I for 10 min at 37°C (Promega, Madison, WI) before blocking. Primary treatment was performed overnight at 4C in block. Secondary treatment was performed with biotinylated anti-rabbit or anti-mouse antibodies (Vector Labs) in block. Tertiary treatment was performed with streptavidin-Cy3 in PBS (JacksonImmuno, West Grove, PA). Slides were stored in VectaShield plus DAPI (Vector Labs).
Antibodies
Hes1 antibody was kindly provided by Dr. Nadean Brown (at the Children’s Hospital Medical Center, Cincinnati, Ohio). We also used activated-Notch1 antibody (Val1744, Cell Signaling, Beverly, MA) and human CD31 antibody (JC70/A, DAKO, Carpinteria, CA).
Data Analysis
Stained tissue sections were imaged using a 40X objective on a Zeiss Axiovert fluorescent microscope (Thornwood, NY) with Intelligent Imaging software (Denver, CO). In BAVM sections, the 3 or 4 vessels with the strongest endothelial cell staining were imaged. Large vessels with a thick media, characteristic of arteries, were imaged. In autopsy control and surgical biopsy control samples, 3 or 4 vessels of similar caliber to the BAVM vessels were imaged. CD31 or non-specific IgG was imaged in the same vessel in adjacent sections. The same exposure time was used for all slides stained with a given antibody and the non-specific IgG control. Individual fluorescent channel intensities were exported as 16-bit TIFF files and analyzed by a blinded examiner using ImageJ. The examiner picked the three cells lining each vessel lumen which showed the most intense Hes1 or activated-Notch1 staining by eye. They then circumscribed the DAPI labeled nuclei of these cells, and measured the mean intensity of Hes1 or activated-Notch1 signal in the circled area. Paraffin sections of brain from Notch4*-Tet (Tie2-tTA:TRE-Notch4*) mutants and genetic controls (Tie2-tTA) were processed in the same way. Hes1 and activated-Notch1 staining in the nuclei of endothelial cells of mouse tissue was normalized to non-specific IgG staining. Staining in human tissue is shown as unadjusted mean intensity values.
Statistical Analysis
Each individual case or control, consisting of at least 3 intensity measurements from each of 3 separate vessels, was processed to provide a mean value and standard error of the mean. A two-tailed Wilcoxon rank sum non-parametric test was performed with STATA-IC (College Station, TX) to determine the significance of the difference between these mean values from the BAVM cases relative to those of the autopsy controls. The same analysis was performed to determine the significance of the difference in mean values between BAVM cases and surgical biopsy controls.
Confocal Imaging of crypt cells
For nuclear localization of Hes1 staining in the crypt cells of human small intestine, sections were imaged using a 63X oil-immersion objective on a Zeiss LSM510 microscope. The same exposure settings were used for Hes1 stained samples and IgG control.
Results
Notch signaling pathway activity is increased in the endothelium of human BAVMs
To examine Notch activity in BAVMs we analyzed the expression of the canonical Notch downstream target Hes1 in endothelium of human BAVM by immunofluorescence. We chose Hes1 because it is a direct transcriptional target of activated Notch both in vitro16, 17 and in vivo18, 19, and because Hes1 antibodies have been well-characterized in immunostaining of Notch gain- and loss-of-function tissue20–24. We examined Hes1 staining in a positive control to verify the specificity of the Hes1 antibody in our brain samples, we examined Hes1 staining in a positive control. In the mouse intestine, Hes1 expression has been well characterized in the nuclei of the crypt cells at the base of the villi in the mouse intestine22, 24–26. We found the Hes1 antibody staining faithfully replicated the established pattern in both paraffin fixed (Figure 1a,b) and fresh frozen (data not shown) mouse large and small intestine. To confirm the specificity of the Hes1 antibody in human tissue, we also stained paraffin-fixed (Figure 1e) or fresh frozen (data not shown) human small intestine, and found that the Hes1 antibody stained the nuclei of crypt cells in the human tissue as well (Figure 1e-3). As a negative control, we performed staining of adjacent sections of small intestine with the same concentration of non-specific IgG, and did not observe similar patterns (Figure 1c,d,f).
Figure 1. Hes1 staining of crypt cell nuclei of the gut confirms antibody specificity.
(a–b) Hes1 antibody positive control. Typical staining pattern shown by immunofluorescence in the crypt cells of mouse large (a) and small intestine (b), demonstrates specificity of the Hes1 antibody. High magnification of the crypt (boxed area in b) shows co-localization of Hes1 and DAPI nuclear label (b-1 to b-2). (c–d) Negative control for Hes1 staining, non-specific IgG in adjacent sections. (e) Typical staining pattern shown by immunofluorescence in the crypt cells of human small intestine, demonstrates the specificity of the Hes1 antibody. High magnification confocal imaging of the crypt shows co-localization of Hes1 and DAPI nuclear label (e-1 to e-3). (f) Negative control with non-specific IgG in an adjacent section to (e) does not show the same staining pattern. Scale bars (a&c) 100 μm, (b&d) 50 μm, (e&f) 100 μm.
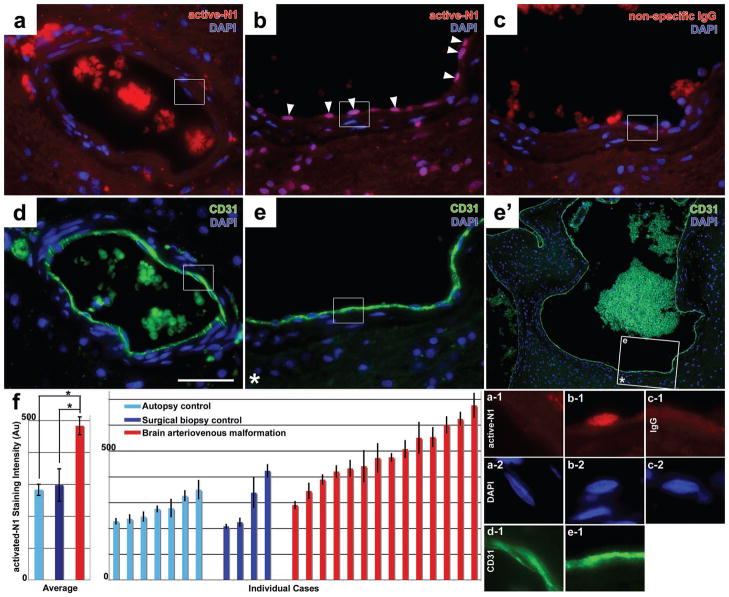
Figure 3. Increased activated-Notch1 staining in the endothelium in human brain arteriovenous malformations (BAVM)s.
(a–b) Immunofluorescent staining for activated-Notch1 in paraffin sections from surgical biopsy controls (a) and human BAVMs (b). Increased activated-Notch1 staining is evident in the endothelium of human BAVMs (arrowheads, b). (c) Negative control for activated-Notch1 antibody staining, non-specific IgG in an adjacent section to (b). (d&e) CD31 stained adjacent sections to (a) and (b). Low-magnification (e′) shows the location of the stained area within a large vascular structure (box with asterisk). (f) High-magnification of the boxed areas shows co-localization of activated-Notch1 staining with the DAPI nuclear label (a-1 to c-1 and a-2 to c-2) in cells lining the CD31 labeled vessels (d-1 & e-1). (f) Quantification of activated-Notch1 staining in BAVM cases and autopsy or surgical biopsy controls (N=14 BAVM samples, N=7 autopsy controls, N=4 biopsy controls; BAVM vs. autopsy controls P=0.0001; BAVM vs. biopsy controls P=0.008). Scale bar is 200 μm. Average represents mean ± SEM of cases. Individual cases represent mean ± SEM of individual endothelial cells in each case.
We then stained paraffin sections of human BAVM by immunofluorescence (Supplemental Table). We detected Hes1 protein in areas of the endothelium of human BAVMs (Figure 2b), where it was found in the nuclei of endothelial cells (ECs), consistent with the nuclear localization of the transcription factor (Figure 2b-1).
Figure 2. Increased Hes1 staining in the endothelium of human brain arteriovenous malformations (BAVM)s.
(a–b) Immunofluorescent staining for Hes1 in paraffin sections from surgical biopsy controls (a) and human BAVMs (b). Increased Hes1 staining is evident in the endothelium of human BAVMs (arrowheads, b). (c) Negative control for Hes1 staining, non-specific IgG in an adjacent section to (b). (d&e) CD31 stained sections adjacent to (a&b), respectively. Low-magnification (e′) shows the location of (e) within a large vascular structure (box with asterisk). High-magnification of the boxed areas in (a–d) shows co-localization of Hes1 staining with the DAPI nuclear label (a-1 to c-1 and a-2 to c-2) in cells lining CD31 labeled vessels (d-1&e-1). (f) Quantification of Hes1 staining in BAVM cases and autopsy or surgical biopsy controls. Scale bars (a–e) 200 μm. In graph (N=12 BAVM samples, N=7 autopsy controls, N=4 biopsy controls; BAVM vs. autopsy controls P=0.0003; BAVM vs. biopsy controls P=0.05). Average represents mean ± SEM of cases. Individual cases represent mean ± SEM of individual endothelial cells in each case.
To determine whether Notch activity was increased in the endothelium of human BAVM, we compared staining intensity to control human brain sections from autopsy (Supplemental Table). We detected little or no Hes1 in autopsy controls, although strong CD31 staining confirmed the endothelium was intact in these samples (data not shown). We quantified this difference by measuring the fluorescent intensity of nuclear Hes1 in the most strongly stained EC nuclei in BAVM samples, and comparing this to controls. We found that the average Hes1 intensity in the strongly stained areas of BAVMs is significantly higher than autopsy controls (Figure 2f; N=12 BAVM samples, N=7 controls; P=0.001). Among individual samples, 9 of 12 BAVM samples had higher mean Hes1 intensity than the most intense surgical biopsy control. As a negative control, we used a non-specific IgG primary antibody on adjacent tissue sections, and did not see a similar staining pattern (Figure 2c). CD31 staining confirmed the integrity of the endothelium in tissue sections from BAVM (Figure 2e), and autopsy controls (data not shown).
Autopsy samples are excellent controls for human BAVM because they are screened for the absence of any detectable brain pathologies, however they are not subject to the same surgical manipulation as BAVM biopsies, and not fixed as quickly. This difference in tissue handling is particularly important because Hes1 can be degraded within hours in some non-endothelial cell types27. Therefore, we also examined levels of Hes1 staining in ECs of surgical brain biopsies from patients without BAVM (Supplemental Table). These surgical biopsies are excellent controls because they are subject to the same manipulation and fixation as BAVM samples. However, brain biopsies are not taken from patients with normal brain function. To minimize the potential effects of brain pathology, we have selected biopsy samples from the least affected patients. The combination of these controls provides the most vigorous baseline expression. We found that, as in the autopsy controls, levels of Hes1 staining in the nuclei of ECs was low or absent (Figure 2a, a-1). We quantified staining intensity in these samples, as we did in the autopsy controls. We found that average Hes1 intensity in BAVM is significantly higher than biopsy controls (Figure 2f; N=12 BAVM samples, N=4 controls; P=0.039). Among individual samples, 9 of 12 BAVM samples had higher mean Hes1 intensity than the most intense surgical biopsy control. As a negative control, we used a non-specific IgG primary antibody on adjacent tissue sections, and did not see a similar staining pattern (Figure 2c). CD31 staining confirmed the integrity of the endothelium in tissue sections from BAVM (Figure 2e) and surgical biopsy controls (Figure 2d).
To examine Notch receptor activation directly, we measured the levels of activated-Notch1 by immunofluorescence in our sample set. We chose to use the activated-Notch1 antibody because it has previously been validated in mouse tissue28, 29, and detects both mouse and human forms of activated-Notch130, since it was raised against a human antigen. As with Hes1, we found that ECs in some of the vessels of BAVM biopsy were strongly positive (Figure 3b). In these ECs, we detected activated-Notch1 in the nucleus, consistent with the nuclear translocation of the activated receptor (Figure 3b-1). As a negative control, we stained adjacent sections of brain with the same concentration of non-specific IgG, and did not observe the same pattern (Figure 3c, c-1). Nuclear staining in autopsy or surgical biopsy controls was not as intense as BAVM samples (Figure 3a, a-1). We quantified differences in activated-Notch1 staining between BAVM samples and autopsy or surgical biopsy controls, and found that average activated-Notch1 intensity in BAVM is significantly higher than autopsy controls (Figure 3f; N=14 BAVM samples, N=7 autopsy controls; P=0.0006), and surgical biopsy controls (Figure 3f; N=4 biopsy controls; P=0.015). Among individual samples, 12 of 14 BAVM samples had higher mean activated-Notch1 intensity than the most intense autopsy control, and 10 of 14 had higher mean activated-Notch1 intensity than the most intense surgical biopsy control.
Notch signaling pathway activity is increased in murine BAVM-like abnormalities caused by endothelial specific expression of Notch4*
We previously reported that endothelial expression of Notch4*, encoding the truncated intracellular domain of Notch4, results in Notch4* activity, indicated by nuclear localization of the intracellular domain of the Notch4 receptor13. However, because Notch4 antibody staining cannot differentiate between the Notch4* transgene and endogenous Notch4, we were unable to determine whether Notch4* expression increased endogenous Notch signaling. We also demonstrated increased expression of Notch downstream genes in whole tissue homogenate from Notch4*-Tet mice12, but the lack of spatial resolution prevented the identification of the cells in which Notch signaling was activated. Here we tested whether endogenous Notch1 activity and expression of Notch downstream target Hes1 is upregulated in the endothelium of Notch4*-Tet mice.
To determine whether expression of Notch4* increased Notch signaling activity in the endothelium during BAVM formation in Notch4*-Tet mice, we examined the expression of the canonical Notch downstream gene Hes1 in paraffin-fixed brain sections from Notch4*-Tet mice with BAVM-like abnormalities. We detected Hes1 in the nuclei of ECs, consistent with the nuclear localization of the transcription factor (Figure 4a, a-1). To control for non-specific staining, we stained an adjacent section with non-specific IgG of the host species used to generate the Hes1 antibody, at the same concentration as the specific primary (Figure 4c, c-1). Nuclear staining in genetic controls was not as intense as in Notch4*-Tet mice (Figure 4b, b-1). Antibody staining intensity was calculated from the ratio of Hes1 staining to non-specific IgG staining. We found an increase in Hes1 signal intensity in the Notch4* expressing mice, relative to controls (Figure 4d; N=5 controls, N=3 Notch4*-Tet mutants; P=0.0253).
Figure 4. Increased Hes1 staining in the endothelium of the Notch4*-Tet mouse model of brain arteriovenous malformation (BAVM)-like lesions.
(a–b) Immunofluorescent staining for Hes1 in paraffin sections from Notch4*-Tet mice (a) and genetic controls (b). Increased Hes1 staining is evident in the endothelium of FITC-lectin perfused vessels in Notch4*-Tet mice. (c) Negative control for Hes1 staining, non-specific IgG in an adjacent section to (a). High-magnification of the boxed areas shows co-localization of Hes1 staining with the DAPI nuclear label (a-1 to c-1 and a-2 to c-2) in cells lining the lectin perfused vessel lumen (a-3 to c-3). (d) Quantification of Hes1 staining relative to non-specific IgG staining in Notch4* expressing mutant mice and controls (N=5 genetic controls, N=3 Notch4*-Tet mutants, values represent mean ± SEM, P=0.0004). Scale bars (a–c) 200 μm.
To determine whether expression of Notch4* increased levels of endogenous Notch signaling, we examined the levels of activated-Notch1 in the endothelium in paraffin-fixed brain sections from Notch4*-Tet mice using an antibody to the activated form of Notch1. We detected activated-Notch1 in the nuclei of ECs, consistent with the nuclear translocation of the activated-Notch1 receptor (Figure 5a, a-1). We controlled for non-specific staining and quantified staining intensity as we had for Hes1 (Figure 5c). Nuclear staining in genetic controls was not as intense as in Notch4*-Tet mice (Figure 5b, b-1). We found increased levels of activated-Notch1 signal intensity in Notch4*-Tet mice, relative to controls (Figure 5d; N=5 controls, N=3 Notch4*-Tet mutants; P=0.0253).
Figure 5. Increased activated-Notch1 staining in the endothelium of the Notch4*-Tet mouse model of brain arteriovenous malformation (BAVM)-like lesions.
(a–c) Immunofluorescent staining for activated-Notch1 in paraffin sections from Notch4*-Tet mice (a) and genetic controls (b). Increased activated-Notch1 staining is evident in the endothelium of FITC-lectin perfused vessels in Notch4*-Tet mice (a). (c) Negative control for activated-Notch1 staining, non-specific IgG in an adjacent section to (a). High-magnification of the boxed areas shows co-localization of activated-Notch1 staining with the DAPI nuclear label (a-1 to c-1 and a-2 to c-2) in cells lining the lectin perfused vessel lumen (a-3 to c-3). (d) Quantification of activated-Notch1 staining relative to non-specific IgG staining in Notch4* expressing mutant mice and controls. (N=5 genetic controls, N=3 Notch4*-Tet mutants, values represent mean ± SEM, P=0.004). Scale bar is 100 μm.
In summary, we show that Notch signaling is increased in human BAVM, using the same tissue preparation, antibodies and quantification that we use to show increased Notch signaling in our Notch4*-Tet transgenic mouse model of the disease.
Discussion
Our study demonstrates that Notch activity is increased in human BAVMs, supporting the hypothesis that Notch activation causes and maintains human BAVM. We found increased levels of the canonical Notch downstream gene Hes1 in the endothelium of human BAVMs relative to levels in autopsy or surgical biopsy controls, as well as increased levels of activated-Notch1 receptor. Furthermore, we demonstrate molecular similarity between the BAVM-like abnormalities of our Notch4*-Tet mouse model and human BAVM, providing molecular validation for this model of the human disease.
Notch signaling is increased in the endothelium of human BAVM
Hes1 is a canonical Notch downstream gene, and increased endothelial Hes1 expression indicates increased endothelial Notch pathway activity. Hes1 is a direct transcriptional target of Notch activity16. In the vascular endothelium, Hes1 expression is regulated by Notch activity. Transfection of human umbilical vein endothelial cells with Notch4* resulted in ~6 fold increase in Hes1 expression by quantitative PCR17. Hes1 expression is also increased by induction of endogenous endothelial Notch signaling in vitro31 and general Notch signaling in vivo32. Conversely, interference with endogenous endothelial Notch signaling decreases Hes1 expression in vitro31 and in vivo18, 19. Therefore, increased Hes1 expression in the endothelium of human BAVMs indicates increased Notch signaling activity.
The specificity of Hes1 staining in BAVM is supported by extensive evaluation in mouse and human tissue. We systematically tested five Hes1 antibodies on frozen and paraffin-fixed tissue, and found that only one gave the expected staining pattern in the positive control, intestinal crypt cells in mouse and human tissue. We chose this positive control because the distinctive crypt-specific Hes1 expression pattern has been repeatedly demonstrated at both the protein and RNA level by several investigators22, 24–26. Demonstrating its dependence on Notch signaling, Hes1 protein expression in the crypt cells is lost when Notch receptors are deleted in the crypt cells, or the gamma-secretase activity required for Notch receptor activation is pharmacologically blocked22, 24. The specificity of staining with this particular Hes1 antibody has been reported in both frozen and paraffin-fixed mouse tissue20, 21, 33. Although the mouse-derived antigen used to generate the antibody is 90% similar to human Hes120, confirmation of Hes1 staining in human crypt cells was a critical step to verify that the antibody detected Hes1 in human as well as mouse tissue. Our finding of increased Hes1 expression in the endothelium of human BAVM is strongly supported by the validation of antibody specificity in positive controls.
Notch1 is a critically important receptor in the endothelium, and the upregulation of Notch1 activity we have demonstrated in human BAVM may be involved in the development and progression of the disease. Notch1 is necessary for vascular development, and subtle changes in Notch1 levels in humans and mice can cause severe vascular defects6. In humans, inheritance of a single mutated loss-of-function allele of Notch1 can cause aortic valve disease and increase the risk of thoracic aorta anuerysm15, 34. Studies in mice demonstrated the importance of endothelial Notch1 signaling, since endothelial specific deletion of just one Notch1 allele significantly impaired blood flow recovery in the hindlimb after the femoral artery was occluded31, and deletion of both alleles is embryonic lethal8, 35. At a cellular level, Notch1 deletion in endothelial cells increases their contribution to angiogenic sprouts rather than retention in existing vessels36. Expression of the constitutively-active intracellular domain of the Notch1 receptor (Notch1-ICD) specifically in the endothelium is also embryonic lethal37(our own unpublished data). We found that even at an adult stage, expression of Notch1* in the endothelium was sufficient to cause vascular malformations12, demonstrating a requirement for Notch signaling homeostasis in adult mice. Therefore, the requirement for tightly regulated Notch1 signaling in the endothelium suggests that the increased activity we observed may disrupt vascular organization.
The cause for upregulated Notch activity in the endothelial cells of BAVM is not yet apparent. Elevated activity may be a secondary effect of BAVM formation. For example, the endothelial cells of BAVMs are exposed to a massive increase in blood flow, which has been shown to increase the expression of Notch ligand Jag1, Notch4 receptor, and downstream genes Hey1 and ephrin-B2 in endothelial cells in vitro38, 39. However, our animal data have demonstrated that increased Notch4 signaling can initiate BAVM-like pathology in mice13, suggesting that the elevated Notch activity may be a causal molecular lesion. Therefore, increased Notch activity seen in human BAVM may not only be a secondary effect of increased blood flow, but may also be an initial molecular pathology.
The increase in Notch activity in the endothelium of human BAVM appears subtle, but this small change is likely sufficient to cause biological effects. Haploinsufficiencies of Notch115, 34 or its ligands Dll135, Dll418, 40, 41 and Jag142 impair vascular function in mice and humans. Of the ligands, Dll4 may be the most critical, since haploinsufficiency of this gene is embryonic lethal18, 40, 41. Such sensitivity in the endothelium to receptor or ligand dosage is rare; Vascular endothelial growth factor A (Vegfa) is the only other protein known to exert such profound vascular effects as a result of haploinsufficiency43, 44. Other biological systems have shown a similar threshold requirement for Notch activity. For example, in left-right differentiation in the chick, a transient increase in extracellular calcium slightly increases Notch receptor activation on the left-side of the developing embryo45, resulting in the subsequent establishment of asymmetry. Similarly, in neuronal development in the fly, small changes in Notch activity between daughter cells are necessary to establish asymmetric fates, and mutations which cause either a general increase or decrease in Notch signaling result in loss of asymmetry46. Therefore, even small changes in the level of Notch activity have the potential to cause severe vascular effects.
Notch activation may be a key regulator of BAVM development
Our study demonstrates increased Notch pathway activation in the endothelium of human BAVM. Until now, the involvement of Notch signaling in the development of human BAVM has not been reported. Genetic understanding of human BAVM has been limited by the sporadic nature of the disease. An exception is the development of BAVM in 10–20% of patients suffering from the autosomal dominant disease Hereditary Hemorrhagic Telangectasia (HHT)47. HHT causes widespread AVMs through many tissues, including the brain, and has been linked to mutations in the TGF-β receptors Alk1 (ACVRL1) and endoglin (ENG)48. Although HHT is implicated in only 2% of all BAVM49, it has been the most studied pathway in human BAVM due to this genetic association. In mouse models, homozygous mutations in these receptors are embryonic-lethal, and heterozygous mutations result in vessel enlargement and hemorrhage, although high-flow arteriovenous shunting characteristic of BAVMs has not been reported50–55. The low penetrence and focal development of BAVMs in HHT suggests that other causes, potentially other signaling pathways, cooperate with the TGF-β mutations to cause this pattern of BAVM development.
Other endothelial signaling pathways appear to be upregulated in human BAVM samples, although animal studies have not demonstrated that these changes induce BAVM. VEGFA expression in the BAVM nidus has been observed at both the RNA and protein level54–58, and is increased at the RNA level in whole tissue homogenate of the BAVM nidus relative to control brain biopsies57. However, while VEGFA expression in the brain results in increased angiogenesis, development of AV shunting has not been reported58–62. Similarly, increased expression of angiopoietin-2 (ANGPT2) has been reported at the RNA and protein level in the BAVM nidus57, 63, but expression of ANGPT2 in the adult brain has not been reported to result in AV shunting60. Gain- and loss-of-function mutations in ANGPT2’s endothelial receptor, TIE2 (TEK), have been associated with venous malformations, but not AVMs64. Integrin alpha V (ITGAV) was also upregulated in the endothelium and smooth muscle-cells of BAVM57. However, no gain-of-function animal studies have been reported. Complete endothelial deletion of Itgav causes no detectable cerebrovascular defects65, although deletion of Itgav in neuronal cells results in dilation of blood vessels and hemorrhage65, suggesting a role in vascular stability through paracrine effects, but not cell autonomous vascular effects. Therefore, the molecular basis of BAVM pathogenesis remains largely unknown.
Increased endothelial Notch signaling is sufficient to induce vascular abnormalities with the hallmarks of BAVM in Notch4*-Tet mice, and an increase in both Hes1 and activated-Notch1 protein in Notch4*-Tet mice demonstrates similarity at the molecular level to the human disease. We have reported that expression of constitutively-active Notch4 receptor in the endothelium of our Notch4*-Tet mice results in enlarged and tortuous BAVM-like vascular abnormalities, shunting of blood, and hemorrhagic stroke13. Here, we demonstrate that Notch activity is increased in the endothelium of human BAVM, as it is in Notch4*-Tet mice. We found similar increases in Hes1 and activated-Notch1 in human BAVM and BAVM-like abnormalities in Notch4*-Tet mice. One key difference between human BAVM and the mouse model is that human BAVMs are focal1, whereas the BAVM-like abnormalities in Notch4*-Tet mice are pervasive13. This is likely a consequence of the transgenic expression of constitutively-active Notch4 throughout the endothelium in the mouse model. Therefore, increased expression of Hes1 and activated-Notch1 demonstrates molecular similarity between BAVM-like abnormalities in Notch4*-Tet mouse model, caused by increased endothelial Notch activity, and human BAVMs.
It is commonly thought that, once they develop, BAVMs not regress, but remain as a constant threat of hemorrhagic stroke66. Our animal study suggests the tantalizing possibility that, in animals at least, BAVM-like lesions are reversible13. The dependence of BAVMs on the activity of molecular signaling pathways is a novel concept, but we have also shown that AVMs that form in the livers of Notch4*-Tet mice regress completely after the Notch4* transgene is suppressed, demonstrating that endogenous machinery exists for the reversal of AVMs once the causative molecular lesion is removed12. Our human data does not demonstrate that increased Notch activity promotes the development and growth of BAVM in humans. Nonetheless, we found that Notch activity is upregulated in large portion of human BAVMs, after their initiation, suggesting that increased Notch activation may be a potential molecular lesion in human BAVM pathogenesis.
In conclusion, this study demonstrates increased activation of endothelial Notch signaling, which we have shown causes BAVM-like abnormalities in mice, in human BAVM. It suggests that activation of Notch signaling is an important molecular candidate in BAVM pathogenesis, and further validates that our animal model provides a platform to study BAVM progression and regression. These findings open a new area of research to advance the knowledge and treatment of this devastating disease.
Supplementary Material
Acknowledgments
We thank Michael Lawton, M.D. for neurosurgical perspective, Nathanael Hevelone for statistical advice, members of our laboratory for helpful discussions, and Natasha Cuk for technical support in the early phase of the project. This work was supported by the Pacific Vascular Research Foundation, the Mildred V. Strouss Trust, and the Frank A. Campini Foundation, to R.A.W. and American Heart Association Predoctoral Fellowship to P.A.M.
Abbreviations
- AV
Arteriovenous
- AVM
Arteriovenous Malformation
- BAVM
Brain Arteriovenous Malformations
- EC
Endothelial Cell
- Notch-ICD
Notch Intracellular Domain
- Notch4*
Constitutively-active Notch4, also known as int3
- Notch4*-Tet
Tie2-tTA; TRE-Notch4* Mutants
- HHT
Hereditary Hemorrhagic Telangiectasia
References
- 1.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704–12. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25(6):416–21. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 3.Stapf C, Mohr JP, Choi JH, Hartmann A, Mast H. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol. 2006;19(1):63–8. doi: 10.1097/01.wco.0000200546.14668.78. [DOI] [PubMed] [Google Scholar]
- 4.Thoma R. Untersuchungen über die Histogenese und Histomechanik des Gefässsytems. Stuttgart: Ferdinand Enke; 1893. [Google Scholar]
- 5.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 6.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134(15):2709–18. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 7.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128(19):3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 8.Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development. 2008;135(22):3755–64. doi: 10.1242/dev.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100(11):1556–68. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 10.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 11.Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci U S A. 1996;93(4):1683–8. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A. 2005;102(28):9884–9. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A. 2008;105(31):10901–6. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–24. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 15.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–4. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 16.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377(6547):355–8. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 17.Shawber CJ, Das I, Francisco E, Kitajewski J. Notch signaling in primary endothelial cells. Ann N Y Acad Sci. 2003;995:162–70. doi: 10.1111/j.1749-6632.2003.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 18.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101(45):15949–54. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dou GR, Wang YC, Hu XB, Hou LH, Wang CM, Xu JF, et al. RBP-J, the transcription factor downstream of Notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. Faseb J. 2008;22(5):1606–17. doi: 10.1096/fj.07-9998com. [DOI] [PubMed] [Google Scholar]
- 20.Lee HY, Wroblewski E, Philips GT, Stair CN, Conley K, Reedy M, et al. Multiple requirements for Hes 1 during early eye formation. Dev Biol. 2005;284(2):464–78. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20(21):3022–35. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444(7122):1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 23.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 24.Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9(4):377–83. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24(1):36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 26.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 27.Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, et al. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298(5594):840–3. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- 28.Geffers I, Serth K, Chapman G, Jaekel R, Schuster-Gossler K, Cordes R, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol. 2007;178(3):465–76. doi: 10.1083/jcb.200702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MH, Kopan R. Long-range, nonautonomous effects of activated Notch1 on tissue homeostasis in the nail. Dev Biol. 2003;263(2):343–59. doi: 10.1016/j.ydbio.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14(3):290–8. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 31.Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100(1):70–8. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetzlaff MT, Yu W, Li M, Zhang P, Finegold M, Mahon K, et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci U S A. 2004;101(10):3338–45. doi: 10.1073/pnas.0307875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaron O, Farhy C, Marquardt T, Applebury M, Ashery-Padan R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development. 2006;133(7):1367–78. doi: 10.1242/dev.02311. [DOI] [PubMed] [Google Scholar]
- 34.McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134(2):290–6. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 35.Limbourg A, Ploom M, Elligsen D, Sorensen I, Ziegelhoeffer T, Gossler A, et al. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100(3):363–71. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 36.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–80. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesh DA, Park KS, Harrington A, Miceli-Libby L, Yoon JK, Liaw L. Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations. Circ Res. 2008;103(4):423–31. doi: 10.1161/CIRCRESAHA.108.177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, et al. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7(1):55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 39.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101(41):14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18(20):2469–73. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18(20):2474–8. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16(3):235–42. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 44.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 45.Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, et al. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427(6970):121–8. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- 46.Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17(1):27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 47.Begbie ME, Wallace GM, Shovlin CL. Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): a view from the 21st century. Postgrad Med J. 2003;79(927):18–24. doi: 10.1136/pmj.79.927.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006;43(2):97–110. doi: 10.1136/jmg.2005.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, Faughnan ME. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2000;21(6):1016–20. [PMC free article] [PubMed] [Google Scholar]
- 50.Bourdeau A, Faughnan ME, McDonald ML, Paterson AD, Wanless IR, Letarte M. Potential role of modifier genes influencing transforming growth factor-beta1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am J Pathol. 2001;158(6):2011–20. doi: 10.1016/s0002-9440(10)64673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217(1):42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 52.Torsney E, Charlton R, Diamond AG, Burn J, Soames JV, Arthur HM. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation. 2003;107(12):1653–7. doi: 10.1161/01.CIR.0000058170.92267.00. [DOI] [PubMed] [Google Scholar]
- 53.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–7. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 54.Bourdeau A, Faughnan ME, Letarte M. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med. 2000;10(7):279–85. doi: 10.1016/s1050-1738(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 55.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12(5):473–82. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 56.Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, et al. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156(3):911–23. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly T, Jr, Stewart CL, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54(2):410–23. doi: 10.1227/01.neu.0000103421.35266.71. discussion 423–5. [DOI] [PubMed] [Google Scholar]
- 58.Yang GY, Xu B, Hashimoto T, Huey M, Chaly T, Jr, Wen R, et al. Induction of focal angiogenesis through adenoviral vector mediated vascular endothelial cell growth factor gene transfer in the mature mouse brain. Angiogenesis. 2003;6(2):151–8. doi: 10.1023/B:AGEN.0000011803.56605.78. [DOI] [PubMed] [Google Scholar]
- 59.Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, et al. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24(2):237–44. doi: 10.1097/01.WCB.0000107730.66603.51. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Y, Lee C, Shen F, Du R, Young WL, Yang GY. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36(7):1533–7. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Shwe Y, Du R, Chen Y, Shen FX, Young WL, et al. Effects of angiopoietin-1 on vascular endothelial growth factor-induced angiogenesis in the mouse brain. Acta Neurochir Suppl. 2006;96:438–43. doi: 10.1007/3-211-30714-1_90. [DOI] [PubMed] [Google Scholar]
- 62.Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295(6):H2250–6. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto T, Lam T, Boudreau NJ, Bollen AW, Lawton MT, Young WL. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res. 2001;89(2):111–3. doi: 10.1161/hh1401.094281. [DOI] [PubMed] [Google Scholar]
- 64.Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41(1):118–24. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132(1):165–76. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 66.ApSimon HT, Reef H, Phadke RV, Popovic EA. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33(12):2794–800. doi: 10.1161/01.str.0000043674.99741.9b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.